Abstract
Purpose
To report a patient with chronic recurrent multifocal osteomyelitis (CRMO) complicated by optic neuropathy and central retinal artery occlusion (CRAO).
Observations
CRMO is a noninfectious, inflammatory bone disorder. It is thought to be an autoimmune condition related to an imbalance of pro- and anti-inflammatory cytokines. Retinal vasculitis has been reported in a patient with CRMO but not CRAO or optic neuropathy.
Conclusions
We expanded the list of ophthalmic involvement of CRMO to include CRAO and optic neuropathy.
Keywords: Chronic recurrent multifocal osteomyelitis, Central retinal artery occlusion, Optic neuropathy
Authorship statement
Michael S. Vaphiades, D.O. Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Investigation, Supervision, Writing- Reviewing and Editing.
Kevin E. Lai, M.D. Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Investigation, Writing- Reviewing and Editing.
Lanning B. Kline, M.D. Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Investigation, Supervision, Writing- Reviewing and Editing.
Brendan Grondines, C.O.A. Conceptualization, Methodology, Writing- Original draft preparation.
Emily Riser M.D. Conceptualization, Methodology.
1. Introduction
Chronic recurrent multifocal osteomyelitis (CRMO) is a rare, noninfectious inflammatory disorder, characterized by lytic bone lesions, accompanied by periodic painful exacerbations and remissions. Typically, it is insidious in onset with swelling and tenderness localized over the affected bone. It may occur with or without fever and is a diagnosis of exclusion following bone biopsy.1, 2, 3 There is a single report in the literature of a patient with CRMO who was found to have funduscopic changes consistent with retinal vasculitis.4 We describe a patient with CRMO and expand the ophthalmic manifestations of this order to include central retinal artery occlusion (CRAO) and optic neuropathy.
2. Case report
A 12-year-old girl developed left-sided lower rib pain. A chest X-ray was normal and she was treated with analgesics. The pain resolved in 1 week. Four months later, the patient spontaneously developed right leg pain without antecedent trauma or illness. An X-ray of her right leg was normal and she was placed in a rigid boot which initially helped, but once out of the boot, the pain returned. This was followed by swelling and a maculopapular, erythematous, nonpruritic rash over her right shin. Magnetic resonance imaging (MRI) showed a stress fracture of the tibia with surrounding inflammation. She was given crutches and instructed to avoid weight bearing.
Two months later, the patient experienced pain in the left medial shin and right wrist. The shin pain worsened to the point where she could not walk. Noncontrast MRI of her left tibia revealed edema in the distal quarter of the tibia without evidence of fracture. She soon reported pain in her right mid-lower shin and the ulnar side of her right distal forearm. At the same time, she developed episodes of fever up to 103° Fahrenheit lasting 24–48 hours and a rash on her legs and abdomen.
The patient was hospitalized and multiple X-rays of her upper and lower extremities showed a periosteal reaction around the distal portion of the left tibia and right fibula as well as the right distal ulna. There was no evidence of fracture. Laboratory testing revealed a white blood cell count of 14,800 cells/mm3 (normal: 4500–13,500 cells/mm3) with normal differential, hemoglobin of 11.9 gm/dL (normal: 12.0–15.0 gm/dL), hematocrit of 35.7% (normal: 35–49%), platelet count of 514,000/mm3 (normal: 150,000–450,000/mm3), positive antinuclear antibodies (ANA) (1:160, speckled pattern), elevated Westergren sedimentation rate (ESR) of 51 mm/hr (normal <10 mm/hr), and elevated C-reactive protein (CRP) of 5.0 mg/dL (normal ≤ 1.0 mg/dL). The patient was treated with nonsteroidal anti-inflammatory drugs, primarily naproxen. Repeat CRP was within normal limits, the repeat ESR was 34 mm/hr, and white blood cell count was 6.8 k/mm3. Tuberculin skin test was negative. Alpha-fetoprotein and human chorionic gonadotropin were within normal limits. A bone scan revealed multiple areas of longitudinal linear uptake in the medial aspect of the right proximal humeral diaphysis, the lateral aspects of the distal bilateral humeral diaphysis, the left proximal forearm, and the distal right radius and ulna. There were additional areas of uptake including the lateral and posterolateral aspects of the ninth ribs, the outer margins of the both mid-femoral diaphyses, the lateral margin of the mid-tibia, and at the junction of the mid and distal thirds of the right tibia and fibula. A bone marrow biopsy taken from the left posterior aspect of the pelvis was unremarkable showing 20 dividing cells with normal chromosomes. A whole-body MRI without contrast demonstrated normal bone marrow signal without findings suggestive of osteomyelitis.
