Abstract
Background
Characterization of aerosol generation during exercise can inform the development of safety recommendations in the face of COVID-19.
Research Question
Does exercise at various intensities produce aerosols in significant quantities?
Study Design and Methods
In this experimental study, subjects were eight healthy volunteers (six men, two women) who were 20 to 63 years old. The 20-minute test protocol of 5 minutes rest, four 3-minute stages of exercise at 25%, 50%, 75%, and 100% of age-predicted heart rate reserve, and 3 minutes active recovery was performed in a clean, controlled environment. Aerosols were measured by four particle counters that were place to surround the subject.
Results
Age averaged 41 ± 14 years. Peak heart rate was 173 ± 17 beat/min (97% predicted); peak maximal oxygen uptake was 33.9 ± 7.5 mL/kg/min; and peak respiratory exchange ratio was 1.22 ± 0.10. Maximal ventilation averaged 120 ± 23 L/min, while cumulative ventilation reached 990 ± 192 L. Concentrations increased exponentially from start to 20 minutes (geometric mean ± geometric SD particles/liter): Fluke >0.3 μm = 66 ± 1.8 → 1605 ± 3.8; 0.3-1.0 μm = 35 ± 2.2 → 1095 ± 4.6; Fluke 1.0-5.0 μm = 21 ± 2.0 → 358 ± 2.3; P-Trak anterior = 637 ± 2.3 → 5148 ± 3.0; P-Trak side = 708 ± 2.7 → 6844 ± 2.7; P-Track back = 519 ± 3.1 → 5853 ± 2.8. All increases were significant at a probability value of <.05. Exercise at or above 50% of predicted heart rate reserve showed statistically significant increases in aerosol concentration.
Interpretation
Our data suggest exercise testing is an aerosol-generating procedure and, by extension, other activities that involve exercise intensities at or above 50% of predicted heart rate reserve. Results can guide recommendations for safety of exercise testing and other indoor exercise activities.
Key Words: aerosol, exercise, exercise testing
Abbreviations: AGP, aerosol generating procedures; HEPA, high-efficiency particulate air; HRR, predicted heart rate reserve; Vo2, oxygen uptake
FOR EDITORIAL COMMENT, SEE PAGE 1174
Concern over potential transmission of SARS-CoV-2 caused curtailment of practice in exercise testing laboratories and cardiac rehabilitation programs; many indoor exercise centers were closed.1, 2, 3, 4
Aerosols are solid particles or liquid droplets suspended in air (or another gas).5 Particles <1.0 μm in diameter remain in suspension, although particles >10 μm likely fall out as droplets at relatively short distances from the mouth during normal breathing; between these sizes, particles are classified as small droplets that may dry out through evaporation to become aerosols.5 Normal breathing generates an air plume that contains aerosols that may contribute to viral shedding.6, 7, 8, 9, 10, 11, 12 Aerosols may remain in suspension up to 3 hours and contaminate distant surfaces.7 , 8
Understandably, viral shedding during exercise in asymptomatic patients who test COVID-19 positive has not been studied, though various exercise settings that include fitness centers,13 , 14 fitness dance classes,15 recreational hockey,16 and high school football17 have been apparent sources of SARS-CoV-2 transmission.
Exercise testing is not listed as an aerosol-generating procedure (AGP),18 likely due to the absence of data that examines aerosol production, although one recent publication has shown increased aerosol concentration in a small gym while four subjects were exercising.19 Studies on lower volume respiratory activity such as breathing, speaking, coughing, or sneezing have demonstrated aerosol production with viable virus or SARS-CoV-2 RNA carrying potential.20, 21, 22, 23, 24, 25, 26, 27 Therefore, we hypothesize that sustained deep and rapid breathing during heavy exercise will generate measurable volumes of aerosols with potential for carrying infective agents like COVID-19 to both near and distant spaces in an exercise laboratory or center.
The aim of our investigation was to describe a method for characterizing aerosol generation during exercise and present initial results.
