Abstract
Purpose
To report on the imaging of internal limiting membrane (ILM) flap following macular hole (MH) surgery.
Observations
Three eyes of 3 patients with baseline Snellen visual acuities (VAs) of 20/250, 20/30, and 20/100 underwent superior wide-base internal limiting membrane flap transposition (SWIFT) for MH. Indocyanine green (ICG) was used for intraoperative staining of the ILM. Following MH surgery, MH closed in all cases and VAs were 20/30, 20/30, and 20/60 respectively. An “en face” ICG fluorescence image of the ILM flap was obtained using infrared confocal scanning laser imaging at 795 nm. ICG fluorescence demonstrated the ILM flap to be intact and in good position with complete coverage of the MH in all cases. An area of hypofluorescence was present superiorly, corresponding to the flap harvest site with absent ILM. ICG hyperfluorescence of varying intensity was present at the MH site in all 3 cases. Folding of the ILM flap was present in one case.
Conclusions and Importance
Following MH surgery, the status of an ILM flap may be evaluated by an “en face” image of the flap obtained by ICG fluorescence imaging. This imaging modality may be valuable in the study of various ILM flap techniques.
Keywords: Macular hole, Internal limiting membrane (ILM) flap, Vitrectomy, Indocyanine green (ICG) fluorescence
1. Introduction
Internal limiting membrane (ILM) flap techniques are frequently used for the treatment of macular holes (MH) at risk of non-closure including large, chronic, or persistent MHs, or MHs associated with high myopia. Michalewska and colleagues described an inverted ILM flap technique and reported improved anatomic closure rate compared to conventional ILM peel.1 Following the original description, a number of variations on ILM flap technique have been reported including free ILM patch grafts and pedicle ILM flaps for eyes with previously removed ILM.2, 3, 4, 5, 6, 7, 8, 9 Intraoperative displacement of ILM flaps by fluid currents is common. Additionally, the ILM flaps may displace during the early postoperative period.
There is little in the literature on the status of the ILM flap following surgery. While optical coherence tomography (OCT) imaging may visualize the ILM in a cross sectional view, it is not suited for localization of the ILM flap or determining if the flap has remained intact. In patients with indocyanine green (ICG)-assisted ILM peel, ICG fluorescence may be demonstrated originating from the residual ILM. Using scanning laser ophthalmoscope infrared imaging, Weinberger et al. reported ICG fluorescence in a patient 6 weeks after pars plana vitrectomy (PPV) and removal of the ILM.10 Subsequently, others reported persistence of ICG fluorescence for many months.11, 12, 13, 14, 15 Most of the fluorescence appears to originate from the residual ILM that was stained with ICG during MH surgery. In a study of outcomes of superior wide-base ILM flap transposition (SWIFT), Tabandeh et al. used ICG fluorescence imaging to evaluate the status of the ILM flap.9
The current report describes “en face” visualization of the ILM flap using postoperative ICG fluorescence imaging in patients who underwent MH surgery with ILM flap.
2. Findings
2.1. Case 1
A 64-year-old female presented with Snellen visual acuity (VA) of 20/150, Fuchs corneal endothelial dystrophy, nuclear sclerosis, and stage 4 MH in the left eye. The patient underwent PPV, ILM peel, and sulfur hexafluoride (SF6) gas tamponade. Three weeks following surgery the VA was 20/250 and MH was open. In view of persistent MH and previously removed ILM, the patient underwent repeat MH surgery that included SWIFT.8,9 The surgical technique involved 23G PPV, staining of residual ILM with ICG (diluted with isotonic dextrose 5% to 1.25 mg/ml for intraocular use), fashioning of a superior ILM flap from the residual ILM, coverage of the MH with the flap, fluid-air exchange, and perfluoropropane (C3F8) gas tamponade.
