Summary
Monocytes and neutrophils are widely distributed throughout the body and play essential roles in health and disease. Here, we present a detailed protocol to isolate polymorphonuclear neutrophils and monocytes from a single sample of human peripheral blood. We have optimized several aspects of the procedure, including the density gradient, timing of each cell processing step, and the buffer/media conditions to preserve cell viability for subsequent functional assays. This protocol is reproducible and can be scaled as required for downstream applications.
For complete details on the use and execution of this protocol, please refer to Cui et al. (2021).
Subject areas: Cell Biology, Cell isolation, Flow Cytometry/Mass Cytometry, Immunology
Graphical abstract

Highlights
-
•
A protocol for isolating neutrophils and monocytes from a single human blood sample
-
•
Optimizes key processing steps to ensure high purity, yield, and viability of cells
-
•
Scalable for a wide range of downstream applications
Monocytes and neutrophils are widely distributed throughout the body and play essential roles in health and disease. Here, we present a detailed protocol to isolate polymorphonuclear neutrophils and monocytes from a single sample of human peripheral blood. We have optimized several aspects of the procedure, including the density gradient, timing of each cell processing step, and the buffer/media conditions to preserve cell viability for subsequent functional assays. This protocol is reproducible and can be scaled as required for downstream applications.
Before you begin
This protocol describes the isolation of monocytes and polymorphonuclear neutrophils (PMNs) from human peripheral blood. The procedure is scalable as required by downstream applications. All reagents should be sterile. It is desirable to perform all steps in a tissue culture hood, particularly if purified cells are to be cultured. If this is not possible, we have found that a clean benchtop will also suffice.
Human studies used in this study were approved by the Institutional Review Board at the University of Chicago (IRB160321).
Equipment and reagent preparation
Timing: 2 min
-
1.
Set the centrifuge to 18°C–22°C.
-
2.
Make sure all reagents, including Ficoll-Paque PLUS, red blood cell (RBC) lysis buffer, HBSS/EDTA, and PBS/EDTA, are at 18°C–22°C.
Note: The efficiency of density gradient separation is greatly affected by temperature. Because this protocol relies on Ficoll-Paque PLUS to separate monocytes and PMNs, the centrifuge and reagents should be equilibrated to 18°C–22°C for optimal separation. Below this optimal temperature, the density of Ficoll-Paque PLUS increases, resulting in fewer RBCs and PMNs in the Ficoll-Paque PLUS layer and higher contamination in the mononuclear layer. Above this optimal temperature, the density of Ficoll-Paque PLUS decreases, resulting in contamination of mononuclear cells in the Ficoll-Paque PLUS layer.
Collection of human peripheral blood
Timing: 10 min
Blood collection from healthy donors and/or patients should be performed on the same day as the isolation by a trained phlebotomist under IRB approval. Exclusion criteria for donors should be considered for each specific study.
-
3.Collect human peripheral blood samples from donors.
-
a.Draw blood into Vacutainer EDTA Tubes (purple cap tube).
-
b.Carefully invert tubes several times immediately upon collection. This step mixes blood with anticoagulant to prevent clot formation.
-
c.Store individual tubes of blood horizontally with gentle shaking on a rack at 18°C–22°C prior to processing.
-
a.
Note: Vacutainer EDTA Tubes are available in variable volumes. The maximum volume of blood collected is lower than the indicated limit. For example, a 10 mL tube holds a maximum of ∼8 mL of blood. Variability in blood volume collected/tube does not compromise isolation.
Note: Immediate isolation of fresh blood produces optimal results. Storage of blood prior to isolation is possible. If >1 h delay between blood draw and separation, tubes should be kept on ice and warmed to 18°C–22°C before separation. Longer delays (24 h) may compromise PMN and monocyte viability.
Preparation of blood and Ficoll-Paque PLUS density gradient
Timing: 10 min
-
4.Prepare LeucosepTM tubes for density gradient centrifugation.
-
a.Combine blood and measure total blood volume.
-
b.Calculate the number of LeucosepTM tubes required by dividing the total blood volume by 15 mL (e.g., 90 mL/15 mL = 6 tubes).Note: LeucosepTM contains a porous barrier, which allows for pre-diluted blood to be transferred without disturbing the underlying Ficoll-Paque PLUS layer. This provides an advantage over a regular Ficoll separation in a 50 mL conical tube.Note: The optimal blood volume per LeucosepTM tube is 15 mL. A lower limit of 10 mL/tube is required to ensure that the PBMC layer forms above the porous barrier post-centrifugation (Figures 1A and 2B). An upper limit of 18 mL/tube is required to ensure that blood is adequately diluted in HBSS/EDTA (see step 5 below). Accordingly, the number of LeucosepTM tubes required is flexible if the volume of blood/tube falls within a range of 10–18 mL/tube.
-
c.Add 15 mL of Ficoll-Paque PLUS per tube (Figure 1B).
-
d.Centrifuge at 1000×g for 30 s.
