Introduction
Enhanced Recovery After Surgery (ERAS) has gained popularity in thoracic surgery especially in the context of lung cancer resections. ERAS pathways are protocolized, perioperative care bundles that are designed to improve outcomes1. For example, this includes interventions like multimodal, opiate-sparing pain control and prompt, protocolized chest tube removal in the peri-operative period. While enhancing outcomes are and should be the primary aim of ERAS, several secondary benefits have also been realized particularly related to the cost-savings associated with accelerated recovery and early discharge2. One challenge to widespread ERAS implementation is that individual components tend to be heterogeneous, varying significantly across hospitals and providers. Further, most studies to date have been small and retrospective, often with historical controls, a caveat to evidence-based implementation3. Another challenge is measuring the impact of individual ERAS components. While single ERAS items may have a modest impact when studied in isolation, combining several elements together often has synergistic effects leaving some uncertainty to which elements are most beneficial4. Nevertheless, and despite these evidential shortcomings, numerous ERAS items specific to lung cancer surgery have emerged for efficient, patient-centered care5.
Key Participants in Successful ERAS Pathway
Fundamental to the success of any ERAS program is a multidisciplinary stakeholder team. This team must enact reliable and reproducible care while also maintaining the ability to continuously adapt and adopt new interventions. This team should span all aspects of patient care, including preoperative, intra-operative, and postoperative periods. The group accompanies the patient through the entire surgical journey from the time of referral to hospital discharge (and even to follow-up)2. At a minimum, this team includes clinic staff, preoperative nurses, anesthesiologists, surgeons, operating room staff, surgical floor team members, pharmacists, and floor nursing. Effective communication between team members is critical to successful implementation.
When implementing an ERAS program, it is important to track patient-level data in order to document and improve efforts. This should encompass all common outcomes, including measures of morbidity, hospital length of stay, patient experience, readmissions, and mortality. Providers should also track compliance with established protocols. Interventions should be assessed in real-time, with the ability to rapidly intervene if adverse outcomes are encountered. Finally, providers should strive to collect less conventional patient-reported outcomes and how these are influenced by ERAS components2,6. These quality of life measures are meaningful to patients and are often overlooked by surgeons.
Required Items in ERAS Pathway
ERAS components can vary significantly across institutions and providers. Further, some components are based on empirical data at best. To help address this issue, the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS) published recent guidelines on ERAS after lung resection in which they reviewed 45 ERAS components and the quality of evidence supporting each5. Several of these items are outlined in Figure 1.
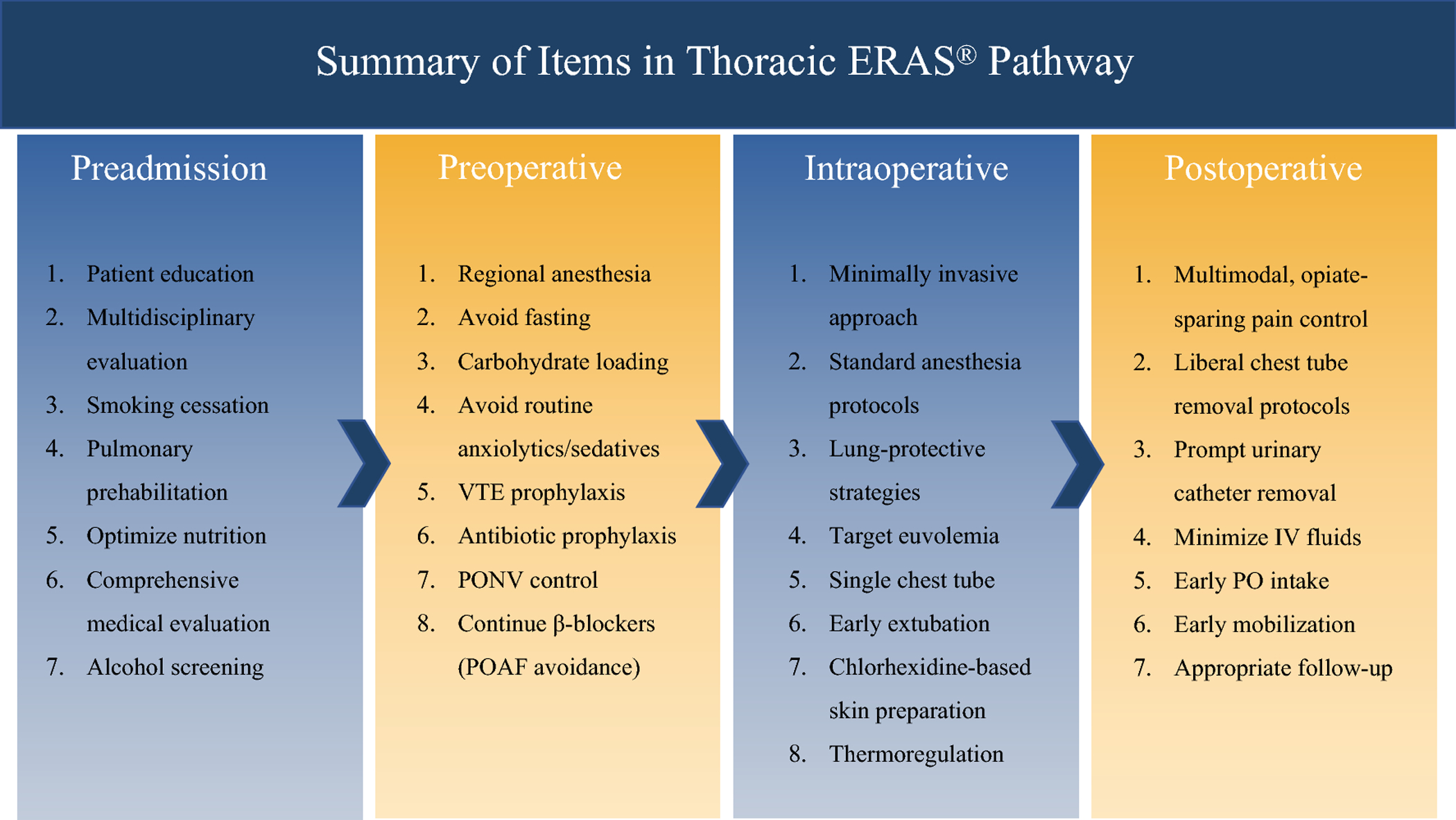
POAF = post-operative atrial fibrillation; PONV = post-operative nausea and vomiting; VTE = venous thromboembolism
Preadmission