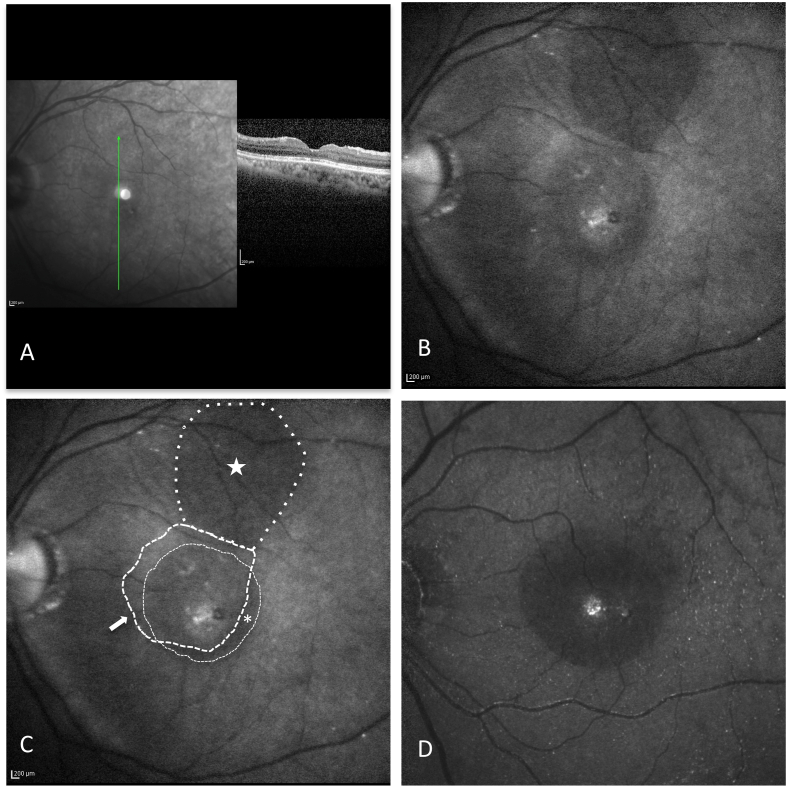
Three months after surgery the VA was 20/175 OS, cataract had progressed, and the MH was closed (Fig. 1A). A confocal laser scanning system (Spectralis, Heidelberg Engineering Inc., Heidelberg, Germany) with the 795 nm ICG filter was used to detect the ICG fluorescence without the intravenous administration of ICG. ICG fluorescence imaging demonstrated the ILM flap to be intact and in good position with complete coverage of the MH site (Fig. 1 B and C). A crescent-shaped hypofluorescent area, representing the area of the original ILM peel not covered by the ILM flap, was notable infero-temporally. Superiorly, an area of ICG hypofluorescence corresponded to the flap harvest site with absent ILM. Granular hyperfluorescence at the MH site and diffuse optic disc fluorescence were present. The patient underwent cataract surgery 10 months after MH surgery. At 1-year follow-up, the MH was closed, VA was 20/30 OS, mild corneal edema and a posterior chamber intraocular lens implant (PCIOL) were present.
Fig. 1.
Case 1: Postoperative OCT and indocyanine green (ICG) imaging after superior wide-based internal limiting membrane flap transposition (SWIFT) for MH.