Methods
Materials
Measurement of respiratory aerosols that are generated by exercise required the design of a special testing environment. We overcame experimental difficulties of attempting to measure exercise-generated aerosols in large rooms such as exercise laboratories, cardiac rehabilitation centers, or fitness centers contaminated with nonrespiratory particles distributed by heating, ventilation, and air conditioning airflow patterns by creating a small, clean environment with controlled airflow (Fig 1 ).
Figure 1.
A, Inside view of Colorado Altitude Training tent and materials. B, The image of a subject exercising inside the clean tent shows external physiologic monitoring equipment and P-Trak 8525 particle counter (Fluke Corporation) behind subject. Black conductance tubing in front of the subject is connected to a second P-Trak, and a third P-Trak is placed to the subject’s side. A Fluke 985 particle counter (TSI Incorporated) is in front of the subject.
A Colorado Altitude Training tent with dimensions of 1.88 × 2.29 × 3.04 m (13.1 m3 = 13,100 L) was attached to intake and outlet high-efficiency particulate air (HEPA) filtered fans H1000V (Abatement Technologies) that generated a maximum flow of 950 cubic feet per minute, which allowed us to achieve particle concentrations of approximately 1000/L within 5 to 10 minutes of filtering at maximum airflow prior each test.
Two types of particle counters were used; Fluke 985 (Fluke Corporation) and P-Trak 8525 (TSI Incorporated) for the aerosol concentration assessment. Both particle counters rely on laser beam technology that counts the number of particles that interrupt the beam, and both particle counters have been used in multiple environmental studies.28, 29, 30, 31 The Fluke counter detects particles in the range of 0.3 to 10.0 μm that are output as six separate channels32; the P-Trak counter registers smaller particles in the range of 0.02 to 1.0 μm output as a single channel.33 For reference, SARS-CoV-2 has a diameter of 0.06 to 0.14 μm and has been detected in significant quantities in aerosols as small as 0.25 to 0.5 μm and as large as 2.5 to 5 μm.34 Because these devices measure particles suspended in air, the term aerosols can apply to the Fluke and P-Trak measurements. Additional monitoring equipment included an MGC Diagnostics Ultima (MGC Diagnostics Corporation) to measure oxygen consumption and ventilation and a Masimo Radical-7 (Masimo) forehead oximeter to measure heart rate.
Subjects
Subjects were eight healthy volunteers (six men, 2 women) who were 20 to 63 years old without baseline cardiovascular disease. Because no treatments were performed and test procedures (cycle exercise) would be no different than what subjects might be doing on a regular basis, only verbal consent was obtained.
Protocol
Tests were conducted according the institutional review board guidelines (IRB 20-004751). After 10 minutes of evacuation of the tent with high airflow HEPA filtering and surface cleaning with Oxivir Tb (Diversity), the subject entered the tent and sat on the exercise bike for 5 minutes of additional cleaning once the tent had been resealed. Fans were then turned off. The test protocol consisted of 5 minutes of resting breathing, 4 × 3-minute stages of exercise at 25%, 50%, 75%, and 100% of age predicted heart rate reserve (HRR), and 3 minutes of cool down (unloaded pedaling). Workload was adjusted by the subject with coaching by research personnel who were monitoring from outside the tent and encouraging constant cadence with resistance augmentation as stages advanced. Although the Colorado Altitude Training tent was essentially airtight, it did have several sealable entry points that might allow air exchange with the non-HEPA filtered room air surrounding the tent. Therefore, we performed a 20-minute passive room monitoring session to determine that air entry would not affect particle concentration significantly during the exercise trials.
Data Collection
Heart rate was measured by forehead oximetry. Oxygen uptake (Vo 2), expired ventilation, and respiratory exchange ratio were measured by the MGC Diagnostics Ultima. Particle concentration was tracked at three locations by P-Trak particle counters: immediately anterior to the exercising subject at mouth level, approximately 1 m behind the subject, and 1.5 m lateral to the exercising subject. A single Fluke particle counter was placed 1 m anterior to the exercising subject. Analyzers were all set on tables at roughly ergometer seat height.
