Abstract
The poorly known majid genus Majella Ortmann, 1893, is revised. The genus was previously known only from one species, M. brevipes Ortmann, 1893, described from Japan and reported from east Africa. Majella brevipes is redescribed and figured in detail from the type and material from the type locality, Sagami Bay in Japan. This species is now restricted to Japan. Specimens from east Africa are herein described as two new species: M. skolopion n. sp. and M. pristis n. sp.; they differ markedly from M. brevipes (now restricted to Japan) in the arrangement of spines on the carapace and pereopods, third maxillipeds, male pleon and gonopods.
Keywords: Spider crab, Majoidea, Japan, Western Indian Ocean, Majella, Systematics, New species
BACKGROUND
Majella Ortmann, 1893 (Majidae: Majoidea) is poorly known and rarely reported. The genus is monotypic, comprising just Majella brevipes Ortmann, 1893, and is thus far, only known from Japan (Ortmann 1893; Sakai 1965 1976) and the Mozambique Channel, off Africa (Griffin 1974). The species has not been well figured, with the original illustrations by Ortmann (1893) being relatively schematic, although Sakai (1965 1976) provided improved figures, including its male first gonopod. The study by Griffin (1974) did not provide any figures of Majella.
The recent collection of fresh material of Majella from Africa requires a re-examination of the available type and topotypic material of M. brevipes to ascertain its identity. The present paper revises Majella, redescribing the genus and species from the type and more recent material, as well as describes two new species from the western Indian Ocean.
MATERIALS AND METHODS
The terminology used here follows that used by Griffin (1966) and Griffin and Tranter (1986) with amendments by Ng and Richer de Forges (2015) and Davie et al. (2015). All measurements are provided in millimetres. The following measurements are used: the maximum carapace length (cl) is the maximum distance between the tips of the pseudorostral and intestinal spines, and the maximum carapace width (cw) is the maximum distance between the tips of the longest branchial spines; the post-pseudorostral carapace length (pcl) is measured from the base of the pseudorostral spines to the posterior margin of the carapace; and the carapace width is measured at the base of the spines (bcw), between the bases of longest branchial spines.
The following abbreviations are used: coll. = collected by; G1 = male first gonopod; G2 = male second gonopod; P2–P5 = pereopods 2–5 (first to fourth ambulatory legs), respectively; stn = station. A detailed description is only done for the type species, with diagnoses provided for the others. Specimens examined are deposited in the Museum of Zoology, Strasbourg (MZS), France; Muséum national d’Histoire naturelle (MNHN), Paris, France; Kanagawa Prefectural Museum of Natural History (KPM), Japan; and Zoological Reference Collection (ZRC), Lee Kong Chian Natural History Museum, National University of Singapore, Singapore.
RESULTS
SYSTEMATIC ACCOUNT
Superfamily Majoidea Samouelle, 1819
Family Majidae Samouelle, 1819
Genus Majella Ortmann, 1893
Majella Ortmann, 1893: 51.
Majella –Sakai 1938: 301; Sakai 1965: 84; Sakai 1976: 235; Griffin and Tranter 1986: 218; Ng et al. 2008: 117.
Type species: Majella brevipes Ortmann, 1893, by monotypy; gender feminine.
Comparative material. Choniognathus reini (Balss, 1924): 1 female (6.3 × 4.0 mm) (ZRC 2020.0373), stn T36, on mud, Cortes, Panglao, Bohol, Visayas, Philippines, 9°43.3'N 123°48.8'E, 123–135 m, coll. PANGLAO 2004, 24 June 2004; 1 female (poor condition) (9.1 × 6.2 mm) (KPM NH 0104599, part), Manazuru Town, Ashigarashimo District, Sagami Bay, Japan, coll. T. Sakai, 1962. Seiitaoides orientalis (Sakai, 1961): 2 males (8.1 × 5.1 mm; 5.5 × 3.2 mm), 2 females (7.5 × 5.0 mm; 7.3 × 4.8 mm) (ZRC 2020.0374), stn T36, sand on echinoderm bed, Cervera Shoal, West Pamilacan island, Bohol, Visayas, Philippines, 9°29.3'N 123°51.5'E, 95–128 m, coll. PANGLAO 2004, 4 July 2004. For comparative material of Eurynome and Kasagia, see Richer de Forges and Ng (2007) and Padate et al. (2019).
Diagnosis: Members all small species, adult females with eggs at cw 9–10 mm. Carapace pyriform, regions well defined by grooves or ridges, but normally obscured by setae; surface smooth or covered by numerous small granules, spines and short stiff and long soft setae, some setae hooked. Pseudorostral spines slender, long, diverging forming V-or broadly U-shaped cleft, with accessory spinules along margin, largest spinules on distal half or proximal third outer margin. Supraorbital eave relatively wide, anterior margin usually with sharp spines or spinules; antorbital spine visible as spine or spinule; intercalated supraocular spine short; postorbital spine longer than antorbital and intercalated supraocular spines in dorsal view, with accessory spinules, small sharp granule present on inner ventral margin, adjacent to suborbital tooth; suborbital tooth clearly separated from postorbital spine and basal antennal article by gaps or clefts. Hepatic lobe distinct, with 2 or 3 prominent spines, all with accessory spinules. Lateral margin with 4 large spines, 1 or 2 epibranchial spines present. Posterolateral margin separated by spine or spinule from posterior carapace margin. Posterior carapace margin with marginal and median spines or spinules; intestinal region not swollen; mesogastric region with 2 short spines, metagastric region with 1 distinct median spine; cardiac region with 1–3 median spines; branchial region with mix of spines, granules, tubercles and/or spinules. Ocular peduncle with sharp distal tubercle. Antennal basal article subquadrate, outer median part with low lobe with tubercles. Epistome subrectangular, wider than long; posterior margin of epistome with triangular lobe with median cleft, separated from sinuous lateral margin by deep cleft. Buccal cavity subtrapezoidal, distal part wider; anteroexternal angle with pterygostomial region marked by serrated flange which brackets anteroexternal angle of merus of third maxilliped when closed; third maxilliped ischium with outer margin lined with sharp tubercles; merus triangular, with anteroexternal angle strongly produced to form large auriculiform structure; exopod outer margin with sharp tubercles. Chelipeds not prominently elongate, not inflated, surfaces covered by short stiff and long soft setae; merus, carpus and chela covered with large and small spines, spinules, tubercles and granules. Ambulatory legs short, with short stiff and long soft setae; margins of merus not carinate, lined with sharp teeth spines or spinules; carpus with dorsal and outer surface lined with spinules or sharp tubercles; dactylo-propodal lock distinct; dactylus falciform, ventral margin usually with 2 sharp submedian granules. Thoracic sternum relatively transversely narrow; surfaces with punctae and small rounded granules; sternites 1 and 2 completely fused to form narrow subtriangular structure, separated from sternite 3 by prominent concave ridge; sternite 3 depressed, fused with sternite 4, demarcated only by lateral incisions, no visible groove present; anterior surface of sternite 4 depressed; sternites 5–8 progressively more narrow, surface of sternites 5–7 medially depressed; small part of sternite 8 visible when pleon closed; sutures between sternites 3–6 medially interrupted; sutures between sternites 6–8 complete; longitudinal groove present between sternites 6–8; sternopleonal cavity deep, reaching to middle part of sternite 4; margin adjacent to telson subcristate, granulate; tubercle of pleonal locking mechanism distinct, on median or distal third part of sternite 5; penis coxal, exiting P5 coxa anterior to condyle. Male pleon narrow; all somites and telson free; somite 1 wider than somite 2, with large median tubercle or spine, visible in dorsal view; somite 2 as wide as somite 3 with swelling on distal margin topped by 4 rounded granules; somite 3 with swelling on distal margin topped by 2 distinct rounded granules, with 1 low granule proximal to them; telson triangular. G1 relatively stout, sinuous or gently curved, groove for G2 on ventral surface; tip relatively sharp to truncate; subdistal setae may be long or absent; G2 very short, with short distal segment. Female pleon longitudinally ovate, all somites and telson free. Vulva large, median, directed obliquely anteriorly inwards towards median line, no vulvar cover.
Remarks: Ortmann (1893: 51) established Majella, differentiating it from Maja Lamarck, 1801, by having a serrate orbital margin, the merus of the third maxilliped being triangular with the external angle prominently produced, and the carpus and palm of the cheliped short and spiny. Only one species was included, Majella brevipes Ortmann, 1893, from Japan. In this paper, two more species are described from the western Indian Ocean. Majella is a very distinctive majid because of its small size, with female adult specimens ovigerous at carapace widths less than 9–10 mm.
Ng and Richer de Forges (2015) revised Maja Lamarck, 1801, and recognised 10 genera, and the differences noted by Ortmann (1893) are still valid. All the genera allied to Maja have the supraorbital eave entire, the merus of the third maxilliped is never projected and the chela is always smooth and unarmed. In addition, the members of these genera have a different arrangement of hepatic, lateral and branchial spines. In addition, the outer margin of the ischium and exopod of the third maxilliped are never spinate like in Majella.
In the Majidae, Majella superficially resembles species of Eurynome Leach, 1814, Seiitaoides Griffin & Tranter, 1986, and Kasagia Richer de Forges & Ng, 2007, especially in the general form of the cheliped (see also Sakai 1965). Eurynome, however, differs from Majella in that the carapace is covered by plates and fungiform tubercles with the margins lined with teeth and spines, the pseudorostral spine is dorsoventrally flattened without accessory granules or spinules, the hepatic lobe marked by a prominent lobe, the basal antennal article is triangular with the margins swollen, the anteroexternal angle of the merus of the third maxilliped is less projecting, the male anterior thoracic sternum is proportionately shorter with the sternopleonal cavity reaching to almost the margin between sternites 3 and 4, and the G1 does not have a subdistal accessory projection (cf. Richer de Forges and Ng 2007: figs. 4B, D, 5A).