A) Postoperative OCT shows U-shaped MH closure with a layer of ILM covering the fovea. B and C) ICG fluorescence imaging demonstrates the ILM flap (white arrow) is intact and in good position with complete coverage of the MH site. A crescent-shaped area (asterisk) of hypofluorescence, representing the area of the original ILM peel not covered by the ILM flap, is present infero-temporally. Superiorly, an area of ICG hypofluorescence (star) is present, corresponding to the flap harvest site with absent ILM. Granular hyperfluorescence at the MH site and mild speckled hyperfluorescence are present. D) ICG fluorescence image from a patient who underwent MH surgery with removal of ILM without a flap is included for comparison. Central circular area of hypofluorescence represents the area of ILM removal. Granular hyperfluorescence at the MH site and scattered speckled hyperfluorescence are present. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.2. Case 2
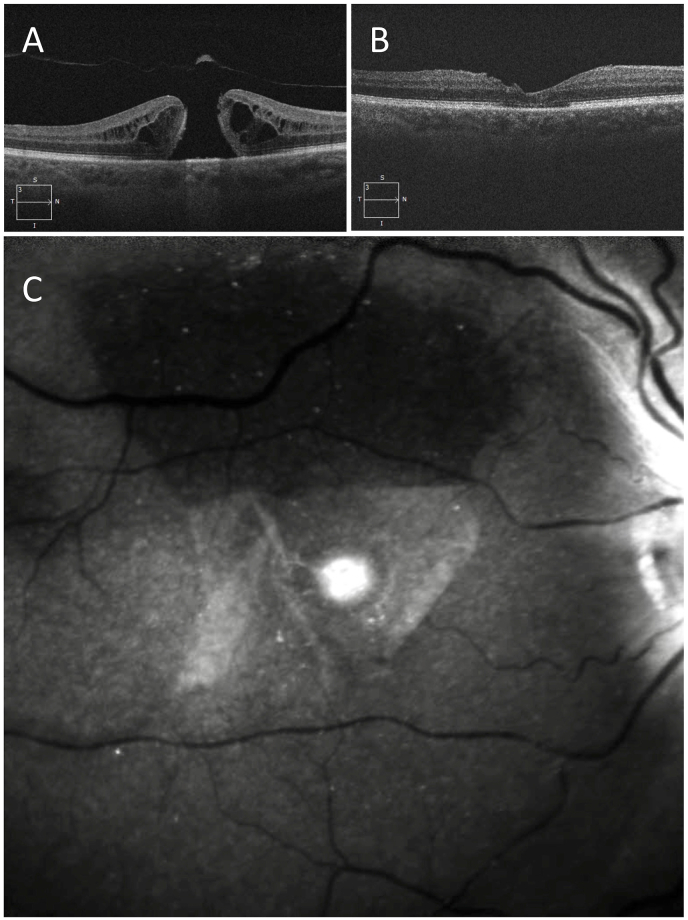
A 71-year-old highly myopic pseudophakic male presented with metamorphopsia and a small central scotoma in the right eye. The VA was 20/30 and a full thickness MH was present in the right eye (Fig. 2 A). Past ocular history was notable for high myopia of −11.0 diopters. The patient underwent MH surgery including PPV, SWIFT and C3F8 gas tamponade. Five months after the surgery the VA was 20/30 OD, and the MH was closed (Fig. 2 B). The ILM flap could be visualized by ICG fluorescence imaging that showed the flap to be intact and in good position with coverage of the MH (Fig. 2 C). An area of ICG hypofluorescence was present superiorly corresponding to the flap harvest site with absent ILM. Hyperfluorescence was present over the MH site, adjacent to the optic disc, and speckled over the posterior pole.
Fig. 2.
Case 2- Pre-operative OCT and post-operative OCT and ICG imaging after SWIFT for MH in a 71-year-old highly myopic pseudophakic patient.
A) Pre-operative OCT shows a full-thickness MH. B) Postoperative OCT shows closed MH with a double layer of ILM superior to the MH corresponding to the flap hinge. C) ICG fluorescence image shows a well-positioned ILM flap covering the MH, and a superior area of hypofluorescence corresponding to the flap harvest site. Hyperfluorescence is present at the MH site.