The patient's left leg pain continued to fluctuate over the next 6 months. A contrasted MRI of the left calf demonstrated an extensive abnormality of the soft tissues overlying the mid-tibial shaft medially, anteriorly and posteriorly. There was a 5 mm rind of tissue extending from the cortical surface circumferentially about the mid-shaft with additional overlying edema. There also was increased T2 signal and enhancement of the bone marrow in this area with evidence of periosteal reaction. The multifocal recurring inflammatory bone involvement was believed to be consistent with a diagnosis of CRMO. This diagnosis was confirmed at another medical institution following review of the patient's clinical course and the diagnostic testing results.
Three years later, the patient experienced decreased vision in her right eye with pain on eye movement. Visual acuity was 20/50, right eye and 20/20, left eye. There was a right relative afferent pupillary defect and the right optic disc was swollen. Fat-suppressed brain and orbital MRI revealed extensive enhancement of the right optic nerve and sheath. Despite no evidence of visual loss in the left eye, there was also mild enhancement of the left optic nerve. The following tests were negative: aquaporin-4-IgG, myelin oligodendrocyte glycoprotein (MOG)-IgG, angiotensin-converting enzyme, Bartonella antibody, Lyme titers, FTA-Abs, p-ANCA, c-ANCA. The patient was initially treated with intravenous corticosteroids and intravenous immunoglobulin followed by an oral corticosteroid taper over 5 months and her acuity improved to 20/20 in the right eye. There was resolution of her optic disc edema over the course of two months.
Seven months after her initial vision loss, her examination demonstrated bilateral optic disc edema, with visual acuity of 20/20 in both eyes. Magnetic resonance imaging revealed enhancement of both optic nerves. A lumbar puncture showed an opening pressure of 12 cm H2O with normal cerebrospinal fluid (CSF) composition including negative aquaporin-4-IgG and repeat MOG-IgG. The patient declined treatment as her vision was stable.
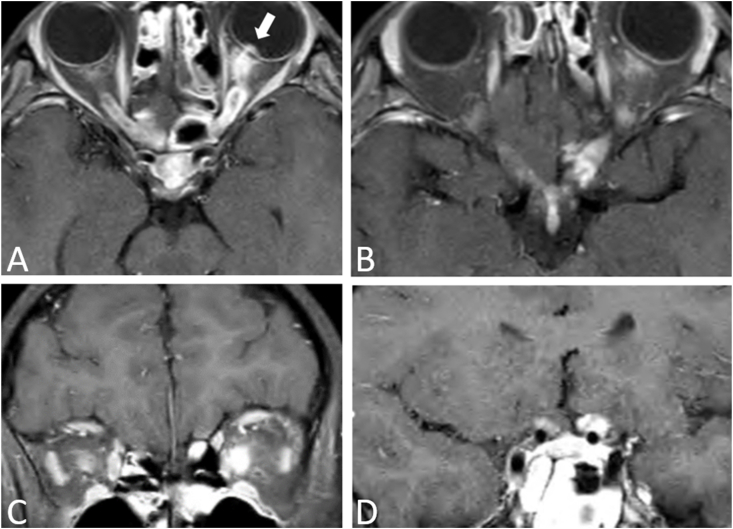
In the next two months, she experienced the onset of diminished vision in her left eye with pain on eye movement. Visual acuity was 20/20, right eye and 20/40, left eye. The right optic disc was pale while the left optic disc was edematous. On MRI, both optic nerves and chiasm demonstrated enhancement (Fig. 1). Treatment consisted of intravenous and oral corticosteroids. Seventeen days later, MRI showed resolution of the optic nerve enhancement.
Fig. 1.
Contrasted axial (A,B) and coronal (C,D) T1 orbital MRI with fat suppression. There is enhancement of both optic nerves (left greater than right) with involvement of the optic chiasm. There also is enhancement of the left optic disc (arrow).