 CRITICAL: Ensure that Ficoll-Paque PLUS is completely below the porous barrier after centrifugation (Figure 1C).Note: The density of Ficoll-Paque PLUS is 1.077 g/mL, which is optimal for isolating mononuclear cells from human blood. Ficoll-Paque PREMIUM, which has density 1.073 g/mL and ideal for isolating lower-density human mononuclear cells, can be used as alternative. LymphoprepTM, which has density of 1.077 g/mL can also be used as direct substitute.
CRITICAL: Ensure that Ficoll-Paque PLUS is completely below the porous barrier after centrifugation (Figure 1C).Note: The density of Ficoll-Paque PLUS is 1.077 g/mL, which is optimal for isolating mononuclear cells from human blood. Ficoll-Paque PREMIUM, which has density 1.073 g/mL and ideal for isolating lower-density human mononuclear cells, can be used as alternative. LymphoprepTM, which has density of 1.077 g/mL can also be used as direct substitute.
-
a.
-
5.Dilute blood with HBSS/EDTA to a final volume of 30 mL per LeucosepTM tube. Blood: HBSS/EDTA ratio of 1:1 is recommended. However, other ratios are acceptable, according to the following limits:
-
a.15 mL of blood (ideal) is mixed with 15 mL of HBSS/EDTA per tube.
-
b.10 mL of blood (lower limit) is mixed with 20 mL of HBSS/EDTA per tube.
-
c.18 mL of blood (upper limit) is mixed with 12 mL of HBSS/EDTA per tube.
-
a.
Note: We refer to this solution as “pre-diluted” blood.
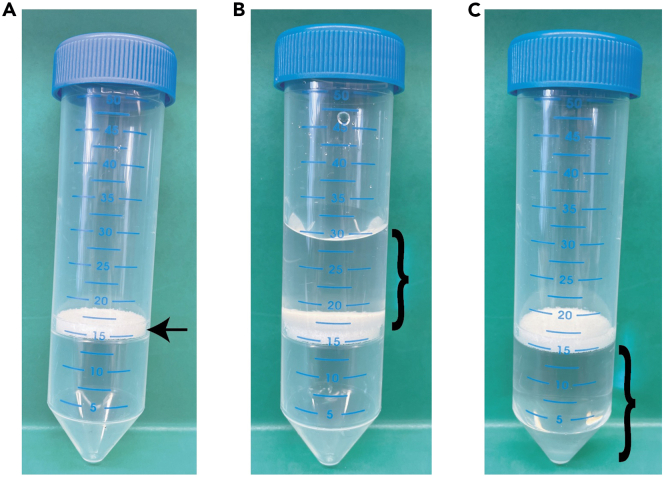
Figure 1.
Preparation of LeucosepTM tubes with Ficoll-Paque PLUS
(A) Photograph of the LeucosepTM tube. Arrow indicates the porous barrier.
(B) Photograph of LeucosepTM tube after adding Ficoll-Paque PLUS on top of the porous barrier.
(C) Photograph of LeucosepTM tube post-centrifugation with Ficoll-Paque PLUS below the porous barrier. }, denotes Ficoll-Paque PLUS.
Figure 2.
Blood separation with Ficoll-Plaque PLUS gradients
(A) Photograph of the LeucosepTM tube filled with the diluted blood sample.
(B) Photograph of a successful separation post-centrifugation with the following layers: (1) plasma, (2) PBMCs, (3) Ficoll-paque PLUS, (4) RBCs and granulocytes mainly comprising PMNs.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-human CD10, PerCP-Cy5.5 (1:100 dilution, for 0.2 million cells) | BD Biosciences | Cat# 563508; RRID: AB_2716629 |
| Mouse anti-human CD16, PE (1:100 dilution, for 0.2 million cells) | Thermo Fisher Scientific | Cat# 12-0168-42; RRID: AB_11043421 |
| Mouse anti-human CD14, APC-Cy7 (1:100 dilution, for 0.2 million cells) | BD Biosciences | Cat# 557831; RRID: AB_396889 |
| Rat anti-human CD11b, FITC (1:100 dilution, for 0.2 million cells) | BD Biosciences | Cat# 562793; RRID: AB_2737798 |
| HBSS without calcium, magnesium, phenol red | Hyclone | Cat# SH30588.02 |
| PBS (1×), pH 7.4 | Thermo Fisher Scientific | Cat# 21-040-CM |
| PBS (10×), pH 7.4 | Thermo Fisher Scientific | Cat# 70011-044 |
| EDTA (0.5 M), pH 8.0, RNase free | Thermo Fisher Scientific | Cat# AM9261 |
| Bovine serum albumin (BSA) | Millipore Sigma | Cat# 9430 |
| Biological samples | ||
| Human peripheral blood | The University of Chicago | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Flow stain buffer | BD Biosciences | Cat # 554657 |
| Ficoll-Paque PLUS | Millipore Sigma | Cat# GE17-1440-03 |
| Calcein Blue AM Viability Dye | Thermo Fisher Scientific | Cat# 65-0855-39 |
| NH4Cl | Millipore Sigma | Cat# A9434 |
| NaHCO3 | Millipore Sigma | Cat# S5761 |
| Critical commercial assays | ||
| CD14 MicroBeads | Miltenyi Biotec | Cat# 130-050-201 |
| Software and algorithms | ||
| FlowJo v.10.4.1 | FlowJo LLC | https://www.flowjo.com/ |
| Other | ||
| BD Vacutainer® EDTA blood collection tubes | Pipette | Cat# 366643 |
| LeucosepTM tube | Greiner Bio-One | Cat# 227290 |
| Ficoll-paque PLUS | Millipore Sigma | Cat# GE17-1440-03 |
| LS column | Miltenyi Biotec | Cat# 130-042-401 |
| QuadroMACS Separator | Miltenyi Biotec | Cat# 130-090-976 |
| Flow tubes | BD Biosciences | Cat# 352008 |
| MACS MultiStand (magnetic stand) | Miltenyi Biotec | Cat# 130-042-303 |