Data Analysis
Vo 2, respiratory exchange ratio, and ventilation data are expressed both as minute volume in liters per minute and cumulative ventilation in liters and are shown at 30-s intervals. Data for particle counts are presented as 30-s averages updated every 10 s for individual subjects and geometric means for the entire group. Fluke device individual output channels are grouped as all particles >0.3 μm, particles 0.3 to 1.0 μm, which overlap P-Trak data, and as larger 1.0- to 5.0-μm particles that also have the potential to carry virus beyond 1 to 2 m from the source.21 Initial examination of data suggested a skewed distribution of particle concentrations among subjects, which suggests the use of geometric vs arithmetic means to best present group data (reducing the effect of extreme values in the small sample size). To test for differences at various time points per exercise intensities compared with baseline rest (time 0), we performed repeat measures analysis of variance with Tukey adjustment for multiple comparisons on the logarithmic transformed values for each particle class and time point with the use of SAS PROC GLM (SAS Institute Inc). Level of significance was set at a probability value of <.05. The SAS Studio software (version 5; SAS Institute Inc) was used for all statistical analyses.
Results
Subjects
Subjects averaged 41 ± 14 years of age. Height averaged 177 ± 7 cm; weight averaged 84 ± 11 kg; and BMI averaged 26.9 ± 2.7 k/m2. Subjects achieved a peak heart rate of 173 ± 17 beats/min (97% predicted). Peak Vo 2 was 33.9 ± 7.5 mL/kg/min with a peak respiratory exchange ratio of 1.22 ± 0.10. Maximal minute volume of ventilation averaged 120 ± 23 L/min; cumulative ventilation during the 20-minute protocol reached 990 ± 192 L. Figure 2 shows key exercise data graphically to illustrate that the protocol achieved the goal of reaching peak Vo 2 in progressive increments.
Figure 2.
Cardiopulmonary exercise data includes heart rate, oxygen uptake, and minute volume of ventilation. BTPS = body temperature and pressure, saturated; HRR = predicted heart rate reserve; Vo2 = oxygen uptake
Aerosol Generation During Exercise
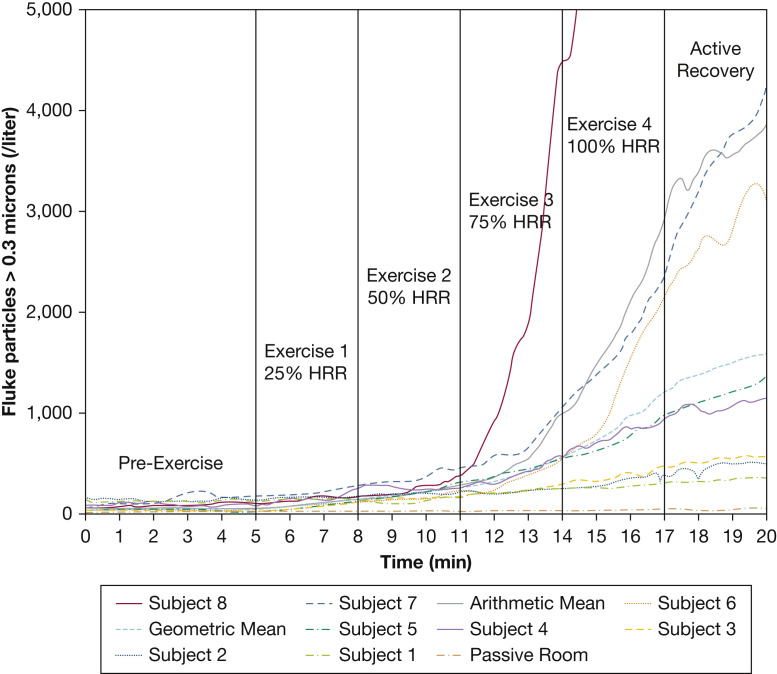
Figure 3 presents individual subject responses to exercise along with passive room monitoring data and both arithmetic and geometric means. Aerosol generation increased consistently in all subjects at higher levels of exercise with wide intersubject heterogeneity. All Fluke particles >0.3 μm are displayed; P-Trak particles were tracked similarly during exercise. Note the dramatic response of subject 8; the Y-axis was truncated at 5,000 particles/L so that trends of other subjects could be better observed. The arithmetic mean was dominated inappropriately by this high aerosol producer, which supports the use of the geometric mean for presenting group data. Passive room monitoring indicated extremely low leakage of particles into the tent during a 20-minute trial.