Over the ensuing four months, the left optic disc swelling gradually resolved and the visual acuity stabilized at 20/20, right eye, and 20/30, left eye. Both optic discs became pale.
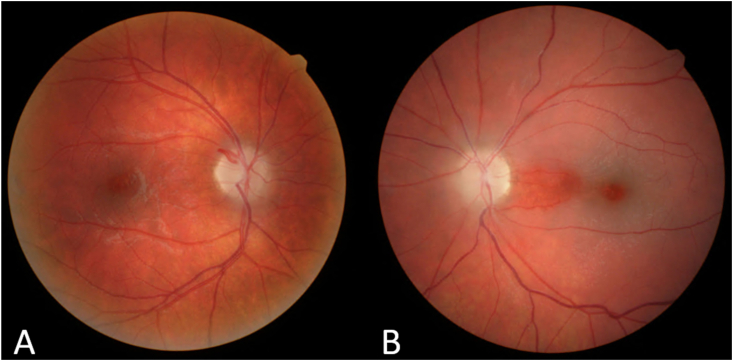
Seventeen months following her initial episode of optic neuropathy, the patient awoke with painless vision loss in her left eye. Visual acuity was 20/20, right eye, and counting fingers, left eye and the appearance of the left fundus was consistent with a CRAO with cilioretinal artery sparing (Fig. 2). Testing included a carotid doppler study, echocardiography and a contrasted fat-suppressed brain and orbital MRI, brain and neck magnetic resonance angiogram (MRA), all of which were normal. Additionally, a number of hematologic tests were performed and proved to be normal, including: complete blood count, ESR 17 mm/hr, Sjogren syndrome Ro antibodies (SSa) and Sjogren syndrome La antibodies (SSb), c-ANCA, p-ANCA, angiotensin-converting enzyme, lysozyme, acquaporin-4-IgG and again MOG-IgG, and hematologic panel for a hypercoagulable state. Lipid panel showed a slightly depressed triglyceride level and elevated high density lipoprotein ratio.
Fig. 2.
The right fundus (A) demonstrates optic disc pallor while the left fundus (B) reveals a central retinal artery occlusion with sparing of the cilioretinal artery distribution.
Eighteen months after her initial visual loss, the patient's vision was 20/20, right eye with normal color vision, and 20/200, left eye, without identification of any of the color plates. Methotrexate was prescribed for presumed autoimmune retinopathy and optic neuropathy related to CRMO and her visual exam has remained stable for the last 6 months.
3. Discussion
Chronic non-bacterial osteomyelitis is an autoinflammatory bone disorder. Its more severe form is CRMO which generally affects female children and adolescents with peak onset between 7 and 12 years of age.2,5,6 The incidence is approximately 1:1,000,000 or 2–5% of all osteomyelitis cases.2 Chronic non-bacterial osteomyelitis is characterized by recurrent attacks of bone inflammation which, at times, leads to bone destruction. This inflammation is associated with pain and often fever and frequently involved bony sites include long bones, the pelvis, vertebral column and the shoulder.1,2 Skin involvement in CRMO is not uncommon,1,3 as in our patient. Establishing the diagnosis of CRMO often is difficult and requires exclusion of infectious, neoplastic and other inflammatory causes of a bone disorder.2,6 The Bristol criteria for CRMO includes bone pain and lytic lesions, sclerosis and new bone formation on plain X-ray or preferably MRI showing bone marrow edema and or bone expansion, lytic areas and periosteal reaction. Also, laboratory testing is without significantly raised CRP.3 Our patient fulfilled this criteria of CRMO.
The pathoetiology of CRMO is not well understood but appears to be a combination of various genetic risk alleles and environmental factors. It is thought that this leads to an imbalance of pro- and anti-inflammatory cytokines, in particular the interleukins. An animal model has been developed in which IL-10-deficient mice develop inflammatory bone loss and synovial inflammation.2,5
We are aware of only one other report of a patient with CRMO who experienced ophthalmic involvement.4 A 37-year-old woman with CRMO complained of a visual disturbance in her left eye. She was found to have a cotton-wool spot in her left fundus and fluorescein angiography demonstrated retinal vascular staining in both eyes. These findings were consistent with retinal vasculitis.