| 2 mL transfer pipette | Fisher | Cat# 13-711-7M |
| 2 mL serological pipette | Millipore Sigma | Cat# SIAL1486-1000EA |
| Polystyrene storage bottles | Thermo Fisher Scientific | Cat# FB12566515 |
| Disposable PES bottle top | Thermo Fisher Scientific | Cat# FB12566510 |
| BD FACS Canto II | BD Biosciences | N/A |
Materials and equipment
10× Red blood cell (RBC) lysis buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| NH4Cl | 1.55 M | 41.3 g |
| NaHCO3 | 120 mM | 5 g |
| 0.5 M EDTA | 1 mM | 1 mL |
| Total | – | 500 mL |
Sterile filter and store at 4°C for up to 2 years.
Note: To make 1 mL of RBC lysis buffer, dilute 10× RBC lysis buffer with ddH20. Store 1× RBC lysis buffer at 4°C.
HBSS/EDTA
| Reagent | Final concentration | Amount |
|---|---|---|
| 1×HBSS | – | 996 mL |
| 0.5 M EDTA | 2 mM | 4 mL |
| Total | – | 1000 mL |
Sterile filter and store at 18°C–22°C until the expiration date of HBSS.
Note: HBSS must not contain magnesium and calcium, which can activate PMNs. Phenol red-free HBSS is preferred.
PBS/EDTA
| Reagent | Final concentration | Amount |
|---|---|---|
| 1×PBS | – | 996 mL |
| 0.5M EDTA | 2 mM | 4 mL |
| Total | – | 1000 mL |
Sterile filter and store at 18°C–22°C until the expiration date of PBS.
Isolation buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 1×PBS | – | 996 mL |
| 0.5M EDTA | 2 mM | 4 mL |
| BSA | 0.1% | 1 g |
| Total | – | 1000 mL |
Sterile filter and store at 4°C for up to 1 month.
Step-by-step method details
Separation of peripheral blood mononuclear cells (PBMCs) and PMNs by Ficoll-Paque PLUS density gradient
Timing: For steps 1–3: ∼1 h
This section uses density gradient centrifugation to separate PBMCs and PMNs for further purification.
-
1.
Gently layer 30 mL of pre-diluted blood into each Ficoll-Paque PLUS-loaded LeucosepTM tube (Figure 2A).
Note: The porous barrier allows for pre-diluted blood to be transferred without disturbing the underlying Ficoll-Paque PLUS layer.
-
2.
Centrifuge tubes at 800×g for 15 min at 18°C–22°C using a swinging bucket rotor with the brake set to “off” or deceleration set to 0.
Note: Turning the brake off helps to maintain the density gradient and avoids the mixing of cell layers.
Note: Depending on the centrifuge, deceleration without the brake can take up to 1 h.
-
3.
Remove tubes from the centrifuge gently so as not to disturb the layers (Figure 2B).
Note: At this point, the protocol diverges. For human monocyte isolation, please refer to steps 4–27. For human PMN isolation, please refer to steps 28–45.
Isolation of the PBMC layer and platelet removal
Timing: For steps 4–16: ∼1 h
This section describes an efficient way to purify viable human monocytes, which can be studied directly or further differentiated into human monocyte-derived macrophages (HMDMs; please refer to Kratz et al. (2014).
Note: Keep the isolation temperature at 18°C–22°C.
-
4.
Carefully aspirate and discard the top plasma layer until ∼5–10 mm above the interphase of the PBMC layer (left, Figure 3A). Be careful not to disturb the PBMC layer.
-
5.
Use a transfer pipette to harvest the PBMC layer. Transfer it into a new 50 mL conical tube (right, Figure 3A).
Note: A transfer pipette is desirable over pouring the layer out for two reasons. First, we have found that the dense PBMC layer can stick to the tube wall. A transfer pipette is better able to dislodge and recover all PBMCs. Second, if one intends to isolate neutrophils from the lower layer (see below), pouring may disrupt this lower layer and require additional centrifugation.
CRITICAL: The PBMC layer from each LeucosepTM tube should not be combined. They should each be transferred into separate 50 mL conical tubes. For example, if you started with 45 mL of blood, you would have three LeucosepTM tubes and three PBMC layers, which should be transferred to three 50 mL conical tubes. If all PMBC layers are combined into one 50 mL conical tube, three washes will not effectively minimize platelet contamination (see steps 6–8).
