Abstract
In order to study the effects of Spirulina, Arthrospira platensis, two cell lines of A549 and HFF were treated with the concentration of IC50 for 24 h. MTT analysis showed that the highest decrease in viability of cells happened at the concentration of 500 μg/ml. The necrosis, releases of LDH, produced DCFH, and Lipid peroxidation were higher in the cancer cell lines in comparison to normal cells. Results showed that the extract affected the cell cycle of the A549 cell line. Also, the algal extract had concentration-dependent antioxidant activity. Also, the production of malonyl dialdehyde was significantly higher in treated cells and there was a significant relationship between produced MDA and ROS. Results showed that A. platensis extract had a remarkable effect on the lung cancer cell cycle and arrest the cell cycle in phase G2; so the cells didn't enter phase M and the proliferation of cancer cells prevented. Furthermore, according to the higher production of ROS and MDA in treated A549 cancer cell lines, it could be concluded that this algal extract could be considered as a natural product with anticancer activity against lung cancer cells.
Keywords Index: Spirulina, Anti-cancer, Lung cancer, Algae, Bioproduct
Highlights
-
•
Arthrospira platensis extract has a concentration-dependent antioxidant effect.
-
•
A. platensis could damage the cancer cells by affecting the cell cycle.
-
•
A. platensis could protect the normal cells by its antioxidant activity.
-
•
A. platensis force the cancer cells to apoptosis through biochemical alterations.
1. Introduction
Lung cancer is one of the most common cancers in the world which possesses the third most common cancer among men and the fourth common cancer among women [1]. All the human tumors have non-activating mutations in genes whose productions act in all cell cycle checkpoints naturally in order to impede the continuation of the cell cycle when it goes wrong or there is a destroyed DNA [2].
Some molecules which control the primary incidences of the cell cycle vastly include cyclin D and kinases dependent on cyclin D (CDKs) [3]. Lung cancer divided into two main groups of small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC includes more than 85% of lung cancers [4].
Oxidative stress is caused by a lack of balance in the body's redox status in which free radicals increase and it leads to tissue damages. The free radicals are mainly divided into two forms, reactive oxygen species (ROS) and reactive nitrogen species (RNS). In the cancer cells, an increase in the level of ROS is the result of metabolic activity, mitochondria malfunction, peroxisome activity, an increase of signaling through the mediation of the receptor, activation of oncogenes, and increased activation in oxidase, cyclooxygenase, lipoxygenase, and thymidine phosphorylase [1].
Antioxidants are generally compounds which control the damage ability activity of reactive oxygen varieties effectively or hinder them. Today antioxidants are acquired through herbs and spices naturally, so their antioxidant properties are analyzed vastly [5].
Spirulina, Arthrospira platensis is a well-known blue-green alga and is a rich source of biological products including antioxidants and granules compounds (phycocyanin, chlorophyll, myxoxanthophyll, beta-carotene, zeaxanthin and xanthophyll), vitamins, proteins, minerals, and various kinds of necessary amino acids [3].
Usage of extracts and medicinal herbs for treating cancers has been noticed so much, and it seems that they are appropriate substitutions for chemical medications. Therefore, the present study has been conducted to investigate the anti-cancer effects of Spirulina blue-green algae extract on the human lung cancer A549 cell line.
2. Experimental
2.1. Algal extract
In order to achieve an algal extraction, a maceration (marinating) method was used. 1 Kg of Arthrospira platensis was purchased from the Noor Daru Co. Iran and dried in a cool dry place (away from sunlight). Then 500 g of it were ground and riddled. 250 ml distilled water was added to 100 g of algal powder and then it was placed in a shaker with 40°-50 °C temperature for 24 h. After filtration, 250 ml of distilled water was poured on the residue again. All the acquired materials were placed into a swinging vaporizer [1]. Dark tar-like sediment was gained. Then the required concentrations (125, 250, 500, 1000, 2000, 4000 μg/ml) of the extract were prepared by solving the sediment in the culture media without serum and filtered through a 0.22 μm pore Millipore filter [1].
2.2. Cell culture
The human Caucasian non-small-cell lung adenocarcinoma A549 cell line and human foreskin fibroblast (HFF) were obtained from the Pasteur Institute, Iran, and cultured in a small flask (25 square centimeters) which was filled with the appropriate culture media according to cell culture conditions brochure from the Pasteur Institute, Iran. In order to see whether there was any kind of viruses or bacterial contaminations, a molecular observation was performed through PCR (The commercially available kit: CinnaGen, Iran), but all the results were negative. Cells were cultured in DMEM contained 10% fetal bovine serum (FBS) in sterile flasks and incubated at 37 °C and humidified atmosphere of 95% air and 5% CO2. Culture media was renewed every 2–3 days and cells were passaged once, after one week [7].