Figure 3.
Individual subject graphs of all Fluke-measured (Fluke Corporation) particles >0.3 microns vs time during the exercise protocol. Arithmetic mean, geometric mean, and passive room clearance data are also plotted. Note: The Y-axis is truncated at 5,000 particles/L so that values for Subject 8 at 17 minutes (16,035 particles/L) and at 20 minutes (19,891 particles/L) are off the scale. HRR = predicted heart rate reserve.
Figures 4 and 5 show the results as continuous graphs of particle concentration vs time. For both measured particle classes, there was a curvilinear increase in concentrations over the 20-minute trial that closely followed cumulative ventilation. From Figure 5, we also saw rapid distribution of aerosols within our tent.
Figure 4.
Concentrations of particles (per liter) counted by the Fluke particle counter (Fluke Corporation) are displayed in the overall range of 0.3 to 5.0 microns with subgroups of particles 0.3 to 1.0 microns and 1.0 to 5.0 microns during the exercise protocol. Data represent geometric means of the data from eight subjects. Cumulative ventilation in liters (Fluke particles 0.3 to 5.0 μm) is also displayed on a separate axis. HRR = predicted heart rate reserve.
Figure 5.
Concentrations of particles 0.02 to 1.0 microns from the P-track sensors (TSI Incorporated) (anterior, side, and back) during the exercise protocol are displayed along with cumulative ventilation. Data represent geometric means of data from eight subjects. Cumulative ventilation in liters is also displayed on a separate axis. HRR = predicted heart rate reserve.
The following data shows a significant increase in concentration (geometric mean ± geometric SD particles/liter) at the start of resting breathing vs 20 minutes later: Fluke > 0.3 μm = 66 ± 1.8 μm → 1605 ± 3.8 μm; 0.3 to 1.0 μm = 35 ± 2.2 μm → 1095 ± 4.6 μm; Fluke 1.0 to 5.0 μm = 21 ± 2.0 μm → 358 ± 2.3 μm; P-Trak anterior = 637 ± 2.3 μm → 5148 ± 3.0 μm; P-Trak side = 708 ± 2.7 μm → 6844 ± 2.7 μm; P-Track back = 519 ± 3.1 μm → 5853 ± 2.8 μm. All increases were significant at a probability value of <.05.
Having confirmed our hypothesis that exercise generates significant quantities of aerosols, we performed exploratory analyses to determine at what levels of exercise significant aerosols are produced. There were no significant differences in the time 0 and 5-min values (representing 5 min of resting breathing) for any particle classes. There was a progressive increase in both arithmetic and geometric mean concentrations for all particle classes with increasing exercise level. For P-Trak particle concentrations that were measured at all three locations, there was a significant increase compared with end of resting breathing for exercise at 75% (time, 14 min) and 100% of HRR (time, 17 min) and cool down (time, 20 min), but not at 25% (time, 8 min) and 50% of HRR (time, 11 min) compared with time 0. All Fluke particle classes increased significantly above the end of resting breathing value at 50%, 75%, 100% HRR and in cool down, but not at 25% HRR.
Arithmetic and geometric means and SD at the end of each phase of the protocol plus statistical summary are provided in Table 1 .
Table 1.