-
6.
Resuspend cells in a small volume first (1 mL) and then top up to 50 mL with PBS/EDTA for each wash.
-
7.
Centrifuge at 300×g at 18°C–22°C for 8 min.
-
8.
Gently aspirate out the supernatant and repeat steps 6–8 at least twice, or until the supernatant is clear (Figure 3B).
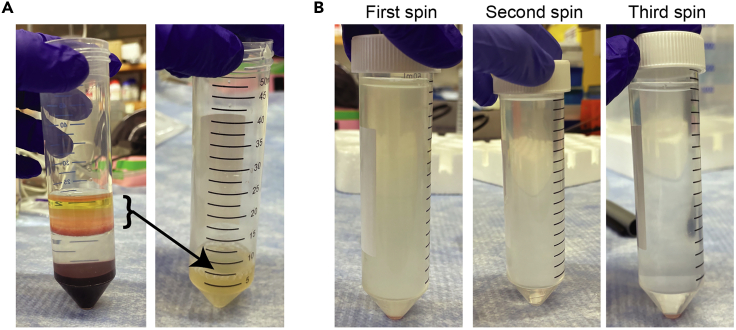
Note: The 300×g centrifugation steps helps to eliminate platelets, which pellet at higher speed spins. The supernatant of each spin should get progressively clearer (Figure 3B). If not, repeat until the supernatant is clear. We empirically selected 300×g because it optimizes recovery of monocytes and platelet removal. Eliminating platelets is important because they can stick to monocytes leading to contamination and affect monocyte viability and/or activation (Inui et al., 2015, Zhou et al., 2012).
-
9.
Combine all cell pellets by resuspension in 1 mL 1× RBC lysis buffer. Transfer cells to a new 50 mL conical tube and add 1× RBC lysis buffer up to a final volume of 10 mL.
Note: 10 mL of 1× RBC lysis buffer is sufficient for PMBCs isolated from <90 mL of undiluted blood. For higher volumes of blood, additional tubes should be used.
-
10.
Incubate at 18°C–22°C for 5 min.
-
11.
Neutralize RBC lysis buffer by adding 10 mL of PBS/EDTA.
-
12.
Centrifuge at 500×g for 5 min at 18°C–22°C.
-
13.
Aspirate the supernatant and resuspend cells in 10 mL isolation buffer per 50 mL blood volume.
-
14.
Count cells.
Note: For cell counting, cell concentration may require adjustment.
-
15.
Count cells and set aside 0.5 million cells for flow cytometry analysis.
-
16.
Perform flow cytometry analysis (see steps 46–50).
Note: The resultant cells are referred to as PBMCs, of which monocytes account for ∼10%–20%.
CRITICAL: Human primary monocytes are generally subdivided into three populations: (1) classical monocytes, which account for majority of the total monocytes and are characterized as CD14+CD16− or CD14++ (high expression) CD16−); (2) intermediate monocytes, which accounts for ∼2%–8% and are characterized as CD14dimCD16+; (3) non-classical monocytes, which account for ∼2%–11% of total monocytes and are characterized as CD14+CD16+ (Ziegler-Heitbrock, 2007; Sampath et al., 2018). Flow cytometry should be used to quantify each population (Figure 4). If the classical monocytes is <80% of the total monocytes, this could indicate an active infection or chronic inflammatory condition in the donor. We have discarded such samples. However, this decision depends on the goals of the study.
Figure 3.
PBMC isolation
(A) Photographs of the PBMC layer with plasma 5–10 mm above it (left), and the appearance of the solution after transferring to a new 50 mL conical tube (right).
(B) Photograph of the turbidity change in the supernatant with each successive wash and 300×g spin.
Figure 4.
Flow cytometry analysis of PBMCs
Flow cytometry gating strategy to analyze PBMCs. CD14 and CD16 were used to distinguish low-density neutrophils (LDNs), non-classical monocytes, intermediate monocytes, and classical monocytes. Gray = negative control; Red = labeled sample.
Isolation of human monocytes
Timing: For steps 17–27: ∼1 h
This section illustrates positive selection of CD14+ monocytes by magnetic activated cell sorting (MACS) from Miltenyi Biotec. Another widely used method is EasySep human monocytes or CD14 positive selection kit from Stem Cell technology. Both methods have a comparable cost, time efficiency, and purity of isolation. But the equipment required (e.g., magnet, separator, etc.) is not interchangeable. Although we have not compared these two methods side by side, there could be functional differences of isolated monocytes for downstream activation. For example, one study showed that monocyte-derived dendritic cells showed better response to tumor antigens and hence better ability to induce T cell cytotoxicity against tumors when monocytes were prepared using MACS from Miltenyi Biotec compared to EasySep from Stem Cell technology (Marques et al., 2018).