2.3. MTT cytotoxicity assay
MTT assay method is based on the fact that metabolically active cells can reduce the MTT by the mitochondrial enzyme succinate dehydrogenase to form insoluble purple formazan crystals that are solubilized subsequently, and thus one can measure the metabolic activity of cells by spectrophotometry. The cytotoxic activity of A. platensis extract was assessed in A549 and HFF cell lines. The cells were plated in 96-well plates (1 × 105 cells per well) in triplicate and incubated overnight at 37 °C. After 24 h, the Spirulina extracts were added from a stock diluted to concentrations of 0, 1, 10, 50, 100, 500, 1000 μg/μl. The negative control consisted of the cell suspension without any treatment of their respective culture medium supplemented with 1% FBS. The cells were then incubated for 24 h. Following incubation, 15 ml of the MTT labeling reagent was added to each well and incubated in a humidified atmosphere at 37 °C for 4 h. Following incubation, 100 ml of the solubilizing reagent, sodium dodecyl sulfate (10%) was added to each well and mixed gently for 1 h at room temperature. The absorbance of each well was measured at 570 nm using a spectrophotometer (BioTek ELx808 microplate reader) and the percent of viability was calculated; furthermore, the mean extract concentration that was cytotoxic to 50% of the studied cells (IC50) was calculated for further experiments.
2.4. Analyzing the level of extract antioxidant activity
In order to analyze the antioxidant activity of the algal extract through 2, 2-diphenyl-1-picrylhydrazyl (DPPH), Brand-Williams et al. [6], method was conducted. DPPH is a stable radical with maximum attraction at 517 nm, so 1.5 ml of various concentrations of the extract (125, 250, 500, 1000, 2000, 4000 μg/ml) with 1.5 ml of 0.004% of DPPH was mixed and placed in a dark place at the room temperature. The absorption of the mixtures was read by spectrophotometer (Uviline 9300, Secomam) at 517 nm wavelength with methanol blank.
The radical control percent of DPPH was calculated according to the following formula:
A: Blank methanol absorption.
B: Sample absorption.
2.5. Treatment of cells with A. platensis extract
Two experimental groups were determined for each cell line: extract-treated and control (without extract). In extract-treated groups, the cells were treated with IC50 aqueous extract of A. platensis (129.5 μg/ml and 366.4 μg/ml for A549 and HFF cell lines respectively) for 24 h [8]. Each cell line had its control group. All experimental groups were studied in a triplicate manner (three wells in the 96-well plate per experiment).
2.6. Study the cell cycle through flow-cytometry
In order to prepare the cells for Flow-cytometric analysis, cells were trypsinized and centrifuged (239×g, 10 min), then the cells were fixed with 70% ice-cold ethanol. PI/RNase (1 μl of Triton x100, 20 μl of Ribonuclease Enzyme and 20 μl of propidium iodide) staining was performed directly before the flow cytometric analysis. The PI fluorescence intensity of individual nuclei was determined and at least 10,000 events were measured within an acquisition rate>60 events/second. The cell cycle analyses were performed with the use of software WIN-MDI software version 2.9 [9].
2.6.1. Assessment of reactive oxygen species (ROS)
The basis of the ROS assay was based on the conversion rate of DCFH-DA (2ʹ, 7ʹ-Dichloro-dihydro-fluorescin Diacetate) into a fluorescent form. The method was based on the manufacturer's kit (Abcam). DCFH-DA was diluted with its own buffer according to the kit instructions. For each category, two test and control samples are required. In both test and control samples, the cells were well pipetaged after trypsinization and washed with PBS, and centrifuged at 1200 rpm for 5 min. Then the supernatant solution was discarded and was added to 0.5 ml DCFH-DA buffer then ROS was measured by flow cytometry (BD FACSCalibur) [10].
2.7. Evaluation of membrane lipid peroxidation
To determine the level of membrane lipid peroxidation, the amount of malonyl dialdehyde as the final product of membrane lipid peroxidation was determined according to the [11], for this purpose 500 × 103 cells were counted from each cell line and centrifuged in two microtubes (test and control). The supernatant was discarded, and then 1.5 ml of 10% trichloroacetic acid was added to the cells in order to precipitate the proteins. After centrifugation, 0.5% thiobarbituric acid was added to the supernatant and placed at 100 °C for 30 min. The MDA level was measured by measuring the absorbance at 532 nm wavelength through spectrophotometry (BioTek ELx808 microplate reader).