In the triangular and strongly auriculiform merus of the third maxilliped, Majella resembles Kasagia Richer de Forges & Ng, 2007, which is represented by two species from the Philippines and Arabian Sea (Richer de Forges and Ng 2007; Padate et al. 2019). Majella, however, differs from Kasagia in several key characters: the dorsal surface of the carapace is covered with large spines and sharp tubercles (Figs. 2B, 3, 4B) (vs. covered with rounded granules and tubercles without long spines in Kasagia; cf. Richer de Forges and Ng 2007: fig. 3A; Padate et al. 2019: figs. 2B, 5B); the supraobital eave is spinate with a strong antorbital spine (Figs. 2B, 3, 4B) (vs. supraobital eave not spinate with no antorbital spine in Kasagia; cf. Richer de Forges and Ng 2007: fig. 3A; Padate et al. 2019: figs. 2B, 5B); the postorbital spine is lobiform in Kasagia; cf. Padate et al. 2019: figs. 2B, 5B); the hepatic region has 3 prominent spines (Figs. 2B, 3, 4B) (vs. with only a distinct lobe in Kasagia; cf. Richer de Forges and Ng 2007: fig. 3A; Padate et al. 2019: figs. 2B, 5B, E); the lateral margin has 4 large spines (Figs. 2B, 3, 4B) (vs. lateral spines are small and low in Kasagia; cf. Richer de Forges and Ng 2007: fig. 3A; Padate et al. 2019: figs. 2B, 5B); the basal antennal article is more quadrate with the distal part narrower and has distal tubercles (Fig. 5B) (vs. triangular and unarmed in Kasagia; cf. Richer de Forges and Ng 2007: figs. 3C, 4A; Padate et al. 2019: figs. 2C, 5C); the auriculiform anteroexternal process of the merus of the third maxilliped is more projected (Fig. 6B) (vs. relatively shorter and lower in Kasagia; cf. Padate et al. 2019: figs. 2C, 5F); the male anterior thoracic sternum and telson are proportionately shorter (Fig. 4F) (vs. more elongate in Kasagia; cf. Richer de Forges and Ng, 2007: figs. 3C, 4A; Padate et al. 2019: figs. 2D, F, 5D); the male chelipeds are short (Figs. 3B, 4A, 5F, G) (vs. elongate in Kasagia; cf. Richer de Forges and Ng 2007: fig. 2A; Padate et al. 2019: figs. 2A, E, 5A, G); the dorsal margin of the ambulatory merus is not carinate (Fig. 5H–K) (vs. distinctly carinate in Kasagia; cf. Padate et al. 2019: figs. 2G, 5H); and the G1 is sinuous to curved with the distal part simple (Fig. 6H–J) (vs. G1 curved with distal part expanded and bifid in Kasagia; cf. Richer de Forges and Ng 2007: fig. 5B, C; Padate et al. 2019: fig. 3A, B); and the G2 has a short distal segment (Fig. 6K) (vs. without a distal segment in Kasagia; cf. Padate et al. 2019: fig. 3C, D).
Two West Pacific species previously placed in Eurynome were separated into a new genus, Seiitaoides, by Griffin and Tranter (1986: 251) as their carapaces are different. Seiitaoides shares with Majella a pseudorostral spine that has accessory granules and spinules, a serrated supraorbital eave, a merus of the third maxilliped that is expanded, and a simple G1 without projections or flanges. Seiitaoides, however, has a short pseudorostrum, prominent gastric, cardiac, intestinal and lateral plates, no hepatic and lateral spines, the merus of the third maxilliped is not strongly expanded, and adult males have proportionately longer chelipeds (cf. Sakai 1961: pl. 4 fig. 2; Sakai 1976: text-fig. 119, pl. 76 fig. 1; Griffin and Tranter 1986: fig. 69b).
Choniognathus Rathbun, 1932, species have previously been placed in Eurynome, but members of this genus are very unusual in that the merus and ischium of the third maxilliped are fused (cf. Sakai 1976: text-fig. 120b). Like, Majella, its members are all small, but Choniognathus species can be distinguished by the pseudorostrum being short, the anteroexternal angle of the merus of the third maxilliped is not expanded, the carapace regions and margins are lined with rounded tubercles and granules, not spines, the hepatic lobe is not spinate, and the chelipeds are short and the chela is not armed (see Balss 1924: pl. 1 fig. 3; Yokoya 1933: text-fig. 57; Sakai 1976: text-fig. 120, pl. 78 fig. 2; Ho et al. 2004: fig. 3F).
Majella brevipes Ortmann, 1893
(Figs. 1–6)
Majella brevipes Ortmann, 1893: 51, pl. 3 fig. 5 (type locality: Sagami Bay, Japan).
Majella brevipes –Sakai 1938: 300; Sakai 1965: 84, text-fig. 12, pl. 37 fig. 4; Sakai 1976: 235, pl. 82 fig. 2; Muraoka 1998: 27; Komai 1999: 85; Ng et al. 2008: 117.
Material examined: Holotype: female (13.2 ×10.9 mm, pcl 11.8 mm, bcw 9.3 mm) (MZS 754), Sagami Bay, Japan, 128–219 m, coll. L. H. P. Döderlein, 1881. Other material: 1 male (9.1 × 7.1 mm, pcl 7.5 mm, bcw 5.7 mm), 1 ovigerous female (10.6 × 8.2 mm, pcl 8.9 mm, bcw 6.8 mm) (KPM NH 0104599), Manazuru Town, Ashigarashimo District, Sagami Bay, Japan, coll. T. Sakai, 1962; 3 females (9.7 × 7.8 mm, pcl 8.6 mm, bcw 6.9 mm; 11.9 × 9.3 mm, pcl 9.9 mm, bcw 8.0 mm; 12.5 × 10.1 mm, pcl 10.7 mm, bcw 8.3 mm) (KPM NH 0104651), Hayama Town, Miura District, Sagami Bay, Japan, coll. T. Sakai, no date; 2 males (9.8 × 7.2 mm, pcl 7.9 mm, bcw 6.2 mm; 8.7 × 6.9 mm, pcl 7.1 mm, bcw 5.6 mm), 2 females (11.5 × 9.4 mm, pcl 9.9 mm, bcw 7.7 mm; 8.3 × 6.6 mm, pcl 7.3 mm, bcw 5.6 mm) (KPM NH 0104665), Hayama Town, Miura District, Sagami Bay, Japan, coll. T. Sakai, no date.
Diagnosis: Carapace surface covered by numerous small granules, spines and short stiff and long soft setae. Supraorbital eave with 4 sharp spines on anterior margin, antorbital spine dentiform, with accessory spinules; intercalated supraocular spine triangular, closely appressed against antorbital and postorbital spines but usually with a narrow space between them; postorbital spine triangular with accessory spinules, lined with 3 short spines in lateral view; suborbital tooth without spine, margin serrated, surface with median sharp tubercle. Hepatic lobe pronounced, with 3 prominent spines. Lateral margin with 4 large spines, all with accessory spinules; first epibranchial spine at level of third and fourth lateral spines, next epibranchial spine subequal in size, spines posterior to first 2 spines smaller, varying in size and number and hard to discern from those on rest of region. Junction of posterolateral and posterior carapace margins usually demarcated by large spine. Posterior carapace margin with marginal and median spines largest, with 2–4 smaller spines between them (sometimes low), arranged marginally and submarginally; intestinal region not well defined, usually with 3 low spines; frontal and protogastric regions relatively smoother, with low rounded granules, not spinular; surfaces of gastric regions with granules and low tubercles of varying sizes; cardiac region with 1 median spine, largest of median longitudinal spines, anterior surface with granules and low tubercles, posterior surface with low spines; branchial region with mix of granules, tubercles, small and low spines. Pterygostomial, subhepatic and sub-branchial regions granulated, ventral surface of hepatic lobe with distinct stout median spine (Figs. 2A, B, 3, 4A–D, 6A). Outer margin of antennular fossa with 4 or 5 low sharp granules, inner margin with 1 or 2 sharp granules subdistally (Fig. 5A, B). Interantennular spine bifurcate, low (Fig. 5B). Antennal basal article with proximal part wider than distal part, outer median part with 2 tubercles, anteroexternal angle with 2 tubercles (Fig. 5A, B). Buccal cavity with anteroexternal angle with pterygostomial region marked by serrated flange (Fig. 5A); third maxilliped ischium outer margin with 3 sharp tubercles, surface with 7 or 8 large rounded granules and scattered smaller ones especially near distal margin, inner proximal angle with sharp tubercle; merus surface with 3 large rounded tubercles and scattered low granules; exopod outer margin with 4 sharp tubercles (Figs. 4E, 5A, 6B). Cheliped basis-ischium with sharp tubercle on ventral margin; merus ventral margin with large spine on proximal third, rest of surface with approximately 10–12 tubercles and granules; dorsal margin with 4 large spines and large oblique spine on distal edge, rest of margin with 6–8 shorter spines and tubercles; carpus with 4 large spines on dorsal margin, 1 large and 2 small spines on outer surface and 3 or 4 sharp tubercles on inner surface (Figs. 4D, 5D–G). Chela with 2 or 3 spines on upper surface (first spine may be small) and large spine on distal edge next to dactylus; 2 large spines and 5 or 6 sharp tubercles on outer surface; with 5 or 6 sharp tubercles on ventral surface; inner surface with scattered small sharp granules; dorsal margin of dactylus and ventral margin of pollex unarmed (Fig. 5D–G). Ambulatory merus with rounded margins, armature as follows: P2 merus with 5 or 6 short spines or spinules on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal submedian row of 4 or 5 sharp granules, ventral and subventral margins with 5 or 6 sharp granules; P3 merus with 7 short spines or spinules on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal submedian row of 3 or 4 small sharp granules, ventral and subventral margins with 3 or 4 sharp granules; P4 merus with 4 or 5 short spines or spinules on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal submedian row of 1 or 2 sharp granules, ventral and subventral margins with 5 or 6 sharp granules; P5 merus with 6 or 7 short spines or spinules on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal submedian row of 2 or 3 sharp granules, ventral and subventral margins with 5 or 6 sharp granules (Fig. 5H–K); carpus smooth on ventral margin, armature as follows: P2 carpus with 5 short dorsal and subdorsal spines; P3 carpus with 4 or 5 short dorsal and subdorsal spines; P4 carpus with 3 or 4 short dorsal and subdorsal spines; P5 carpus with 1 or 2 short dorsal and subdorsal spines (Fig. 5H–K); propodus smooth, unarmed (Fig. 6C); dactylus ventral margin usually with 2 sharp submedian granules, occasionally with small sharp granule just before chitinous distal part (Fig. 5H–K). Tubercle of pleonal locking mechanism on median part of sternite 5. Male pleonal somite 1 adjacent surface with 7–9 smaller sharp tubercles or granules, margins granulated; somite 2 semicircular; somite 3 rectangular, lateral margins sinuous, swelling on distal margin topped by 2 distinct rounded granules, with 1 low granule proximal to them; somite 4 trapezoidal, margins gently concave, swelling on distal margin topped by 2 indistinct rounded granules; somite 5 subrectangular with concave margins, low swelling on distal margin topped by 2 almost indiscernible rounded granules; somite 6 subquadrate with convex lateral margins, surface smooth; telson as long as broad, with sinuous lateral margins (Figs. 4F, 6D). G1 relatively stout, sinuous, tip relatively sharp; subdistal setae very long, dense (Fig. 6E, F, H–J).
