Abstract
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), has affected millions of people globally. It was declared a pandemic by the World Health Organization in March 2020. The hyperinflammatory response to the entry of SARS-CoV-2 into the host through angiotensin-converting enzyme 2 is the result of a “cytokine storm” and the high oxidative stress responsible for the associated symptomatology. Not only respiratory symptoms are reported, but gastrointestinal symptoms (diarrhea, vomiting, and nausea) and liver abnormalities (high levels of aspartate aminotransferase, alanine aminotransferase transaminases, and bilirubin) are observed in at least 30% of patients. Reduced food intake and a delay in medical services may lead to malnutrition, which increases mortality and poor outcomes. This review provides some strategies to identify malnutrition and establishes nutritional approaches for the management of COVID-19 and liver injury, taking energy and nutrient requirements and their impact on the immune response into account. The roles of certain phytochemicals in the prevention of the disease or as promising target drugs in the treatment of this disease are also considered.
Keywords: COVID-19, Infection, Liver, Nutrition therapy, Phytochemicals
Core Tip: The Coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), was declared a pandemic by the World Health Organization in 2020. It has since affected millions of persons worldwide. In some cases, entry of the virus into the cell leads to a hyperinflammatory response, giving rise to all of the associated symptomatology. Liver abnormalities have been detected frequently during the course of COVID-19, with or without related symptoms. Herein we discuss the infectious process, the response to the immune system and to the oxidative stress induced, and provide a brief guideline for nutritional therapy in patients affected by SARS-CoV-2 and liver injury.
INTRODUCTION
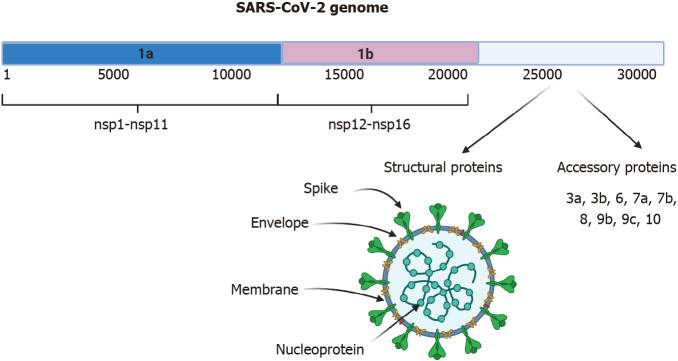
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) belongs to the family of coronaviruses; the genome is +ssRNA, which is nonsegmented and has a size of 30 kb[1,2]. In the cytoplasm of the host cell, it can be translated immediately into protein, as occurs with messenger (m)RNA. The genome encodes 16 nonstructural proteins (nsps) that are not part of the virion and have various functions in genome replication, protein processing, viral assembly, the exit of viral progeny, and others[3]. It also codes for four structural proteins, including spike (S), nucleocapsid (N), membrane (M), and envelope (E) that are required to constitute the complete virus particle (Figure 1)[2,4]. The origin of the disease may be from BatCov RaTG13 (GenBank: MN996532), a relatively close virus and one that was isolated from horseshoe bats[5]. SARS-CoV-2 causes the disease known as coronavirus disease 2019 (COVID-19). Since March 2020, a pandemic has been declared, and up to December 31, 2020, 81475053 cases were confirmed and 1798050 deaths had been reported. The global lethality rate is 2.22%[6].
Figure 1.
Genome and structure of severe acute respiratory syndrome-coronavirus-2. The 30-kb severe acute respiratory syndrome-coronavirus-2 virus genome is shown. It possesses different open reading frames (ORFs). ORF 1a generates the nonstructural proteins (nsp) from nsp1-nsp11, ORF 1b generates proteins nsp12-nsp16. The spike, envelope, membrane, and nucleoprotein structural proteins and accessory proteins are generated from the 3’ region. Created with BioRender.com. SARS-CoV-2: Severe acute respiratory syndrome-coronavirus-2.
SARS-CoV-2 can affect various organs that express the entry receptor. The main receptor described is angiotensin-converting enzyme 2 (ACE2)[1]. Distribution of the ACE2 protein was investigated by immunohistochemistry, and the protein was found present in endothelial cells from small and large arteries and veins, arterial smooth muscle cells, myofibroblasts, the membrane of fat cells in various organs, and in the basal layer of nonkeratinizing squamous epithelium. Importantly, ACE2 is expressed in nasal and oral mucosae and the nasopharynx in type I and type II alveolar epithelial cells in normal lungs, which explains the respiratory tract infection. Gastrointestinal manifestations can be explained by the presence of the receptor in smooth muscle cells and in the endothelium of vessels in the stomach, small intestine, and colon. In addition, the receptor can be found in the brush border of enterocytes in the small intestine, including the duodenum, jejunum, and ileum, but not in enterocytes of the colon. In the kidney, weak glomerular visceral ACE2 staining was observed, whereas parietal epithelial cells were moderately positive. In the skin, ACE2 was present in the basal cell layer of the epidermis extending to the basal cell layer of hair follicles. Smooth muscle cells surrounding the sebaceous glands were also positive for ACE2. In the brain, ACE2 receptors were found in only endothelial and smooth muscle cells[7]. Some of the extra respiratory manifestations can be explained by the previous findings. However, several alterations, such as those related to the liver, require explanation. The underlying mechanisms of acute liver injury associated with COVID-19 have yet to be determined. The possible mechanisms of damage are described below.
PATHOPHISIOLOGY IN COVID-19 INFECTION
Viral receptor
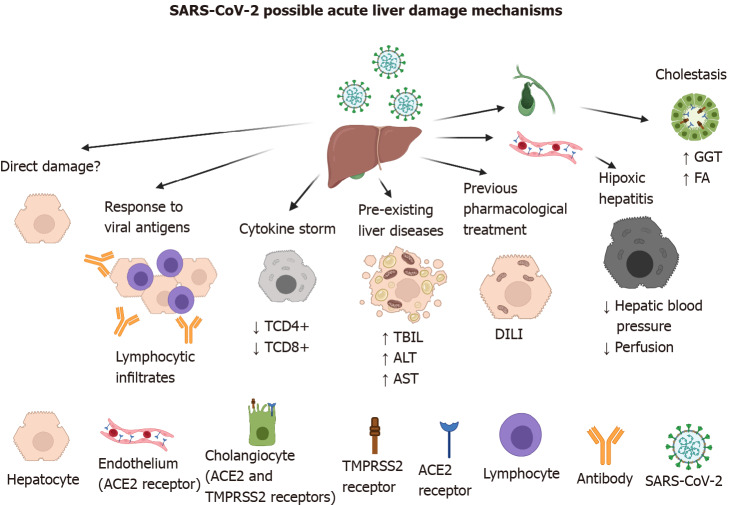
The ACE2 receptor has been described as the main entry receptor for the SARS-CoV and SARS-CoV-2 viruses, and it is abundantly expressed in the cells of the biliary system (i.e. cholangiocytes)[7]. Thus, it has been implicated in elevated levels of alkaline phosphatase and gamma-glutamyl transferase (GGT). However, those changes have been found in a small number of patients[8], compared with elevated levels of aminotransferases directly related to functions of hepatocytes that do not express the ACE2 receptor (Figure 2)[9].
Figure 2.
Possible mechanisms of acute liver damage by severe acute respiratory syndrome-coronavirus-2 infection. Direct damage to the hepatocyte is does not occur because it does not possess the viral entry receptor. However, indirect damage-associated with the immune response against viral antigens, cytokine storm, pre-existing liver diseases such as hepatitis A, B, and C viruses infection, and treatment with hepatotoxic drugs like acetaminophen, remdesivir, lopinavir, etc. has been characterized. In severely affected patients, oxygen deficiency can give rise to hypoxic hepatitis. Severe acute respiratory syndrome-coronavirus-2 damage to cholangiocytes has been documented, because they both possess the angiotensin-converting enzyme 2 and transmembrane protease serine 2 receptors. Created with BioRender.com. ACE2: Angiotensin-converting enzyme 2; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; DILI: Drug-induced liver injury; FA: Alkaline phosphatase; GGT: Gamma-glutamyl transferase;SARS-CoV-2: Severe acute respiratory syndrome-coronavirus-2; TBIL: Total bilirubin; TMPRSS2: Transmembrane protease serine 2.
Direct damage of liver cells by SARS-CoV-2 has been reported, based on ultrastructural changes and SARS-CoV-2 viral particles observed in the cytoplasm of hepatocytes by transmission electron microscopy of liver biopsies of two COVID-19 who subsequently died, one of acute respiratory distress syndrome (ARDS) and the other of septic shock distress syndrome[10]. In a subsequent letter to the editor[11], multiple observations were made indicating that the elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were not sufficient for the condition to be called acute liver injury, the number of biopsies performed were those of two patients, the characteristics of liver cell renewal could be mistaken for hepatic injury, “corona-like” particles could have been intrahepatic cholesterol crystals, and lamellations, or “crown-like” structures are observed in patients with nonalcoholic fatty liver disease.
In addition to the above observations, we would ask how the virus enters cells that do not have the receptor? In the case of cells with high phagocytic capacity that could swallow the virus, viral replication capacity would have to be investigated by the detection of nonstructural proteins. In the case of suspected viral reservoir cells, detecting at least some of the viral structural proteins would require staining, as has been reported for other viruses[12].
On the other hand, SARS-CoV-2 can use transmembrane protease serine 2 (TMPRSS2), which has not been detected in the liver, to enter cells. However, other transmembrane serine proteases have been detected, in particular furin and hepsin (in Huh7-25-CD81 cells)[13]. In three-dimensional (3D) culture systems, liver bile duct-derived progenitor cells form “liver ductal organoids” that retain their tissue-of-origin commitment and genetic stability. In a SARS-CoV-2 infection model with human liver ductal organoids, cholangiocytes expressing ACE2 and TMPRSS2 were preserved ex vivo in long-term culture. In addition, the expression of TMPRSS2 mRNA was found in a subset of hepatocytes and cholangiocytes[14]. In other experiments, human liver ductal organoids showed increased expression of viral mRNA 24 h after being infected with SARS-CoV-2[15]. The experimental evidence suggests that viral receptors are not static and that they can be regulated by mechanisms that involve the presence of the virus and have not yet been described.
Response against viral antigens
Damage to the liver is known to be caused by hepatotropic viruses that replicate in the liver (e.g., hepatitis A, B, C, and E viruses). However, some viruses that attack the respiratory tract can cause liver damage, from small alterations in transaminases to fulminant liver failure, for example, the influenza virus[16,17], parvovirus[18], and respiratory syncytial virus bronchiolitis[19]. In general, hepatitis is thought to be a consequence of an immune response to viral antigens and to the loss of regulation of the inflammatory response. SARS-CoV-2 infection has been associated with hepatitis characterized by focal lobular lymphocytic infiltrates[20]. SARS-CoV-2 viral antigens were recently detected in liver as nucleocapsids and spike proteins[21].
Cytokine storm
The first line of antiviral defense is the innate immune response, initiated by the production of interferon (IFN) type 1 or IFN-α/β[22,23]. IFN-α/β binds to its cellular receptor and initiates autocrine and paracrine signaling to stimulate the expression of genes involved in the antiviral response (interferon-stimulated genes, ISG), in order to build resistance to infection and limit the spread of the virus. However, poor interferon response and high viral replication have been characterized in severe cases of COVID-19[24]. High viral replication stimulates an exaggerated systemic inflammatory response that is related to a sustained elevation of interleukin (IL)-1β, IL-2, IL-7, IL-8, IL-9, IL-10, IL-17, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM)-CSF, IFN-γ, TNF-α, interferon γ-induced protein 10 kDa (IP-10), monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)1α, and MIP-1β, and decreases in CD4+ T cell (TCD4+) and TCD8+ cell counts[25-27]. The latter could contribute to altered liver function[28] and to activation of the coagulation cascade[29,30], and an alteration of iron homeostasis with elevated ferritin levels[31].
Pre-existing liver diseases
Correlations between the severity of COVID-19 and chronic diseases such as diabetes mellitus, arterial hypertension, obesity, renal alterations, and cardiovascular disease have been clearly described[32-34]. Regarding the impact of liver diseases on the progression of COVID-19, ALT and AST activity, and the concentration of C-reactive protein (CRP) have been related to the prediction of disease severity. The prediction of mortality was correlated with liver failure, total bilirubin, platelet count, and serum albumin concentration. Chronic hepatitis B and chronic liver disease were not related to disease severity, the requirement for treatment in the intensive care unit (ICU), or mortality[35]. In another study, 17 inactive hepatitis B virus carriers with SARS-CoV-2 co-infection were found to have abnormal liver function tests (total bilirubin, ALT, and AST)[36]. Thus, it is not clear whether pre-existing liver diseases, such as viral hepatitis, are associated with the severity of COVID-19 infection[37].
Previous pharmacological treatment
The liver contributes mainly to the elimination of lipophilic drugs by increasing their solubility, therefore facilitating their elimination through the cytochrome P450 isoenzyme complex. Therefore, it is important to inquire whether patients diagnosed with COVID-19 are using medications for the treatment of chronic diseases or even anti-influenza or antipyretic drugs[38]. Drugs that are especially potentially hepatotoxic, such as acetaminophen, lopinavir/ritonavir, remdesivir, corticosteroids, and immune modulators, should be taken into account[39] because they can predispose to drug-induced liver injury.
Hypoxic hepatitis
Ischemic hepatitis, also known as hypoxic hepatitis or liver shock, is defined as extensive and potentially severe predominantly centrilobular hepatocellular necrosis resulting from a significant decrease in hepatic perfusion. In patients with severe COVID-19, mechanical ventilation, or hemodynamic disturbance and the occurrence of a sudden drop in systemic blood pressure, can lead to a reduction in hepatic blood pressure, decreased perfusion, and hepatocellular hypoxia. The pathogenesis involves hepatic ischemia and hepatic venous congestion because of elevated central venous pressure, which can predispose the hepatocytes to irreversible hypoxic injury[39,40]. Additionally, the hypoxia can directly affect the tissue–oxygen demand[41].
LIVER MANIFESTATIONS IN COVID-19
Various hepatic manifestations associated with COVID-19 have been documented, ranging from mild enzyme disruption to acute hepatic injury (Figure 3). The manifestations can be grouped into enzymatic (ALT and AST), metabolic (hypoglycemia and hyperammonemia), secretory (hyperbilirubinemia), synthetic (hypoalbuminemia and prothrombin time), and degradation (D-dimer) dysfunctions. Cholestasis with impaired GGT and alkaline phosphatase (FA) is rare, but it is suggested that it may be caused by the dysfunction of the bile duct cells rather than by liver cell damage[8].
Figure 3.
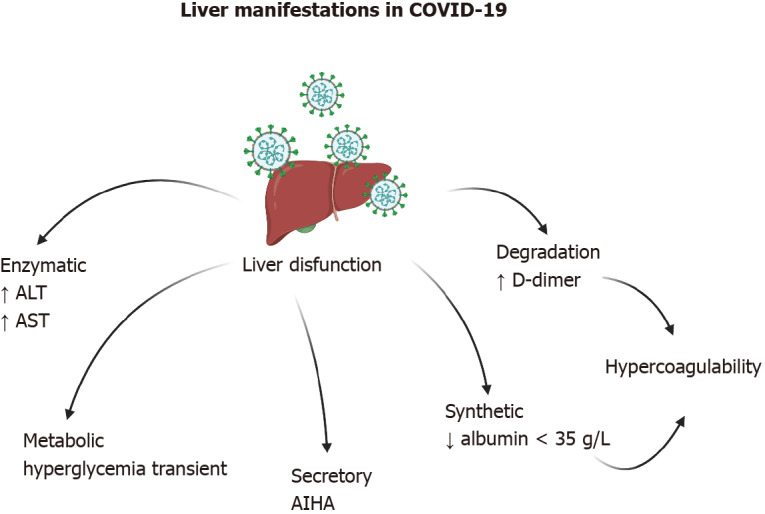
Liver manifestations in coronavirus disease 2019 are quite varied. Increased transaminases and hypercoagulability have been observed in the majority of patients. In rare cases, autoimmune hemolytic anemia has been observed. Created with BioRender.com. AIHA: autoimmune hemolytic anemia; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; COVID-19: Coronavirus disease 2019.
Enzymatic liver dysfunction
Liver enzyme abnormalities in patients with COVID-19 are usually transient and are associated with disease severity. Alanine and aspartate aminotransferases (ALT and AST) are generally found to be elevated in the serum and are more often observed in hospitalized patients[33,42-44] than in patients with subclinical disease. Acute hepatic injury can be diagnosed by an ALT at 10 times the upper reference limit and by AST at three times the appropriate upper reference limit[45].
Metabolic liver dysfunction
Hypoglycemia and hyperammonemia suggest liver failure and have not been found in COVID-19 patients, but there is a hypothesis that coronaviruses can cause a transient dysfunction of pancreatic beta cells[46], leading to acute hyperglycemia and relative insulin deficiency. The hypothesis is supported by a previous study including 39 patients with SARS and without a history of diabetes. Twenty of the patients developed diabetes, all but two temporarily. In addition, ACE2 has been identified in the pancreas of patients with SARS[47].
Secretory liver dysfunction (hyperbilirubinemia)
Regarding bilirubin, no relationship has been directly associated with SARS-CoV-2. However, there are reports of autoimmune hemolytic anemia (AIHA) in patients with symptomatic COVID-19[48,49]. Additionally, there is a report of a patient without clear evidence of SARS-CoV-2 infection for 2 wk and a subsequent positive PCR test. The final diagnosis was AIHA secondary to COVID-19)[50].