2.8. Analysis of necrosis and apoptosis using flow-cytometry
In order to evaluate the necrosis and apoptosis rates of studied cells, the flow-cytometry method conducted based on the fact that in necrosis, the cell membrane has been damaged in contrast with apoptotic cells, which this difference could be used alongside DNA-dyes to distinguish between these two groups. For evaluation of the necrosis and apoptosis rates in algal extract-treated cells, cells were trypsinized, centrifuged at 1200 g, washed with 5 ml PB, and centrifuged again, 1 ml of buffer added to the cells, pipetaged, 5 μl of annexin v added, and incubated in dark for 15 min. Finally, 4 μl of propidium iodide added and the cells evaluated by flow-cytometry [12]. Results of the flow-cytometer then analyzed using WIN-MDI software.
2.9. Production of LDH
In order to measure the production of LDH, the German Society of Clinical Chemistry and Biochemistry (DGKC) method was followed using the PARS AZMOON kit (Iran). In this method, the enzyme activity is determined according to the change in NADH concentration:
Lactate dehydrogenase is oxidized by NADH activity. In the process, the reduction of NAD to NADH is directly proportional which can be measured by the photometric method [13].
2.10. Analyzing the data
The statistical analysis of the data was performed through the one-way ANOVA and it was followed by the Tukey test; the correlation between amounts was determined using Pearson's test, all statistical analyses was performed through SPSS software version 19. The charts were drawn by Graph Pad Prism 5. All presented data is provided as mean ± SD.
3. Results
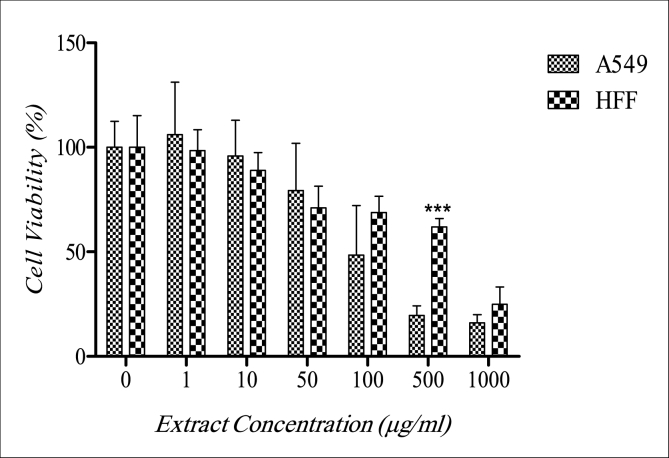
Results of the MTT analysis showed that the 24 h IC50 concentration of S. platensis extract for treated cell lines of HFF and A549 was 366.4 μg/ml and 129.5 μg/ml, respectively. Results showed that the viability of A549 cells had a reverse relation to the concentration of the algal extract, which means with the increase in extract concentration, the viability of A549 cells was decreased. On the other hand, a decrease in viability of HFF cells after treatment with algal extract at the concentrations of 1 and 10 μg/ml was not significant (Fig. 1).
Fig. 1.
Effects of A. platensis extract on A549 and HFF cells, at concentration of 500 μg/ml the viability of A549 cells was significantly decreased compare to HFF cells, (p ≤ 0.001: ***).
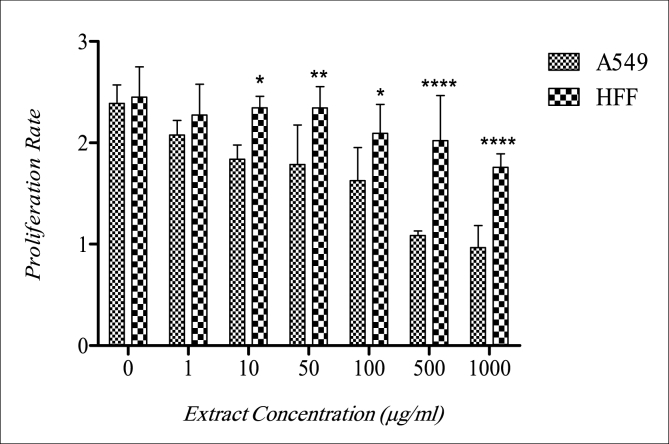
Results of the effects of A. platensis showed that at a concentration of 500 μg/ml viability of A549 cells was significantly decreased compared to HFF cells (Fig. 1). Results also showed that the proliferation of A549 and HFF cells after 24 h of treatment with A. platensis extract was inhibited especially at concentrations of 500 and 1000 μg/ml (Fig. 2). It seems that with the increase in the concentration of the extract, the proliferation rate of the A549 decreased compare to the control group. In HFF cells, only at the 1000 μg/ml concentration, the proliferation rate was significantly decreased.