Arithmetic Mean, Geometric Mean, and Geometric SD of Eight Subjects at the End of Each Study Phase for P-Track (TSI Incorporated) Front, Side, and Back Particle Counts per Liter and Fluke (Fluke Corporation) Particle Counts per Liter for Particles >0.3 μm, 0.3 to 1.0 μm, and 1.0 to 5.0 μm
| Time, min |
Phase |
P-Trak Measured Particles |
Fluke Measured Particles |
||
|---|---|---|---|---|---|
| Arithmetic Mean ± SD | Geometric Mean ± SD | Arithmetic Mean ± SD | Geometric Mean ± geometric SD | ||
| Anterior Sensor | >0.3 μm | ||||
| 0 | Start rest | 849 ± 607 | 637 ± 2.3 | 77 ± 48 | 66 ± 1.8 |
| 5 | End rest | 971 ± 968 | 642 ± 2.6 | 90 ± 54 | 73 ± 2.1 |
| Predicted heart rate reserve | |||||
| 8 | 25% | 925 ± 602 | 707 ± 2.5 | 173 ± 61 | 164 ± 1.4 |
| 11 | 50% | 1,200 ± 472 | 1,125 ± 1.5 | 277 ± 102 | 261 ± 1.4a |
| 14 | 75% | 1,862 ± 891 | 1,726 ± 1.5a | 1,007 ± 1,432 | 604 ± 2.6a |
| 17 | 100% | 5,725 ± 6,895 | 3,702 ± 2.5a | 2,961 ± 5,340 | 1,213 ± 2.6a |
| 20 | Cool-down | 10,100 ± 15,875 | 5,148 ± 3.0a | 3,902 ± 6,606 | 1,605 ± 3.8a |
| Side Sensor | 0.3-1.0 μm | ||||
| 0 | Start rest | 975 ± 628 | 708 ± 2.7 | 45 ± 36 | 35 ± 2.2 |
| 5 | End rest | 1,175 ± 759 | 935 ± 2.2 | 56 ± 36 | 42 ± 2.5 |
| Predicted heart rate reserve | |||||
| 8 | 25% | 1,538 ± 833 | 1,365 ± 1.7 | 107 ± 52 | 98 ± 1.6 |
| 11 | 50% | 2,513 ± 2,975 | 1,592 ± 2.7 | 172 ± 70 | 160 ± 1.5a |
| 14 | 75% | 3,263 ± 2,590 | 2,633 ± 1.9a | 803 ± 1,346 | 385 ± 3.0a |
| 17 | 100% | 6,388 ± 6,975 | 4,402 ± 2.4a | 2,547 ± 5,082 | 799 ± 4.4a |
| 20 | Cool-down | 11,706 ± 16,467 | 6,844 ± 2.7a | 3,388 ± 6,301 | 1095 ± 4.6a |
| Back Sensor | 1.0-5.0 μm | ||||
| 0 | Start rest | 775 ± 548 | 519 ± 3.1 | 25 ± 16 | 21 ± 2.0 |
| 5 | End rest | 596 ± 448 | 447 ± 2.7 | 28 ± 14 | 24 ± 1.7 |
| Predicted heart rate reserve | |||||
| 8 | 25% | 992 ± 891 | 684 ± 2.8 | 48 ± 17 | 45 ± 1.4 |
| 11 | 50% | 1,417 ± 1,005 | 1,200 ± 1.8 | 80 ± 32 | 75 ± 1.5a |
| 14 | 75% | 2,454 ± 1,530 | 2,116 ± 1.8a | 168 ± 97 | 147 ± 1.8a |
| 17 | 100% | 4,225 ± 4,114 | 3,092 ± 2.2a | 369 ± 284 | 288 ± 2.1a |
| 20 | Cool-down | 10,150 ± 13,404 | 5,853 ± 2.8a | 470 ± 354 | 358 ± 2.3a |
Indicates significantly different at P < .05 from geometric mean at 0 minutes (start rest).
Although the P-Trak and Fluke particle counters are not intended for precise quantification (accurate to ±15%), we attempted to estimate rate of aerosol production. Assuming even distribution of particles throughout the tent and estimating the free space in the tent (total volume–subject and equipment) to be 12,000 L, the increase in particle concentration with exercise gives a rough estimate of 18,384,000 Fluke-measured aerosols ≥0.3 μm added to the tent during the 20-min protocol. Rates of aerosol generation can then be estimated for each 3-min exercise stage. Exercise at 50% HRR theoretically generated Fluke-measured aerosols ≥0.3 μm at 6,467/s. These numbers increase to 22,867/s at 75% HRR and 40,600/s at 100% HRR. We emphasize that these are very approximate estimates that are intended to show only the order of magnitude at which aerosols are generated during exercise at various levels.