In addition to positive selection, plastic adhesion and negative selection are two other methods used in the field. Plastic adhesion takes advantage of monocytes’ fast and strong adherence to tissue culture plates, while negative selection labels all other cell types except monocytes for magnetic selection. However, plastic adhesion results in lower yield and less than 50% purity. Negative selection can result in heavy contamination with platelets (Nielsen et al., 2020). This is consistent with our observations and hence we prefer positive selection and present a detailed protocol here. Admittedly, negative selection provides the advantage of avoiding the attachment of microbeads to monocytes, which might affects their function for downstream application (Bhattacharjee et al., 2017). And platelet removal cocktail in combination with negative selection (e.g., monocyte negative isolation kit, EasySep from Stem Cell) could circumvent the contamination issue. Nevertheless, we have not observed changes of expression level of CCR2 or CX3CR1, chemokine receptors that have been associated with activation, pre- and post-positive selection nor any hampered functionality of isolated monocytes.
CRITICAL: From this point forward, samples should be kept at 4°C to preserve monocyte viability. Adjust the centrifuge temperature to 4°C and keep all buffers on ice.
-
17.
Centrifuge cells at 500×g for 5 min.
-
18.
Gently aspirate the supernatant and add 80 μL isolation buffer + 20 μL CD14 MicroBeads for up to each 10 million cells. If 10–20 million cells, adjust to 160 μL isolation buffer + 40 μL CD14 MicroBeads. If 20–30 million cells, adjust to 240 μL isolation buffer + 60 μL CD14 MicroBeads.
CRITICAL: Mix the MicroBeads thoroughly (by gently vortexing or pipetting) to create a homogenous solution immediately before adding to cells.
-
19.
Gently mix the CD14 MicroBeads with cells and incubate for 15 min at 4°C.
CRITICAL: For best results, incubate samples in a 4°C fridge, not on ice, which is lower than 4°C and could have variable temperature.
-
20.
Top up to 30 mL with isolation buffer and centrifuge at 500×g for 5 min at 4°C.
-
21.
While samples are spinning, set up the magnetic stand and column.
Note: For <10 million cells, an MS column should be used. For >10 million cells, an LS column should be used. The procedure below describes the use of an LS column as an example. Refer to the manufacturer’s instructions for MS columns: https://www.miltenyibiotec.com/US-en/products/ms-columns.html#gref.
CRITICAL: Place the columns tightly in the magnet. Be aware that the LS column has a front and back face and should be oriented properly into the magnet with the front face out (Figure 5A). Place a tube or reservoir below the column to collect flowthrough and washes (Figure 5A).
-
22.
Equilibrate the column by passing 3 mL isolation buffer through it.
-
23.
Remove samples from the centrifuge and aspirate the supernatant. Resuspend pellet in 1 mL isolation buffer (for up to 106 cells) and transfer to the column.
Note: The manufacture’s instruction is to use one LS column for 107–2 × 109 total cells. We found that a maximum of 1 × 108 PBMCs per LS column is ideal for an efficient positive selection (i.e., won’t clog the column).
-
24.
After the cell suspension passes through the column, wash with 3 mL isolation buffer three times.
-
25.
Remove the column from the magnetic rack and place it in a 15 mL conical tube for collecting monocytes (Figure 5B).
-
26.
Add 5 mL isolation buffer and immediately push the solution through the column with a plunger into a collection tube (Figure 5C).
-
27.
Count cells and save 0.2 million for flow cytometry analysis.
Figure 5.
Magnetic column set up to obtain CD14+ cells
(A) Photograph of setting up the LS column on a magnetic stand.
(B) LS column transferred to a 15 mL conical tube.
(C) Elution of monocytes with a plunger.
Isolation of human PMNs
Timing: For step 28–45: 1–2 h
This section describes an efficient way to remove red blood cells and purify human PMNs.
CRITICAL: Keep the temperature at 18°C–22°C for the entire isolation as human PMNs can be activated by temperature changes.
-
28.
Aspirate the layer above the porous barrier.
-
29.
Remove the porous barrier with a 2 mL sterile serological pipette and tweezers (Figure 6A, Methods video S1).
CRITICAL: If the bottom RBC layer is disturbed during barrier removal (Figure 6B), centrifuge the tube at 500×g for 5 min at 18°C–22°C with the brake off. If the RBC layer is undisturbed, proceed to step 30.
-
30.
Aspirate and discard the Ficoll-Plaque PLUS gradient carefully without disturbing the dark red pellet.
-
31.
Top up to 50 mL with 1×RBC lysis buffer for each tube (tube 0).
-
32.
Centrifuge tubes at 500×g for 5 min at 18°C–22°C (tube 0).
CRITICAL: Centrifugation here will not pellet all PMNs. Therefore, once centrifugation is complete, we do not aspirate any media. Instead, we perform additional RBC lysis steps (33–34) by dividing tube 0 into three tubes (Figure 7).
-
33.
Directly transfer top 20 mL from this tube (tube 0) into a new tube (new tube 1), and the next 20 mL out into another new tube (new tube 2).
-
34.
Add 30 mL and 40 mL 1×RBC lysis buffer to new tubes 1/2, and tube 0 respectively.
-
35.