Fig. 2.
Comparison of Proliferation rate in A549 and HFF cells after treatment with multiple concentrations of A. platensis (p ≤ 0.05: *; p ≤ 0.01: **; p ≤ 0.0001: ****).
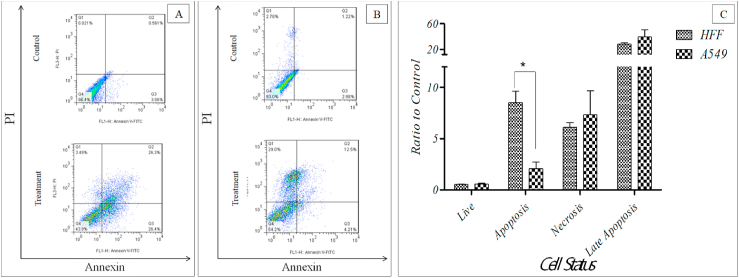
Flow-cytometry results showed that after treatment of cells with A. platensis extract, the viability of cells was 51.7% and 55.23% for HFF and A549 cells respectively (Viability of cells in the control group, without algal extract, was 96.06% and 95.6% for HFF and A549, respectively) (Fig. 3). Results showed that the incidence of apoptosis was increased and the algal extract caused cell death in treated cells through triggering apoptosis. Apoptotic cells of control groups (without algal extract) were 2.47% and 1.35% for HFF and A549 cells respectively. After the treatment of cells with A. platensis extract for 24 h, the apoptotic cells measured as 2.03% and 2.79% for HFF and A549 cells respectively. On the other hand, necrotic cells of the control group (without algal extract) were measured as 0.63% and 2.444% for HFF and A549 cells but after treatment with algal extract, the necrotic cells of experimental groups measured as 3.85% and 17.93% for HFF and A549 cells respectively, which showed an increase in the incidence of necrosis too. Generally, the necrosis was higher in the cancer cell line of A549 and apoptosis was dominant in normal cells of HFF (Fig. 3).
Fig. 3.
Type and measure of cell death in HFF cells (A) and A549 cells (B) horizontal axis showing fluorescent of Annexin and vertical axis showing Propidium Iodide staining. Comparison of amount and type of cell death in A549 and HFF cells treated with algal extract and control group (C) (p ≤ 0.05: *). (Q1: Necrotic cells; Q2: cells at final stages of apoptosis; Q3: cells at beginning of apoptosis; Q4: healthy cells).
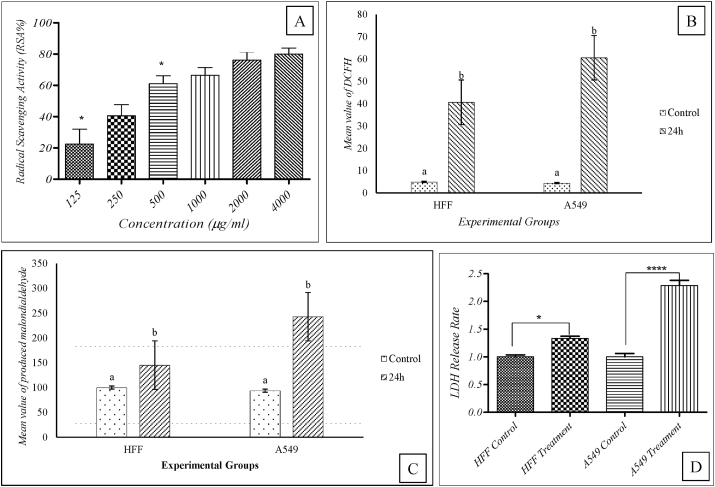
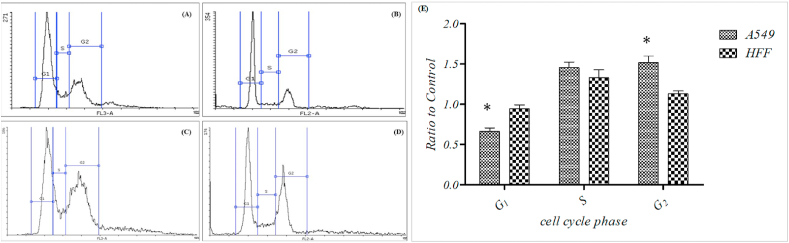
The results showed that the A. platensis extract antioxidant activity was dependent on concentration so that the control percentage of DPPH radical from 22.51% in 125 μg/ml concentration will increase to 79.96% in 4000 μg/ml density. No significant differences between experimental concentrations observed, except for the first lowest concentration of 125 μg/ml to the next higher concentration of 500 μg/ml (Fig. 5). Results of the flow-cytometry analysis (Fig. 4) showed that in the control group, the presence of cells in different phases of the cell cycle was as follows: G1>G2>S for the A549 cell line; and G1>G2>S for the HFF cell line. Also, results showed that the presence of cells in different phases of the cell cycle for the algal extract-treated groups were as follows: G2>G1>S for the A549 cell line and G1>G2>S for the HFF cell line.