2.3. Case 3
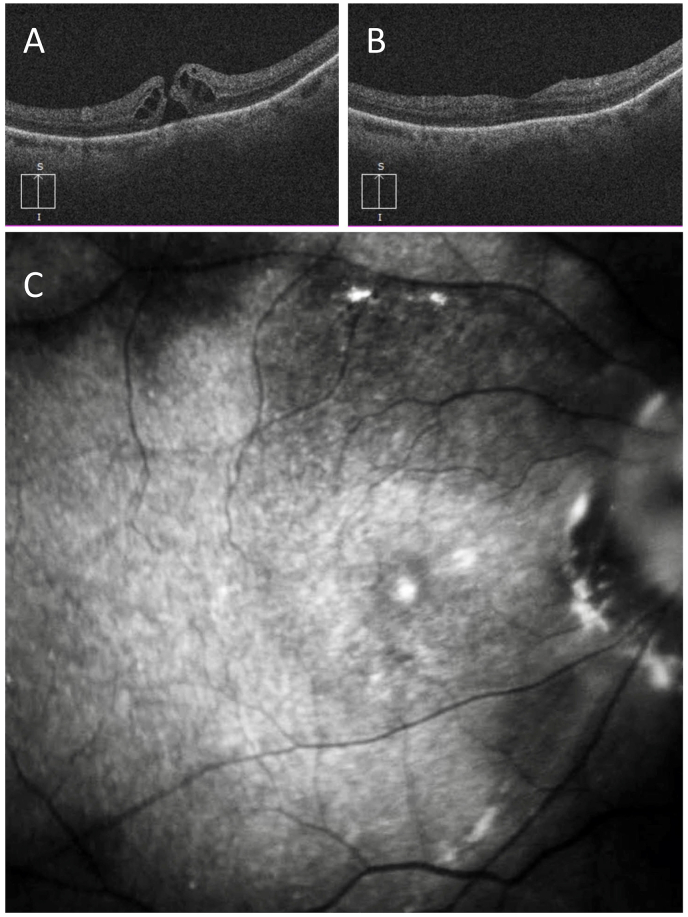
A 66-year-old female presented with reduced vision, VA 20/100, and a large full thickness MH with an inner diameter of 570 μm and a basal diameter of 1230 μm in the right eye (Fig. 3 A). The patient underwent MH surgery including PPV, SWIFT, and C3F8 gas tamponade. Four months after surgery the VA was 20/60 OD, the MH was closed, and nuclear sclerotic cataract was present. OCT demonstrated closed MH with mild irregularities on the inner retina surface temporal to the MH (Fig. 3 B). ICG fluorescence imaging showed the ILM flap to be intact and in good position covering the MH completely. ILM folds were present, predominantly involving the temporal side of the flap, sparing the center (Fig. 3 C). An area of ICG hypofluorescence was present superiorly corresponding to the flap harvest site with absent ILM. The residual ILM showed a diffuse ICG fluorescence over the posterior pole with some overlying speckled hyperfluorescence. A circular area of hyperfluorescence corresponded to the site of the MH. ICG hyperfluorescence was also detected at and around the optic disc.
Fig. 3.
Case 3- Pre-operative OCT and post-operative OCT and ICG imaging after SWIFT for MH in a 67-year-old patient with a large full-thickness MH with an inner diameter of 570 μm and a basal diameter of 1230 μm.
A) Preoperative OCT shows a large full-thickness MH. B) Postoperative OCT shows closed MH with mild irregularities on the retina surface temporal to the MH. C) ICG fluorescence image shows the ILM flap is covering the MH. ILM folds are present on the temporal aspect of the flap sparing the center. Superiorly, the area of hypofluorescence corresponds to the flap harvest site. Hyperfluorescence is present at the MH site.
3. Discussion
Primary MH closure rates of over 90% have been reported for idiopathic macular holes. Large MHs, chronic MHs, myopic MHs, and persistent MHs are associated with lower closure rates. Removal of ILM has been advocated for many types of MHs and is now widely practiced. Michalewska and colleagues described a surgical technique for the coverage of the MH by an inverted ILM flap and reported improved closure rate.1 A number of variations on the ILM flap technique, including a temporal inverted flap technique have been described.5 Distal flap techniques are useful in eyes with previously removed ILM but are susceptible to displacement.2,3,6,7 Postoperative status of ILM flaps has not been studied in detail due to difficulties in visualization of the flap by conventional imaging modalities including OCT. Optical coherence tomography visualizes segments of the ILM flap on a cross-sectional view but not the entirety of the flap. “En face” imaging of the ILM flap would be valuable in the study of flap location and integrity.