Incubate at 18°C–22°C for 5 min.
CRITICAL: If the number of tubes generated above exceeds the capacity of the centrifuge, stagger the resuspension step to avoid prolonged exposure to RBC lysis buffer, which will also lyse PMNs and lead to the appearance of a ‘gooey’ cell pellet. In this case, the PMN prep is compromised, and a new blood sample should be obtained.
-
36.
Centrifuge tubes at 500×g for 5 min at 18°C–22°C.
-
37.
Gently remove supernatant and resuspend pellet in 1 mL 1×RBC lysis buffer.
-
38.
Combine up to six tubes together and top up to 50 mL with 1×RBC lysis buffer.
-
39.
Incubate tubes at 18°C–22°C for 5 min.
-
40.
Centrifuge tubes at 500×g for 5 min at 18°C–22°C.
CRITICAL: If cell pellets are white or contain a thin red ring above a white pellet, proceed to step 41. If cell pellets remain red, repeat steps 38–40 to further eliminate RBCs.
Note: RBC susceptibility to lysis varies between donors.
-
41.
Aspirate the supernatant and wash the cell pellet with 15 mL PBS.
-
42.
Centrifuge at 500×g for 5 min at 18°C–22°C.
-
43.
Gently remove supernatant.
-
44.
Resuspend cells in PBS (∼1–5 million cells/mL). Typically, 50 mL of blood yields ∼50–200 million PMNs.
Note: Depending on the cell counting method, you may need to adjust your cell concentration to obtain an accurate cell count.
-
45.
Set aside 0.5 million PMNs at 18°C–22°C for flow cytometry analysis.
Figure 6.
Removal of the porous barrier
(A) Photographs of removing the porous barrier.
(B) Examples of solutions following porous barrier removal; undisturbed RBC layer (left), disturbed RBC layer (right).
Figure 7.
Experimental flow of RBC lysis buffer steps
Examination of the purity of human monocytes and PMNs
Timing: For step 46–50: 0.5–1 h
This section describes a method for assessing monocyte and PMN purity by flow cytometry.
-
46.
Stain 0.2 million purified monocytes or PMNs with the following panels in a flow tube at 18°C–22°C for 15 min or 4°C for 30 min.
Note: For monocytes, use anti-CD14, anti-CD11b (monocyte markers), and a cell viability dye (calcein blue, cBlue, 10 μM final concentration, stains live cells positive). For PMNs, use anti-CD16, anti-CD10 (mature neutrophil markers [Marini et al., 2017]), and cBlue. 1 uL of each antibody tested here could be used for 0.2 million cells. Refer to manufacturer’s instructions for concentration for other antibodies catalogs that are not listed here. Because only cell surface markers are being tested, no fixation is required.
Note: Use 0.2 million cells for negative and/or isotype control.
-
47.
Add 700 μL of flow staining buffer to each flow tube.
-
48.
Centrifuge at 500×g for 5 min at 18°C–22°C.
-
49.
Gently discard the supernatant.
-
50.
Add 300 μL flow staining buffer and analyze in a flow cytometer.
CRITICAL: PMNs have a very short half-life (∼7 h) in healthy individuals, and they will naturally cycle to apoptosis (Saverymuttu et al., 1985; Dancey et al., 1976). Following isolation, we observed that they started undergoing apoptosis within 2 h and almost all are dead within 24 h. Long-term cell culture is not possible with these cells.
Expected outcomes
A successful isolation from 50 mL of human blood should yield ∼8–15 million monocytes and ∼50–200 million PMNs. Both isolated cell types should have >90% viability and >90% purity. For monocytes, purity is assessed by quantifying CD11b+CD14+ cells (Figure 8A). For PMNs, purity is assessed by quantifying CD16+CD10+ cells (Figure 8B). Purified cells are ready for immediate analysis or for further functional studies (e.g., differentiation of monocytes into HMDMs, induction of PMN apoptosis or netosis, etc.).
Optional: Additional cell markers can be used to further assess purity. For monocytes, contaminating cells may include T-cells, B-cells, low-density neutrophils (LDNs), and NK cells which are also present in the PBMC layer (Kleiveland, 2015). For PMNs, contaminating cells may include basophils, eosinophils, and mast cells, which are also present in the granulocyte layer (Beenhouwer, 2010).
Figure 8.
Flow cytometry analysis
Example of flow cytometric analysis of purified human monocytes (A) and PMNs (B). Gray = negative control; Red = labeled sample.
Limitations
This protocol is most suitable for purifying PMNs and monocytes from freshly collected human blood. For other blood-derived starting materials (e.g., buffy coat, leukocyte reduction system chambers (LRSC)), optimization may be required. In addition, this protocol isolates high-density neutrophils (HDNs), which is the mature polymorphonuclear population present in the granulocyte layer. Low-density neutrophils (LDNs), which are phenotypically and functionally distinct from HDNs, reside in the PBMC layer (Sagiv et al., 2015; Silvestre-Roig et al., 2019). For isolation of LDNs, refer to the above references. Lastly, this protocol isolates CD14+ monocytes, which comprise both classical monocytes (∼90%, CD14+CD16−) and non-classical monocytes (∼10%, CD14+CD16+). Isolation of specific monocyte populations requires additional methods (e.g., classical monocyte isolation kit; Mitenyi Biotec).