Fig. 5.
(A) Results of the extract antioxidant activity level of A. platensis in various concentrations: statistical analysis showed that there was no significant difference between different concentrations except for the 125 μg/ml and 500 μg/ml. (B) The amount of DCFH produced in two experimental cell lines (A549 cells compared to HFF cells). Control groups and treated with IC50 concentration of S. platensis extract at 24 h (P ≤ 0/01). (C) The amount of malondialdehyde produced in A549 cells compared to HFF cells treated with IC50 concentration of Spirulina extract at 24 h (p ≤ 0.001). (D): Releases of LDH in HFF and A549 cells treated with algal extract in compare to control group (without extract).
Fig. 4.
Diagram (A) shows the accumulation of the cancer cells control group of the A549 in various phases of cell cycle and Diagram (B) shows the accumulation of the normal cells control group of the HFF in various phases of the cell cycle and Diagram (C) shows the accumulation of cancer cells treatment group A549 in various phases of the cell cycle. Diagram (D) shows the accumulation of the treatment group HFF cells in various phases of the cell cycle. Diagram (E) shows the presence of cells in each phase of the cell cycle compared to the control group. Two cell lines were compared.
The percentage of cells in each cell cycle phase is shown in Fig. 4. As clear in Fig. 4 the presence of cells in phase G1 was decreased for both A549 and HFF cell lines in control and treated groups, and the percentage of cells in Phases S and G2 was increased for both A549 and HFF cell lines in control and treated groups.
Statistical analysis showed that the decreased mean number of cells in the A549 cell line (%) between the control and treated group was significant (p < 0.05) (Fig. 2). Results also showed that the increase in the mean number of A549 cells in the G2 phase was significantly different between control and treated groups (p < 0.05), the same value for HFF cells was not significantly different (Fig. 4).
Conducting flow-cytometry on experimental groups showed that the amount of DCFH as an indicator for ROS production in A549 cells was higher than the HFF cells (Fig. 5). Statistical analysis showed that there was a significant difference (p ≤ 0.01) in the amount of ROS produced in the algal extract-treated cells compared to the control group. Results of the measurement of the lipid peroxidation showed that the production of malonyl dialdehyde was significantly higher in algal extract-treated cells compare to the control group. Results also showed that the lipid peroxidation was higher in A549 cells compared to the HFF cells (Fig. 5).
In the present study, the Pearson correlation was used to investigate the relationship between MDA and ROS. According to Table 1, the correlation coefficient between the MDA and ROS variables was significant (p < 0.05). In other words, there was a significant relationship between produced MDA and ROS.
Table 1.
Correlation test results for the research variables.
| Variable name | MDA | ROS | |
|---|---|---|---|
| MDA | Pearson | 1.000 | 0.933 |
| Significant value | 0.000 | ||
| ROS | Pearson | 0.933 | 1.000 |
| Significant value | 0.000 | ||
Results also showed that the releasing rate of LDH by cells was increased in both cell lines of A549 and HFF cells after treatment with the algal extract. In fact, the releases of LDH by A549 were significantly higher compared to the HFF cell line which showed the difference in cell death type between the cancer cell line and normal cells. Releases of LDH were 1.33 and 2.29 times higher than the control group in HFF and A549 algal extract-treated cells respectively (Fig. 5).
4. Discussion
Blue-green algae (Cyanobacteria) have been extensively described as potential sources for the protection of the human respiratory system [14,15]. Previous studies demonstrated that Spirulina contains some components with anticancer activity against lung carcinoma cells [16]. Therefore, the present study investigates the anti-cancer effects of A. platensis extract on human lung cancer cell line A549 along with human foreskin fibroblasts HFF.
In the present study, A. platensis antioxidant activity was observed. Results showed that the A. platensis antioxidant activity in absorbing the free radical (DPPH) will increase through increasing the concentration of the algal extract. The best concentration which can be used as an effective antioxidant with minimum toxicity determined as 500 μg/ml. Safari et al. [17] observed the antioxidant properties of a green algae Chaetomorpha sp. And a brown algae Colpomenia sp. The results showed that the green algae had higher antioxidant activity and higher absorption of radicals compared to the brown species. Gunes et al. [18], also observed the effects of A. platensis extract and antioxidant activity in skin cream in order to amend the skin after a surgery and they reported that the maximum antioxidant activity and the treatment of the extract were in the range of 0.001%–1%.