Synthetic liver dysfunction (hypoalbuminemia) elevated prothrombin time
Albumin is a protein synthesized in the liver and has a serum half-life of approximately 21 d[51]. In the majority of cases, a decrease in serum albumin can be explained by two factors, either a decrease in its synthesis in the liver) or an increase in renal permeability. However, the prevalence of hypoalbuminemia (< 35 g/L) in COVID-19 patients was increased in critically ill patients in an ICU, but liver damage or albuminuria were not detected. hypoalbuminemia was associated with high-sensitivity C-Reactive protein (hs-CRP) and elevated D-dimer, which is considered to be a marker of thrombin. Therefore, an attempt was made to establish a cause–effect relationship between hypoalbuminemia and hypercoagulability, in that albumin possesses anticoagulant and antiplatelet activity[52]. In a separate study, it was determined that hypoalbuminemia (< 35 g/L) on hospital admission increased the risk of death in COVID-19 patients by at least six-fold. Lower albumin levels on hospital admission can predict the outcome of COVID-19 independently of other known indicators, such as lymphocyte count or comorbidities[32,53].
D-dimer levels
D-dimer is the end-product of fibrin degradation, and it serves as a serological indicator of the activation of coagulation and of the fibrinolytic system. D-dimer levels have been reported as elevated in patients with COVID-19 and have been used as a tool for the prediction of disease severity in 178 studies[54]. In one study, it was determined that elevated D-dimer levels predicted in-hospital mortality in patients with COVID-19[55]. However, the usefulness of D-dimer is diminished by poor reporting of the values. The majority of publications did not identify either the assay manufacturer or the D-dimer product used for the determination. The majority of the authors did not identify whether D-dimer values were reported as D-dimer units or fibrinogen equivalent units (FEU), which differ by approximately ×2. The studies did not report normal cutoff values, and the units of comparison were not the same[56].
IMMUNE SYSTEM AND OXIDATIVE STRESS
Response of the immune system in infection by SARS-CoV-2
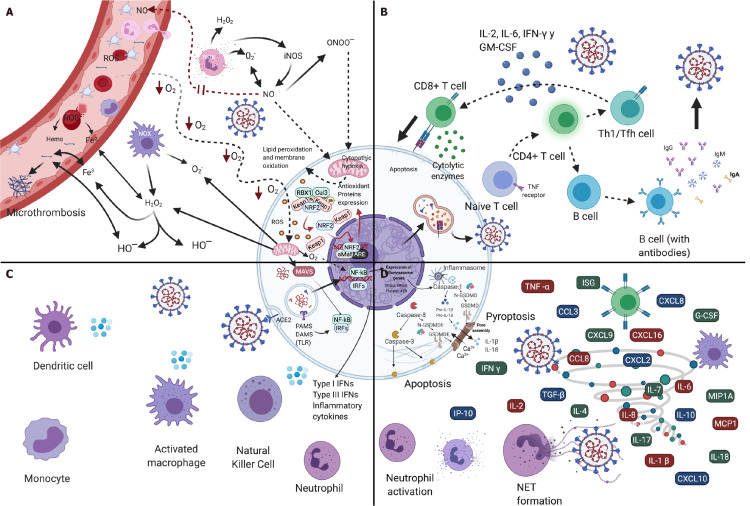
The innate immunological system functions as the first line of defense of the host against infection by SARS-CoV-2; it is crucial for identifying and eliminating the infected cells, and at the same time, for coordinating an adaptive immunity response[57]. The immune response of the host in cases of COVID-19 can be described as an early local immune response (antiviral defense) and a later local/systemic response phase, followed by uncontrolled inflammatory responses and cytokine storm syndromes (Figure 4)[58]. Because SARS-CoV-2 initially affects the upper respiratory tract, its first interactions with the immunological system during the inductive and effector phases, should take place predominantly on the surfaces of the respiratory and oral mucosae[57,59]. Exposure to the virus antigens causes immunoglobulin (Ig)A-mediated responses in the mucosa[60,61] that can be accompanied by the systemic production of IgA, but the systemic production can be absent, transitory, or delayed[61]. The response involving IgA antibodies of the mucosa that maintains an essentially noninflammatory medium[59] and can be particularly prevalent in young people with a mild infection by SARS-CoV-2 without evidence of pneumonia[61].
Figure 4.
Immune response and oxidative stress in severe acute respiratory syndrome-coronavirus-2 infection. A: Oxidative stress; B: Adaptive immune response; C: Innate immune response; D: Cytokine storm. Created with BioRender.com.
If the immunological system of the individual does not counteract the virus during the initial phase of exposure through a rapid early response, the virus advances to the lower respiratory tract (LRT)[62,63]. Once the virus reaches terminal respiratory pathways and the alveoli, B and T cells are activated, which results in the production of specific anti-SARS-CoV-2 antibodies[61], with the predominance of an inflammatory environment dominated by IgG[59]. The S and N proteins are the two principal antigens of the coronavirus that induce the production of Ig[64,65]. The IgA, IgM, and IgG antibodies against the N and S proteins, and the IgM and IgG antibodies against the protein receptor-binding domain, as well as the presence of neutralizing antibodies (nAbs) against SARS-CoV-2 are positive from day 1 after the appearance of symptoms[64]. The antibody levels, especially IgG, increase during the disease course, while a limited increase of IgA and IgM is observed[64]. The antibodies against the S1 and N antigens persist for at least 3 mo after the infection[60,65]. In addition, higher levels of nAbs as well as IgG and IgM antibodies and anti-S1 and -N antibodies have been observed in patients with very severe symptoms[9]. In addition, depletion of memory B cells of IgM was associated with worse outcomes, including a higher mortality rate and a greater risk of developing superimposed infections[66].
In response to viral invasion, the innate immunological system recognizes viral nucleic acids by host recognition-pattern (HRP) receptors, which are expressed in innate immune cells (e.g., neutrophils, dendritic cells, epithelial cells, and macrophages)[67], and by toll-like receptors (TLRs) and retinoic acid-inducible gene I-like (NOD-like) receptors (NLRs) (Figure 4C). For the production of cytokines and the induction of an antiviral state[57,58], as a response to specific pathogen-associated molecular patterns and damage-associated molecular patterns (DAMPs)[57,68]. HRP receptors can activate antiviral responses in neighboring cells and recruit innate and adaptive immune cells and the participation of phagocytes such as macrophages and neutrophils, as well as natural killer (NK) cells[59,68].
Once the virus was detected by an HRP receptor, intracellular signaling pathways are triggered that activate interferon regulatory factor 3 (IRF3) and the nuclear factor kappa beta (NF-κB) signaling cascade[58]. The initial phase of the production of interferon (IFN) mediated by IRF3 performs the initial phase of the innate immune response to detect and brake viral replication[69]. Viral detection stimulates the production of type-1 and type-3 IFN, which results in the expression of interferon-simulated genes ISG (IRF1, IFI44L, and IFIT3) and antiviral genes (OAS3 and ADAR)[70], and the release of large amounts of inflammatory procytokines like interferon gamma (IFN-γ), interleukin (IL)-1RA, IL-6, IL-8, IL-10, and IL-19, monocyte chemoattractant protein 1 (MCP-1), MCP-2, and MCP-3, bonding with C-X-C motif chemokines, including CXCL9, CXCL10, and CXCL5, and tumor necrosis factor alpha (TNF-α)[71]. Stimulation of TLRs activates NF-κΒ, giving rise to the production of inflammatory markers deriving from monocytes (IL-1, TNF-α, and IL-6) to control the infection by means of direct antiviral pathways and the recruitment of other leukocytes[67]. Signaling is elevated 24 h after infection by SARS-CoV-2, leading to the progressive loss of the pulmonary alveolar epithelial function[62]. The activation of complement, and especially of the C5a/C5aR1 axis, was also implicated in the pulmonary pathology of COVID-19[71].
HRP receptors, particularly the nucleotide-binding oligomerization domain-like (NOD)-like receptor (NLR) family, subsequent to the SARS-CoV-2 infection, assemble a multiprotein complex called inflammasome NLRP3, which results in the activation of caspase-1. Activated caspase-1 splits from the pro-interleukin IL-1β, pro-IL-18, and gasdermin D (GSDMD) and releases the GSDMD N-terminal fragment that can be oligomerized within membranes to form membrane pores and “pyroptosis”[57]. The excision of caspase-1-dependent GSDMD leads to the release of IL-1β and IL-18[72], which are key mediators of the inflammatory response, and their increase in the plasma have been correlated with COVID-19 mortality or severity[73]. Apoptosis, another type of cell death, also occurs during SARS-CoV-2 infection and is driven by the excision of the caspase-8, -9, -10 initiators of the executioner caspase-3 and -7. Apoptotic caspase-3 activates gasdermin E (GSDME) to induce the lytic form of cell death; the protein ORF3a of the SARS-CoV-2 virus also induces the excision of caspase-8 and -9 and causes apoptosis[57]. The activation associated with the death pathways of the inflammatory cells can give rise to critical tissue damage, severe inflammation, and lactate dehydrogenase (LDH), which is a marker of cell death that has high concentrations in COVID-19 patients. The LDH concentration is considered a predictive factor for the early recognition of pulmonary lesions in severe cases[74].
Macrophages are a key respiratory system. They produce chemokines and IFN-β. Infected dendritic cells (DCs) produce antiviral cytokines, like IFN-α and IFN-β; the proinflammatory cytokine TNF, IL-6, and high levels of the inflammatory chemokines CCL3, CCL5, CCL2, and CXCL10. The cytokines/chemokines are key factors for the chemotaxis of neutrophils, monocytes, and activated T cells[75]. Activated neutrophils, whose main function is the elimination of pathogens and dendrites by means of phagocytosis[67], release leukotrienes and reactive oxygen species (ROS) that induce local damage to pneumocytes and the endothelium, which in turn leads directly to acute lung lesions[68].
Neutrophils can also develop DNA networks called neutrophilic extracellular traps (NETs)[68] through the process of NETosis, or the release of nucleic acids enveloped in histones, which retain viral particles and promote the inactivation of the viral infection and cytokine production to restrict replication of the virus[67]. NETosis is conditioned to the production of ROS by means of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. In addition to the physical containment promoted by NETosis, NETs contain proteases and cytotoxic enzymes that permit the neutrophils to centralize lethal proteins at the sites of infection. A variety of stimuli, including toxic factors, viruses, and proinflammatory cytokines such as TNF-α and IL-8, can drive neutrophils to release NETs (Figure 4D)[67]. The uncontrolled production of NETs is correlated with the severity of the disease and the extension of the pulmonary lesion, with acute respiratory insufficiency and multiple organic dysfunction syndromes[76]. NETs also contribute to the formation of thrombi, or immunothrombosis, which can amplify the production of cytokines[68]. The inflammatory process comprises a triggering of thrombotic complications that are usually observed in patients with COVID-19, and immunothrombotic dysregulation appears to be an important marker of the severity of the disease[77,78]. In SARS-CoV-2, elevation of the neutrophil/ lymphocyte ratio (NLR), a marker of infection and systemic inflammation, suggests a poor disease prognosis. In addition, patients with COVID-19 have the lowest lymphocyte count and the highest neutrophil count and NLR during severe disease[67,79,80].
The inflammatory responses in the respiratory system intended to eliminate SARS-CoV-2 result in the generation of metabolic-acid waste material that, together with an increase in respiratory muscular work, lead to the development of metabolic acidosis. Metabolic acidosis compromises adaptive cellular immunity and the efficient eradication of SARS-CoV-2[81]. Activated neutrophils and the T lymphocytes depend mainly on glycolytic metabolism for their proliferation, differentiation, and function, which results in the accumulation of lactic acid[81]. A low pH induces anergy in CD8+ T cells, suppresses NK cells, and inhibits the function of CD4+ T cells. Acidosis also increases the levels of circulating glucocorticoids; thus, their anti-inflammatory and immunosuppressor properties compromise immunity against viruses to an even greater degree[81].
Infection by SARS-CoV-2 promotes mechanisms that antagonize proinflammatory signals, particularly the signaling of IFN-I and IFN-III, but increases the expression of chemokines and proinflammatory cytokines in order to counteract the host’s innate immune response[57,58,63]. Thus, the expansion and early differentiation of T cells depend on the direct action of IFN-I[82]. The descending production of interferons promotes intracellular antiviral defenses in neighboring epithelial cells that can limit viral dissemination, while the release of IL-6 and IL-1β from other immune cells promotes neutrophil recruitment and immune cell activation[68].
The three most critical components of the adaptive immune responses are viral protein-specific CD4+ T cells, CD8+ T cells, and nAbs. The nAbs produced by B cells can bind to and neutralize the extracellular SARS-CoV-2 proteins. If the Abs cannot prevent the virus from entering cells, cytotoxic CD8+ T cells are called upon to destroy the cells directly infected with their granules[83,84]. Pulmonary cytotoxic CD8+ T cells recognize and induce apoptosis in cells infected through direct mechanisms (i.e. cell–cell contact) and indirect mechanisms with the participation of the perforin and granzyme-secreted cytolytic enzymes, as well as with the cytokines IFN-γ and TNF-α[85]. However, cytotoxic cells by nature do not prevent the infection, they destroy already infected cells, thus reducing propagation of the infection (Figure 4B)[59]. Transitory increases of the CD8 effector T and memory T cells constitute an effective and efficient response during early viral infection[81]. High counts of CD8+ T cells in the lungs are correlated with better control of SARS-CoV-2[80,85].
CD4+ T cells, the third arm, are auxiliaries and coordinators of the production of Abs and of the activation of the cytotoxic CD8+ T cells[83,84]. After being infected with SARS-CoV-2, CD4+ T cells are activated and differentiate to Th1 cells or circulating T follicular helper T cells (Tfh)[68,80] that secrete proinflammatory cytokines, such as IL-2, IL-6, IFN-γ, and granulocyte-macrophage colony-stimulating factor (GM-CSF)[68] that participate in the activation, proliferation, and differentiation of cytotoxic T lymphocytes. In addition, elevated levels of cytokines secreted by Th2 cells, such as IL-4 and IL-10), which inhibit Th1 inflammatory responses have been reported[86]. The severity of SARS-CoV-2 infection has been related to diminished adaptive immunity responses, mainly because of depletion of T cells and lymphopenia[80], alteration of the differentiation of T follicular helper (Tfh) cells[63,87], low levels of CD8+ NK cells[83], CD4+ auxiliary T cells[87], and memory T cells[84]. However, Abs by themselves do not correlate with the severity of the disease[64,83]. It is probable that the level of inflammation and the amount of proinflammatory cytokines are associated with the activation and depletion of T cells, but it has not yet been determined whether the early response reaches a state of depletion in individuals with severe hyperinflammation[63,70].
Lymphocytes gradually decrease as the disease advances, which results in immunosuppression manifested as atrophy of the organs of the immune system, secondary infection, and multiple organ dysfunction syndrome[75]. Lymphopenia is consistent with overrepresentation of nonfunctional T lymphocytes, with increased percentages of virgin Th lymphocytes (i.e. CD45RA+, CXCR3−, CCR4−, CCR6−, and CCR10−) and a persistent low frequency of markers associated with effector memory T cells, TFH cells, and regulatory T cells (Tregs)[80]. Lymphocytopenia is negatively correlated with inflammatory biochemical parameters (ferritin, fibrinogen, PCR, D-dimer, LDH) and the percentage of lymphocytes and positively correlated with the neutrophil count[80]. From an immunological point of view, lymphopenia could depend on the possibly dysfunctional deactivation of dendritic cells and on the increased concentration of cytokines such as TNF-α, IL-6, and IL-10, which act as negative regulators of the proliferation and survival of the T lymphocytes[88]. The production of acute-phase proteins such as ferritin and CRP, in addition to affecting the equilibrium of pro- and anticoagulant pathways (i.e. increasing D-dimer), can induce lymphocyte apoptosis[82].
The host capacity to generate efficient T cell responses after infection by SARS-CoV-2 probably depends on the directed epitopes, the presence or absence of pre-existing cross-reactive T cells, and genetic factors such as the human lymphocyte antigen (HLA) type, and the repertory of T cell receptors (TCRs)[85]. Given that activated T cells in elderly persons and in those with chronic disease present reduced responses to IFN-I, a longer time is needed to generate effective adaptive immune responses because of the deterioration of the immune functions such as the production of virgin T cells and memory T cells, which diminish with aging[84], and present asynchronous immune responses with high Ab levels and weak T cell responses[83]. Delayed activation of SARS-CoV-2-specific T cells and a reduction of the clarification of the virus increase the risk of cytokine storm, the earlier appearance of severe disease, and increased mortality[68].
In contrast with innate immune responses, which are produced before the infection and participate fully in the elimination of the virus, the adaptive immune responses begin 4-7 d after infection. If the body does not generate effective adaptive antiviral responses in time to eliminate the virus, the innate immune responses will be maintained, but without eliminating the virus in an effective manner and even leading to systemic inflammatory responses and the uncontrolled release of inflammatory cytokines[68].