Troubleshooting
Problem 1
Low yield of monocytes (i.e., <8 million cells from 50 mL of blood).
Potential solution
One potential cause is that the temperature of Ficoll density gradient separation is suboptimal, refer to steps 1–2 in the before you begin section. If the temperature of the centrifuge and/or reagents is above the optimal range (18°C–22°C), the density of Ficoll-Paque PLUS decreases, which results in monocyte loss from the PBMC layer. Ensure sufficient time for temperature equilibration of reagents and centrifuges. Another potential cause is insufficient dilution of blood with HBSS/EDTA (ideally 1:1), refer to step 5 in the before you begin section. This results in high hematocrit, which may result in monocyte loss from the PBMC layer due to trapping by RBCs. Make sure that blood is diluted sufficiently. Another potential cause is suboptimal positive selection with CD14 MicroBeads. This could be due to several reasons. First, if the PBMC cell count is underestimated, the amount of CD14 MicroBeads added may be insufficient to bind all CD14+ cells, refer to step 14 in the step-by-step method details section. Second, if MicroBeads were not thoroughly mixed prior to incubation, it may lower binding to CD14+ cells, refer to step 19 in the step-by-step method details section. Third, if the binding of CD14 MicroBeads to cells occurs below 4°C (e.g., incubation on ice) then 15 min may be insufficient to bind all CD14+ cells, refer to step 19 in the step-by-step method details section. Fourth, excessive numbers of clumped cells may bind non-specifically to CD14 MicroBeads and diminish yield, refer to step 23 in the step-by-step method details section. Clumped cells can be removed using a 40 μm filter.
Problem 2
Low yield of PMNs (i.e., <50 million cells from 50 mL of blood).
Potential solution
One potential cause is that the Ficoll-Paque PLUS density gradient separation is suboptimal. This could be due to several reasons. First, if the temperature of the centrifuge and/or reagents is below the optimal range (18°C–22°C), the density of Ficoll-Paque PLUS increases, which results in fewer RBCs and PMNs in the Ficoll-Paque PLUS layer, refer to steps 1–2 in the before you begin section. Ensure sufficient time for temperature equilibration of reagents and centrifuges. Second, adequate time and g-force are required to ensure complete sedimentation of granulocytes and RBCs. We found that 800×g for 15min during density gradient centrifugation is optimal, refer to step 2 in the step-by-step method details section. Third, a vibration of the rotor or failure to turn the brake off can lead to disruption of the gradient, mixing of the cell layers, and loss of cells, refer to step 2 in the step-by-step method details section. Another potential cause is due to excessive RBC lysis, refer to steps 31–40 in the step-by-step method details section. Because there are many RBC lysis steps involved, an excessive amount of RBC lysis buffer may result in the lysis of PMNs (visualized as a ‘gooey’ pellet). Be cautious about the incubation time and volume of RBC lysis buffer used.
Problem 3
Low purity (i.e., <90%) of monocytes.
Potential solution
One reason could be due to excessive dead or clumped cells, which may bind non-specifically to CD14 MicroBeads, refer to step 23 in the step-by-step method details section. This affects not only monocyte yield, but also purity. We have found that the cell viability of PBMCs is typically high (>95%); accordingly the presence of dead cells may reflect poor execution of previous steps in the protocol. Dead cell removal kits can be used, but often lead to substantial loss of live monocytes. Clumped cells can be avoided by resuspending PBMCs in small volumes first during the wash steps. They can also be effectively removed by passing cells through a 40 μm filter. Another reason is that binding to CD14 MicroBeads was performed for too long or at too high a temperature, both of which could lead to non-specific cell binding, refer to step 14 in the step-by-step method details section. Be cautious about temperature and time.
Problem 4
Low purity (i.e., <90%) of PMNs.
Potential solution
There are two potential sources of contamination in isolated PMNs. One is other granulocytes, which include basophils, eosinophils, and mast cells. Because the procedure to isolate PMNs described here relies on Ficoll-Paque PLUS density gradient centrifugation, the bottom cell layer contains all granulocytes. In healthy donors, PMNs account for ∼ 95% of total granulocytes populations. However, donors with allergic or inflammatory conditions may have higher levels of other granulocytes (i.e., >5%) (Alberts et al., 2014). In this case, we have discarded the samples. While positive selection methods can be used to further purify PMNs, longer purification methods lead to reduced PMN viability (PMNs have a short half-life) and the functional status of PMNs could be influenced by the underlying condition of the donor. The second source of contamination is mononuclear cells. This can occur if the temperature of the centrifuge and reagents is below the optimal range (18°C–22°C) during Ficoll-Paque PLUS density gradient centrifugation, refer to steps 1–2 in the before you begin section. Be cautious about temperature.
Problem 5
Low viability of isolated cells.