In another research conducted by Al-Qahtani and Al-Binobead [3] regarding the antioxidant effects and anti-hepatotoxic Spirulina against the toxicity caused by d-galactosamine in rats showed that the animals were treated by a feeding diet containing Spirulina with 6% and 9% concentrations. The extract with a 9% concentration was more effective to protect them against liver damages caused by toxicity. According to the results of the present and previous studies, it could be concluded that the antioxidant activity of A. platensis increased with an increase in concentration.
Results of the present study showed that A. platensis affects the human lung cancer cell line A549 by increasing the production of ROS and peroxidation of membrane lipids compare to the control group and the normal HFF cells. Alishahi et al. [19] observed the effects of astaxanthin of Donalila salina on cell membrane lipid peroxidation and declared it reduced lipid peroxidation. According to another study by Kim et al. [20], Spirulina can increase the activity of glutathione peroxidase and glutathione reductase enzymes in the liver, thereby reducing the damage caused by oxidative stress.
In a study by Kim et al. [20], Spirulina reduces the damage caused by oxidative stress, which is inconsistent with the results of the present study. Research on the protective effects of Spirulina against mycotoxin-induced toxicity showed that Spirulina significantly reduced mycotoxin-induced oxidative stress by decreasing lipid peroxidation and increasing glutathione [21].
In the study of Upasani et al. [21], Spirulina reduces mycotoxin-induced oxidative stress by decreasing lipid peroxidation, which is inconsistent with the results of the present study. In a study by Connan et al. [22], it was observed that the production of reactive oxygen species in alga is correlated with environmental stresses such as high light intensity, heavy metals, high salt concentration, UV radiation, and etc.
According to the analysis of the flow-cytometry results in both HFF and A549 cell lines of control and treatment groups in the present study, the control group cells were found in G1, G2, and S, in order of G1>G2>S; And the algae extract effect on the A549 cells of the treatment group is in a way that it decreases the power of cell propagation of the cancer cell in phase G1 from 55.65% to 36.87% and in phase G2 it has increased from 25.19% to 38.24%. Ping et al. [23] observed the capability of Spirulina extract raw protein effect on skin fibroblast CCD-986SK cells. Results showed that due to the proteins of such extract, it can cause an increase in the cell density in phases S and G2.
Results of the present study showed A. platensis extract had anti-proliferative activity against A549 cells, but HFF cells showed no significant differences in control and algal extract treated groups. Previous studies demonstrated that Spirulina has a cytotoxicity effect and could be claimed as anticancer algae. For instance, it's been documented that Spirulina extract had cytotoxic effects on HCT116 colon carcinoma and HEPG2 hepatocellular carcinoma Zaid et al. [24], Kasumi-1 human acute leukemia and K-562 chronic myelogenous leukemia Flores Hernandez and Khandual [25], and CM human chronic myelogenous Ramzi et al. [26] cell lines. The results of such studies also documented that the effects of Spirulina significantly dependent on the origin of the cancer cell lines.
Furthermore, the results of the present study showed that the presence of HFF cells in different stages of the cell cycle was not affected by the A. platensis extract. This result is consistent with the previously published studies showing that Spirulina has no effects on normal cells, and even in some cases had protective effects on normal cells. For example, it's been clear that Spirulina sp. extract had protective effects on mouse BV-2 normal microglial cells Chen et al. [27], normal 3T3 mouse fibroblasts Chu et al. [28], murine bone marrow Hayashi et al. [29], and human stem cells Bachstetter et al. [30]. According to the results of the present study along with the previous studies, it seems that Spirulina extract has significant inhibitory effects on cancer cells and at the same time protects the normal cells. Therefore, Spirulina could be a potential and promising factor for possible treatments of cancer cells.
5. Conclusion
The results of the present study showed that Arthrospira platensis extract had an antioxidant activity depending on the concentration. This extract can have remarkable effects on the lung cancer cell cycle so that it can stop the cells in phase G2, consequently, the cells won't enter phase M and it stops the proliferation of the cancer cells.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Elham Tajvidi: Software, Investigation, Formal analysis, Resources, Writing – original draft, Visualization, Project administration. Nikta Nahavandizadeh: Software, Investigation, Formal analysis, Resources, Writing – original draft, Visualization, Funding acquisition. Maryam Pournaderi: Software, Investigation, Formal analysis, Resources, Funding acquisition. Azin Zargar Pourrashid: Software, Investigation, Formal analysis, Resources, Funding acquisition. Fatemeh Bossaghzadeh: Methodology, Data curation. Zahra Khoshnood: Conceptualization, Methodology, Validation, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration.