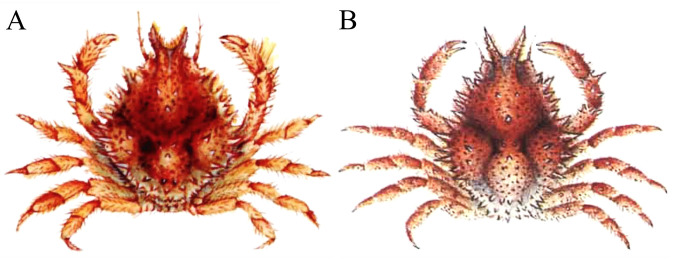
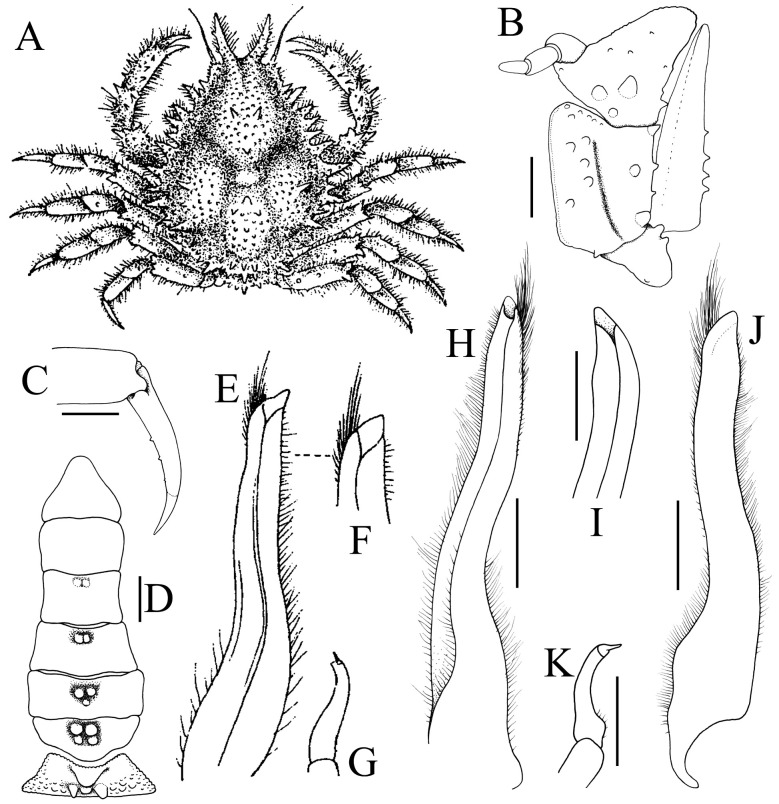
Fig. 1.
Majella brevipes Ortmann, 1893, colour in life. A, after Sakai (1965: pl. 37 fig. 4); B, after Sakai (1976: pl. 82 fig. 2).
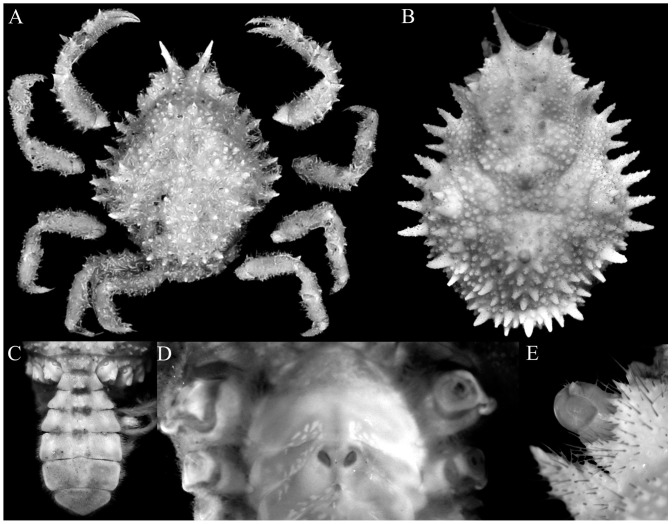
Fig. 2.
Majella brevipes Ortmann, 1893. A, holotype female (cl 13.2 mm, cw 10.9 mm) (MZS 754); B–D, ovigerous female (cl 10.6 mm, cw 8.2 mm) (KPM NH 0104599); E, female (cl 11.5 mm, cw 9.4 mm) (KPM NH 0104665). A, overall dorsal view; B, dorsal view of carapace; C, pleon; D, sternopleonal cavity and vulvae; E, left eye. A, courtesy of Marie Meister.
Fig. 3.
Majella brevipes Ortmann, 1893. A, male (cl 9.8 mm, cw 7.2 mm) (KPM NH 0104665); B, male (cl 8.7 mm, cw 6.9 mm) (KPM NH 0104665); C, female (cl 11.5 mm, cw 9.4 mm) (KPM NH 0104665); D, female (cl 8.3 mm, cw 6.6 mm) (KPM NH 0104665). A, D, dorsal view of carapace; B, C, overall dorsal view.
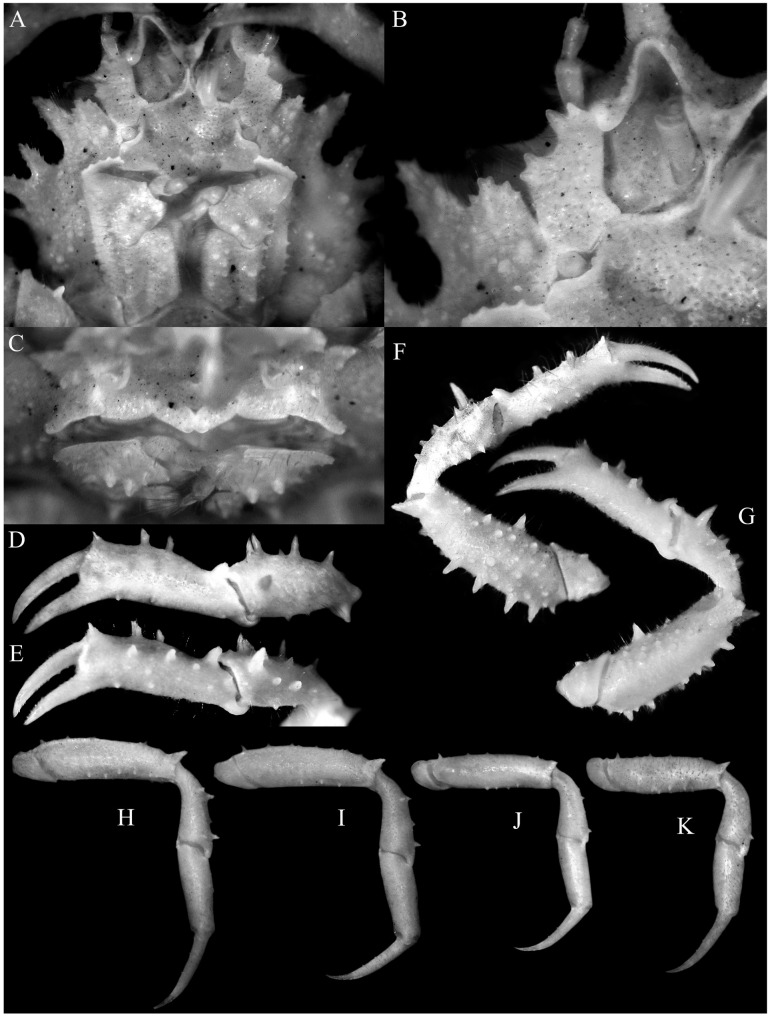
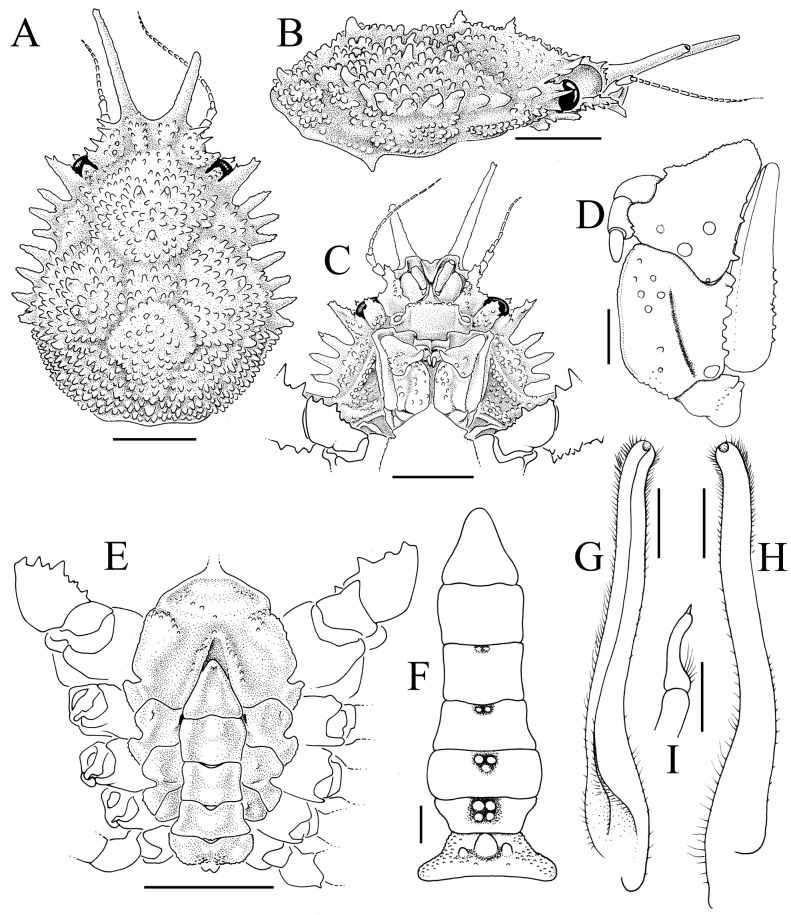
Fig. 4.