Indocyanine green fluorescence originating from the residual ILM may be detected postoperatively by infrared fundus imaging in eyes that have undergone staining of the ILM by ICG during MH surgery. Weinberger et al. used scanning laser ophthalmoscope imaging at 795 nm to evaluate ICG fluorescence in a patient 6 weeks after ICG-assisted removal of ILM.10 The authors noted persistent ICG signal along the vascular arcades and in the foveal center. In a study of 17 patients with MH or epiretinal membrane who underwent PPV and ICG-assisted removal of ILM with ICG, Tadayoni et al. noted diffuse fluorescence of the fundus, the optic disc, and the peripapillary area that lasted for many months.15 In eyes that underwent surgery for MH, a granular fluorescence was present at the macular center. They postulated that ICG binds to the residual ILM, the retinal nerve fiber layers, and possibly to some components of the ganglion cell axons. Others have subsequently reported on residual ICG fluorescence patterns after MH surgery.11, 12, 13, 14 Although the fluorescence pattern is somewhat variable, most cases demonstrate diffuse hyperfluorescence from residual ILM, a central area of hypofluorescence corresponding to the area of removed ILM, a smaller area of granular hyperfluorescence at the RPE level within the bed of the MH, optic disc and NFL hyperfluorescence, and a variable degree of stardust or speckled hyperfluorescence scattered over the macula. In a study of outcomes of SWIFT for MH, Tabandeh et al. used ICG fluorescence imaging to evaluate the status of the ILM flap.9 They noted that using the imaging technique it was possible to postoperatively visualize the ILM flap and the harvest site in eyes that had undergone ICG-assisted ILM flap surgery.
In the present case series, an ‘en face” image of the ILM flap was obtained by ICG fluorescence imaging. Case 1 was a persistent MH with history of previous MH surgery and ILM removal, case 2 was a MH associated with high myopia, and case 3 was a large MH. All 3 cases showed the ILM flap to be intact with good coverage of the MH. Case 3 demonstrated folding of the ILM flap. Other findings included hypofluorescence representing the flap harvest site and the area of ILM peel not covered by the ILM flap. The area corresponding to overlap of the ILM flap with the residual ILM, the original MH site, and the optic disc demonstrated a variable degree of hyperfluorescence.
The ICG fluorescence may be detected up to 2 years after surgery, however, the fluorescence signal fades with time. The optimal time for ICG imaging is between 2 and 4 months after the surgery as by this time the gas tamponade has resolved and the ICG fluorescence is still strong. In cases that present with persistent MH, preoperative ICG imaging allows evaluation of the ILM status and planning of the surgical approach for reoperation.
4. Conclusion
Postoperative visualization of ILM flap by ICG fluorescence imaging is a non-invasive imaging modality that provides an “en face” image of the ILM flap, MH, and the neuroretina, allowing the study of the ILM flap performed by various surgical techniques.
Patient consent
Consent to publish the report was not obtained. This report does not contain any personal information that could lead to the identification of the patients.
Author contributions
Homayoun Tabandeh: Conceptualization, Methodology, Data curation, Manuscript preparation, reviewing and editing.
Acknowledgements and Disclosures
Homayoun Tabandeh: None relevant.