A pillar of preoperative ERAS management is patient education. This education should set expectations – focusing on the postoperative course – while also addressing patient fear and anxiety. Patient counselling is an important yet simplistic intervention, providing benefit with little risk of harm5. Another tenant of good ERAS management is perioperative nutrition. Specific to the preoperative setting, clinicians should assess for signs of malnutrition like weight loss which can be common in the setting of malignancy. Screening tools like the Nutritional Risk Score (NRS) and the Subjective Global Assessment (SGA) in conjunction with other objective measures like the ESPEN criteria (>10–15% wight loss within 6 months, body mass index <18.5 kg/m2, or serum albumin <30 g/l) may guide providers on whether to delay surgery to optimize nutritional parameters. Unique to thoracic surgery is the high incidence of smoking. Active smoking is associated with a higher risk of several postoperative complications. Providers not only should screen for smoking but also offer cessation assistance. Generally, patients should abstain from smoking for at least 4 weeks prior to an operation. Most of this literature has relied on self-reported smoking histories as opposed to objective measures like exhaled carbon monoxide levels and/or urine/salivary cotinine levels8. Even if objective evidence of persistent smoking were collected, the decision to delay surgery (for cessation purposes) must be carefully weighed against the risks of disease progression9. Another important intervention is pulmonary prehabilitation. Preoperative exercise programs have low level of evidence but may be beneficial especially to elderly patients with borderline lung function. Objective criteria for which patients may benefit most from prehabilitation are lacking10. In general, patients should be carefully screened for surgical candidacy based on their functional status and efforts should be made to optimize physical fitness. Other ERAS components to consider in the preoperative phase are comprehensive medical evaluation and optimization, screening for additional comorbidities (like anemia), and evaluation by a multidisciplinary team. While the ESTS/ERAS Society guidelines do not explicitly mention multidisciplinary team evaluation, it has been our experience that patient evaluation by anesthesiology and in some cases an expert tumor-board is particularly beneficial in patients with malignancy.
Preoperative
Perhaps the most notable recommendation of ERAS in the preoperative period is the avoidance of excessive fasting. While patients have traditionally been encouraged to fast starting the night before an operation, newer recommendations limit fasting for solid foods to 6 hours and clear liquids to 2 hours5. Another lower-evidence suggestion is carbohydrate loading. The benefits of this have been observed mostly in other fields, but carbohydrate loading is thought to achieve a “metabolically fed state” potentially resulting in fewer postoperative complications5. Another recommendation is the continuance of beta-blocking medications; abrupt cessation of these medications is believed to unduly increase an already high risk for postoperative atrial fibrillation (POAF). These medications should be resumed promptly in the postoperative phase. POAF prophylaxis is not otherwise recommended. Additional preoperative ERAS components to consider are routine antibiotic prophylaxis, routine venous thromboembolism (VTE) prophylaxis, and avoidance of anxiolytic or sedating medications (given for the purposes of reducing preoperative anxiety). Effective communication between the preoperative staff and operating room personnel is also essential to allow for a seamless transition to the OR and to ensure that all ERAS items are completed in a timely manner.
Intraoperative
Intraoperative ERAS items can be roughly divided between the anesthesiology and surgery teams. From the anesthesiologist perspective, a primary aim of any ERAS pathway is standardized anesthesia protocols. This should include regional anesthesia when possible (particularly paravertebral blockade as opposed to epidural catheterization). Paravertebral blockade has been associated with fewer post-operative respiratory complications compared to epidural catheterization, while maintaining equivalent pain control5. Additionally, fast acting anesthetic agents should be used to help facilitate prompt extubation. Lung protective ventilation strategies are preferred and patient fluid status should target euvolemia. Monitoring patient temperature is also mandatory to avoid hypothermia.