Majella brevipes Ortmann, 1893, male (cl 9.1 mm, cw 7.1 mm) (KPM NH 0104599). A, overall dorsal view; B, dorsal view of carapace; C, anterior part of carapace; D, right lateral view of cephalothorax; E, left third maxilliped; F, anterior thoracic sternum and pleon.
Fig. 5.
Majella brevipes Ortmann, 1893, male (cl 9.1 mm, cw 7.1 mm) (KPM NH 0104599). A, antennules, antennae, epistome, buccal cavity and third maxillipeds; B, right antenna; C, posterior margin of epistome; D, E, outer views of left chela and carpus; F, dorsal view of left cheliped; G, ventral view of left cheliped; H–K, right P2–P5, respectively (setae denuded, all to same scale).
Fig. 6.
Majella brevipes. A, overall dorsal view; B, left third maxilliped; C, right P5 propodus and dactylus; D, pleon; E, right G1; F, distal part of right G1; G, right G2; H, left G1 (ventral view); I, distal part of left G1 (subventral view, setae not drawn); J, left G1 (dorsal view); J, left G2. A, E–G after Sakai (1965: text-fig. 12). Scale bars: B, C, D, H–K = 0.5 mm.
Description: Small species, ovigerous females with eggs at cw 9–10 mm. Carapace pyriform, regions well defined by grooves but normally obscured by setae; surface covered by numerous small granules, spines and short stiff and long soft setae; pseudorostral spines slender, long, prominently diverging, forming V-or broadly U-shaped cleft, with accessory spinules along margin, largest spinules on distal half outer margin; supraorbital eave wide, anterior margin usually with 4 sharp spines, antorbital spine dentiform, with accessory spinules; intercalated supraocular spine short, triangular, closely appressed against antorbital and postorbital spines but usually with a narrow space between them; postorbital spine longer than antorbital and intercalated supraocular spines in dorsal view, triangular with accessory spinules, lined with 3 short spines in lateral view, small sharp tubercle present on inner ventral margin, adjacent to suborbital tooth; suborbital tooth without spine, margin serrated, clearly separated from postorbital spine and basal antennal article by narrow gap, surface with median sharp tubercle; hepatic lobe pronounced, with 3 prominent spines, all with accessory spinules; lateral margin with 4 large spines, all with accessory spinules; first branchial spine (epibranchial) at level of third and fourth lateral spines, next epibranchial spine subequal in size, spines posterior to first 2 spines smaller, varying in size and number and hard to discern from those on rest of region; posterolateral margin gradually curving to meet posterior carapace margin, usually demarcated by large spine; posterior carapace margin with marginal and median spines largest, with 2–4 smaller spines between them (sometimes low), arranged marginally and submarginally; intestinal region not swollen, not well defined usually with 3 low spines; frontal and protogastric regions relatively smoother, with low rounded granules, not spinular; mesogastric region with 2 short spines, metagastric region with 1 distinct median spine, surfaces of gastric regions with granules and low tubercles of varying sizes; cardiac region with 1 median spine, largest of median longitudinal spines, anterior surface with granules and low tubercles, posterior surface with low spines; branchial region with mix of granules, tubercles, small and low spines; pterygostomial, subhepatic and sub-branchial regions granulated, ventral surface of hepatic lobe with distinct stout median spine (Figs. 2A, B, 3, 4A–D, 6A). Eye tightly fitting into cylindrical orbit, peduncle with sharp distal tubercle (Fig. 2E). Antennules large, folding obliquely; distal outer margin of fossa with 4 or 5 low sharp granules, inner margin with 1 or 2 sharp granules subdistally (Fig. 5A, B). Interantennular spine bifurcate, low (Fig. 5B). Antennae relatively short; nephridiophore (green gland) large, partially raised, with low bracket on ventral margin formed fused articles 1 and 2, partially bracketed by inner proximal margin of suborbital tooth; basal article (fused articles 3 and 4) subquadrate with proximal part wider than distal part, outer median part with low lobe with 2 tubercles, anteroexternal angle with 2 tubercles; articles 5 and 6 short, subcylindrical (Fig. 5A, B). Epistome subrectangular, wider than long; posterior margin of epistome with triangular lobe with median cleft, separated from sinuous lateral margin by deep cleft (Fig. 5A, C). Buccal cavity subtrapezoidal, distal part wider; anteroexternal angle with pterygostomial region marked by serrated flange which brackets anteroexternal angle of merus of third maxilliped when closed (Fig. 5A).
Third maxilliped filling most of buccal cavity when closed, leaving narrow longitudinal gap medially (Fig. 5A); basis separated from ischium by shallow groove, with 2 tubercles on inner margin adjacent to exopod; ischium subquadrate, outer margin longer than inner margin; outer margin with 3 sharp tubercles; surface with 7 or 8 large rounded granules and scattered smaller ones especially near distal margin, inner proximal angle with sharp tubercle; median longitudinal sulcus deep; merus triangular, with anteroexternal angle strongly produced to form large auriculiform structure, surface with 3 large rounded tubercles and scattered low granules; carpus round, dactylus shorter than cylindrical propodus; exopod stout, strongly tapering towards sharp distal part, reaching to tip of auriculiform part of merus, outer margin with 4 sharp tubercles, flagellum short, about half width of merus (Figs. 4E, 5A, 6B).
Chelipeds not prominently elongate, not inflated, surfaces covered by short stiff and long soft setae (Figs. 2A, 3B, 4A, D, 5F, G). Basis-ischium with sharp tubercle on ventral margin (Fig. 5F, G). Merus trigonal in cross-section, ventral margin with large spine on proximal third, rest of surface with approximately 10–12 tubercles and granules; dorsal margin with 4 large spines and large oblique spine on distal edge, rest of margin with 6–8 shorter spines and tubercles (Figs. 4D, 5F, G). Carpus with 4 large spines on dorsal margin, 1 large and 2 small spines on outer surface and 3 or 4 sharp tubercles on inner surface (Figs. 4D, 5D–G). Chela with 2 or 3 spines on upper surface (first one may be small) and large spine on distal edge next to dactylus; 2 large spines and 5 or 6 sharp tubercles on outer surface; with 5 or 6 sharp tubercles on ventral surface; inner surface with scattered small sharp granules; fingers shorter than palm, bent downwards, cutting edges with low teeth, dorsal margin of dactylus and ventral margin of pollex unarmed (Fig. 5D–G).
Ambulatory legs short, P2 longest, P5 shortest, short stiff and long soft setae (Figs. 2A, 4A, 5H–K). Merus with rounded margins, not carinate, armature as follows: P2 merus with 5 or 6 short spines or spinules on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal submedian row of 4 or 5 sharp granules, ventral and subventral margins with 5 or 6 sharp granules; P3 merus with 7 short spines or spinules on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal submedian row of 3 or 4 small sharp granules, ventral and subventral margins with 3 or 4 sharp granules; P4 merus with 4 or 5 short spines or spinules on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal submedian row of 1 or 2 sharp granules, ventral and subventral margins with 5 or 6 sharp granules; P5 merus with 6 or 7 short spines or spinules on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal submedian row of 2 or 3 sharp granules, ventral and subventral margins with 5 or 6 sharp granules (Fig. 5H–K). Carpus smooth on ventral margin, armature as follows: P2 carpus with 5 short dorsal and subdorsal spines; P3 carpus with 4 or 5 short dorsal and subdorsal spines; P4 carpus with 3 or 4 short dorsal and subdorsal spines; P5 carpus with 1 or 2 short dorsal and subdorsal spines (Fig. 5H–K). Propodus smooth, unarmed; dactylo-propodal lock distinct (Fig. 6C). Dactylus falciform, ventral margin usually with 2 sharp submedian granules, occasionally with very small sharp granule just before chitinous distal part (Fig. 5H–K).
Thoracic sternum relatively transversely narrow; surfaces with punctae and small rounded granules (Fig. 4F). Sternites 1 and 2 completely fused to form narrow subtriangular structure, separated from sternite 3 by prominent concave ridge; sternite 3 depressed, fused with sternite 4, demarcated only by lateral incisions, no visible groove present; anterior surface of sternite 4 depressed; sternites 5–8 progressively more narrow, surface of sternites 5–7 medially depressed; small part of sternite 8 visible when pleon closed (Fig. 4F). Sutures between sternites 3–6 medially interrupted; sutures between sternites 6–8 complete; longitudinal groove present between sternites 6–8. Sternopleonal cavity deep, reaching to middle part of sternite 4; margin adjacent to telson subcristate, granulate; tubercle of pleonal locking mechanism distinct, on median part of sternite 5; penis coxal, exiting P5 coxa anterior to condyle.