Discussion
Our study demonstrated significant aerosol generation during exercise. We detected limited aerosol generation during resting breathing and only low levels during exercise at 25% of HRR (not significant). Aerosols increased modestly at 50% HRR (significant in Fluke particle classes). Aerosols in all particle classes rose rapidly at 75% of HRR (likely above the anaerobic threshold) and continued to increase in a more exponential manner at 100% of HRR and during active recovery. This can be likely explained by aerosol accumulation from lower exercise levels coupled with greater aerosol production at higher levels of ventilation. For this reason, we show cumulative, not instantaneous, ventilation as a reference; particle concentrations appear to track similarly to cumulative ventilation across the time frame of the protocol. We hypothesize that greater aerosol production at higher levels of exercise was likely related to greater expiratory force, creating greater sheer stress in the airways, combined with larger tidal volumes that indicate more complete emptying of the lungs.35, 36 It is reasonable to assume an 10- to 15-fold higher minute volume of expired ventilation for maximal exercise vs sitting with lower (10% vs 20% to 30%) dead space ventilation and higher peak flow (5 to 10 vs 1 to 2 L/s),37 each factor contributing to higher aerosol generation.
Our small, clean tent allowed us to achieve a low background particle concentration of 66 particles/L measured by the Fluke sensor vs 3000-5000/L in our clinical exercise testing laboratories. Even small increases in particle concentrations at low intensity exercise could be discerned readily. The larger size of the exercise laboratory (approximately 50,000 L) compared with the tent (13,100 L) and ambient airflow generated by the heating, ventilation, and air conditioning system would have been further impediments to the measurement of aerosols. A recent paper by Doggett et al38 was unable to detect a significant increase in particle concentration during extubation, a known AGP, against a background of concentration of >13,000 particles/L.
Although we could not determine a respiratory source for all aerosols, our data suggest it was the predominant source during exercise. Other possible nonrespiratory components generated from subject dander or clothing should not increase in proportion to ventilation or resistance to pedaling the cycle ergometer; the pedaling cadence remained relatively similar, from low to high resistance with no substantial change in aerosol generation at initial stages of the protocol and light breathing. There was additional cleaning for 5 minutes by the HEPA filtered fans with the subject in the tent before resting data collection.
Despite no ambient air flow in the clean tent other than what was generated by pedaling the exercise bike, aerosols quickly dispersed evenly around the tent, likely due to Brownian movement of air molecules striking the aerosols that contributed to a quick equalization of concentration across the internal space of the tent.39 , 40 This observation has implications for safety in indoor exercise environments and for potential aerosol mitigation efforts.
Although there was a consistent increase in aerosol generation by all subjects, we also documented significant intersubject heterogeneity. Given our relatively small number of subjects, we cannot say whether subject 8 represents a high point in a normal distribution or an outlie, a potential “super spreader” as was found in the SARS-1 experience.41
There was more variability in P-Trak vs Fluke measurements, likely due to smaller sample volumes (1.0 L/min for P-Trak vs 2.83 L/min for Fluke) and smaller particles sizes that required growth in a cloud chamber. The Fluke 985 appeared to be particularly better suited to counting the low levels of particles during resting breathing and exercise at 25% HRR. Moreover, many of the P-Trak measured particles in the range of 0.02 to 0.3 μm, which was below the threshold of measurement by Fluke, may be too small to carry virus.20 , 33 Therefore, we did most of our analyses with Fluke data, although P-Trak data were useful in showing rapid distribution of particles during exercise and confirming the close association of aerosol generation with cumulative ventilation.
We might address how exercise compares with other activities. However, it is challenging to compare results from one study to another due to diverse measurement technologies used, different sampling protocols, and different background air flow. Also, we measured aerosol generation over a 20-minute time frame, whereas other reports focused on much shorter time frames or single events (like a cough).
Asadi et al42 reported that reading in a loud voice generated 53 particles/s in the range of 0.5 to 10.0 μm. We estimate that exercise at just 50% of HRR generated >3,7000 particles/s in that size range, although results may not be directly comparable because Asadi et al used an aerodynamic particle sizer TSI Model 321 vs our Fluke 985 device and had a very different means of capturing aerosols.