Our patient had an extensive evaluation to exclude other disorders that also may be associated with CRAO and optic neuropathy. Etiologies excluded were autoimmune (sarcoidosis, Behcet), hematologic (plasma cell dyscrasia), neoplastic (sarcoma) and infectious (tuberculosis, syphilis). During the initial episode of visual loss, the patient had enhancement of the left optic nerve despite no evidence of optic neuropathy in the left eye. At another point in time, our patient experienced several months of stable 20/20 vision despite having bilateral optic disc edema and enhancement on MRI and normal lumbar puncture. This suggests that there may be a subclinical inflammatory phase prior to vision loss.
Patients with vasculitis-induced optic neuropathy have been reported to have extensive optic nerve and chiasm enhancement on MRI, similar to inflammatory or demyelinating optic neuropathies.7,8 High in the differential diagnosis of the recurrent bilateral optic neuropathy with optic nerve enhancement on MRI is neuromyelitis optica spectrum disorder (NMOSD) and myelin oligodendrocyte glycoprotein associated disease (MOGAD). Our patient had been tested for the aquaporin-4-IgG and MOG-IgG in blood and CSF, both of which were negative. The patient's optic nerve enhancement involving the full length of the optic nerve on MRI is similar to patients with MOGAD.9 Thus, it might be useful to include CRMO in the differential diagnosis of NMOSD and MOGAD.
In conclusion, we have expanded the spectrum of ophthalmic complications of CRMO to include CRAO and optic neuropathy. While this bone disorder is rare, it behooves clinicians caring for these patients to be attuned to any visual complaints.
Patient consent
Written consent to publish this case has not been obtained. This report does not contain any personal identifying information.
Acknowledgment and disclosures
This work was supported in part by an unrestricted grant from the Research to Prevent Blindness, Inc. N.Y., N.Y.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Gicchino M.F., Diplomatico M., Granato C. Chronic recurrent multifocal osteomyelitis: a case report. Ital J Pediatr. 2018;44(1):26. doi: 10.1186/s13052-018-0463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buch K., Thuesen A.C.B., Brøns C., Schwarz P. Chronic non-bacterial osteomyelitis: a review. Calcif Tissue Int. 2019;104(5):544–553. doi: 10.1007/s00223-018-0495-0. [DOI] [PubMed] [Google Scholar]
- 3.Roderick M.R., Sen E.S., Ramanan A.V. Chronic recurrent multifocal osteomyelitis in children and adults: current understanding and areas for development. Rheumatology. 2018;57(1):41–48. doi: 10.1093/rheumatology/kex066. [DOI] [PubMed] [Google Scholar]
- 4.Shanmugam V.K., Phillpotts M., Brady T. Retinal vasculitis with chronic recurrent multifocal osteomyelitis: a case report and review of the literature. BMC Rheumatol. 2019;3:29. doi: 10.1186/s41927-019-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann S.R., Kapplusch F., Girschick H.J. Chronic recurrent multifocal osteomyelitis (CRMO): presentation, pathogenesis, and treatment. Curr Osteoporos Rep. 2017;15(6):542–554. doi: 10.1007/s11914-017-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nepal P., Alam S.I., Sajid S., Sapire J., Ojili V. Rare presentation of chronic recurrent multifocal osteomyelitis of the Iliac wing mimicking Ewing's sarcoma. SA J Radiol. 2021;25(1):2030. doi: 10.4102/sajr.v25i1.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sklar E.M., Schatz N.J., Glaser J.S., Post M.J., ten Hove M. MR of vasculitis-induced optic neuropathy. AJNR Am J Neuroradiol. 1996;17(1):121–128. [PMC free article] [PubMed] [Google Scholar]
- 8.Siatkowski R.M., Scott I.U., Verm A.M. Optic neuropathy and chiasmopathy in the diagnosis of systemic lupus erythematosus. J Neuro Ophthalmol. 2001;21(3):193–198. doi: 10.1097/00041327-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Prasad S., Chen J. What you need to know about AQP4, MOG, and NMOSD. Semin Neurol. 2019;39(6):718–731. doi: 10.1055/s-0039-3399505. [DOI] [PubMed] [Google Scholar]