The inflammatory cytokine storm, also known as the cytokine release syndrome, is a severe excessive immune response caused by positive biofeedback circuit damage of immune cells by the cytokines[67,75,87]. The formation of a cytokine storm leads to a “suicide attack” that not only limits additional propagation of the virus, but also induces secondary tissue damage[68]. The marked release of proinflammatory cytokines causes lymphopenia, lymphocyte dysfunction, granulocyte and monocyte anomalies[58], coagulation disorders such as capillary extravasation syndrome, formation of thrombi, and even the combined immunodeficiency syndrome[76,81]. A series of destructive effects on tissues, including destabilization of the interactions among endothelial cells, damage to the vascular barrier, diffuse alveolar damage characterized by the formation of hyaline membranes[13], ARDS[57,68], tissue toxicities that affect the respiratory, hematological, gastrointestinal, cardiovascular, renal, hepatic, and neurological systems[89], multiorgan failure[80] and, ultimately death may occur[58,68,75,76]. Despite the large number of studies much of the physiology of the immune response in COVID-19 has yet to be described.
Oxidative stress and infection by SARS-CoV-2
Oxidative stress is the result of disequilibrium between the oxidant system, which consists principally of free radicals, ROS), and reactive nitrogen species (RNS), and the antioxidant systems that neutralize the free radicals[90]. Reactive oxygen and nitrogen species (RONS) are characterized by unpaired valance electrons, obliging them to react with diverse biological molecules[90,91]. ROS comprise the hydroxyl (OH) radicals, superoxide anion (O2−), singlet oxygen (¹O2), hydrogen peroxide (H2O2), and ozone (O3). RNS include nitric oxide (NO), peroxynitrite (ONOO−), nitrosyl cation (NO+), the nitrosyl anion (NO−), and nitrose acid (NH2O2)[90]. Under physiological conditions, the reactive species play an important role in cellular signaling (redox signaling) and the regulation of cytokines, and growth factors such as immunomodulators, cellular differentiation, and others. However, when the equilibrium of oxidant agents and antioxidant systems is disturbed, harmful effects are generated[90,91]. The damage caused by free radicals affects cellular membranes by lipid peroxidation, oxidation, protein denaturalization, DNA damage that can induce inflammatory immune responses and increase the risk of mutations and tumorigenesis, and apoptosis[90]. In general, hydroxyl radicals are highly reactive and are responsible for the greatest cellular damage modification of biomolecules induced by ROS. H2O2 is considered the least harmful and can travel to and penetrate cell membranes, and the superoxide is intermediately harmful[92].
In the pathology of COVID-19, the cytokine storm is an important source of endogenous oxidative stress, and excessive production of ROS that in turn stimulates the increased release of cytokines, causing an exaggeration of the already initiated inflammatory responses (Figure 4A)[93-97]. The interaction of ROS and cytokines generates a self-sustaining cycle involving the cytokine storm and the production of oxidative stress that eventually leads to a high pulmonary protein exudate with a low hemoglobin carrier, the generation of free radicals and proteases, and an increase in the permeability and entry of edematous fluid into the alveoli. The results in deficient gas exchange in the lungs, pulmonary hypoxia, cytopathic hypoxia, damage to the epithelium, acute pulmonary lesions, disseminated coagulation, multiorgan failure and death in patients with COVID-19[95-98].
The cytokine storm with hyperinflammation accompanied by cytopenia and hyperferritinemia is known to generate ROS, by means of the Fenton reaction (Fe²+ + H2O2→ Fe³+ + HO- + HO-). Additionally, the cytokines and endotoxins stimulate an isoform of nitric oxide synthase (iNOS), the inducible isoform NO, which stimulates the production of NO that in turn reacts with the superoxide to yield peroxynitrite (ONOO−)[90,96]. Both peroxynitrite and NO are toxic to mitochondria, producing dysfunctional mitochondria that, in turn, result in cytopathic hypoxia[96,99]. In addition, they cause a possible oxidative storm with all of the harmful effects of RONS, in particular the peroxidation of lipids and oxidation of membrane proteins that contribute to the transformation and hyalinization of the pulmonary alveolar membranes, with lethal respiratory difficulty[90].
SARS-CoV-2 activates oxidant-sensitive pathways through inflammatory responses following activation of the NF-κΒ pathway[93]. The reduction of oxygen saturation leads to the generation of superoxide radicals and H2O2 by the mitochondria. Hydrogen peroxide triggers the expression of genes that positively regulate proinflammatory cytokines, such as IL-1, IL-6, and TNF-α, and inducible nitric oxide synthase (iNOS) by means of the activation of the (iNOS) NF-κΒ pathway[94,96,98,100]. That, together with the ROS, activates NLRP3 inflammasomes[101]. IFN-γ, IL-1β, IL-2, IL-6, and TNF-α stimulate the generation of NO[96]. IL-6 and TNF-α give rise to superoxide generation in neutrophils, and hydrogen peroxide stimulates the generation of IL-6[98,102]. In cyclical fashion, the proinflammatory cytokines activate macrophages, neutrophils, and endothelial cells through NADPH oxidase (NOx) to produce more superoxide and H2O2. Patients with COVID-19 exhibit an overactivation of NOx2, a mechanism that favors ischemic events related to thrombotic events and associated with severe disease[103].
At the same time, the IFN-γ pathways are activated by oxidative stress induced by the inflammation intended to combat the infection by the virus[33]. Circulation of the inflammatory cytokines and ROS damage erythrocytes, leading to the generation of heme and free iron and diminish the circulating nitric oxide (NO), which worsens the existing ischemia of the organs. Deterioration of the mitochondria leads to cytopathic hypoxia, which results in a partial reduction of oxygen with the generation of ROS and the reduced energy production[98]. In addition, macrophages and activated neutrophils produce respiratory bursts that generate superoxide radicals and H2O2[94,98] that maintain the oxidative stress[98].
Poorly coordinated iron, especially in the presence of high concentrations of oxygen and reducers have the potential to generate hydrogen peroxide, superoxide, and hydroxyl radicals in the lung[92]. The radical superoxide anion reduces Fe (III) to Fe (II) that, in the presence of H2O2, produces hydroxyl radicals (•OH), which are extremely toxic and promote the formation of lipid peroxidases in the cell membrane and the oxidation of proteins, causing cell death by apoptosis[94]. Hydroxyl radicals plus free iron convert soluble plasma fibrinogen into abnormal fibrin clots in the form of enzymatic degradation-resistant dense and entangled deposits, leading to microthrombosis in the vascular system and in the microcirculation[95,96].
Oxidative damage resulting from SARS-CoV-2 infection can produce viral mutations that affect the immunological response. In addition, overproduction of ROS suppresses T-lymphocyte responses and results in weakened adaptive immunity[94], altering the structure and function of the circulating lymphocytes, principally TCD4+, with selective depletion reduced antiviral activity of CD8+[94] T cells. In general, the host response to stress and to combat an inflammatory condition is marked by a strong increase of the cortisol level. Cortisol supports the mechanisms of the host immunological defense in a permissive manner, and high levels of cortisol suppress inflammation and prevent tissue damage[93]. However, in the case of severe COVID-19, patients can develop a corticosteroid insufficiency related to a critical disease[93]. It is known that overproduction of ROS and the weakening of antioxidants are needed for viral replication and the subsequent disease associated with the virus[97]. Viral infections alter antioxidant mechanisms leading to an unbalanced oxidative-antioxidant state and consequent oxidative cellular damage. Exposure to various pro-oxidants generally leads to activation of nuclear factor erythroid 2-related factor 2 (Nrf2) and to an increase of the expression of components of the antioxidant response. However, respiratory virus infections have also been associated with inhibition of the Nrf2 pathways that leads to inflammation and oxidative damage[97]. Nrf2 is a transcription factor responsible for the adaptation of cells and tissues including alveolar epithelium, endothelium, and macrophages to electrophilic or oxidative stress. Under normal conditions, Nrf2 is found in the cytoplasm bound to its inhibitor Keap1, which is directed to Nrf2 for ubiquination and later degradation. In the presence of electrophiles or ROS, the Keap1–Nrf2 complex dissociates and Nrf2 migrates to the nucleus where it stimulates the transcription of target genes with sequences of antioxidant response elements in their promoters[104]. Nrf2 controls the expression of the genes that participate in the antioxidant response, redox homeostasis, and the biogenesis of the mitochondria, etc. In addition, Nrf2 functions as a transcription repressor that inhibits the expression of inflammatory cytokines in the macrophages (e.g., IL-1β, IL-6, and TNF-α)[95]. SARS-CoV-2 can interfere with the equilibrium between the transcription factor NF-κB involved in the expression of cytokines and in the activation of Nrf2, responsible for the expression of antioxidant enzymes[96], including hemoxygenase 1 (HO-1), superoxide dismutase 1 (SOD1), superoxide dismutase 3 (SOD3), glutathione S-transferase (GST), catalase (CAT), and glutathione peroxidase (GPx)[96]. Nrf2 also regulates the increase in the production of the antioxidant enzymes NAD(P)H and quinone oxidoreductase (NQO1), and enzymes needed for the biosynthesis of glutathione, which functions as the main cellular antioxidant[95]. In patients with SARS-CoV-2 infection, deficiencies in systems protection against free radicals, as well as deficits in superoxide dismutases (SODs), CAT, and reduced glutathione (GSH) have been described[94].
Oxidative stress is already increased in the elderly and people with diabetes and chronic cardiovascular diseases[90,91]. Increase in the stress level in response to viral infection affords a possible explanation of the severity of COVID-19 in such patients. In addition, elderly individuals may particularly vulnerable to infection by SARS-CoV-2 because the level and the activity of Nrf2 diminish with age[98]. Therefore, aging is not only associated with alterations in the response to adaptive immunity, but also to a proinflammatory state in the host[97].
NUTRITIONAL MANAGEMENT AND APPROACHES
Malnutrition in patients with COVID-19 infection
SARS-COV-2 infection and the resulting COVID-19 may cause multiorgan failure in addition to respiratory symptoms, including gastrointestinal (GI) and liver dysfunction, which can be complicated in the elderly or in the presence of comorbidities. There are complications associated with prolonged ICU stays; thus, the longer the ICU stay, the greater the risk of malnutrition. Malnutrition, mostly undernutrition, is the result of inadequate food intake or altered nutrient assimilation, and when it is caused by disease, different degrees of acute or chronic inflammation contribute to the state. Inflammation induces the elevation of resting expenditure, anorexia, and reduced intake. Such catabolic conditions modify body composition, with waste of muscle mass that alters functionality along and clinical outcomes[105]. Indeed, malnutrition may develop as a result of the physiological effects of SARS-COV-2, management procedures such as ventilation. Limited access of patients to direct consultations with healthcare professionals because of confinement impairs the identification of risk factors for malnutrition as well detection of the reduced food intake. Unplanned weight loss and the diminution of muscle mass induce a catabolic state in the patient, with an impact on mobility and, in turn, a poor quality of life and an increased risk of mortality, in addition to the complication of polymorbidities. As a consequence of malnutrition, immune function can be reduced, respiratory and muscle strength may be impaired, which delay recovery and result in longer hospital stays with poor outcomes. Thus, the additional use of healthcare services increases costs.
Based on the previous statement, malnutrition should be identified as an early step in the assessment of patients with SARS-CoV-2 infection, especially in patients with a high risk of mortality or of poor outcomes, such as older adults and polymorbid individuals. The identification of malnutrition is crucial for establishing effective nutritional support in order to improve food consumption, nutritional status, the patient’s health prognosis, and even to prevent the occurrence of COVID-19 in the future. Particularly in the COVID-19 crisis, food and nutrient absorption is often impaired by nausea, vomiting, and diarrhea, the main GI symptoms, which lead to enhanced malnutrition. The international meta-analysis of McClave et al[106] of 47 studies in 10890 patients who were analyzed to determine the prevalence of liver and GI manifestations resulting from COVID-19 established consultative management of patients. Nausea/vomiting was a very frequent GI manifestation with a pooled prevalence of 7.8% (95%CI, 7.1%-8-5%) followed by diarrhea in 7.7% (95%CI, 7.2%-8.2%) and abdominal pain in 2.7% (95%CI, 2.0-3.4%). Moreover, ALT had a pooled prevalence of 15% (95%CI, 13.6%-16.5%) and AST had a prevalence of 15% (95%CI, 13.6%-15.4%) as manifestations of liver disorders. A cohort study of patients with COVID-19 in Hong Kong revealed that GI symptoms were present in 17.6% of patients and that diarrhea appeared to be the most common symptom. In the meta-analysis, the RNA virus was detected in 48.1% (95%CI, 38.3%-57-9%) of stool samples, and 70.3% (95%CI, 49.6%-85.1%) of the samples were collected after respiratory samples were found to be negative. Therefore, good management must be considered as a low risk of infection through endoscopic procedures or saving stool samples[107]. Thus, it is suggested that, prior to the administration of any treatment, it is necessary to evaluate the nutritional status of every infected patient.
In clinical practice, malnutrition is assessed with various tools (Table 1). The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends using the Malnutrition Universal Screening Tool (MUST) for the identification of malnutrition in patients diagnosed with COVID-19[100]. The MUST instrument was designed for persons at risk of malnutrition, not only in those who are underweight but also in obese individuals. In patients with SARS-COV-2, loss of appetite is very frequent because of symptoms such as shortness of breath, loss of taste and smell, muscle pain, fatigue, and general discomfort. Related symptoms may contribute to insufficient achievement of energy and nutrient demands, thus promoting unplanned weight loss. To perform the MUST assessment, simple objective measurements of weight and height are needed to estimate the body mass index (BMI) in addition to weight at 3-6 mo previously. The acute disease effect denoted no nutritional intake for more than 5 d. However, even if conditions do not allow for obtaining objective measurements, screening can be carried out utilizing other measurement options, such as ulna length to estimate height and mid-upper-arm circumference. The latter are very useful for subjective BMI estimation in that healthcare personal and professionals, the patients’ family members, or the patients themselves are able to obtain them. Nevertheless, if the patients are in the ICU or at home, and if it is not possible to obtain the measurements in any manner, the second step comprises the subjective criteria. The patient is asked to seek information related to reduced food consumption during the last 5 d or more, apparent weight loss visible in clothes and jewelry, changes in smell and taste perception if the cause of the reduced food intake is due to related symptoms in COVID-19 described previously, clinical management (ventilation, medication, and sedation), or because of the influence of physiological factors (social restriction, anxiety, and depression). The subjective criteria are useful to estimate a low, medium, or high risk of malnutrition under different circumstances, considering the limitations of infection-control restrictions instituted by local policies. It is highly recommended that patients remaining at home also perform a complete MUST assessment following progress after the implementation of nutritional strategies.
Table 1.
Tools for malnutrition detection in patients with coronavirus disease 2019 and liver injury
| Tool | Target patients | Criteria |
| MUST | Low weight | Objective criteria: |
| Objective measures: weight and height to obtain BMI | ||
| Other measures (optional): ulna length and mid upper arm circumference | ||
| Weight loss in las 3-6 mo | ||
| Obese patients | Subjective criteria: | |
| Reduced food intake in last 5 d: clinical management, psychological factors | ||
| Weight loss appearance (clothes, jewelry) | ||
| NRS-2002 | Hospitalized individuals | BMI |
| Weight loss within 3 mo | ||
| Reduced dietary intake in last week | ||
| NUTRIC score | Hospitalized patients at ICU | Age |
| Days hospitalized or in the ICU | ||
| Number of comorbidities | ||
| IL-6 levels (optional) | ||
| APACHE II score | ||
| SOFA score | ||
| APACHE II score | Patients at ICU (predicting mortality) | Age |
| Temperature | ||
| Mean arterial pressure | ||
| pH | ||
| Heart rate/pulse | ||
| Respiratory rate | ||
| Sodium, potassium levels | ||
| Creatinine | ||
| Acute renal failure | ||
| SOFA score | Patients at ICU (estimation of mortality) | PaO2 |
| FiO2 | ||
| Medical ventilation | ||
| Platelets level | ||
| Glasgow Coma Scale | ||
| Bilirubin levels | ||
| Mean arterial pressure or administration of vasoactive agents required | ||
| Creatinine levels | ||
| Is a COVID-19 patient? | ||
| GLIM | Individuals at risk in general | Phenotypic criteria: |
| Weight loss | ||
| Low BMI | ||
| Loss of muscle mass | ||
| Etiologic criteria: | ||
| Reduced food intake or assimilation | ||
| Presence of disease or inflammation | ||
| NRF-NPT | Detection of malnutrition in liver patients disease | Unplanned weight loss in las 3-6 mo |
| BMI | ||
| Reduced dietary intake and uncompleted meals |
BMI: Body mass index; GLIM: Global Leadership Initiative of Malnutrition; ICU: Intensive care unit; MUST: Malnutrition Universal Screening Tool; NRF-NPT: Royal Free Hospital-Nutritional Prioritizing Tool. NRS-2002: Nutrition Risk Screening-2002; NUTRIC: Nutrition risk in the critically ill; SOFA: Sequential Organ Failure Assessment.
Several tools developed for assessment of hospitalized patients have been applied in COVID-19. Nutrition Risk Screening-2002 (NRS-2002) criteria, also recommended by ESPEN, predict malnutrition in hospitalized individuals and are recommended by the American College of Gastroenterology (ACG) guidelines for nutrition therapy[106]. NRS-2002 takes into account BMI; weight loss within the past 3 mo; reduced dietary intake in the previous week, and an ICU stay. In addition, the Nutrition Risk in the Critically ill (NUTRIC) score[108-111] is designed for patients, particularly for those in the ICU, and for those who can benefit from nutritional therapy considering their age, days hospitalized or time in the ICU, number of comorbidities, IL-6 Levels (optional). The Acute Physiology And Health Evaluation II (APACHE II) score[112,113], which is the most widely used tool for predicting mortality in the ICU, and the Sequential Organ Failure Assessment (SOFA) score[114,115], which estimates ICU mortality based on clinical data and laboratory results have both been used in COVID-19 clinical trials[116].