References
- 1.Michalewska Z., Michalewski J., Adelman R.A., Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117:2018–2025. doi: 10.1016/j.ophtha.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Michalewska Z., Michalewski J., Dulczewska-Cichecka K., Adelman R.A., Nawrocki J. Temporal inverted internal limiting membrane flap technique versus classic inverted internal limiting membrane flap technique: a Comparative Study. Retina. 2015;35:1844–1850. doi: 10.1097/IAE.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 3.De Novelli F.J., Preti R.C., Ribeiro Monteiro M.L., Pelayes D.E., Junqueira Nobrega M., Takahashi W.Y. Autologous internal limiting membrane fragment transplantation for large, chronic, and refractory macular holes. Ophthalmic Res. 2015;55:45–52. doi: 10.1159/000440767. [DOI] [PubMed] [Google Scholar]
- 4.Gekka T., Watanabe A., Ohkuma Y. Pedicle internal limiting membrane transposition flap technique for refractory macular hole. Ophthalmic Surg Lasers Imaging Retina. 2015;46:1045–1046. doi: 10.3928/23258160-20151027-10. [DOI] [PubMed] [Google Scholar]
- 5.Koo E.B., Smiddy W.E. Internal limiting membrane flap technique for macular holes: is it ready for prime time? Retina. 2018;38:865–869. doi: 10.1097/IAE.0000000000002149. [DOI] [PubMed] [Google Scholar]
- 6.Morizane Y., Shiraga F., Kimura S. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol. 2014;157:861–869 e1. doi: 10.1016/j.ajo.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Wang L.P., Sun W.T., Lei C.L., Deng J. Clinical outcomes with large macular holes using the tiled transplantation internal limiting membrane pedicle flap technique. Int J Ophthalmol. 2019;12:246–251. doi: 10.18240/ijo.2019.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabandeh H. American Society of Retinal Specialists; Boston: 2017. RETINAWS: When the Going Gets Tough, the Tough Get Going- Challenging Cases in Vitreoretinal Surgery.https://eyetube.net/series/retinaws-asrs-2017/ilm-flap--wmkek Accessed. [Google Scholar]
- 9.Tabandeh H., Morozov A., Rezaei K.A., Boyer D.S. Superior wide-base internal limiting membrane flap transposition (SWIFT) for macular holes: flap status and outcomes. Ophthalmol Retina. 2021;5:317–323. doi: 10.1016/j.oret.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Weinberger A.W., Kirchhof B., Mazinani B.E., Schrage N.F. Persistent indocyanine green (ICG) fluorescence 6 weeks after intraocular ICG administration for macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2001;239:388–390. doi: 10.1007/s004170100267. [DOI] [PubMed] [Google Scholar]
- 11.Tadayoni R., Paques M., Girmens J.F., Massin P., Gaudric A. Persistence of fundus fluorescence after use of indocyanine green for macular surgery. Ophthalmology. 2003;110:604–608. doi: 10.1016/S0161-6420(02)01761-X. [DOI] [PubMed] [Google Scholar]
- 12.Kersey T.L., Bolton A., Patel C.K. Serial autofluorescence imaging over two years following indocyanine green-assisted internal limiting membrane peel for macular hole. Clin Exp Ophthalmol. 2005;33:538–539. doi: 10.1111/j.1442-9071.2005.01078.x. [DOI] [PubMed] [Google Scholar]
- 13.Sayanagi K., Ikuno Y., Soga K. Residual indocyanine green fluorescence pattern after vitrectomy for idiopathic macular hole with internal limiting membrane peeling. Br J Ophthalmol. 2007;91:939–944. doi: 10.1136/bjo.2006.108746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayanagi K., Ikuno Y., Soga K., Sawa M., Tano Y. Residual indocyanine green fluorescence pattern after vitrectomy with internal limiting membrane peeling in high myopia. Am J Ophthalmol. 2007;144:600–607. doi: 10.1016/j.ajo.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 15.Sekiryu T., Iida T. Long-term observation of fundus infrared fluorescence after indocyanine green-assisted vitrectomy. Retina. 2007;27:190–197. doi: 10.1097/01.iae.0000237079.08886.87. [DOI] [PubMed] [Google Scholar]