From the surgeon perspective, ERAS should truly start from the operative planning stage. It is essential that, when possible, a minimally invasive approach to resection is employed. A thoracotomy is a morbid incision which significantly impacts patient quality of life and several postoperative complications. In the setting of malignancy, patients should receive an oncologic operation, paying particular attention to adequate resection and lymph node sampling. All standard operative protocols should be followed including appropriate prophylaxis and antimicrobial skin preparation. Finally, surgeons should strive to place a single chest tube. This allows for sufficient drainage while expediting postoperative removal and recovery.
Postoperative
Perhaps the most important ERAS component in any field is the early initiation of multimodal, opiate-sparing pain control (including regional anesthesia). This is particularly important if a thoracotomy is required. Patients should be encouraged to mobilize early. Early mobilization can be more easily achieved with prompt chest tube removal using liberal, standardized protocols. Urinary drainage catheters, if required, should also be removed promptly (although epidural anesthesia could affect urinary retention). Finally, intravenous fluids should be discontinued as soon as possible in the postoperative course in favor of an oral diet. Throughout the postoperative course, patients should continue to be evaluated by a multidisciplinary team (including physical and occupational therapists as needed) with attention to appropriate discharge planning. Early follow-up with patients, although not explicitly mentioned in most guidelines, is also ideal to expediently address potential complications and to reduce readmissions.
Barriers and Facilitators to ERAS Pathway
Several barriers exist to ERAS implementation, one of which is compliance. Simply having a pathway is important but actually implementing that pathway can be challenging. A recent study demonstrated that compliance with ERAS components was independently associated with reduced mortality11. Perfect compliance to ERAS pathways is not possible for a variety of clinical realities; however, optimizing compliance to as many pathway components as possible is clearly important. Facilitators to higher compliance, at least in our experience, include easily accessible protocols and strong communication within the multidisciplinary team. Another barrier to ERAS implementation is the relatively sparse evidence upon which interventions are currently based. Some concepts that could help mitigate this lack of evidence include (1) forming international, multi-institutional ERAS collaboratives (like those in other fields), (2) collecting prospective (and possibly randomized) data, and (3) mandating specific ERAS elements as a standard to high quality lung cancer care (and tracking these on a national level). Another potential barrier stems from complexities associated with different operations; for example, ERAS may differ significantly between open and thoracoscopic approaches1. Regardless, several of these barriers can be lessened by higher quality, multi-institutional evidence.
In summary, ERAS after lung cancer resection is important for optimizing patient outcomes. Several ERAS components exist with proven benefit although further research in this field is required.
Checklist and Flow Diagram
Figure 1 is an example flow diagram of ERAS components that should be considered in a typical pathway. Additional information regarding the ERAS Society and ESTS guidelines are available at: https://doi.org/10.1093/ejcts/ezy301.
Funding:
Funded in part by NIH 5T32HL007776-25 (BTH)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: None
References
- 1.Semenkovich TR, Hudson JL, Subramanian M, Kozower BD. Enhanced Recovery After Surgery (ERAS) in Thoracic Surgery. Semin Thorac Cardiovasc Surg. 2018;30(3):342–349. doi: 10.1053/j.semtcvs.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 2.Medbery RL, Fernandez FG, Khullar OV. ERAS and patient reported outcomes in thoracic surgery: A review of current data. J Thorac Dis. 2019;11(1):S976–S986. doi: 10.21037/jtd.2019.04.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Haren RM, Atay SM. Enhancing the study of enhanced recovery after thoracic surgery: Methodology and population-based approaches for the future. J Thorac Dis. 2019;11(Suppl 4):S612–S618. doi: 10.21037/jtd.2019.01.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248(2):189–198. doi: 10.1097/SLA.0b013e31817f2c1a [DOI] [PubMed] [Google Scholar]
- 5.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: Recommendations of the Enhanced Recovery after Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothoracic Surg. 2019;55(1):91–115. doi: 10.1093/ejcts/ezy301 [DOI] [PubMed] [Google Scholar]
- 6.Comacchio GM, Monaci N, Verderi E, Schiavon M, Rea F. Enhanced recovery after elective surgery for lung cancer patients: Analysis of current pathways and perspectives. J Thorac Dis. 2019;11(Suppl 4):S515–S522. doi: 10.21037/jtd.2019.01.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weimann A, Braga M, Carli F, et al. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr. 2017;36(3):623–650. doi: 10.1016/j.clnu.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 8.Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. 2014;2014(3). doi: 10.1002/14651858.CD002294.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samson P, Patel A, Garrett T, et al. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage i non-small cell lung cancer. Ann Thorac Surg. 2015;99(6):1906–1913. doi: 10.1016/j.athoracsur.2015.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. 2017;2017(6). doi: 10.1002/14651858.CD012020.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg. 2018;155(4):1843–1852. doi: 10.1016/j.jtcvs.2017.10.151 [DOI] [PubMed] [Google Scholar]