Recently, the Global Leadership Initiative on Malnutrition (GLIM), which includes the leading clinical nutrition societies worldwide, achieved a consensus with the purpose of establishing global criteria for the diagnosis of malnutrition in clinical practice. Such a consensus is a two-step approach. The first consists of the identification of “at risk” status by validated screening tools, and the second is the assessment for the diagnosis and categorization of the severity of the malnutrition[117]. According to this, three phenotypic criteria were highlighted: weight loss; reduced muscle mass, and low BMI, along with two etiological criteria, reduced food intake or assimilation and the presence of disease or inflammation. Therefore, the diagnosis of malnutrition requires at least one phenotypic and one etiologic criterion. The stratification of the phenotypic metric considers stage 1 (moderate) and stage 2 (severe)[117].
In patients with liver damage caused by COVID-19, the risk of malnutrition may be increased by the presence of GI-associated abnormalities. In severe cases of COVID-19 infection, liver injury has most commonly been observed. At the same time, patients with previous liver damage represent more critical cases of COVID-19[118]. The incidence of liver injury ranges from 14%-53%[8]. Approximately one-third of the infected population reported altered aminotransferase levels. Liver damage may affect glucose, amino acid, and lipid metabolism, and result in poor clearance of lactate and protein catabolism as a consequence of hyperaminoacidemia, and hyperammonemia, which contributes to malnutrition. On the other hand, it is crucial to determine whether the liver injury is a consequence of pre-existing liver injury (cirrhosis, viral hepatitis, NASH, and ASH) or the result of COVID-19 infection and/or the reported drug-induced liver injury. In conjunction with the MUST and NRS-2002 tools for malnutrition assessment, ESPEN recommends the Royal Free Hospital-Nutritional Prioritizing tool (RFH-NPT), which has been developed for the detection of malnutrition in patients with liver disease[119]. RFH-NPT is a validated tool very similar to NRS-2002, but it has demonstrated greater sensitivity for the identification of malnutrition in liver diseases. The screening tool considers nearly the same indicators, namely unplanned weight loss in the previous 3-6 mo, BMI, reduced dietary intake, and uncompleted meals. The stratification scale is based scores of 1 (low risk), 2 (moderate risk), and 2-7 (high risk)[120]. Thus, in patients with COVID-19 and liver damage, the use of at least two of these options for the detection of malnutrition could be reasonable.
Nutrition strategies for liver injury in COVID-19
In COVID-19, disease complications are basically the result of a hyperinflammatory response mediated by cytokine storm and several immune-related stimuli mentioned above. Thus, nutrition treatment should be designed to strengthen the immune system during the COVID-19 crisis, providing nutrients that relieve inflammation and oxidative stress. Whether liver injury emerges as a consequence of COVID-19 or pre-existing before infection, nutrition strategies should also correct liver and GI manifestations as related symptoms to prevent or treat the associated malnutrition.
Energy and nutrient recommendations
Liver injury associated with COVID-19, as in any disease, could enhance inflammation, which alters the metabolic rate. In critically ill patients, it is recommended to estimate energy needs by means of indirect calorimetry considering all of the conditions for sterility during the measurement procedure. Significant increases in resting energy expenditure (REE) in patients with acute liver failure (ALF, 18%-30%)[121], alcoholic hepatitis (55%)[122] and alcoholic cirrhosis (26%)[123] have been reported. Hypermetabolism reported in patients with liver cirrhosis (> 30%)[124] could be related to effect of delay in the improvement of body composition on clinical outcomes. Hence, because of the individual variability in liver damage, REE should be measured by indirect calorimetry. Recently, the estimation of REE with a less-expensive handheld calorimeter method based on a respiratory quotient of 0.85, and which is very accurate for REE has been proposed[125]. However, when accessibility to calorimetric equipment is limited, prediction equations for the estimation of energy expenditure could be employed. A summary of caloric and nutrient recommendations is shown in Table 2. Caloric intake should be 1500-2000 kcal/d for normal maintenance by oral diets, with an increase of 400-500 kcals under conditions of stress or in an infection crisis[126]. ESPEN guidelines[100] suggest 27 kcal/kg body weight (Bw)/day in polymorbid patients and in patients > 65 years of age. For low-weight or older patients, it is suggested to achieve 30 kcal/kg Bw/day. In malnourished cirrhotic patients with muscle depletion, the energy supply must provide 30-35 kcal/kg Bw/day. Contrariwise, in overweight or obese patients with liver disease, the prognosis may be worse. In such cases, obesity has been associated with portal hypertension[127]. For that reason, an increased energy intake is not recommended. Nonetheless, all energy recommendations must be adjusted individually, taking into account disease severity, mobility, physical activity, and tolerance. In severely underweight patients, the energy supply must be carefully administered in order to prevent the refeeding syndrome, which is very common in such patients. Whenever possible, oral feeding should be the first energy and nutrient-supply option. When oral feeding is not feasible, support nutrition therapy by nasogastric tube through enteral nutrition (EN) or parenteral nutrition (PN) should be available as the next step.
Table 2.
Energy and nutrient recommendations for patients with coronavirus disease 2019 with or without liver injury
| Energy/Nutrient | Criteria | Recommendation |
| Estimation of REE | All individuals with COVID-19 | Estimation by indirect calorimetry |
| Prediction equations | ||
| Calories | Normal oral diets | 1500-2000 kcals/d |
| Increase 400-500 kcals in stress or infection crisis | ||
| Polymorbid, old patients > 65 yr | 27 kcals/kg Bw/day | |
| Low weight, older patients | 30 kcals/kg Bw/day | |
| Malnourish chronic patients and muscle depletion | 30-35 kcals/kg Bw/day | |
| -Patients with COVID-19 outside ICU | ONS with low oral intolerance: | |
| 150-400 kcals/service | ||
| 70-100 g protein/service | ||
| Carbohydrates, fiber, PUFAs, vitamins, minerals, probiotics | ||
| Consuming for a month | ||
| Protein | Normal individuals (prevent loss and muscle mass) | 1 g/kg Bw/day |
| 70-100 g/d | ||
| Form animal (milk, yogurt, meat, fish, chicken, cheese) and vegetable sources (beans, soy, nuts, peas) | ||
| Patients with liver cirrhosis sarcopenic | 1.2-1.5 g/kg Bw/day | |
| Obese sarcopenic | Oral supplementation of BCAA 0.20-0.25 g/kg Bw/day or 30 g/d | |
| Glutamine and arginine supplementation | ||
| Carbohydrates/fat | Patients with COVID-19 without respiratory impairment | Ratio 70:30 carbohydrates/fat |
| Medium and low glycemic | ||
| Fiber 25-30 g/d | ||
| PUFAs: DHA, EPA, ALA | ||
| Patients with ventilator support | Ratio 50:50 carbohydrates/fat | |
| Vitamins | All individuals with COVID-19 | A, C, D, E, folate, B6 and B12 (monitoring in patients with liver abnormalities) |
| Minerals | All individuals with COVID-19 | Zinc, copper, selenium (monitoring in patients with liver abnormalities) |
| Critically ill patients | EN after 24-36 h. after ICU admission | |
| Initiate with trophic low-dose (10-20 mL/h.) | ||
| Polymeric formula: 15-20 kcals/kg Bw and 1.2-2.0 g/kg Bw/day of protein vitamins, minerals, fiber, probiotics | ||
| Provide 70%-80% needs in over 1 wk | ||
| Sever obese patients BMI > 50 | Energy 22-25 kcals/kg IBW | |
| Protein 2 g/kg per day (Class I, II) or 2.5 g/kg IBW/day of (Class III) | ||
| Vitamins, minerals, fiber, probiotics | ||
| Gastric intolerance individuals | Use prokinetics | |
| Post-pyloric feeding in persistence intolerance or at high risk of aspiration | ||
| Patients with no GI feasible | PN recommended | |
| Poor nutrition status | Limit the use of omega-6 soy-based ILE during first week | |
| Prolonged stay at ICU | Mixture of lipids such as olive oil based ILE or SMOF (soy, medium chain triglycerides, olive oil, fish oil) |
ALA: Alpha linoleic acid; BCAA: Branch chain amino acids; DHA: Docosahexaenoic acid; EN: Enteral nutrition; EPA: Eicosanoid acid; GI: Gastrointestinal; IBW: Ideal body weight; ICU: Intensive care unit; ILE: Intravenous lipid emulsion. ONS: Oral nutritional supplements; PN: Parenteral nutrition; PUFAs: Polyunsaturated fatty acids; REE: Resting energy expenditure.
Proteins in the diet are known to be crucial nutrients for gut-associated lymphoid tissue, as are active immunoglobulins, which act against infection in the gut mucosa[128]. The consumption of high-value proteins that contain essential amino acids is associated with immune responses, for example, adequate production of antibodies; activation of T and B lymphocytes, macrophages, and NK cells; and the production of cytokines and other immune elements that prevent infectious diseases[129]. In addition, in order to prevent body weight loss and muscle mass, > 1 g/kg Bw/day of protein intake is recommended for patients with anorexia or reduced food intake. In general, 1 g/kg Bw/day is sufficient to meet the requirement, but protein intake should be adapted to individual needs according to disease status, physical activity, and tolerance. Overall, 70-100 g/d of protein are acceptable in the oral diet, preferably consumed from animal (milk, yogurt, meat, fish, chicken, and cheese) and vegetable sources (beans, soy, nuts, and peas)[126]. Oral nutrition supplements (ONS) are a fairly good option when the goals of oral feeding are not fully achieved. In the latter cases, ONS are suggested to supply 150-400 kcals and 15-30 g of protein and should be consumed for at least 1 mo[100,126].
In liver injury, nutritional approaches must be attended to according to the type and grade of the lesion(s). In patients with acute liver failure, nutrition therapy must be centered on providing sufficient energy in the form of glucose and fatty acids along with vitamins and mineral elements, preventing hypoglycemia and hypertriglyceridemia, and protein and amino acid sources adequate to avoid catabolism and to promote protein synthesis. Patients with liver cirrhosis are very likely to experience malnutrition and muscle wasting because of a decrease in protein synthesis and increased total protein breakdown. The recommendation is 1.2-1.5 g/kg Bw/day of protein to ameliorate protein synthesis in patients with sarcopenia, including those with obese sarcopenia, preferably with high-quality proteins from animal and vegetable sources in oral diets. In those with poor tolerance of protein, vegetable sources are acceptable[119]. It is preferable to distribute the intake in 3-5 meals a day, avoiding long starvation periods. A late evening snack of protein and carbohydrates has been shown to be very effective in improving nitrogen balance in cirrhotic patients[130,131]. Oral supplementation with isoleucine, leucine, valine, and branched chain amino acids (BCAA) has been useful for patients with intolerance to protein with liver encephalopathy. In liver dysfunction, low BCAA levels are regularly observed in addition to high levels of tryptophan, aromatic amino acids, and sulfur-containing amino acids. The imbalance of amino acids may induce liver encephalopathy. Hence, the administration of 0.20-0.25 g/kg Bw/day or 30 g/d of BCAA has beneficial effects on the patient’s mental state after episodes of encephalopathy and on improvement of protein metabolism[132,133]. In addition, BCAA supplementation in protein-restricted diets has been associated with improvement of the intestinal immune defense by increasing the levels of jejunal and ileal immunoglobulins and protecting duodenal villous morphology[134]. Some amino acids are responsible for modulating the immune response; for instance, glutamine activates protein participation with extracellular signal-regulated kinases (ERKs) and c-Jun N-terminal (JNKS) kinases in signal transduction, triggering transcription factor activation, and the expression of genes regulating the proliferation of lymphocytes, macrophages, and neutrophils. Moreover, glutamine induces the expression of some cytokines, including IL-6, TNF-α, and IFN-γ, and certain surface markers of lymphocytes[135]. Under catabolic and hypercatabolic conditions, glutamine has an essential role in metabolism; therefore, it is widely utilized in clinical nutrition under conditions of immunosuppression[135,136]. On the other hand, arginine and its downstream metabolites citrulline and ornithine are involved in T cell activation, and promoting and modulating innate and adaptive immune responses[137].
During liver injury, the metabolism of glucose is altered, the glucose oxidation rate is reduced, and the glucose production rate in the liver is low because of the depletion of glycogen stores despite increased gluconeogenesis. Glucose deposition in skeletal muscle and liver as glycogen is impaired. At the same time, glucose uptake in tissues is reduced because of insulin resistance in response to high secretion and reduced degradation[138,139]. Lipid metabolism reflects an augmented rate of lipid oxidation parallel to insulin resistance. Additionally, the plasma levels of essential and polyunsaturated fatty acids are reduced in relation to the nutritional status and to the severity of the injury[140]. In patients with COVID-19, the ratio of carbohydrates to fat should fall within the range of 70:30 for patients without respiratory impairment and within 50:50 in patients on ventilatory support[141]. Nonetheless, it is noteworthy to take into consideration the previously mentioned changes in glucose and lipid metabolism in patients with liver disorders. In oral diets, low glycemic index foods, such as vegetables, whole grains, and legumes, are preferable rather than those with high glycemic/glycemic loads, which have been associated with the immediate increase of the inflammatory cytokines IL-6 and TNF-α[89] and CRP[142]. In parallel, fatty acids (FAs) have also been associated with the immune response, impacting on macrophages, epithelial cells, lymphocytes, neutrophils, dendritic cells, and lymphoid cells[143]. The consumption of saturated and trans-FAs from processed and fried foods has been correlated with the elevation of hs-CRP, IL-6, and TNF-α[144]. In influenza A virus infection, a diet rich in saturated FAs has been associated with impaired immune viral response, resulting in higher viral loads in the lung and heart, inducing inflammation and tissue damage[145]. Contrariwise, polyunsaturated FAs, particularly omega-3 FAs, have a potent anti-inflammatory effect. Omega-3 FA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), consumed mainly from fish and seafoods, including α-linolenic acid (ALA) consumed from plant sources, have been reported to initiate anti-inflammatory signaling[143]. In epithelial cells in the lungs, gut, and skin, omega-3 FAs are capable of activating nuclear and transmembrane receptors and can restore a compromised barrier defense and reducing the production of anti-inflammatory mediators such as IL-1β, TNF-α, interferon gamma (IFN-γ), and lipopolysaccharides (LPSs)[69]. Also, they reduce the expression of ERK1/2 MAPK, NF-κB, and COX-2, the main signaling pathways of the inflammatory response[69]. In viral infections, supplementation with omega-3 FAs appears to inhibit the production of TNF-α, IL-1β, IL-6, and IL-8 and reduce the production of ROS. In critically ill patients in the ICU, the addition of fish oil rich in omega-3/antioxidants in the enteral formula appears to enhance the response to oxygen therapy, improve clinical outcomes, and shorten ICU stays[146,147].
For patients outside the ICU, nutrition treatment should begin early, within 24-48 h of hospitalization, orally and mainly in older and polymorbid patients, whose nutritional situation may be compromised. The use of ONS could be fully applicable in patients with COVID-19 and liver injury in order to prevent or treat malnutrition and reestablish liver function. ONS are recommended to meet energy, macronutrient (carbohydrate, protein, and lipids) and micronutrient (vitamins and mineral elements) needs. Fiber and probiotics are suggested to promote optimal intestinal function. The increase of dietary fiber from whole grains benefits gut microbiome composition and is also correlated with reducing both systemic and gut inflammation by reducing IL-6, TNF-α, and hs-CRP, and by increasing short chain fatty acids (SCFAs)[74]. SCFA are produced by gut microbiota as a result of dietary fermentation. They are potential activators of anti-inflammatory signaling cascades and inhibitors of proinflammatory cytokines, as well as the reduced expression of NF-kB[72]. Furthermore, dietary fiber has been reported to promote healthy gut microbiota, which is related to inhibition of systemic inflammation and enhanced mucosal thickness, protecting the gut barrier from the infiltration of pathogens[77]. Dietary fiber is also thought to influence the gut microbiome and respiratory function, and it is noteworthy that, in some cases, the macrophage response to respiratory viruses is linked to the composition of the gut microbiome[148].
Vitamins and minerals in nutrition therapy
Some vitamins and mineral elements have attracted special attention because of the potential benefit that they may have during the COVID-19 crisis. In general, treatment with vitamins A, C, D, and E, folate, vitamin B6 and B12, and minerals including zinc, selenium, copper, iron, and calcium is recommended as ONS as well as by consumption of natural food sources in the diet. To date, there is no consistent evidence to support the idea that the previously mentioned vitamins and minerals possess a potential effect in preventing or treating the COVID-19. Nonetheless, in agreement with previous data regarding their effect on reducing flu symptoms and possessing antioxidant benefits, the consumption of vitamin C is suggested for consumption within a range of 1-2 g in persons at risk of respiratory infections[149]. Vitamin C exerts an influence on the immune system and on the production, development, and maturation of lymphocytes, and in the promotion of phagocytosis and chemotaxis of leukocytes during infection[82,150]. In pneumonia, vitamin C has been found to reduce ROS and inflammation by inhibiting the activation of NF-κB that, in turn, decreases IL-1, IL-8, IL-6, and TNF-α production, and reduces DNA damage and LPS-induced ROS[82]. In various trials that investigated the treatment of sepsis and ARDS vitamin C was found to reduce TNF-α and IL-1β and increase CAT, SOD, and glutathione levels[151]; in that regard, vitamin C supplementation could be beneficial in COVID-19.