Pleon narrow; all somites and telson free (Figs. 4F, 6D). Somite 1 yoke-like slightly wider than somite 2, with large median tubercle or spine, visible in dorsal view, adjacent surface with 7–9 smaller sharp tubercles or granules, margins granulated; somite 2 semicircular, as wide as somite 3, swelling on distal margin topped by 4 rounded granules; somite 3 rectangular, lateral margins sinuous, swelling on distal margin topped by 2 distinct rounded granules, with 1 low granule proximal to them; somite 4 trapezoidal, margins gently concave, swelling on distal margin topped by 2 indistinct rounded granules; somite 5 subrectangular with concave margins, low swelling on distal margin topped by 2 almost indiscernible rounded granules; somite 6 subquadrate with convex lateral margins, surface smooth; telson triangular, as long as broad, with sinuous lateral margins (Figs. 4F, 6D).
G1 relatively stout, sinuous, groove for G2 on ventral surface; tip relatively sharp; subdistal setae very long, dense (Fig. 6E, F, H–J). G2 very short, with short distal segment (Fig. 6G, K).
Female: Pleon longitudinally ovate; somite 1 trapezoidal, surface with large median subtruncate tubercle on distal margin, flanked by 2 smaller rounded tubercles, rest of surface with several small rounded granules; somite 2 trapezoidal, with 4 small rounded median granules on distal margin; somites 3–5 mostly smooth except for 2 low median granules on distal margin for each; somite 6 broadly rectangular with gently convex lateral margins; telson broadly triangular with gently sinuous lateral margin (Fig. 2C). Vulva large, median, directed obliquely anteriorly inwards towards median line, no visible vulvar cover (Fig. 2D).
Variation: The degree of spination on the carapace varies somewhat, with small specimens generally less spinose, especially on the posterior surface (Fig. 3D).
Colouration: The species is reddish-brown with patches of white in life (Fig. 1).
Remarks: Ortmann (1893: 51, pl. 3, fig. 5, 5a, i) described Majella brevipes from Japan in Sagami Bay from only one female collected between 129–219 m. His figures are rather schematic, but some key characters are obvious: a small crab with a spiny hepatic area and lateral border, pseudorostral spines with accessory spines, and a peculiar triangular shaped merus of the third maxilliped. Through the courtesy of Marie Meister, photographs of the type specimen in MZS were examined and there is no doubt it is conspecific with topotypic material from Sagami Bay in KPM. The type specimen is not in good condition and is not well cleaned but all the diagnostic characters are visible (Fig. 2A). The left third maxilliped is missing, which was the one that was figured by Ortmann (1893: fig. 5i) but the right is still intact.
Sakai (1965; 1976) redescribed the species from fresh material he had collected. Sakai (1965: 84) listed many specimens from various parts of Sagami Bay: 11 females, Amadaiba Kannonzuka-dashi, 50–85 m; 1 male, 12 females, Amadaiba Aoyarna-dashi, 80–120 m; and 1 male, 18 females, west of Jogashima, 85–120 m. Sakai (1976: 235) subsequently recorded two other lots of specimens: 2 males and 4 females from the Emperor’s Collection from Amadaiba Kannonzuka-dashi, Aoyama-dashi and west of Jogashima in Sagami Bay, from 50–120 m depth; and 2 males and 5 females he dredged off Mitsuishi, Manazuru, from 85 m. Muraoka (1998: 27) listed several Sakai lots in the Kanagawa Museum and these are re-examined here. Only two specimens (a male and a female) in lot KPM NH 0104599 actually belong to Majella brevipes. One poorly preserved specimen (pleon, maxillipeds and most of the pereopods missing), a female (cl 9.1 mm, cw 6.2 mm) was identified as Choniognathus reini (Balss, 1924) instead. Once the specimen was cleaned of thick mud and detritus, its carapace features are diagnostic. This species is also known from Sagami Bay, Japan (Sakai 1965; 1976).
Biology: In Sagami Bay, it occurs between depths of 50 and 219 m in soft substrate sand, mud and/or shell detritus.
Distribution: Majella brevipes is endemic to Sagami Bay and has not been recorded outside Japan. Griffin (1974: 20) recorded the species from the Mozambique Channel by the “Anton Bruun” vessel, but his record is almost certainly incorrect and is here referred to as M. skolopion n. sp. (see discussion for that species). Griffin and Tranter (1986: 218), however, seem to have overlooked this paper when they wrote that the species was “restricted to Japan”.
Majella skolopion n. sp.
(Figs. 7–9)
urn:lsid:zoobank.org:act:C63C2533-C758-4118-AA19-90A00785F5F2
Majella brevipes –Griffin 1974: 20. (not Majella brevipes Ortmann, 1893).
Material examined: Holotype: male (10.3 × 6.9 mm, pcl 7.7 mm, bcw 6.0 mm) (MNHN-IU-2017-8767), stn CP3130, Mozambique Channel, 25°52'S 33°75'E, 112–127 m, coll. MAINBAZA Cruise, 9 April 2009.
Diagnosis: Carapace surface covered by numerous small granules, spines and short stiff and long soft setae. Pseudorostral spines forming V-shaped cleft, with largest accessory spinules on distal outer margin. Supraorbital eave with anterior margin with 3 sharp spines, antorbital spine sharp, with accessory spinules; intercalated supraocular spine short, subtruncate, closely appressed against antorbital and postorbital spines but with a narrow space between them; postorbital spine subtriangular with accessory spinules, lined with 3 short spines in lateral view; suborbital tooth without spine, margin serrated, separated from postorbital spine and basal antennal article by narrow gap, surface with cluster of 3 median tubercles. Hepatic lobe pronounced, with 3 prominent spines. Lateral margin with 4 large spines, all with accessory spinules; first epibranchial spine at level of first and second lateral spines, next epibranchial spine smaller, tubercles posterior to first 2 spines smaller, varying in size and number and most of which are hard to discern from low granules on rest of region. Posterolateral margin gradually curving to meet posterior carapace margin, usually demarcated by tubercle. Posterior carapace margin with median spine largest; intestinal region granulated; frontal and protogastric regions relatively smoother, with low rounded granules, not spinular; surfaces of gastric regions with granules and low tubercles of varying sizes; cardiac region with 1 median spine, largest of median longitudinal spines, anterior surface with granules and low tubercles, posterior surface granulated; branchial region with mix of sharp tubercles and granules. Pterygostomial, subhepatic and sub-branchial regions granulated, ventral surface of hepatic lobe with 3 tubercles (Figs. 7, 9A–C). Outer margin of antennular fossa with 3 or 4 low granules, inner margin with 4 or 5 low granules (Fig. 7E). Interantennular spine bifurcate, low (Fig. 8A). Antennal basal article with proximal part wider than distal part, outer median part with 4 or 5 tubercles, anteroexternal angle with 2 or 3 tubercles (Figs. 7E, 8A). Buccal cavity with anteroexternal angle with pterygostomial region marked by weakly serrated flange (Fig. 7E); yhird maxilliped ischium outer margin with 7 sharp tubercles, surface with 7 or 8 small rounded granules and scattered smaller ones, inner proximal angle with short sharp tubercle; merus surface with 2 large and 1 smaller rounded tubercles and smaller low granules; exopod outer margin with 7 sharp tubercles (Figs. 7E, 9D). Cheliped basis-ischium with 2 sharp tubercles on ventral margin; merus ventral margin with 2 large spines, rest of surface with approximately 12–14 tubercles and granules; dorsal margin with 3 or 4 large spines and large oblique spine on distal edge, rest of margin with 8–10 shorter spines and tubercles; carpus with 3 large spines on dorsal margin, 2 large spines on outer surface and 2 sharp tubercles on inner surface, rest of surface with scattered granules, some sharp (Figs. 7A, B, D, 8C–F). Chela with 2 spines on upper surface and several small sharp granules; 3 large spines and scattered sharp tubercles on outer surface; with 10–12 spinules on ventral surface; inner surface with scattered small sharp granules; proximal part of dorsal margin of dactylus and ventral margin of pollex with 3 or 4 spinules (Fig. 8C–F). Ambulatory merus with rounded margins, armature as follows: P2 merus with 5 short spines on dorsal margin (excluding 1 distal spine), first spine recurved, outer surface unarmed, ventral and subventral margins with 10 or 11 sharp granules; P3 merus with 5 short spines or spinules on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface unarmed, ventral and subventral margins with 10 or 11 sharp granules; P4 merus with 3 or 4 short spines on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface unarmed, ventral and subventral margins with 6 or 7 sharp granules; P5 merus with 3 short spines on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal submedian row of 2 granules, ventral and subventral margins with 7 or 8 sharp granules; carpus smooth on ventral margin, dorsal margin with 3 short dorsal and subdorsal spines; propodus smooth, unarmed; dactylus ventral margin usually with 2 sharp submedian granules, occasionally with very small sharp granule just before chitinous distal part (Figs. 7A, 8G–K). Tubercle of pleonal locking mechanism on median part of sternite 5. Male pleonal somite 1 with a smaller lateral tubercle, adjacent surface with numerous small granules; somite 2 subtrapezoidal; somite 3 rectangular, lateral margins gently convex, swelling on distal margin topped by 2 distinct rounded granules, with 1 low granule proximal to them; somite 4 trapezoidal, margins gently concave, swelling on distal margin topped by 2 indistinct rounded granules; somite 5 subrectangular with concave margins, low swelling on distal margin topped by 2 almost indiscernible rounded granules; somite 6 subquadrate with gently convex lateral margins, surface smooth; telson slightly longer than wide, with gently concave lateral margins (Figs. 8B, 9E, F). G1 gently sinuous, tip rounded; subdistal setae short (Fig. 9G, H).