Potential solution
The timing of cell isolation is essential for maintaining cell viability. For optimal results, freshly collected blood should be processed immediately and the isolation should be performed efficiently, refer to step 3 in the before you begin section. Substantial delays (>4 h) in processing blood after collection can result in loss of cell viability. This is particularly problematic for PMNs because they have a very short half-life (∼7–24 h). In addition, the temperature, pH, and composition of buffers, as well as the timing of RBC lysis steps are essential. For example, buffers for PMN isolation should be at 18°C–22°C and void of magnesium or calcium, which can activate PMNs and induce apoptosis.
Resource availability
Lead contact
Further information and requests should be directed to and will be fulfilled by the lead contact, Lev Becker: levb@uchicago.edu.
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We would like to thank the healthy blood donors for their collaboration. This work was supported by Virginia and D.K. Ludwig Fund for Cancer Research, Ruth Bruch Triple Negative Breast Cancer Research Award, Janet D. Rowley Discovery Fund, and J. Clifford Moos Award.
Author contributions
C.C. and L.B. conceived and designed the experiments and wrote the manuscript; C.C., K.Q.S., and K.M.B. performed the experiments. All authors critically reviewed the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100845.
Contributor Information
Chang Cui, Email: changc@uchicago.edu.
Lev Becker, Email: levb@uchicago.edu.
Data and code availability
This study did not generate data sets and/or code.
References
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. Sixth Edition. Garland Science; 2014. Molecular Biology of the Cell. [Google Scholar]
- Beenhouwer D.O. In: Essential Concepts in Molecular Pathology. Coleman W., Tsongalis G., editors. Elsevier; 2010. Molecular basis of diseases of immunity; pp. 205–216. [Google Scholar]
- Bhattacharjee J., Das B., Mishra A., Sahay P., Upadhyay P. Monocytes isolated by positive and negative magnetic sorting techniques show different molecular characteristics and immunophenotypic behaviour. F1000Res. 2017;6:2045. doi: 10.12688/f1000research.12802.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C., Chakraborty K., Tang X.A., Zhou G., Schoenfelt K.Q., Becker K.M., Hoffman A., Chang Y.-F., Blank A., Reardon C.A. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184 doi: 10.1016/j.cell.2021.04.016. 3163–3177.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancey J.T., Deubelbeiss K.A., Harker L.A., Finch C.A. Neutrophil kinetics in man. J. Clin. Invest. 1976;58:705–715. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui M., Tazawa K., Kishi Y., Takai T. Platelets convert peripheral blood circulating monocytes to regulatory cells via immunoglobulin G and activating-type Fcγ receptors. BMC Immunol. 2015;16:20. doi: 10.1186/s12865-015-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiveland C.R. In: The Impact of Food Bioactives on Health. Verhoeckx K., Cotter P., López-Expósito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H., editors. Springer International Publishing; 2015. Peripheral blood mononuclear cells; pp. 161–167. [Google Scholar]
- Kratz M., Coats B.R., Hisert K.B., Hagman D., Mutskov V., Peris E., Schoenfelt K.Q., Kuzma J.N., Larson I., Billing P.S. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini O., Costa S., Bevilacqua D., Calzetti F., Tamassia N., Spina C., De Sabata D., Tinazzi E., Lunardi C., Scupoli M.T. Mature CD10+ and immature CD10- neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood. 2017;129:1343–1356. doi: 10.1182/blood-2016-04-713206. [DOI] [PubMed] [Google Scholar]
- Marques G.S., Silva Z., Videira P.A. Antitumor efficacy of human monocyte-derived dendritic cells: comparing effects of two monocyte isolation methods. Biol. Proced. Online. 2018;20:4. doi: 10.1186/s12575-018-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.C., Andersen M.N., Møller H.J. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology. 2020;159:63–74. doi: 10.1111/imm.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv J.Y., Michaeli J., Assi S., Mishalian I., Kisos H., Levy L., Damti P., Lumbroso D., Polyansky L., Sionov R.V. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- Sampath P., Moideen K., Ranganathan U.D., Bethunaickan R. Monocyte subsets: phenotypes and function in tuberculosis infection. Front. Immunol. 2018;9:1726. doi: 10.3389/fimmu.2018.01726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saverymuttu S.H., Peters A.M., Keshavarzian A., Reavy H.J., Lavender J.P. The kinetics of 111indium distribution following injection of 111indium labelled autologous granulocytes in man. Br. J. Haematol. 1985;61:675–685. doi: 10.1111/j.1365-2141.1985.tb02882.x. [DOI] [PubMed] [Google Scholar]
- Silvestre-Roig C., Fridlender Z.G., Glogauer M., Scapini P. Neutrophil diversity in health and disease. Trends Immunol. 2019;40:565–583. doi: 10.1016/j.it.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Somasundaram R., Nederhof R.F., Dijkstra G., Faber K.N., Peppelenbosch M.P., Fuhler G.M. Impact of human granulocyte and monocyte isolation procedures on functional studies. Clin. Vaccine Immunol. 2012;19:1065–1074. doi: 10.1128/CVI.05715-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate data sets and/or code.