Regarding vitamin D, recent investigations have pointed out its role in preventing infections, including COVID-19. Vitamin D has been described as possessing anti-inflammatory and immunomodulatory effects and may interfere with viral replication, probably by its effects on innate and adaptive responses by the modulation of defensins and cathelicidins and reduction of the Th1 helper cell response[152]. Furthermore, vitamin D participates in the activation of regulatory T cells and Th2 cells along with a decrease of proinflammatory cytokines, such as TNFα and IFNγ, which are involved in the pathogenesis of ARDS, by Th1 cells[153]. Vitamin D deficiency is correlated with an increase of susceptibility to infections and increased autoimmunity; its benefits are thus highlighted in persons with low levels of vitamin D[154]. One of the possible mechanisms by which vitamin D could impair the entry of COVID-19 into host cells is by suppressing the adhesion molecule CD26/DDP4, a key element for viral entry[155]. Another possible mechanism of lung protection is through regulation of the renin–angiotensin system to inhibit viral entry and replication[156].
It appears that the role of vitamin A in preventing the effects of COVID-19 is not fully understood, but it is known that retinoic acid, the most active retinoid form of vitamin A, has an impact on the production of IL-1β and IL-1 receptor antagonists by means of alveolar macrophages and the consequent infiltration of neutrophils into the lungs during the course of ARDS. Retinoic acid contributes to surfactant production, which may be another way to protect against ARDS. Meanwhile, carotenes attenuate ROS production, diminishing the level of oxidative stress in the lungs. Under conditions of vitamin A deficiency, epithelial damage is often observed during viral infections, as is the increase of susceptibility of the host to respiratory viruses like influenza and SARS-CoV[156].
The most relevant activity of vitamin E is it antioxidant activity, which protects against free radicals like superoxides. In addition, the vitamin E effect is associated with the immune response of lymphocytes, NK cells, and neutrophils, which decline in the elderly[157]. Vitamin E supplementation in older adults was shown to enhance immune cell functions including neutrophil chemotaxis and phagocytosis, NK cell activity, and mitogen-induced lymphocyte proliferation[158].
With regard to the vitamin B group, vitamins B6, B9 (folate), and B12 are considered relevant to immune function in the context of the COVID-19 pandemic framework[159]. Low concentrations of B6 influence on lymphocyte maturation, antibody responses, and the cytotoxic activity of NK cells. Folate intervenes in the response of Th1 and NK cells and in optimal antibody production[160]. Two mechanisms have been proposed by which folate may blunt the COVID-19 infection. The first is the significance of the homocysteine-mediated trans-sulfuration pathway and of ferroptotic stress that leads to cell death[88]. The second is the probable disruption of furin protease activity involved in SARS-CoV-2 spike protein cleavage, as mentioned previously[161]. Vitamin B12 also participates in antibody responses and circulating lymphocyte levels. In DNA synthesis, which is important for cell replication, B12 restores an abnormal ratio of CD4+/CD8+ T cells, and its deficiency may be associated with the impairment of the immune response to viruses and bacteria in conjunction with suppressed NK cells[86]. Hence, the consumption of folate, B6, and B12, is recommended, and they should be integrated within nutrition therapy.
The trace minerals zinc, copper, and selenium are intricately involved in immune function. Zinc is highlighted because of its immunomodulatory effects on NK cell activity and macrophage and neutrophil function, complementary activity, and T cell-mediated function. Antioxidant activity has been attributed to trace minerals because they are cofactors of antioxidant metalloenzymes such as SOD. Zinc deficiency is implicated in the systemic activation of NF-κΒ[162]. Zinc might reduce viral replication and attenuate GI and respiratory symptoms related to COVID-19. Additionally, zinc at low doses (2 µmol/L) can prevent SARS-CoV replication by inhibiting SARS RNA polymerase[163]. Increased intracellular levels of zinc enhance the therapeutic effect of some drugs employed in the treatment of COVID-19, such as chloroquine, disulfiram, and tetracycline[164]. Recent evidence supports the idea that maintaining optimal levels of zinc is fundamental for resistance in the host response in COVID-19 and for preventing other viral infections[165]. A clinical study of four patients treated with oral administration of a high dose of a zinc salt, based on previous data that zinc shortens the duration of a cold, reported rapid improvement of the symptomatology[166]. Therefore, alone or in combination with other drugs, zinc is being investigated in several trials to determine its effectiveness in the prevention or treatment of COVID-19.
Selenium is considered another important trace element with antioxidant and anti-inflammatory effects. Selenium, together with vitamin D deficiency, probably impairs immune responses to COVID-19 and increases the severity of the disease[167]. In contrast, other clinical studies reveal that the recovery rate in surviving patients was related to increased selenium levels in patients with COVID-19[168,169]. Selenium is intricately involved in a wide range of immune activities, such as T cell activation, maturation, differentiation, and proliferation. It exerts antiviral activity by regulating the TCD4+ response, modulating TCD8+ and NK cells with cytotoxic activity, and it has a crucial role in the production of antibodies[170]. It is thought that a selenium deficiency has an influence on the high incidence of thromboembolism in patients critically ill with COVID-19 in the ICU[171]. A possible explanation is that low selenium levels are correlated with an abnormal thromboxane A2-to-prostacyclin I2 ratio, which is implicated in vasoconstriction and coagulation[172]. However, further studies are needed for clarification[170].
Copper protects DNA integrity by reducing oxidative damage, and its deficiency alters the immune response and promotes infectious diseases. Copper appears to participate in immune cell differentiation and to inhibit viral replication. It exerts an influence on the optimal functioning of NK cells, lymphocytes, neutrophils, macrophages, and monocytes. Supplementation with high doses of copper (7.8 mg/d) was shown to improve the activity of antioxidant enzymes and to activate SOD, ceruloplasmin, and benzylamine oxidase. However, the high dose affected neutrophils, serum IL-2R, and the levels of anti-influenza virus antibodies[173]. In this framework, copper has been proposed as a target element, together with others, such as zinc, in the treatment of COVID-19[174]. The antiviral properties attributed to copper would act directly with the virus by blocking structural proteins or by improving immune cell functioning[159]. Following this line of reasoning, optimal plasma levels of copper can elevate innate and adaptive immune responses to promote best action against SARS-CoV-2. Therefore, it must be part of regimens for COVID-19 prevention and therapy[175].
Nutritional support in critically ill patients with COVID-19 and liver injury
The hyperinflammatory response is one of the main characteristics of patients critically ill with COVID-19. Accordingly, the GI tract could play a central role in modulation of the inflammatory response, as it is the largest immune organ in the body, with the greatest microbiome. Nutritional therapy through EN strengthens gut barrier defenses and the microbial burden by providing luminal nutrients that impair dysbiosis and stimulate the anti-inflammatory response[176]. EN nutrition should start early, within 24-36 h after admission to the ICU or within 12 h of mechanical ventilation. To avoid refeeding syndrome, EN should be initiated with low trophic doses (10-20 mL/h) of a standard isosmotic polymeric formula that supplies 15-20 kcal/kg Bw and 1.2-2.0 g/kg Bw/day of protein, considering the actual body weight. The goal is to provide 70-80% of energy needs during 1 wk. In obese individuals with a BMI of 30-50, EN provides 11-14 kcals/kg Bw/day; for patients with severe obesity with a BMI >50 energy 22-25 kcal/kg Bw and 2 g/kg/d (classes I and II) or 2.5 g/kg/d (class III), taking into account the ideal body weight (IBW)[102]. Various complications may occur in patients with EN, such as GI disorders (e.g., diarrhea, vomiting, and nausea) and mechanical disorders related to intubation and metabolic disorders (e.g., hyperglycemia and an electrolyte imbalance). Thereafter, close monitoring is required to prevent difficulties[177]. In patients with gastric intolerance, prokinetics may alleviate symptoms and promote motility, but post-pyloric feeding should be performed in the persistence of intolerance or in patients with a high risk of aspiration[118].
For patients in whom GI feeding is not feasible, PN should be considered. Patients with a poor nutrition status, significant intolerance to EN, or those with a prolonged length-of-stay in the ICU, are potential candidates for PN. To avoid proinflammatory infusion, it is suggested to limit the use of omega-6 soy-based intravenous lipid emulsion (ILE) in week 1 or to change to a mixture of lipids, such as olive oil-based ILE or to soy, medium chain triglycerides, olive oil, and fish oil (SMOF)[102]. PN complications include catheter-related bloodstream infections and PN liver-associated damage[99]. At present, there are no reported cases associated with these complications. Nevertheless, the background must be taken into account for patients with sustained PN[126].
Certain other aspects must be taken into consideration when determining nutritional support in the patient. For instance, in liver injury, the primary treatment goal is against the COVID-19 infection, using antivirals, antibiotics, symptomatic support, oxygen therapy, and anti-inflammatory drugs, etc. It has been reported that some of the antivirals and drugs employed to treat this infection did cause liver damage[55,178], and clinicians should consider reducing the dose or discontinuing the drug(s). In patients with severe COVID-19, AST, ALT, total bilirubin, albumin, and certain other liver function indicators are significantly increased. However, the indices gradually returned to normal levels during recovery. With moderate liver damage, the organ function was restored without special treatment[179]. Consequently, moderate-to-severe cases require the regular monitoring of liver indicators, including AST, ALT, alkaline phosphatase, bilirubin, GGT, albumin, and prothrombin time. In severe cases, hepatoprotective drugs should be administered with caution[8] and the patient should probably be referred to the hepatology service for further specialized management. Mildly elevated indicators may not require specific liver treatment or hepatoprotective drugs[118].
ROLE OF PHYTOCHEMICALS IN COVID-19 THERAPY
The outbreak of COVID-19 has revolutionized medical science, and has been accompanied by the absence of a definitive treatment in addition to the unknown long-term effectiveness of recently approved vaccines. That has encouraged researchers to design target drugs in the fight against COVID-19 and also to seek further therapeutic options. Phytochemicals are molecules that are biologically active and that generally derive from plants or vegetable sources. Phytochemicals provide benefits when they are consumed in foods, supplements, or as medicinal ingredients. For several years, these compounds have been extensively studied from different perspectives. To date, 5000 bioactive compounds have been identified and classified into different groups depending their chemical features or biological activities. The groups include polyphenolic compounds, carotenoids, alkaloids, and sulfur/nitrogen compounds. Each group comprises a wide variety of molecules with high biological potential. The most predominant biological activities are the result of antioxidant, antitumor, anticancer, anti-inflammatory, antimutagenic, detoxifying, and immunomodulatory properties[180,181]. Moreover, various phytochemicals have antiviral activity that is shown to be more effective than that of antivirals, in addition to being generally better tolerated and having fewer adverse effects[182]. Two modalities have been proposed by which phytochemicals may induce protection against the virus, including (1) direct interaction with the structure of the virus and (2), enhancement of endogenous cytoprotective systems. In both cases, some of the compounds have proven to be effective. For the purpose of this review, some of these will be discussed briefly within the framework of COVID-19 and liver injury.
Direct interaction with the virus
From structural studies of SARS-CoV-2, it has been possible to determine the major drug targets, which include 3-chymotrypsin-like protease (3CLpro), papain-like protease (PLpro), RNA-dependent RNA polymerase, and spike (S) proteins. The S proteins are considered potential therapeutic targets because the virus attaches itself to the ACE2 receptor through these proteins, leading to fusion with the host cell. Subsequently, the virus releases its genetic material for RNA replication, building new viruses. As the proteins participate in the process, are attractive targets of antiviral drugs and phytochemicals[183].
Previous studies of SARS-CoV and Middle East Respiratory Syndrome provided relevant information for understanding the behavior of SARS-CoV-2 and the effectiveness of natural compounds that inhibit viral activity. Among the compounds that appear to possess effective antiviral activity against coronaviruses silvestrol, tryptanthrin, scutellarein, saikosaponin B2, quercetin, myricetin, caffeic acid, isobavachalcone, psoralidin, and lectins. The majority are extracted from natural herbs that are very commonly used in traditional medicines. Many of the bioactive compounds depend on enzyme inhibition for their antiviral effects[184]. It appears that polyphenols are the most effective antiviral compounds. For example, quercetin inhibits the protease 3CLpro, which has a crucial role in viral transcription. The inhibitory concentration (IC50) of quercetin was reported to be 8.6 ± 3.2 μmol/L against SARS-CoV PLpro[185]. Quercetin has shown to be effective in inhibiting the influenza virus, poliovirus, adenovirus, Epstein-Barr virus, and hepatitis C virus, among others[182].
A few computer modeling studies have been performed to screen the targeted biding sites of phytochemicals with SARS-CoV-2. Recently, Kurman et al[76] used molecular docking or molecular dynamics (MD) software to predict phytochemical interactions with Nps15, a nidoviral RNA uridylate-specific endoribonuclease (NendoU) protein commonly present in coronaviruses, and responsible for interfering with the innate immune response. It was found that 50 phytochemicals had the potential to bond with Nsp15 and obstruct viral replication. The phytochemicals with the effective bonding potential were sarsasapogenin, ursonic acid, curcumin, ajmalicine, novobiocin, silymarin and aranotin, piperine, gingerol, rosmarinic acid, and alpha terpinyl acetate, suggestive of potential use as inhibitors of SARS-CoV-2 replication. In another MD simulation study, phytochemicals from the Aryurvedic medicinal plants Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy), and Ocimuvingm sanctum (Tulsi), identified as anolide V, somniferine, tinocordiside, vicenin, ursolic acid, and isoorientin 4’-O-glucoside 2’’-O-p-hydroxybenzoagte, potentially inhibited the main protease Mpro or the 3Clpro of SARS-CoV-2. The authors suggested that such active compounds were safe, and that the drug-likeness test and their absorption, distribution, metabolism, excretion, and toxicity (ADMET) profile predicted their potential effectiveness[186]. In an in-silico MD study of 32 phytocompounds, Swargiary et al[187] found that amentoflavone and gallocatechin gallate were potential inhibitors of 3CLpro and PLpro and might be promising drug candidates for the treatment of SARS-CoV-2. However, the findings of in-silico studies require support by in vivo research to determine their mechanism of action.
Activation of cytoprotective systems
In addition, phytochemicals can also induce protection against SARS-CoV-2 by activating cytoprotective systems. A variety of these compounds have been shown to trigger signaling pathways related to endogenous defenses such as Nrf2/Keap1/ARE. Nuclear factor erythroid 2-related factor 1 (Nrf2) is a nuclear transcription factor coded by the NFE2L2 gene. It is known as the master regulator of cytoprotection and antioxidant responses[188]. Nrf2 impacts approximately 250 genes involved in antioxidant responses, detoxification, cell growth, proliferation, differentiation, energy metabolism, and apoptosis. Under homeostatic conditions, Nrf2 binds to its negative regulator Keap1 in the cytosol, but the electrophilic molecules that result, such as ROS, encourage Nrf2 dissociation from Keap1 and further translocation into the nucleus to bind the specific DNA sequence. The antioxidant response elements (ARE) elicit the expression of several genes involved in cell protection, including antioxidant enzymes, phase-II detoxifying enzymes, NAD(P)H quinone oxidoreductase 1 (NQO1), and the enzymes involved in glutathione synthesis and metabolism via musculo-aponeurotic fibrosarcoma proteins (s-Maf)[189]. The redox-sensitive Nrf2/Keap1/ARE signaling pathway has a fundamental role in preserving homeostasis caused by oxidative stress, inflammation, diabetes, cardiovascular diseases, cancer, neurodegeneration, liver-kidney injury, and aging[190]. According to the evidence, some phytochemicals possess the ability to activate the Nrf2/Keap1/ARE pathway. They are called “Nrf2 bioactivators”, but few have been broadly studied. Sulphoraphane (SFN), an isothiocyanate found in Brassica species like broccoli, is the most potent Nrf2 bioactivator. Taking into account the “CD value”, which refers to the amount of a certain compound necessary to double NQO1 activity in murine hepatoma cells (hepg2)[191], SFN requires 0.2 µmol/L, and other phytochemicals, such as quercetin, require, 2.5 µmol/L, with curcumin requiring 2.7 µmol/L, and silymarin, 3.5 µmol/L. It is important to bear in mind that the lower the amount required, the more potent the phytochemical is considered to be. Other phytochemicals have been found to activate Nrf2, including andrographolides, quercetin, β-carotene, genistein, lutein, resveratrol, and zeaxanthin[192]. SFN activates nuclear respiratory factor (NRF) by means of a direct reaction with the electrophilic isothiocyanate group, changing the covalent linking of the Keap1 cysteine residues. SFN has thus been extensively employed in several protocols for the treatment of inflammation, cancer, diabetes; and cardiovascular, neurodegenerative, lung, hepatic, and kidney damage. SFN is considered to be an anticancer, antiproliferative, antioxidant, and anti-inflammatory agent[193,194]. The Nrf2/Keap1/ARE pathway is ubiquitously active, which may be the reason why certain phytochemicals could be promising protectors under different clinical situations.
To our knowledge, silymarin (SM) could be one of the most remarkable phytochemicals from the COVID-19 and liver injury perspective. SM is a blend of seven flavonolignans (65%-80%) obtained from the extract of Silybum marianum. The main flavonolignan is silybin (50%-70%) and its isoforms silybin A and silybin B. Isosilybin, silychristin, isosilychristin, and silydianin and their isoforms are the remaining flavonolignan constituents. SM has been used as a traditional medicine since ancient times by physicians and herbalists to treat acute and chronic liver abnormalities because of its antioxidant, anti-inflammatory, and antibiotic activities[101]. The mechanisms of action of SM are not yet fully elucidated, but involve triggering the Nrf2/Keap1/ARE signaling pathway. Various in vitro and in vivo trials have demonstrated the efficacy of SM in Nrf2 activation. Likewise, in SFN, the reported benefits are not only found in liver injury models[195,196] but have also been described in lung[197], neurodegenerative[198], cardio–renal[199], gastric ulcer[200], and neurotoxicity[201] models, among others. Furthermore, an in-silico MD study revealed the affinity of six SM flavonolignans (silybin A, silybin B, isosilybin A, isosilychristin, taxifolin, and silydianin) for bonding Keap1 by electrostatic and van der Waals interactions, as well as hydrogen bonds. That could partially explain the ability of SM to induce the activation of the Nrf2/Keap1/ARE signaling pathway[202].