References
- 1.Czerwonka A., Kaławaj K., Sławińska-Brych A., Lemieszek M.K., Bartnik M., Wojtanowski K.K., Zdzisińska B., Rzeski W. Anticancer effect of the water extracts of a commercial Spirulina (Arthrospira platensis) product on the human lung cancer A549 cell line. Biomed. Pharmacother. 2018;106:292–302. doi: 10.1016/j.biopha.2018.06.116. [DOI] [PubMed] [Google Scholar]
- 2.Afkhami-Ardakani M., Hasanzadeh S., Shahrooz R., Delirezh N., Malekinejad H. Antioxidant effects of Spirulina platensis (Arthrospira platensis) on cyclophosphamide-induced testicular injury in rats. Vet. Res. Forum. 2018;9(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Qahtani W.H., Binobead M.A. Anti-inflammatory, antioxidant and antihepatotoxic effects of Spirulina platensis against d-galactosamine induced hepatotoxicity in rats. Saudi J. Biol. Sci. 2019;26(4):647–652. doi: 10.1016/j.sjbs.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akhtar N., Bansal J.G. Risk factors of lung cancer in nonsmoker. Curr. Probl. Canc. 2017;41(5):328–339. doi: 10.1016/j.currproblcancer.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Premkumar K., Pachiappan A., Abraham S.K., Santhiya S.T., Gopinath P.M., Ramesh A. Effect of Spirulina fusiformis on cyclophosphamide and mitomycin-C induced genotoxicity and oxidative stress in mice. Fitoterapia. 2001;72(8):906–911. doi: 10.1016/s0367-326x(01)00340-9. [DOI] [PubMed] [Google Scholar]
- 6.Brand-Williams W., Cuvelier M.E., Berset C.L. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 1995;28(1):25–30. [Google Scholar]
- 7.Arthur E., Greene R., Silver K., Krug M. Preservation of cell cultures by freezing in liquid nitrogen vapor. Proc. Soc. Experim. Biol. Med. 1964;116(2):462–467. doi: 10.3181/00379727-116-29280. [DOI] [PubMed] [Google Scholar]
- 8.Silva A.S., Moreira L.M., de Magalhães W.T., Farias W.L., Rocha M.V.P., Bastos A.K.P., Joece J. Extraction of biomolecules from Spirulina platensis using non-conventional processes and harmless solvents. J. Environ. Chem. Eng. 2017;5(3):2101–2106. [Google Scholar]
- 9.Banafa Roshan S., Liu Y.Y., Chen H.J., Chen M.J., Yang G.X., He G.Y. Fucoidan induces G1 phase arrest and apoptosis through caspases-dependent pathway and ROS induction in human breast cancer MCF-7 cells. J. Huazhong. Uni. Sci. Technol. Med. Sci. 2013;33(5):717–724. doi: 10.1007/s11596-013-1186-8. [DOI] [PubMed] [Google Scholar]
- 10.Eruslanov E., Kusmartsev S. Advanced Protocols in Oxidative Stress II. Springer; 2010. Identification of ROS using oxidized DCFDA and flow-cytometry; pp. 57–72. [DOI] [PubMed] [Google Scholar]
- 11.Reilly C.A., Aust S.D. Measurement of lipid peroxidation. Curr. Protoc. Toxicol. 1999;1(2.4):1–13. doi: 10.1002/0471140856.tx0204s00. [DOI] [PubMed] [Google Scholar]
- 12.Nicoletti I., Migliorati G., Pagiacci M., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 13.Bathaei S.Z., Hoshyar R., Miri H., Sadeghizadeh M. Anticancer effects of crocetin in both human adenocarcinoma gastric cancer cells and rat model of gastric cancer. Biochem. Cell. Biol. 2013;91:397–403. doi: 10.1139/bcb-2013-0014. [DOI] [PubMed] [Google Scholar]
- 14.McCarty M.F., O'Keefe J.H., DiNicolantonio J.J. Carvedilol and spirulina may provide important health protection to smokers and other nicotine addicts: a call for pertinent research. Mo. Med. 2015;112:72–75. [PMC free article] [PubMed] [Google Scholar]