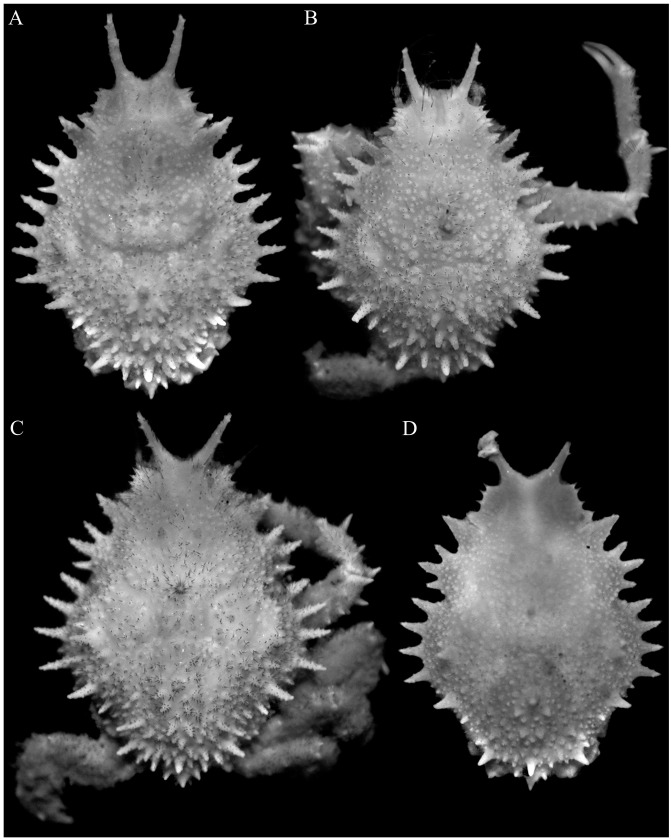
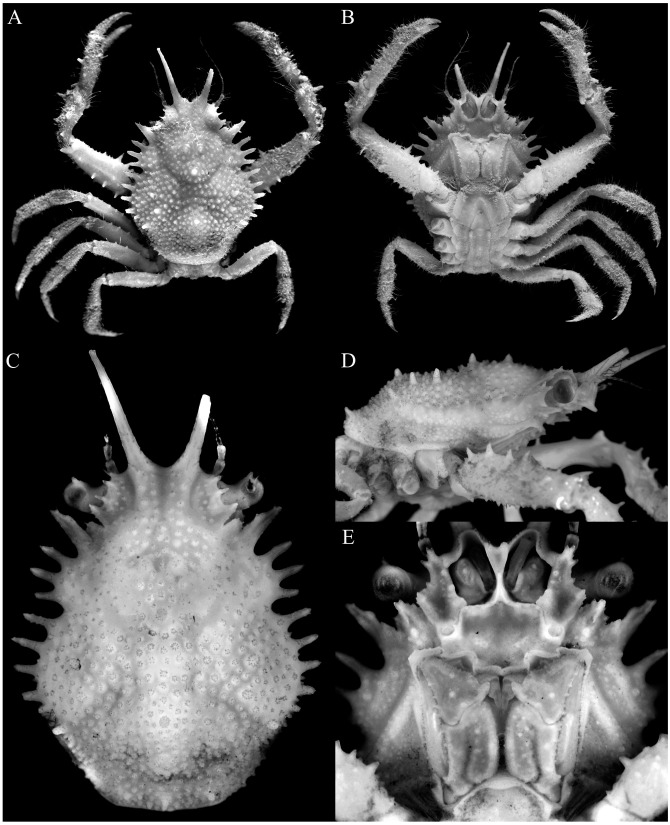
Fig. 7.
Majella skolopion n. sp., holotype male (cl 10.3 mm, cw 6.9 mm) (MNHN-IU-2017-8767). A, overall dorsal view; B, ventral view of cephalothorax; C, dorsal view of carapace; D, right lateral view of cephalothorax; E, antennules, antennae, epistome, buccal cavity and third maxillipeds.
Fig. 8.
Majella skolopion n. sp., holotype male (cl 10.3 mm, cw 6.9 mm) (MNHN-IU-2017-8767). A, right antenna; B, anterior thoracic sternum and pleon; C, outer views of right chela and carpus; D, dorsal views of right chela and carpus; E, dorsal view of right cheliped; F, ventral view of right cheliped; G–J, left P2–P5, respectively (setae denuded, all to same scale); K, right P5 (setae denuded, same scale as G–J).
Fig. 9.
Majella skolopion n. sp., holotype male (cl 10.3 mm, cw 6.9 mm) (MNHN-IU-2017-8767). A, dorsal view of carapace; B, right lateral view of carapace; C, antennules, antennae, epistome, buccal cavity and third maxillipeds; D, left third maxilliped; E, anterior thoracic sternum and pleon; F, pleon; G, left G1 (ventral view); H, left G1 (dorsal view); I, left G2. Scale bars: A–C, E = 2.0 mm; D, F–I = 0.5 mm.
Etymology: The name is derived from the Greek σκόλοφρον (skólophron) for thorn or spike, alluding to the spiny appearance of the species. The name is used as a noun in apposition.
Remarks: Griffin (1974) recorded Majella brevipes from three males collected from the Mozambique Channel by the cruise of the “Anton Bruun”. He did note, however, that “there is obvious variation in the relative size of the spines, in the spinulation, and in the shape and length of the rostrum. The rostrum is much longer in the largest male (more than one-half postrostral length) than in the other specimens (less than one-third postrostral length). There are several spinules on the dorsal and ventral edges of all the ambulatory meri and a few on the carpi, whereas Sakai states that only the fourth legs are tuberculate. In the largest specimen the spines on the gastric, cardiac, and dorsal surface of the branchial regions are very low, whereas in the other specimens there are prominent spines in these regions as described by Sakai” (Griffin 1974: 20). As discussed above, Majella brevipes does have all the ambulatory meri armed with small spines or tubercles along the dorsal and ventral margins. The lower density and relatively shorter spines on the carapace recorded by Griffin (1974) actually agree very well with the condition observed in the holotype of M. skolopion n. sp., which was also collected from the Mozambique Channel. This material is regarded as conspecific.
In M. brevipes, all the spines are distinctly serrulated, with the accessory spinules prominent even on the hepatic and lateral carapace spines (Figs. 2B, 3, 4B, C) (vs. the accessory spinules are distinctly fewer and lower in M. skolopion n. sp.; Fig. 7C); the dorsal carapace surface, especially the branchial, cardiac and intestinal regions as well as the posterior carapace margin are covered by numerous spines in M. brevipes with the posterior part appearing prominently spinose (Figs. 2B, 3, 4B–D) (vs. covered only by short granules and tubercles blunt or rounded in M. skolopion n. sp., with the posterior carapace margin appearing almost entire; Fig. 7C, D); there are usually 4 spinules on the margin of the supraocular eave in M. brevipes (Figs. 2B, 3, 4B, C) (vs. with 3 spines in M. skolopion n. sp.; Fig. 7C); the intercalated supraocular spine is more triangular in shape in M. brevipes (Figs. 2B, 3, 4B, C) (vs. subtruncate in M. skolopion n. sp.; Fig. 7C); the postocular spine is relatively more triangular in shape in M. brevipes (Figs. 2B, 3, 4B, C) (vs. proportionately longer and more slender in M. skolopion n. sp.; Fig. 7C); the first two epibranchial spines are distinct (Figs. 2B, 3, 4B, D) (vs. with epibranchial spines very low or absent in M. skolopion n. sp.; Fig. 7A, C, D); the basal antennal article is relatively more quadrate with the distal half shorter (Fig. 5B) (vs. basal antennal article longer, with the distal half elongate in M. skolopion n. sp.; Fig. 7A); the ventral margin of the chela has only scattered small rounded granule or smooth (Fig. 5D, E) (vs. with many small spines in M. skolopion n. sp.; Fig. 8D); the male telson is relatively shorter with the lateral margins gently concave (Figs. 4F, 6D) (vs. more elongate in M. skolopion n. sp. with the lateral margins almost straight; Figs. 8B, 9F); and the G1 is has a rounded tip, with long subdistal setae (Fig. 6E, F, H–J) (vs. tip subtruncate, with short setae in M. skolopion n. sp.; Fig. 9G, H).
Majella pristis n. sp.
(Figs. 10–12)
urn:lsid:zoobank.org:act:E66680BE-9A39-4FF8-9486-A9C7C627BB4D
Material examined: Holotype: male (7.0 × 4.4 mm, pcl 5.2 mm, bcw 3.8 mm) (MNHN-IU-2010-78), stn DW3230, northwest of Madagascar, 13°25'S 47°57'E, 71–158 m, coll. MIRIKY Cruise, 3 July 2009.