Moreover, recent investigations have identified SM antiviral activity against hepatitis C and B, dengue, influenza, Chikungunya, and HIV[203]. SM even proved to inhibit the entry and attachment of enterovirus 71 (EV-A71), which is responsible for causing hand, food, and mouth disease in childhood, more effectively than the positive control, balcalein[204]. Together with previously mentioned results of MD studies with regard to potential SM and COVID-19 inhibition, it is suggested that SM or its flavonolignans should be considered as very promising drugs for the treatment of COVID-19 and liver injury.
Curcumin is another phytochemical with potential for treating SARS-CoV-2 and liver damage. Curcumin is the main polyphenol in turmeric, present the rhizome of Curcuma longa and other species. In traditional medicine, it is used as an anti-inflammatory, antiseptic, analgesic, antimalarial, and as an insect repellent[205]. Current evidence supports the ability of curcumin to attenuate oxidative stress produced by ROS and RNS. Curcumin induces the antioxidant enzymes GST and γ-Glutamyl cysteine ligase (γ-GCL) and, at the same time, inhibits ROS-producing enzymes (oxidases, lipooxygenase, xanthine hydrogenase, and cyclooxygenase) and removes peroxide radicals, hydrogen peroxide, hydroxyl radicals, oxygen, and nitric oxide. The activities are associated with regulation of the Nrf2/Keap1/ARE pathway in addition to the modulation of other transcription factors, such as NF-kB and AP1[206]. In rat hepatic stellate-T6 (HSC-T6) cells, curcumin reduced the levels of malonaldehyde (MDA), and ROS treated with glucose oxidase, upregulated the nuclear translocation of Nrf2, augmenting glutathione levels, and reduced the expression of smooth muscle α-actin and extracellular matrix molecules[207]. In a rat model of arthritis, curcumin reduced inflammation by increasing methionine sulfoxide reductase A and the antioxidant enzymes CAT, SOD, and GPx[208]. In macrophages and RAW264.7 cells, curcumin protected cells from stress by inducing H2O2, triggering the Nrf2/Keap1/ ARE pathway and the expression of antioxidant enzymes, thus promoting cell survival[209]. Additional evidence that supports the effect of curcumin on Nrf2 activation, curcumin is also described as an antiviral. Curcumin interacts with viral proteins on the cell surface, impairing entry of the virus. It also interacts with RNA polymerase and other proteins to hamper replication and may also interact with protease Mpro[210,211]. In the previously mentioned MD study, curcumin was an effective inhibitor of the SARS-CoV-2 Nps15 protein. The hyperinflammatory response initiated in the “cytokine storm” is also weakened by curcumin, which interferes in the NF-κΒ and MAPK inflammatory pathways to reduce the expression of the proinflammatory cytokines IFN-γ, monocyte chemoattractant protein 1 (MCP)-1, IL-6, IL-10, and TNF-α[212]. Curcumin appears to prevent apoptosis and lung fibrosis by inhibiting the TGF-β pathway[213]. The envelope protein of SARS-CoV-2 is the smallest of the four structural proteins of the virus and it is known to participate in the viral entry process. We have performed some in-silico studies to investigate that protein.
Molecular modeling of the SARS-CoV-2 envelope protein
The three-dimensional model of the SARS-CoV-2 envelope protein of was built using Modeller 9.23 Software and considering the crystal structure of the transmembrane region of the envelope protein of SARS-CoV (PDB: 5X29). The resulting model represents a homo-pentamer of the E protein of SARS-CoV-2.
Docking analysis of the compounds of interest
Docking studies were used to predict the binding affinity of the compounds of interest and the SARS-CoV-2 envelope protein. Results of the analysis of silibylin, curcumina, and sulfuraphane were performed with Autodock 4.2 (Table 3 and Figure 5). The input files were prepared with the AutoDock Tools 1.5.2 graphic interface[214]. A grid box of 120 Å × 120 Å × 120 Å and a grid spacing of 0.375 Å was used for the docking studies. The Lamarckian genetic algorithm was selected for the sampling score, considering a randomized initial population of 100 and a maximum number of energy evaluations of 107.
Table 3.
Curcumina, silibylin, sulfuraphane evaluation by Autodock 4.2
| Ligand | Binding energy (kcal/mol) | kI µmol/L | Residue interactions |
| Curcumine | -4.6 | 428.13 | Phe 23, Val 25, Leu 28, Phe 26, Thr 30, Val 26 |
| Silybin | -5.73 | 62.57 | Phe 23, Phe 26, Val 29 |
| Sulforaphane | -3.75 | 1.79 | Val 29, Phe 26, Val 25, Ala 22, Phe 23, Leu 27 |
Figure 5.
Graphic representation of the binding poses of the ligands on the envelope protein of severe acute respiratory syndrome-coronavirus-2. Images were carried out using Pymol. Homo-pentamer of the E protein of severe acute respiratory syndrome-coronavirus-2 is shown in green, and the ligand is shown in yellow. Surrounded residues are shown in different colors and labeled as indicated. A: Curcumine; B: Silybin; C: Sulforaphane.
CONCLUSION
The current pandemic situation caused by COVID-19 infection has generated global changes in not only human health, but in all economic, political, and social levels. The lack of an effective vaccine that prevents COVID-19 infections and the absence of an effective treatment that reduces multiorgan consequences, only allows us to establish guidelines and recommendations based on direct experience with patients and observing what works and what does not and to make decisions supported by science. In this review, we attempted to provide an outline of how hyperinflammatory and oxidative stress responses to COVID-19 infection may cause liver injury or worsen a pre-existing one. GI-related symptoms, such as diarrhea, vomiting, nausea, and reduced food intake, along with a delay in medical attention, promote weight loss. Malnutrition increases mortality, hospital stay, and poor outcomes, in addition to high economic costs. The detection of malnutrition is thus crucial for establishing optimal nutritional therapy. the nutritional guidelines for patients with SARS-CoV-2 infection and liver injury are based on the statements of international clinical nutrition associations and the currently available evidence. Maintaining an optimal nutritional state is fundamental for preventing infection or overcoming SARS-CoV-2. Herein, the key role of providing sufficient energy, protein, lipids, and carbohydrates is clarified. Furthermore, the function of vitamins and trace minerals impacting the immune response is explained. To complete the nutritional approaches, we considered the use of phytochemicals as potential components of the therapy because of the possible benefits that they might offer during the COVID-19 crisis. The interactions (i.e. possible antiviral effects) of phytochemicals such curcumine, sulphoraphane, and silybin with the viral envelope protein have been demonstrated in-silico. The latter is an open door to researchers in the race to find tools to help overcome this pandemic in the best possible manner.
ACKNOWLEDGEMENTS
Nancy Vargas-Mendoza and Marcelo Angeles-Valencia are scholarship holders for their postgraduate studies by the Consejo Nacional de Ciencia y Tecnología (CONACyT) and the BEIFI program, Instituto Politécnico Nacional, Mexico.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflicting interests.
Manuscript source: Invited manuscript
Peer-review started: January 27, 2021
First decision: May 13, 2021
Article in press: July 19, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tumminello G S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Yuan YY
Contributor Information
Nancy Vargas-Mendoza, Laboratorio de Medicina de Conservacion, Instituto Politécnico Nacional, México 11340, Mexico.
Jazmín García-Machorro, Laboratorio de Medicina de Conservacion, Instituto Politécnico Nacional, México 11340, Mexico.
Marcelo Angeles-Valencia, Laboratorio de Medicina de Conservacion, Instituto Politécnico Nacional, México 11340, Mexico.
Marlet Martínez-Archundia, Laboratorio de Diseño y Desarrollo de Nuevos Fármacos e Innovación Biotécnológica, Instituto Politécnico Nacional, México 11340, Mexico.
Eduardo Osiris Madrigal-Santillán, Escuela Superior de Medicina, Instituto Politécnico Nacional, México 11340, Mexico.
Ángel Morales-González, Escuela Superior de Cómputo, Instituto Politécnico Nacional, México 07738, Mexico.
Liliana Anguiano-Robledo, Laboratorio de Farmacología Molecular, Instituto Politécnico Nacional, México 11340, Mexico.
José A Morales-González, Laboratorio Medicina de Conservación, Escuela Superior de Medicina, Instituto Politécnico Nacional, México 11340, Mexico. jmorales101@yahoo.com.mx.
References
- 1.Mittal A, Manjunath K, Ranjan RK, Kaushik S, Kumar S, Verma V. COVID-19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 2020;16:e1008762. doi: 10.1371/journal.ppat.1008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palacios Cruz M, Santos E, Velázquez Cervantes MA, León Juárez M. COVID-19, a worldwide public health emergency. Rev Clin Esp. 2020 doi: 10.1016/j.rceng.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimoto FK. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. Protein J. 2020;39:198–216. doi: 10.1007/s10930-020-09901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Coronavirus disease (COVID-19) pandemic. 2020. [cited 31 December 2020]. Available from: https://www.who.int/es/emergencies/diseases/novel-coronavirus-2019 .
- 7.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philips CA, Ahamed R, Augustine P. SARS-CoV-2 related liver impairment - perception may not be the reality. J Hepatol. 2020;73:991–992. doi: 10.1016/j.jhep.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedro KD, Henderson AJ, Agosto LM. Mechanisms of HIV-1 cell-to-cell transmission and the establishment of the latent reservoir. Virus Res. 2019;265:115–121. doi: 10.1016/j.virusres.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esumi M, Ishibashi M, Yamaguchi H, Nakajima S, Tai Y, Kikuta S, Sugitani M, Takayama T, Tahara M, Takeda M, Wakita T. Transmembrane serine protease TMPRSS2 activates hepatitis C virus infection. Hepatology. 2015;61:437–446. doi: 10.1002/hep.27426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038–2040. doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polakos NK, Cornejo JC, Murray DA, Wright KO, Treanor JJ, Crispe IN, Topham DJ, Pierce RH. Kupffer cell-dependent hepatitis occurs during influenza infection. Am J Pathol. 2006;168:1169–1178; quiz 1404-1405. doi: 10.2353/ajpath.2006.050875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton A. Presumed acute fatty liver of pregnancy following influenza A hepatitis. Obstet Med. 2017;10:186–188. doi: 10.1177/1753495X17695173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardi DS, Romero Y, Mertz LE, Douglas DD. Hepatitis-associated aplastic anemia and acute parvovirus B19 infection: a report of two cases and a review of the literature. Am J Gastroenterol. 1998;93:468–470. doi: 10.1111/j.1572-0241.1998.468_1.x. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhut M, Thorburn K, Ahmed T. Transaminase levels in ventilated children with respiratory syncytial virus bronchiolitis. Intensive Care Med. 2004;30:931–934. doi: 10.1007/s00134-004-2236-2. [DOI] [PubMed] [Google Scholar]
- 20.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung CCL, Goh D, Lim X, Tien TZ, Lim JCT, Nerurkar SN, Shihleone L, Cheow PC, Chan CY, Koh YX, Tan TT, Kalimuddin S, David Tai WM, Ng JL, Hong Low JG, Yeong J, Hon Lim TK. Residual SARS-CoV-2 viral antigens detected in gastrointestinal and hepatic tissues from two recovered COVID-19 patients. 2020 Preprint. Available from: medRxiv:2020.10.28.20219014.
- 22.Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouayad A. Innate immune evasion by SARS-CoV-2: Comparison with SARS-CoV. Rev Med Virol. 2020;30:1–9. doi: 10.1002/rmv.2135. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13–24. doi: 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Xiao SY. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92:1491–1494. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- 29.de Lucena TMC, da Silva Santos AF, de Lima BR, de Albuquerque Borborema ME, de Azevêdo Silva J. Mechanism of inflammatory response in associated comorbidities in COVID-19. Diabetes Metab Syndr. 2020;14:597–600. doi: 10.1016/j.dsx.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edeas M, Saleh J, Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020;97:303–305. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, Tang C, Sang L, Liu J, Ni Z, Hu Y, Liu L, Shan H, Lei C, Peng Y, Wei L, Liu Y, Peng P, Wang J, Chen Z, Li G, Zheng Z, Qiu S, Luo J, Ye C, Zhu S, Liu X, Cheng L, Ye F, Zheng J, Zhang N, Li Y, He J, Li S, Zhong N Medical Treatment Expert Group for COVID-19. Risk Factors of Fatal Outcome in Hospitalized Subjects With Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest. 2020;158:97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zádori N, Váncsa S, Farkas N, Hegyi P, Erőss B KETLAK Study Group. The negative impact of comorbidities on the disease course of COVID-19. Intensive Care Med. 2020;46:1784–1786. doi: 10.1007/s00134-020-06161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Váncsa S, Hegyi PJ, Zádori N, Szakó L, Vörhendi N, Ocskay K, Földi M, Dembrovszky F, Dömötör ZR, Jánosi K, Rakonczay Z Jr, Hartmann P, Horváth T, Erőss B, Kiss S, Szakács Z, Németh D, Hegyi P, Pár G. Pre-existing Liver Diseases and On-Admission Liver-Related Laboratory Tests in COVID-19: A Prognostic Accuracy Meta-Analysis With Systematic Review. Front Med (Lausanne) 2020;7:572115. doi: 10.3389/fmed.2020.572115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Y, Yuan J, Long Q, Hu J, Deng H, Zhao Z, Chen J, Lu M, Huang A. Patients with SARS-CoV-2 and HBV co-infection are at risk of greater liver injury. Genes Dis. 2021;8:484–492. doi: 10.1016/j.gendis.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferron PJ, Gicquel T, Mégarbane B, Clément B, Fromenty B. Treatments in Covid-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury? Biochimie. 2020;179:266–274. doi: 10.1016/j.biochi.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kavoliuniene A, Vaitiekiene A, Cesnaite G. Congestive hepatopathy and hypoxic hepatitis in heart failure: a cardiologist's point of view. Int J Cardiol. 2013;166:554–558. doi: 10.1016/j.ijcard.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Salehi B, Calina D, Docea AO, Koirala N, Aryal S, Lombardo D, Pasqua L, Taheri Y, Marina Salgado Castillo C, Martorell M, Martins N, Iriti M, Suleria HAR, Sharifi-Rad J. Curcumin's Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J Clin Med. 2020;9 doi: 10.3390/jcm9020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509–519. doi: 10.1177/2050640620924157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46:2050–2068. doi: 10.1093/clinchem/46.12.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ilias I, Zabuliene L. Hyperglycemia and the novel Covid-19 infection: Possible pathophysiologic mechanisms. Med Hypotheses. 2020;139:109699. doi: 10.1016/j.mehy.2020.109699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez C, Kim J, Pandey A, Huang T, DeLoughery TG. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br J Haematol. 2020;190:31–32. doi: 10.1111/bjh.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, Merabet F, Mekinian A, Braun T, Damaj G, Delmer A, Cymbalista F. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190:29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jawed M, Hart E, Saeed M. Haemolytic anaemia: a consequence of COVID-19. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-238118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothschild MA, Oratz M, Schreiber SS. Serum albumin. Hepatology. 1988;8:385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- 52.Violi F, Ceccarelli G, Cangemi R, Alessandri F, D'Ettorre G, Oliva A, Pastori D, Loffredo L, Pignatelli P, Ruberto F, Venditti M, Pugliese F, Mastroianni CM. Hypoalbuminemia, Coagulopathy, and Vascular Disease in COVID-19. Circ Res. 2020;127:400–401. doi: 10.1161/CIRCRESAHA.120.317173. [DOI] [PubMed] [Google Scholar]
- 53.Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, Lin S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92:2152–2158. doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.U.S. NIH National Library of Medicine. COVID-19 Information. 2020. [cited 31 December 2020]. Available from: https://www.clinicaltrials.gov/
- 55.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Favaloro EJ, Thachil J. Reporting of D-dimer data in COVID-19: some confusion and potential for misinformation. Clin Chem Lab Med. 2020;58:1191–1199. doi: 10.1515/cclm-2020-0573. [DOI] [PubMed] [Google Scholar]