- 15.Ismail M., Hossain M.F., Tanu A.R., Shekhar H.U. Effect of spirulina intervention on oxidative stress, antioxidant status, and lipid profile in chronic obstructive pulmonary disease patients. Biomed. Res. Int. 2015;2015:1–7. doi: 10.1155/2015/486120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B., Gao M.H., Chu X.M., Teng L., Lv C.Y., Yang P., Yin Q.F. The synergistic antitumor effects of all-trans retinoic acid and C-phycocyanin on the lung cancer A549 cells in vitro and in vivo. Eur. J. Pharmacol. 2015;749:107–114. doi: 10.1016/j.ejphar.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Safari P., Rezaei M., Shaviklo A.R., Garmsiri A., Babakhani A. In vitro antioxidative activity and total phenolic content determination of two Persian Gulf seaweed species Chaetomorpha sp and Colpomenia sinuosa. J. Mar. Sci. Technol. 2015;14(1):64–77. [Google Scholar]
- 18.Gunes S., Tamburaci S., Dalay M.C., Deliloglu Gurhan I. In vitro evaluation of Spirulina platensis extract incorporated skin cream with its wound healing and antioxidant activities. Pharmaceut. Biol. 2017;55(1):1824–1832. doi: 10.1080/13880209.2017.1331249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alishahi M., Karamifar M., Mesbah M., Zarei M. Comparison of the effect of Astaxanthin and Dunaliella salina algae on skin carotenoid, lipid peroxidation and coloration of Heros severus. J. Vet. Res. 2014;69(1):95–102. [Google Scholar]
- 20.Kim M.Y., Cheong S.H., Lee J.H., Kim M.J., Sok D.E., Kim M.R. Spirulina improves antioxidant status by reducing oxidative stress in rabbits fed a high-cholesterol diet. J. Med. Food. 2009;13(2):420–426. doi: 10.1089/jmf.2009.1215. [DOI] [PubMed] [Google Scholar]
- 21.Upasani C.D., Khera A., Balararnan R. Effect of lead with vitamin E, C, or Spirulina on malondialdehyde, conjugated dienes and hydroperoxides in rat. J. Exp. Biol. 2001;39(1):70–74. [PubMed] [Google Scholar]
- 22.Connan S., Delisle F., Deslandes E., Gall E.A. Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot. Mar. 2006;49(1):39–46. [Google Scholar]
- 23.Ping L., Jeong-Wook C., Min-Kyeong L., Youn-Hee C., Taek-Jeong N. Wound healing potential of spirulina protein on CCD-986sk cells. Mar. Drugs. 2019;17(2):130. doi: 10.3390/md17020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaid A.A.A., Hammad D.M., Sharaf E.M. Antioxidant and anticancer activity of Spirulina platensis water extracts. Int. J. Pharmacol. 2015;11:846–851. [Google Scholar]
- 25.Flores Hernandez F.Y., Khandual S., Ramirez Lopez I.G. Cytotoxic effect of Spirulina platensis extracts on human acute leukemia Kasumi-1 and chronic myelogenous leukemia K-562 cell lines. Asian. Pacific J. Tropical. Biomed. 2017;7:14–19. [Google Scholar]
- 26.Ramzi G.A., Puneeth H.R., Madhu C.S., Sharada A.C. Antagonistic effects of combination of flaxseed oil and Spirulina platensis oil on their biological properties. Int. J. Pharmaceut. Pharmacol. Sci. 2015;7:122–127. [Google Scholar]
- 27.Chen J.C., Liu K.S., Yang T.J., Hwang J.H., Chan Y.C., Lee I.T. Spirulina and Cphycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr. Neurosci. 2012;15(6):252–256. doi: 10.1179/1476830512Y.0000000020. [DOI] [PubMed] [Google Scholar]
- 28.Chu W.L., Lim Y.W., Radhakrishnan A.K., Lim P.E. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals, BMC Complement. BMC Compl. Alternative Med. 2010;10:53. doi: 10.1186/1472-6882-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi O., Ono S., Ishii K., Shi Y.H., Hirahashi T., Katoh T. Enhancement of proliferation and differentiation in bone marrow hematopoietic cells by Spirulina (Arthrospira) platensis in mice. J. Appl. Phycol. 2006;18:47–56. [Google Scholar]
- 30.Bachstetter A.D., Jernberg J., Schlunk A., Vila J.L., Hudson C., Cole M.J., Shytle R.D., Tan J., Sanberg P.R., Sanberg C.D., Borlongan C., Kaneko Y., Tajiri N., Gemma C., Bickford P.C. Spirulina promotes stem cell genesis and protects against LPS induced declines in neural stem cell proliferation. PloS One. 2010;5(5):1–11. doi: 10.1371/journal.pone.0010496. [DOI] [PMC free article] [PubMed] [Google Scholar]