Diagnosis: Carapace with regions defined by ridges; surface mostly smooth and apparently glabrous; pseudorostral spines forming broadly U-shaped cleft, with largest accessory spinules on proximal third outer margin; supraorbital eave with anterior margin lined with 5–7 sharp spinules, antorbital spine short; intercalated supraocular spine short, triangular, separated from antorbital and postorbital spines by V-shaped clefts; postorbital spine subtruncate with accessory spinules, with 3 short triangular lobes in lateral view; suborbital tooth triangular, clearly separated from postorbital spine and basal antennal article by V-shaped clefts. Hepatic lobe low but visible, with 2 short spines. Lateral margin with 4 simple spines; first epibranchial spine at level of third lateral spine, next epibranchial spine smaller, with spinule between epibranchial and lateral spines. Posterolateral margin converging to posterior carapace margin, with 2 spines and large spine at area adjacent to intestinal region; intestinal region with median spine. Posterior carapace margin and area just behind it with 2 marginal and 1 median spines; regions demarcated by prominent ridges, angles usually with spines; protogastric regions with 2 short spines and oblique longitudinal row of sharp granules; surfaces of gastric regions smooth; cardiac region with 3 median spines arranged in longitudinal row, median one longest, surface smooth; in addition to epibranchial spines, branchial region with 4 transverse short spines, surface smooth. Pterygostomial region with 2 sharp tubercles, otherwise smooth; subhepatic and sub-branchial regions smooth, ventral surface of hepatic lobe with 2 short sharp tubercles (Figs. 10, 11A). Outer margin of antennular fossa with 4 or 5 sharp granules, inner margin with 8 or 9 low sharp granules (Fig. 10E). Interantennular spine unispinate, with small accessory spinule at base (Fig. 10B). Antennal basal article with proximal part gradually tapering to distal part, outer median part with 2 tubercles, outer lateral margin serrated, anteroexternal angle with 1 large sharp tubercle (Fig. 11A). Buccal cavity with anteroexternal angle with pterygostomial region marked by gently serrated flange (Fig. 10E); third maxilliped ischium outer margin with 5 sharp tubercles, surface with 3 large rounded granules and scattered smaller ones; merus surface with 3 or 4 tubercles and scattered small granules; exopod outer margin with 9 or 10 sharp tubercles or granules (Figs. 11B, 12A). Cheliped basis-ischium with 5 sharp tubercles on ventral margin; merus ventral margin with 1 or 2 large spines rest of surface with approximately 14–16 tubercles and granules; dorsal margin with 4 or 5 large spines and large oblique spine on distal edge, rest of margin with several shorter spines and tubercles, outer surface with longitudinal ridge with 3 spines; carpus with 4 large spines on dorsal margin, 2 large spines on outer surface and 3 sharp tubercles on inner surface (Figs. 10A, 11D, E). Chela with 3 spines on upper surface and spine on distal edge next to dactylus; 1 or 2 large spines and 5 or 6 sharp tubercles on outer surface; with 6 or 7 sharp tubercles on ventral surface; inner surface with scattered small sharp granules; dorsal margin of dactylus and ventral margin of pollex with 3–5 sharp granules (Fig. 11D, E). Ambulatory merus, carpus and propodus with sharp teeth, armature as follows: P2 merus with 6 sharp teeth or spines on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal ridge with 1 proximal recurved spine and 1 subproximal spine, each ventral margin with 8 or 9 sharp teeth or spines; P3 merus with 4 or 5 sharp teeth or spines on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal ridge with 1 proximal recurved spine, 1 subproximal spine and 1 median spine, each ventral margin with 6 sharp teeth or spines; P4 merus with 5 sharp teeth or spines on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal ridge with 1 proximal recurved spine, 1 subproximal spine and 1 low median spine, each ventral margin with 7 sharp teeth or spines; P5 merus with 5 sharp teeth or spines on dorsal margin (excluding 1 large distal spine), first spine recurved, outer surface with longitudinal ridge with 1 proximal recurved spine and 1 median spine, each ventral margin with 6 or 7 sharp teeth or spines; P2 carpus with 5 dorsal spines, 3 median spines on outer surface and no ventral spines; P3 carpus with 4 dorsal spines, 3 median spines on outer surface and no ventral spines; P4 carpus with 4 dorsal spines, 3 median spines on outer surface and 3 ventral spines; P5 carpus with 4 dorsal spines, 3 median spines on outer surface and 2 ventral spines; P2 propodus dorsal margin with 2 rows of 4 spines each, ventral margin with 4 spines; P3 propodus dorsal margin with 2 rows of 3 spines each, ventral margin with 3 spines; P4 propodus dorsal margin with 2 rows of 4 or 5 spines each, ventral margin with 6 spines; P5 propodus dorsal margin with 2 rows of 3 or 4 spines each, ventral margin with 5 spines; dactylus ventral margin with 2 low sharp granules on distal half (Figs. 10A, 11F, G). Tubercle of pleonal locking mechanism on distal third of sternite 5. Male pleonal somite 1 adjacent surface with 1 sharp tubercles and scattered very small granules; somite 2 semicircular; somite 3 subrectangular, lateral margins convex, swelling on submedian part topped by 2 distinct rounded granules, with 2 low granules proximal to them; somite 4 trapezoidal, margins gently concave, swelling on distal margin topped by 2 low rounded granules; somite 5 subquadrate with concave margins, low swelling on distal margin topped by 2 almost indiscernible rounded granules; somite 6 subquadrate with gently sinuous lateral margins, surface smooth; telson longer than broad, with almost straight lateral margins (Figs. 11C, 12B). G1 relatively stout, gently curved, tip subtruncate; subdistal setae without long setae (Fig. 12D, E).
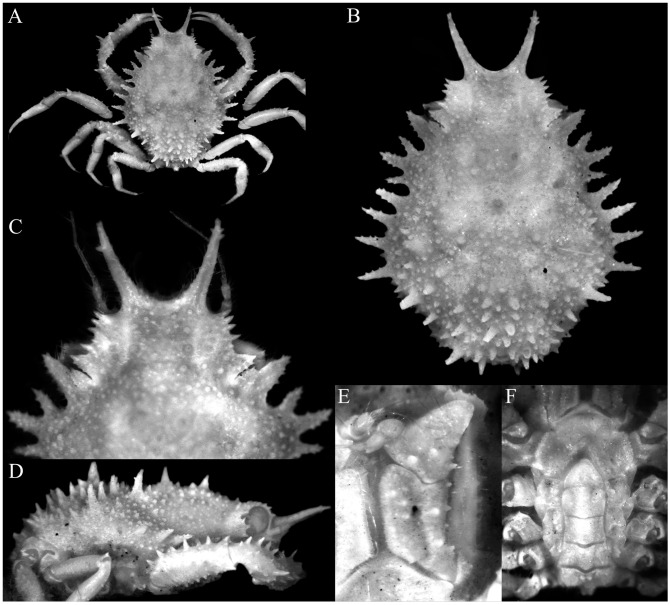
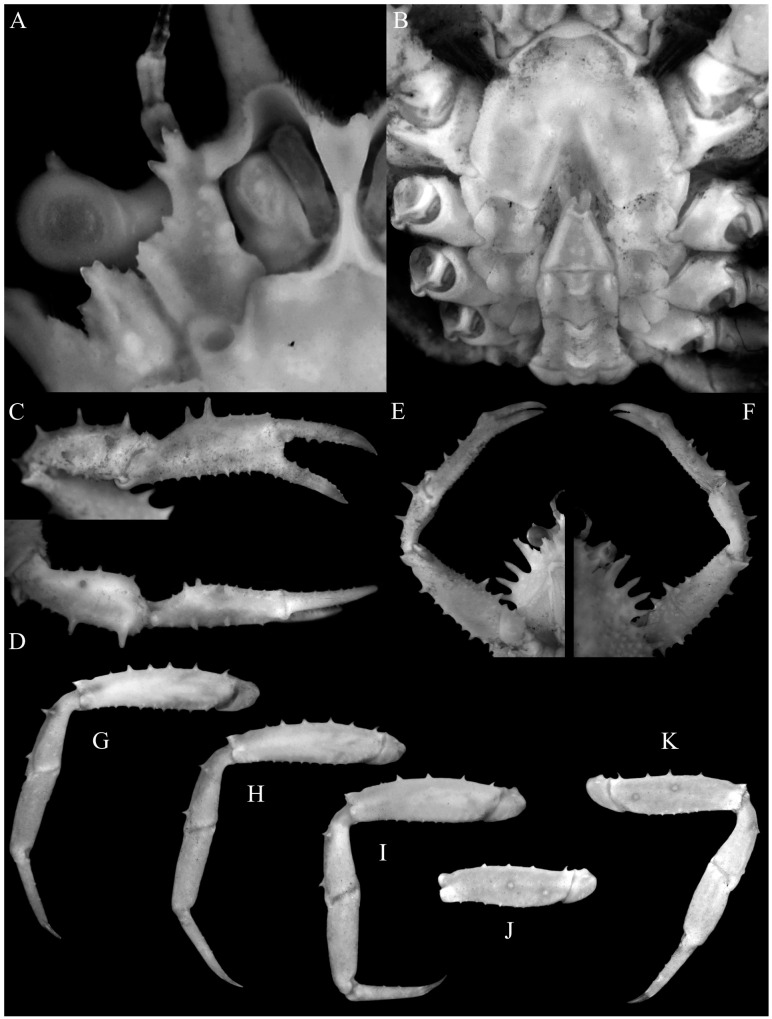
Fig. 10.
Majella pristis n. sp., holotype male (cl 7.0 mm, cw 4.4 mm) (MNHN-IU-2010-78). A, overall dorsal view; B, dorsal view of carapace (right intercalated supraocular spine broken); C, right lateral view of cephalothorax; D, anterior part of carapace (right intercalated supraocular spine broken); E, antennules, antennae and epistome.
Fig. 11.
Majella pristis n. sp., holotype male (cl 7.0 mm, cw 4.4 mm) (MNHN-IU-2010-78). A, right antenna; B, right third maxilliped; C, anterior thoracic sternum and pleon; D, outer views of left chela, carpus and merus; E, outer views of right chela, carpus and merus; F, right P3 (setae denudecd); G, left P5 (setae denuded).
Fig. 12.
Majella pristis n. sp., holotype male (cl 7.0 mm, cw 4.4 mm) (MNHN-IU-2010-78). A, left third maxilliped; B, pleon; C, pleonal somite 1 (in dorsal view); D, left G1 (ventral view); E, left G1 (dorsal view); F, left G2. Scale bars: A, B = 0.5 mm; D–F = 0.25 mm.
Etymology: The name is derived from the Greek πρίων (príōn) for saw, alluding to the general appearance of the carapace margins of the new species. The name is used as a noun.
Remarks: The present new species from the northwest coast of Madagascar is peculiar in many aspects and its placement in Majella is provisional, mainly because it has more characters for this genus than any other majid (the pseudorostral spines have accessory spinules, the anterior margin of the supraorbital eave has small spines, there is a low antorbital spine, the intercalated supraorbital spine is small and tightly appressed with the adjacent spines, the postorbital spine is prominent, the basal antennal article is subquadrate, the merus and ischium of the third maxilliped are free with the distal external angle of the merus prominently elongate and auriculiform, the outer margin of the exopod of the third maxilliped has prominent spines, the margin of the hepatic lobe is lined with spines, the male anterior thoracic sternum is relatively narrow, and the male pleon is relatively slender).