- 57.Lee S, Channappanavar R, Kanneganti TD. Coronaviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Trends Immunol. 2020;41:1083–1099. doi: 10.1016/j.it.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu BM, Hill HR. Role of Host Immune and Inflammatory Responses in COVID-19 Cases with Underlying Primary Immunodeficiency: A Review. J Interferon Cytokine Res. 2020;40:549–554. doi: 10.1089/jir.2020.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell MW, Moldoveanu Z, Ogra PL, Mestecky J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front Immunol. 2020;11:611337. doi: 10.3389/fimmu.2020.611337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isho B, Abe KT, Zuo M, Jamal AJ, Rathod B, Wang JH, Li Z, Chao G, Rojas OL, Bang YM, Pu A, Christie-Holmes N, Gervais C, Ceccarelli D, Samavarchi-Tehrani P, Guvenc F, Budylowski P, Li A, Paterson A, Yue FY, Marin LM, Caldwell L, Wrana JL, Colwill K, Sicheri F, Mubareka S, Gray-Owen SD, Drews SJ, Siqueira WL, Barrios-Rodiles M, Ostrowski M, Rini JM, Durocher Y, McGeer AJ, Gommerman JL, Gingras AC. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cervia C, Nilsson J, Zurbuchen Y, Valaperti A, Schreiner J, Wolfensberger A, Raeber ME, Adamo S, Weigang S, Emmenegger M, Hasler S, Bosshard PP, De Cecco E, Bächli E, Rudiger A, Stüssi-Helbling M, Huber LC, Zinkernagel AS, Schaer DJ, Aguzzi A, Kochs G, Held U, Probst-Müller E, Rampini SK, Boyman O. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild vs severe COVID-19. J Allergy Clin Immunol. 2021;147:545–557.e9. doi: 10.1016/j.jaci.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chakraborty J, Banerjee I, Vaishya R, Ghosh S. Bioengineered in Vitro Tissue Models to Study SARS-CoV-2 Pathogenesis and Therapeutic Validation. ACS Biomater Sci Eng. 2020;6:6540. doi: 10.1021/acsbiomaterials.0c01226. [DOI] [PubMed] [Google Scholar]
- 63.Karlsson AC, Humbert M, Buggert M. The known unknowns of T cell immunity to COVID-19. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe8063. [DOI] [PubMed] [Google Scholar]
- 64.Ren L, Zhang L, Chang , Wang J, Hu Y, Chen H, Guo L, Wu C, Wang C, Wang Y, Wang G, Yang S, Dela Cruz CS, Sharma L, Wang L, Zhang D. The kinetics of humoral response and its relationship with the disease severity in COVID-19. Commun Biol. 2020;3:780. doi: 10.1038/s42003-020-01526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hashem AM, Algaissi A, Almahboub SA, Alfaleh MA, Abujamel TS, Alamri SS, Alluhaybi KA, Hobani HI, AlHarbi RH, Alsulaiman RM, ElAssouli MZ, Hala S, Alharbi NK, Alhabbab RY, AlSaieedi AA, Abdulaal WH, Bukhari A, Al-Somali AA, Alofi FS, Khogeer AA, Pain A, Alkayyal AA, Almontashiri NAM, Ahmad BM, Li X. Early Humoral Response Correlates with Disease Severity and Outcomes in COVID-19 Patients. Viruses. 2020;12 doi: 10.3390/v12121390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lenti MV, Aronico N, Pellegrino I, Boveri E, Giuffrida P, Borrelli de Andreis F, Morbini P, Vanelli L, Pasini A, Ubezio C, Melazzini F, Rascaroli A, Antoci V, Merli S, Di Terlizzi F, Sabatini U, Cambiè G, Tenore A, Picone C, Vanoli A, Arcaini L, Baldanti F, Paulli M, Corazza GR, Di Sabatino A. Depletion of circulating IgM memory B cells predicts unfavourable outcome in COVID-19. Sci Rep. 2020;10:20836. doi: 10.1038/s41598-020-77945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borges L, Pithon-Curi TC, Curi R, Hatanaka E. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Mediators Inflamm. 2020;2020:8829674. doi: 10.1155/2020/8829674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang L, Yin Z, Hu Y, Mei H. Controlling Cytokine Storm Is Vital in COVID-19. Front Immunol. 2020;11:570993. doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desai N, Neyaz A, Szabolcs A, Shih AR, Chen JH, Thapar V, Nieman LT, Solovyov A, Mehta A, Lieb DJ, Kulkarni AS, Jaicks C, Xu KH, Raabe MJ, Pinto CJ, Juric D, Chebib I, Colvin RB, Kim AY, Monroe R, Warren SE, Danaher P, Reeves JW, Gong J, Rueckert EH, Greenbaum BD, Hacohen N, Lagana SM, Rivera MN, Sholl LM, Stone JR, Ting DT, Deshpande V. Temporal and spatial heterogeneity of host response to SARS-CoV-2 pulmonary infection. Nat Commun. 2020;11:6319. doi: 10.1038/s41467-020-20139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blot M, Bour JB, Quenot JP, Bourredjem A, Nguyen M, Guy J, Monier S, Georges M, Large A, Dargent A, Guilhem A, Mouries-Martin S, Barben J, Bouhemad B, Charles PE, Chavanet P, Binquet C, Piroth L LYMPHONIE study group. The dysregulated innate immune response in severe COVID-19 pneumonia that could drive poorer outcome. J Transl Med. 2020;18:457. doi: 10.1186/s12967-020-02646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 73.Rodrigues TS, Sa KS, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Goncalves AV, Perucello DB, Andrade WA, Castro R, Veras FP, Toller-Kawahisa JE, Nascimento DC, de Lima MH, Silva CM, Caetite DB, Martins RB, Castro IA, Pontelli MC, de Barros FC, do Amaral NB, Giannini MC, Bonjorno LP, Lopes MIF, Benatti MN, Santana RC, Vilar FC, Auxiliadora-Martins M, Luppino-Assad R, de Almeida SC, de Oliveira FR, Batah SS, Siyuan L, Cunha TM, Alves-Filho JC, Cunha FQ, Cunha LD, Frantz FG, Kohlsdorf T, Fabro AT, Arruda E, de Oliveira RD, Louzada-Junior P, Zamboni DS. Inflammasome activation in COVID-19 patients. 2020 Preprint. [Google Scholar]
- 74.Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2013;98:872–884. doi: 10.3945/ajcn.113.064659. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Chen Y, Meng Z. Immunomodulation for Severe COVID-19 Pneumonia: The State of the Art. Front Immunol. 2020;11:577442. doi: 10.3389/fimmu.2020.577442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar S, Kashyap P, Chowdhury S, Kumar S, Panwar A, Kumar A. Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine. 2021;85:153317. doi: 10.1016/j.phymed.2020.153317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Myhrstad MCW, Tunsjø H, Charnock C, Telle-Hansen VH. Dietary Fiber, Gut Microbiota, and Metabolic Regulation-Current Status in Human Randomized Trials. Nutrients. 2020;12 doi: 10.3390/nu12030859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, Scherer C, Rudelius M, Zoller M, Höchter D, Keppler O, Teupser D, Zwißler B, von Bergwelt-Baildon M, Kääb S, Massberg S, Pekayvaz K, Stark K. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun S, Cai X, Wang H, He G, Lin Y, Lu B, Chen C, Pan Y, Hu X. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. 2020;507:174–180. doi: 10.1016/j.cca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutiérrez-Bautista JF, Rodriguez-Nicolas A, Rosales-Castillo A, Jiménez P, Garrido F, Anderson P, Ruiz-Cabello F, López-Ruz MÁ. Negative Clinical Evolution in COVID-19 Patients Is Frequently Accompanied With an Increased Proportion of Undifferentiated Th Cells and a Strong Underrepresentation of the Th1 Subset. Front Immunol. 2020;11:596553. doi: 10.3389/fimmu.2020.596553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liao WH, Yang GG, Henneberg M. The renin-angiotensin-aldosterone system inhibitors in COVID-19: from acidosis to ventilation and immunity. Swiss Med Wkly. 2020;150:w20444. doi: 10.4414/smw.2020.20444. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Luo G, Yuan J, Wang Y, Yang X, Wang X, Li G, Liu Z, Zhong N. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014;2014:426740. doi: 10.1155/2014/426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi Y, Zhou G, Li Q. Asynchronous actions of immune responses in COVID-19 patients. Signal Transduct Target Ther. 2020;5:284. doi: 10.1038/s41392-020-00424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalpakci Y, Hacibekiroglu T, Trak G, Karacaer C, Demirci T, Kocayigit H, Sunu C, Varim C, Falay M. Comparative evaluation of memory T cells in COVID-19 patients and the predictive role of CD4+CD8+ double positive T lymphocytes as a new marker. Rev Assoc Med Bras (1992) 2020;66:1666–1672. doi: 10.1590/1806-9282.66.12.1666. [DOI] [PubMed] [Google Scholar]
- 85.Shomuradova AS, Vagida MS, Sheetikov SA, Zornikova KV, Kiryukhin D, Titov A, Peshkova IO, Khmelevskaya A, Dianov DV, Malasheva M, Shmelev A, Serdyuk Y, Bagaev DV, Pivnyuk A, Shcherbinin DS, Maleeva AV, Shakirova NT, Pilunov A, Malko DB, Khamaganova EG, Biderman B, Ivanov A, Shugay M, Efimov GA. SARS-CoV-2 Epitopes Are Recognized by a Public and Diverse Repertoire of Human T Cell Receptors. Immunity. 2020;53:1245–1257.e5. doi: 10.1016/j.immuni.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saeed F, Nadeem M, Ahmed RS, Tahir Nadeem M, Arshad MS, Ullah A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds – a review. Food Agric Immunol . 2016;27:205. [Google Scholar]
- 87.Bacher P, Rosati E, Esser D, Martini GR, Saggau C, Schiminsky E, Dargvainiene J, Schröder I, Wieters I, Khodamoradi Y, Eberhardt F, Vehreschild MJGT, Neb H, Sonntagbauer M, Conrad C, Tran F, Rosenstiel P, Markewitz R, Wandinger KP, Augustin M, Rybniker J, Kochanek M, Leypoldt F, Cornely OA, Koehler P, Franke A, Scheffold A. Low-Avidity CD4+ T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity. 2020;53:1258–1271.e5. doi: 10.1016/j.immuni.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh Y, Gupta G, Kazmi I, Al-Abbasi FA, Negi P, Chellappan DK, Dua K. SARS CoV-2 aggravates cellular metabolism mediated complications in COVID-19 infection. Dermatol Ther. 2020;33:e13871. doi: 10.1111/dth.13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bulló M, Casas R, Portillo MP, Basora J, Estruch R, García-Arellano A, Lasa A, Juanola-Falgarona M, Arós F, Salas-Salvadó J. Dietary glycemic index/load and peripheral adipokines and inflammatory markers in elderly subjects at high cardiovascular risk. Nutr Metab Cardiovasc Dis. 2013;23:443–450. doi: 10.1016/j.numecd.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Ntyonga-Pono MP. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan Afr Med J. 2020;35:12. doi: 10.11604/pamj.2020.35.2.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suhail S, Zajac J, Fossum C, Lowater H, McCracken C, Severson N, Laatsch B, Narkiewicz-Jodko A, Johnson B, Liebau J, Bhattacharyya S, Hati S. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020;39:644–656. doi: 10.1007/s10930-020-09935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muhoberac BB. What Can Cellular Redox, Iron, and Reactive Oxygen Species Suggest About the Mechanisms and Potential Therapy of COVID-19? Front Cell Infect Microbiol. 2020;10:569709. doi: 10.3389/fcimb.2020.569709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dutta S, Sengupta P. SARS-CoV-2 infection, oxidative stress and male reproductive hormones: can testicular-adrenal crosstalk be ruled-out? J Basic Clin Physiol Pharmacol. 2020;31 doi: 10.1515/jbcpp-2020-0205. [DOI] [PubMed] [Google Scholar]
- 94.Bakadia BM, Boni BOO, Ahmed AAQ, Yang G. The impact of oxidative stress damage induced by the environmental stressors on COVID-19. Life Sci. 2021;264:118653. doi: 10.1016/j.lfs.2020.118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zinovkin RA, Grebenchikov OA. Transcription Factor Nrf2 as a Potential Therapeutic Target for Prevention of Cytokine Storm in COVID-19 Patients. Biochemistry (Mosc) 2020;85:833–837. doi: 10.1134/S0006297920070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Delgado-Roche L, Mesta F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch Med Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu J. Tackle the free radicals damage in COVID-19. Nitric Oxide. 2020;102:39–41. doi: 10.1016/j.niox.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu ZW, Li YS. Pathogenesis and treatment of parenteral nutrition-associated liver disease. Hepatobiliary Pancreat Dis Int. 2012;11:586–593. doi: 10.1016/s1499-3872(12)60229-x. [DOI] [PubMed] [Google Scholar]
- 100.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, Pirlich M, Singer P endorsed by the ESPEN Council. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Federico A, Dallio M, Loguercio C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules. 2017;22 doi: 10.3390/molecules22020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patel JJ, Martindale RG, McClave SA. Relevant Nutrition Therapy in COVID-19 and the Constraints on Its Delivery by a Unique Disease Process. Nutr Clin Pract. 2020;35:792–799. doi: 10.1002/ncp.10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Violi F, Oliva A, Cangemi R, Ceccarelli G, Pignatelli P, Carnevale R, Cammisotto V, Lichtner M, Alessandri F, De Angelis M, Miele MC, D'Ettorre G, Ruberto F, Venditti M, Pugliese F, Mastroianni CM. Nox2 activation in Covid-19. Redox Biol. 2020;36:101655. doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 105.Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MA, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49–64. doi: 10.1016/j.clnu.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 106.McClave SA, DiBaise JK, Mullin GE, Martindale RG. ACG Clinical Guideline: Nutrition Therapy in the Adult Hospitalized Patient. Am J Gastroenterol. 2016;111:315–334; quiz 335. doi: 10.1038/ajg.2016.28. [DOI] [PubMed] [Google Scholar]
- 107.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15:R268. doi: 10.1186/cc10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalaiselvan MS, Renuka MK, Arunkumar AS. Use of Nutrition Risk in Critically ill (NUTRIC) Score to Assess Nutritional Risk in Mechanically Ventilated Patients: A Prospective Observational Study. Indian J Crit Care Med. 2017;21:253–256. doi: 10.4103/ijccm.IJCCM_24_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rosa M, Heyland DK, Fernandes D, Rabito EI, Oliveira ML, Marcadenti A. Translation and adaptation of the NUTRIC Score to identify critically ill patients who benefit the most from nutrition therapy. Clin Nutr ESPEN. 2016;14:31–36. doi: 10.1016/j.clnesp.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 111.Compher C, Chittams J, Sammarco T, Nicolo M, Heyland DK. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit Care Med. 2017;45:156–163. doi: 10.1097/CCM.0000000000002083. [DOI] [PubMed] [Google Scholar]
- 112.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 113.Capuzzo M, Valpondi V, Sgarbi A, Bortolazzi S, Pavoni V, Gilli G, Candini G, Gritti G, Alvisi R. Validation of severity scoring systems SAPS II and APACHE II in a single-center population. Intensive Care Med. 2000;26:1779–1785. doi: 10.1007/s001340000715. [DOI] [PubMed] [Google Scholar]
- 114.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 115.Cárdenas-Turanzas M, Ensor J, Wakefield C, Zhang K, Wallace SK, Price KJ, Nates JL. Cross-validation of a Sequential Organ Failure Assessment score-based model to predict mortality in patients with cancer admitted to the intensive care unit. J Crit Care. 2012;27:673–680. doi: 10.1016/j.jcrc.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 116.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats AJS, Crivelli AN, Evans DC, Gramlich L, Fuchs-Tarlovsky V, Keller H, Llido L, Malone A, Mogensen KM, Morley JE, Muscaritoli M, Nyulasi I, Pirlich M, Pisprasert V, de van der Schueren MAE, Siltharm S, Singer P, Tappenden K, Velasco N, Waitzberg D, Yamwong P, Yu J, Van Gossum A, Compher C GLIM Core Leadership Committee, GLIM Working Group. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10:207–217. doi: 10.1002/jcsm.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aguila EJT, Cua IHY, Dumagpi JEL, Francisco CPD, Raymundo NTV, Sy-Janairo MLL, Cabral-Prodigalidad PAI, Lontok MAD. COVID-19 and its effects on the digestive system and endoscopy practice. JGH Open. 2020;4:324–331. doi: 10.1002/jgh3.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arora S, Mattina C, McAnenny C, O'Sullivan N, McGeeney L, Calder N, Gatiss G, Davidson B, Morgan MY. The development and validation of a nutritional prioritising tool for use in patients with chronic liver disease. J Hepatol. 2012;56:S241. [Google Scholar]
- 121.Schneeweiss B, Pammer J, Ratheiser K, Schneider B, Madl C, Kramer L, Kranz A, Ferenci P, Druml W, Grimm G. Energy metabolism in acute hepatic failure. Gastroenterology. 1993;105:1515–1521. doi: 10.1016/0016-5085(93)90159-a. [DOI] [PubMed] [Google Scholar]
- 122.John WJ, Phillips R, Ott L, Adams LJ, McClain CJ. Resting energy expenditure in patients with alcoholic hepatitis. JPEN J Parenter Enteral Nutr. 1989;13:124–127. doi: 10.1177/0148607189013002124. [DOI] [PubMed] [Google Scholar]
- 123.Levine JA, Harris MM, Morgan MY. Energy expenditure in chronic alcohol abuse. Eur J Clin Invest. 2000;30:779–786. doi: 10.1046/j.1365-2362.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 124.Müller MJ, Böttcher J, Selberg O, Weselmann S, Böker KH, Schwarze M, von zur Mühlen A, Manns MP. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. 1999;69:1194–1201. doi: 10.1093/ajcn/69.6.1194. [DOI] [PubMed] [Google Scholar]
- 125.Glass C, Hipskind P, Cole D, Lopez R, Dasarathy S. Handheld calorimeter is a valid instrument to quantify resting energy expenditure in hospitalized cirrhotic patients: a prospective study. Nutr Clin Pract. 2012;27:677–688. doi: 10.1177/0884533612446195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aguila EJT, Cua IHY, Fontanilla JAC, Yabut VLM, Causing MFP. Gastrointestinal Manifestations of COVID-19: Impact on Nutrition Practices. Nutr Clin Pract. 2020;35:800–805. doi: 10.1002/ncp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, Calleja JL, Bañares R, García-Pagán JC, Mesonero F, Bosch J Ciberehd SportDiet Collaborative Group. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65:1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 128.Amaral JF, Foschetti DA, Assis FA, Menezes JS, Vaz NM, Faria AM. Immunoglobulin production is impaired in protein-deprived mice and can be restored by dietary protein supplementation. Braz J Med Biol Res. 2006;39:1581–1586. doi: 10.1590/s0100-879x2006001200009. [DOI] [PubMed] [Google Scholar]