Majella pristis n. sp., however, has a unique pattern of spines connected by low but distinct ridges, with most of the surface appearing almost smooth (Fig. 10A–C); and the margins of the ambulatory meri, carpi and propodi are lined with sharp dentiform spines, with a longitudinal ridge on the outer median surface of the merus (Fig. 11F). In addition, the G1 is gently curved with the tip truncate and without setae (Fig. 12D, E) rather than sinuous with the distal parts setose like in other Majella species (Fig. 6H, J).
The unusual carapace and ambulatory leg characters suggest that M. pristis n. sp. may need to be assigned to a new genus. With this species being described from only one small subadult male, this decision is postponed until more material is available for examination.
DISCUSSION
The spider crabs of the family Majidae s. str. (sensu Ng et al. 2008; Windsor and Felder 2014) is a diverse group of brachyurans found mainly in the Indo-Pacific, with 40 recognised genera. Of these, 12 are monotypic. Majella was one of the most poorly known genera, with one species known only from Sagami Bay in Japan and the Mozambique Channel in Africa. A re-examination of the type female of M. brevipes, a good series of specimens from the topotypic locality and fresh specimens from Africa, now show that three species can be recognised. Two new species, M. pristis n. sp. and M. skolopion n. sp., are described from a suite of morphological characters. The unusual morphological characters of M. pristis n. sp. suggests that it may need to be transferred to a new genus, but more material is needed to confirm this
The present study also shows that while Majella has a carapace that superficially resembles majids like Maja and its allies (see Ng and Richer de Forges 2015), which it has been compared to in the past, it is actually morphologically closest to Eurynome, Choniognathus, Seiitaoides and Kasagia. These genera share characters in the orbits, antennae, epistome, buccal cavity, third maxilliped and pereopods, but Majella differs in several key aspects.
CONCLUSIONS
In our study, the type species of the previously monotypic and poorly known genus Majella, M. brevipes, is revised. Two new species, M. pristis n. sp. and M. skolopion n. sp., are described from the western Indian Ocean based on evidence from morphological characters (carapace, ventral frontal region, pleon and gonopod characters).
Acknowledgments
This work and the new species name were registered with ZooBank under urn: lsid:zoobank.org:pub:6B8439CC-D51F-44C1-85D6-59781CD886EA. The authors are grateful to Marie Meister (MZS) for checking on the type of Majella brevipes and taking excellent photographs of the specimen for our study. We also thank Takehiro Sato (KPM) for kindly sending the Sakai material of the species for the study, and Paula Martin-Lefèvre and Laure Corbari (MNHN) for the material from the Indian Ocean. These colleagues did this during the worst pandemic in recent memories, and we are most grateful for their diligence and kindness. We also thank Jean-François Dejouannet (MNHN) for rendering the excellent figures of Majella skolopion (Fig. 9A–C, E), as well as the Malagasy crew helped by fishing master Jean-François Barazer and scientists on board the research vessel Miriky who collected the samples from the Indian Ocean. The Mozambique material came from the MAINBAZA cruise under MNHN (Philippe Bouchet, chief scientist) and Instituto Espanol de Oceanografia, as part of the Mozambique-Madagascar expeditions on board Vizconde de Eza with Pro-Natura International funded by Total Foundation, Prince Albert II of Monaco Foundation and Stavros Niarchos Foundation.
Footnotes
Authors’ contributions: BRdF processed the MNHN samples, performed the morphological comparison, and drafted the manuscript. PKLN processed the KPM samples, performed the morphological comparison with the types, and drafted the manuscript. LBY processed the MNHN samples, photographed the specimens, and formatted the manuscript. All authors read and approved the final manuscript.
Competing interests: The authors declare that they have no conflict of interests.
Availability of data and materials: All specimens are deposited in museum collections stated in the paper.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Balss H. 1924. Ostasiatische Decapoden. V. Die Oxyrhynchen und Schlussteil. Archiv für Naturgeschichte 90(A5):20–84.
- Davie PJF, Guinot D, Ng PKL. 2015. Anatomy and functional morphology of Brachyura. In: Castro P, Davie PJF, Guinot D, Schram FR, von Vaupel Klein JC (eds) Treatise on Zoology –Anatomy, Taxonomy, Biology. The Crustacea. Volume 9, Part C-I. Brill, Leiden, pp. 11–164.
- Griffin DJG. 1966. A review of the Australian majid spider crabs (Crustacea, Brachyura). Australian Zoologist 13(3):259–298.
- Griffin DJG. 1974. Spider crabs (Crustacea: Brachyura: Majidae) from the International Indian Ocean Expedition 1963-64. Smithsonian Contributions to Zoology 182:i-iv, 1–35.
- Griffin DJG, Tranter HA. 1986. The Decapoda Brachyura of the Siboga expedition. Part VIII: Majidae. Siboga Expeditie Monografie 39:1–335.
- Ho P-H, Ng PKL, Chan TY, Lee D-A. 2004. New records of 31 species of brachyuran crabs from the joint Taiwan-France Expeditions, “TAIWAN 2000” and “TAIWAN 2001”, off deep waters in Taiwan. Crustaceana 77(6):641–668. doi:10.1163/1568540041958617.
- Komai T. 1999. Decapod Crustacea collected by L. Döderlein in Japan and reported by Ortmann (1890–1894) in the collection of the Musée Zoologique, Strasbourg. In: Nishikawa T (ed.) Preliminary taxonomic and historical studies on Prof. Ludwig Döderlein’s collection of Japanese animals made in 1880-81 and deposited at several European museums. Report of Activities in 1997-98 supported by Grant-in-Aid for International Scientific Research (Field Research) No. 09041155, pp. 53–101.
- Lamarck JBPA de. 1801. Systême des animaux vertèbres, ou Tableau général des classes, des orders et des genres de ces animaux; Présentant leurs caractères essentiels et leur distribution, d’après la considération de leurs rapports naturels et leur organisation, et suivant l’arrangement établis dans les galleries du Muséum d’Histoire Naturelle, parmi leur dépouilles conservées; Précédé du discours d’ouverture du cours de zoologie, donné dans le Muséum National d’Historie Naturelle l’an 8 de la République. Déterville, Paris, Volume 6, pp. i–viii + 1–432.
- Leach WE. 1814. Crustaceology. In: Webster D (ed) The Edinburgh Encyclopædia. Vol. 9(1). Balfour, Edinburgh, pl. 221.
- Muraoka K. 1998. Catalogue of the Brachyuran and Anomuran Crabs donated by Prof. Tune Sakai to the Kanagawa Prefectural Museum. Catalogue of the Collection in the Kanagawa Prefectural Museum of Natural History, Odawara, Kanagawa, Japan 11:1–67.
- Ng PKL, Guinot D, Davie PJF. 2008. Systema Brachyurorum: Part I. An annotated checklist of extant brachyuran crabs of the world. Raffles B Zool, Suppl 17:1–286.
- Ng PKL, Richer de Forges B. 2015. Revision of the spider crab genus Maja Lamarck, 1801 (Crustacea: Brachyura: Majoidea: Majidae), with descriptions of seven new genera and 17 new species from the Atlantic and Indo-West Pacific. Raffles B Zool 63:110–225.
- Ortmann AE. 1893. Abtheilung: Brachyura (Brachyura genuina Boas), I. Unterabtheilung: Majoidea und Cancroidea, 1: Section Portuninea. Die Decapoden-Krebse des Strassburger Museums, mit besonderer Berücksichtigung der von Herrn Dr. Döderlein bei Japan und bei den Liu-Kiu-Inseln gesammelten und zur Zeit im Strassburger Museum aufbewahrten Formen. Theil VI [= Part 6]. Zoologische Jahrbücher, Abtheilung für Systematik, Geographie, und Biologie der Thiere 7(I):23–88, pl. 3.
- Padate VP, Manjebrayakath H, Ng PKL. 2019. Kasagia sudhakari, a new species of deep-sea spider crab (Crustacea: Brachyura: Majidae) from the southeastern Arabian Sea. Mar Bio Res 15(3):290–296. doi:10.1080/17451000.2019.1637527.
- Rathbun MJ. 1932. Preliminary descriptions of new species of Japanese crabs. P Biol Soc Wash 45:29–38.
- Richer de Forges B, Ng PKL. 2007. On a new genus and new species of deep-water spider crab from the Philippines (Crustacea, Decapoda, Brachyura, Majidae). Zootaxa 1644:59–68. doi:10.11646/zootaxa.1644.1.3.
- Sakai T. 1938. Studies on the Crabs of Japan. III. Brachygnatha, Oxyrhyncha. Yokendo, Tokyo, Japan.
- Sakai T. 1961. New species of Japanese crabs from the collection of His Majesty the Emperor of Japan. Crustaceana 3(2):131–150, pls. 3, 4. doi:10.1163/156854061X00635.
- Sakai T. 1965. The Crabs of the Sagami Bay collected by His Majesty the Emperor of Japan. Maruzen Co., Tokyo, Japan.
- Sakai T. 1976. Crabs of Japan and the Adjacent Seas. In three volumes; English Text, pp. xxix + 773, Japanese Text, pp. 1–461, Plates volume, pp. 1–16, pls. 1–251. Kodansha Ltd., Tokyo, Japan.
- Samouelle G. 1819. The Entomologist’s Useful Compendium, or an Introduction to the Knowledge of the British Insects. Thomas Boys, London, UK.
- Windsor AM, Felder DL. 2014. Molecular phylogenetics and taxonomic reanalysis of the family Mithracidae MacLeay (Decapoda: Brachyura: Majoidea). Invertebr Syst 28:145–173. doi:10.1071/IS13011.
- Yokoya Y. 1933. On the Distribution of Decapod Crustaceans inhabiting the Continental Shelf around Japan, chiefly based upon the Materials collected by S. S. Sôyô-Maru, during the Year [sic] 1923–1930. Journal of the College of Agriculture, Tokyo Imperial University 12(1):1–226.