- 129.Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 130.Swart GR, Zillikens MC, van Vuure JK, van den Berg JW. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. BMJ. 1989;299:1202–1203. doi: 10.1136/bmj.299.6709.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zillikens MC, van den Berg JW, Wattimena JL, Rietveld T, Swart GR. Nocturnal oral glucose supplementation. The effects on protein metabolism in cirrhotic patients and in healthy controls. J Hepatol. 1993;17:377–383. doi: 10.1016/s0168-8278(05)80221-1. [DOI] [PubMed] [Google Scholar]
- 132.Les I, Doval E, García-Martínez R, Planas M, Cárdenas G, Gómez P, Flavià M, Jacas C, Mínguez B, Vergara M, Soriano G, Vila C, Esteban R, Córdoba J. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011;106:1081–1088. doi: 10.1038/ajg.2011.9. [DOI] [PubMed] [Google Scholar]
- 133.Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. doi: 10.1002/14651858.CD001939.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ren M, Zhang SH, Zeng XF, Liu H, Qiao SY. Branched-chain Amino Acids are Beneficial to Maintain Growth Performance and Intestinal Immune-related Function in Weaned Piglets Fed Protein Restricted Diet. Asian-Australas J Anim Sci. 2015;28:1742–1750. doi: 10.5713/ajas.14.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018;10 doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shah AM, Wang Z, Ma J. Glutamine Metabolism and Its Role in Immunity, a Comprehensive Review. Animals (Basel) 2020;10 doi: 10.3390/ani10020326. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 137.Kim SH, Roszik J, Grimm EA, Ekmekcioglu S. Impact of l-Arginine Metabolism on Immune Response and Anticancer Immunotherapy. Front Oncol. 2018;8:67. doi: 10.3389/fonc.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Selberg O, Burchert W, vd Hoff J, Meyer GJ, Hundeshagen H, Radoch E, Balks HJ, Müller MJ. Insulin resistance in liver cirrhosis. Positron-emission tomography scan analysis of skeletal muscle glucose metabolism. J Clin Invest. 1993;91:1897–1902. doi: 10.1172/JCI116407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Müller MJ, Willmann O, Rieger A, Fenk A, Selberg O, Lautz HU, Bürger M, Balks HJ, von zur Mühlen A, Schmidt FW. Mechanism of insulin resistance associated with liver cirrhosis. Gastroenterology. 1992;102:2033–2041. doi: 10.1016/0016-5085(92)90329-w. [DOI] [PubMed] [Google Scholar]
- 140.Croci I, Byrne NM, Choquette S, Hills AP, Chachay VS, Clouston AD, O'Moore-Sullivan TM, Macdonald GA, Prins JB, Hickman IJ. Whole-body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut. 2013;62:1625–1633. doi: 10.1136/gutjnl-2012-302789. [DOI] [PubMed] [Google Scholar]
- 141.Gomes F, Schuetz P, Bounoure L, Austin P, Ballesteros-Pomar M, Cederholm T, Fletcher J, Laviano A, Norman K, Poulia KA, Ravasco P, Schneider SM, Stanga Z, Weekes CE, Bischoff SC. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37:336–353. doi: 10.1016/j.clnu.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 142.Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang CY, Noar K, Song X, Lampe JW. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142:369–374. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Radzikowska U, Rinaldi AO, Çelebi Sözener Z, Karaguzel D, Wojcik M, Cypryk K, Akdis M, Akdis CA, Sokolowska M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients. 2019;11 doi: 10.3390/nu11122990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Innis SM. Dietary lipids in early development: relevance to obesity, immune and inflammatory disorders. Curr Opin Endocrinol Diabetes Obes. 2007;14:359–364. doi: 10.1097/MED.0b013e3282be90b9. [DOI] [PubMed] [Google Scholar]
- 145.Siegers JY, Novakovic B, Hulme KD, Marshall RJ, Bloxham CJ, Thomas WG, Reichelt ME, Leijten L, van Run P, Knox K, Sokolowski KA, Tse BWC, Chew KY, Christ AN, Howe G, Bruxner TJC, Karolyi M, Pawelka E, Koch RM, Bellmann-Weiler R, Burkert F, Weiss G, Samanta RJ, Openshaw PJM, Bielefeldt-Ohmann H, van Riel D, Short KR. A High-Fat Diet Increases Influenza A Virus-Associated Cardiovascular Damage. J Infect Dis. 2020;222:820–831. doi: 10.1093/infdis/jiaa159. [DOI] [PubMed] [Google Scholar]
- 146.Singer P, Shapiro H. Enteral omega-3 in acute respiratory distress syndrome. Curr Opin Clin Nutr Metab Care. 2009;12:123–128. doi: 10.1097/MCO.0b013e328322e70f. [DOI] [PubMed] [Google Scholar]
- 147.Dushianthan A, Cusack R, Burgess VA, Grocott MP, Calder PC. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst Rev. 2019;1:CD012041. doi: 10.1002/14651858.CD012041.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schenck LP, Surette MG, Bowdish DM. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016;590:3705–3720. doi: 10.1002/1873-3468.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients. 2020;12 doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hemilä H. Vitamin C and Infections. Nutrients. 2017;9 doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Erol N, Saglam L, Saglam YS, Erol HS, Altun S, Aktas MS, Halici MB. The Protection Potential of Antioxidant Vitamins Against Acute Respiratory Distress Syndrome: a Rat Trial. Inflammation. 2019;42:1585–1594. doi: 10.1007/s10753-019-01020-2. [DOI] [PubMed] [Google Scholar]
- 152.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12 doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, Holford P, Thornton CA, Whitaker IS. Could Vitamins Help in the Fight Against COVID-19? Nutrients. 2020;12 doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14:283–289. doi: 10.2174/1871530314666140922143950. [DOI] [PubMed] [Google Scholar]
- 158.Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12 doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Galmés S, Serra F, Palou A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients. 2020;12 doi: 10.3390/nu12092738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, Potter JD, Ulrich CM. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136:189–194. doi: 10.1093/jn/136.1.189. [DOI] [PubMed] [Google Scholar]
- 161.Sheybani Z, Dokoohaki MH, Negahdaripour M, Dehdashti M, Zolghadr AR. The Role of Folic Acid in the Management of Respiratory Disease Caused by COVID-19. ChemRexiv. 2020 [Google Scholar]
- 162.Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Doboszewska U, Wlaź P, Nowak G, Młyniec K. Targeting zinc metalloenzymes in coronavirus disease 2019. Br J Pharmacol. 2020;177:4887–4898. doi: 10.1111/bph.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Razzaque MS. COVID-19 Pandemic: Can Maintaining Optimal Zinc Balance Enhance Host Resistance? Tohoku J Exp Med. 2020;251:175–181. doi: 10.1620/tjem.251.175. [DOI] [PubMed] [Google Scholar]
- 166.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int J Infect Dis. 2020;99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Im JH, Je YS, Baek J, Chung MH, Kwon HY, Lee JS. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moghaddam A, Heller RA, Sun Q, Seelig J, Cherkezov A, Seibert L, Hackler J, Seemann P, Diegmann J, Pilz M, Bachmann M, Minich WB, Schomburg L. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients. 2020;12 doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bae M, Kim H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules. 2020;25 doi: 10.3390/molecules25225346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Haberland A, Neubert K, Kruse I, Behne D, Schimk I. Consequences of long-term selenium-deficient diet on the prostacyclin and thromboxane release from rat aorta. Biol Trace Elem Res. 2001;81:71–78. doi: 10.1385/bter:81:1:71. [DOI] [PubMed] [Google Scholar]
- 173.Turnlund JR, Jacob RA, Keen CL, Strain JJ, Kelley DS, Domek JM, Keyes WR, Ensunsa JL, Lykkesfeldt J, Coulter J. Long-term high copper intake: effects on indexes of copper status, antioxidant status, and immune function in young men. Am J Clin Nutr. 2004;79:1037–1044. doi: 10.1093/ajcn/79.6.1037. [DOI] [PubMed] [Google Scholar]
- 174.Andreou A, Trantza S, Filippou D, Sipsas N, Tsiodras S. COVID-19: The Potential Role of Copper and N-acetylcysteine (NAC) in a Combination of Candidate Antiviral Treatments Against SARS-CoV-2. In Vivo. 2020;34:1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Raha S, Mallick R, Basak S, Duttaroy AK. Is copper beneficial for COVID-19 patients? Med Hypotheses. 2020;142:109814. doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Martindale R, Patel JJ, Taylor B, Arabi YM, Warren M, McClave SA. Nutrition Therapy in Critically Ill Patients With Coronavirus Disease 2019. JPEN J Parenter Enteral Nutr. 2020;44:1174–1184. doi: 10.1002/jpen.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bodoky G, Kent-Smith L. Basics in clinical nutrition: Complications of enteral nutrition. E Spen Eur E J Clin Nutr Metab. 2009;4 [Google Scholar]
- 178.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World J Gastroenterol. 2020;26:2286–2293. doi: 10.3748/wjg.v26.i19.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.López-Romero D, Izquierdo-Vega JA, Morales-González JA, Madrigal-Bujaidar E, Chamorro-Cevallos G, Sánchez-Gutiérrez M, Betanzos-Cabrera G, Alvarez-Gonzalez I, Morales-González Á, Madrigal-Santillán E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 2: Plants, Vegetables, and Natural Resin. Nutrients. 2018;10 doi: 10.3390/nu10121954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Izquierdo-Vega JA, Morales-González JA, SánchezGutiérrez M, Betanzos-Cabrera G, Sosa-Delgado SM, Sumaya-Martínez MT, Morales-González Á, Paniagua-Pérez R, Madrigal-Bujaidar E, Madrigal-Santillán E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 1: Fruits and Polysaccharides. Nutrients. 2017;9 doi: 10.3390/nu9020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ben-Shabat S, Yarmolinsky L, Porat D, Dahan A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv Transl Res. 2020;10:354–367. doi: 10.1007/s13346-019-00691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, Wang Q, Xu Y, Li M, Li X, Zheng M, Chen L, Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mani JS, Johnson JB, Steel JC, Broszczak DA, Neilsen PM, Walsh KB, Naiker M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020;284:197989. doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Park JY, Yuk HJ, Ryu HW, Lim SH, Kim KS, Park KH, Ryu YB, Lee WS. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhib Med Chem. 2017;32:504–515. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shree P, Mishra P, Selvaraj C, Singh SK, Chaube R, Garg N, Tripathi YB. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants - Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi) - a molecular docking study. J Biomol Struct Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1810778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Swargiary A, Mahmud S, Saleh MA. Screening of phytochemicals as potent inhibitor of 3-chymotrypsin and papain-like proteases of SARS-CoV2: an in silico approach to combat COVID-19. J Biomol Struct Dyn. 2020:1–15. doi: 10.1080/07391102.2020.1835729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 190.Tu W, Wang H, Li S, Liu Q, Sha H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019;10:637–651. doi: 10.14336/AD.2018.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Houghton CA, Fassett RG, Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev. 2013;71:709–726. doi: 10.1111/nure.12060. [DOI] [PubMed] [Google Scholar]
- 192.Houghton CA, Fassett RG, Coombes JS. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician's Expectation Be Matched by the Reality? Oxid Med Cell Longev. 2016;2016:7857186. doi: 10.1155/2016/7857186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.de Figueiredo SM, Binda NS, Nogueira-Machado JA, Vieira-Filho SA, Caligiorne RB. The antioxidant properties of organosulfur compounds (sulforaphane) Recent Pat Endocr Metab Immune Drug Discov. 2015;9:24–39. doi: 10.2174/1872214809666150505164138. [DOI] [PubMed] [Google Scholar]
- 194.Kamal MM, Akter S, Lin CN, Nazzal S. Sulforaphane as an anticancer molecule: mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch Pharm Res. 2020;43:371–384. doi: 10.1007/s12272-020-01225-2. [DOI] [PubMed] [Google Scholar]
- 195.Liu Y, Yu Q, Chen Y. Effect of silibinin on CFLAR-JNK pathway in oleic acid-treated HepG2 cells. Biomed Pharmacother. 2018;108:716–723. doi: 10.1016/j.biopha.2018.09.089. [DOI] [PubMed] [Google Scholar]
- 196.Ou Q, Weng Y, Wang S, Zhao Y, Zhang F, Zhou J, Wu X. Silybin Alleviates Hepatic Steatosis and Fibrosis in NASH Mice by Inhibiting Oxidative Stress and Involvement with the Nf-κB Pathway. Dig Dis Sci. 2018;63:3398–3408. doi: 10.1007/s10620-018-5268-0. [DOI] [PubMed] [Google Scholar]
- 197.Zhao F, Shi D, Li T, Li L, Zhao M. Silymarin attenuates paraquat-induced lung injury via Nrf2-mediated pathway in vivo and in vitro. Clin Exp Pharmacol Physiol. 2015;42:988–998. doi: 10.1111/1440-1681.12448. [DOI] [PubMed] [Google Scholar]
- 198.Zhou J, Chao G, Li Y, Wu M, Zhong S, Feng Z. Activation of NRF2/ARE by isosilybin alleviates Aβ25-35-induced oxidative stress injury in HT-22 cells. Neurosci Lett. 2016;632:92–97. doi: 10.1016/j.neulet.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 199.Abdelsalam HM, Samak MA, Alsemeh AE. Synergistic therapeutic effects of Vitis vinifera extract and Silymarin on experimentally induced cardiorenal injury: The pertinent role of Nrf2. Biomed Pharmacother. 2019;110:37–46. doi: 10.1016/j.biopha.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 200.Arafa Keshk W, Zahran SM, Katary MA, Abd-Elaziz Ali D. Modulatory effect of silymarin on nuclear factor-erythroid-2-related factor 2 regulated redox status, nuclear factor-κB mediated inflammation and apoptosis in experimental gastric ulcer. Chem Biol Interact. 2017;273:266–272. doi: 10.1016/j.cbi.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 201.Li L, Sun HY, Liu W, Zhao HY, Shao ML. Silymarin protects against acrylamide-induced neurotoxicity via Nrf2 signalling in PC12 cells. Food Chem Toxicol. 2017;102:93–101. doi: 10.1016/j.fct.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 202.Vargas-Mendoza N, Morales-González Á, Morales-Martínez M, Soriano-Ursúa MA, Delgado-Olivares L, Sandoval-Gallegos EM, Madrigal-Bujaidar E, Álvarez-González I, Madrigal-Santillán E, Morales-Gonzalez JA. Flavolignans from Silymarin as Nrf2 Bioactivators and Their Therapeutic Applications. Biomedicines. 2020;8 doi: 10.3390/biomedicines8050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu CH, Jassey A, Hsu HY, Lin LT. Antiviral Activities of Silymarin and Derivatives. Molecules. 2019;24 doi: 10.3390/molecules24081552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lalani SS, Anasir MI, Poh CL. Antiviral activity of silymarin in comparison with baicalein against EV-A71. BMC Complement Med Ther. 2020;20:97. doi: 10.1186/s12906-020-2880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lestari ML, Indrayanto G. Curcumin. Profiles Drug Subst Excip Relat Methodol. 2014;39:113–204. doi: 10.1016/B978-0-12-800173-8.00003-9. [DOI] [PubMed] [Google Scholar]
- 206.Asouri M, Ataee R, Ahmadi AA, Amini A, Moshaei MR. Antioxidant and free radical scavenging activities of curcumin. Asian J Chem. 2013;25:7593. [Google Scholar]
- 207.Liu Z, Dou W, Zheng Y, Wen Q, Qin M, Wang X, Tang H, Zhang R, Lv D, Wang J, Zhao S. Curcumin upregulates Nrf2 nuclear translocation and protects rat hepatic stellate cells against oxidative stress. Mol Med Rep. 2016;13:1717–1724. doi: 10.3892/mmr.2015.4690. [DOI] [PubMed] [Google Scholar]
- 208.Meshkibaf MH, Maleknia M, Noroozi S. Effect of curcumin on gene expression and protein level of methionine sulfoxide reductase A (MSRA), SOD, CAT and GPx in Freund's adjuvant inflammation-induced male rats. J Inflamm Res. 2019;12:241–249. doi: 10.2147/JIR.S212577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H, Huang X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. 2019;14:e0216711. doi: 10.1371/journal.pone.0216711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zahedipour F, Hosseini SA, Sathyapalan T, Majeed M, Jamialahmadi T, Al-Rasadi K, Banach M, Sahebkar A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;34:2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mathew D, Hsu WL. Antiviral potential of curcumin. J Functional Foods. 2018;40:692. [Google Scholar]
- 212.Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A. Immune modulation by curcumin: The role of interleukin-10. Crit Rev Food Sci Nutr. 2019;59:89–101. doi: 10.1080/10408398.2017.1358139. [DOI] [PubMed] [Google Scholar]
- 213.Avasarala S, Zhang F, Liu G, Wang R, London SD, London L. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PLoS One. 2013;8:e57285. doi: 10.1371/journal.pone.0057285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]