The World Health Organization has documented increased risk of transmission of coronaviruses with AGP.43 Recognized AGPs include tracheal intubation, noninvasive ventilation, tracheotomy, CPR, manual ventilation before intubation, bronchoscopy, and positive pressure ventilation treatments.19 , 44 , 45 Brown et al46 reported that a single volitional cough produced an average of 134 ± 77 particles in the range of 0.3 to 10.0 μm that produced an average concentration of 732 ± 418 particles/L in a small sampling funnel over a 12-s sampling period. Bake et al6 estimated a similar 678 particles/L for coughing based on data presented by Chao et al.47 Compared with coughing, tracheal intubation generated a 500-fold lower (1.4/L) and extubation a 35-fold lower (21/L) average particle concentration over a 5-minute measurement period.46 In contrast, we calculate that a single breath during exercise at just 50% of HRR was generating approximately 18,000 particles in that size range; exercise at 100% of HRR generated aerosols at approximately six times that rate. Thus, our data indicate that exercise testing produces aerosols (many with COVID-19 virus-carrying potential) in far greater quantity than other known AGPs. Size of the exercise laboratory, ambient airflow rates and patterns, and staff personal protective equipment will modify potential risk and exposure.
Helgeson et al19 described exercise at three levels for 10 minutes each at an average of 61%, 79%, and 91% of predicted peak heart rate by four subjects exercising simultaneously (while wearing masks). At the end of the 30-minute exercise, concentration of all Fluke-measured particles could be calculated as 2,058 particles/L at a location central to the four subjects, slightly more than the geometric mean of 1,650 particles/L that was achieved in our trials with a single subject exercising. Their environment was much larger (473.2 vs 13.1 m3) and had ambient airflow of 6.3 vs 0 air changes per hour in our experimental set-up. An exact mathematic comparison of our findings with theirs is problematic, but it is reassuring that the peak aerosol concentrations that were achieved were of the same order of magnitude.
Addressing the generalizability of our results to standard noncardiopulmonary exercise testing, we thought it unlikely that the mouthpiece and pneumotach used in the measurement of Vo 2 were significantly affecting aerosol generation, and we had comparative data on five subjects with the use of the identical protocol without any gas analysis system or masking. Figure 6 confirms the similarity of response. At no time point were there significant differences between the two testing conditions.
Figure 6.
Concentrations of particles (per liter) measured by the Fluke particle counter (Fluke Corporation) are displayed in the overall range of >0.3 microns with subgroups of particles 0.3 to 1.0 microns and 1.0 to 5.0 microns during the exercise protocol. Data represent means of the data from five subjects comparing a no mask trial vs a trial measuring oxygen uptake. HRR = predicted heart rate reserve; Vo2 = oxygen uptake.
Interpretation
Limitations
We report several limitations. First, we are measuring aerosol generation, not demonstrating respiratory viral transmission, because the latter is dependent on several factors that include distance from the source of aerosols, duration of the exposure, symptoms and viral load, and host susceptibility.7 At this time, the number of virus-containing particles shed by an asymptomatic infected subject at different levels of ventilation is unknown, as is the number virus-containing particles necessary for causing infection in the airways of another person.
Second, we acknowledge that there are more precise particle quantification devices available, but we believe it is unlikely that different measurement technology would have significant impact in our findings. Finally, we acknowledge that data represent a limited number of healthy subjects. Heart failure, asthma, or other forms of pulmonary disease or seasonal allergies might affect significantly the quantity and size distribution of respiratory aerosols that are generated during exercise.
Conclusions
First, we demonstrate the feasibility of an innovative method for characterization of aerosol generation during exercise. Second, our data show that significant concentrations of aerosols are generated during exercise in all measured sizes of particles that are exponentially related to exercise intensity and volume of ventilation. Many of the aerosols are in the size range shown to carry SARS-CoV-2.7 , 21 , 26 , 34 Third, aerosols distribute quickly and evenly within our aerosol laboratory. Fourth, intersubject aerosol production during exercise was significantly heterogeneous in a small sample population.
Based on our data, exercise testing should be considered a potential AGP and, by extension, so should other kinds of physical activity, especially those that involve exercise intensities >50% of HRR. Future research should be continued to guide evidence-based recommendations for safety exercise testing laboratories and indoor exercise centers in the face of COVID-19.
Take-home Points.
Study Question: Does exercise at various intensities produce aerosols in significant quantities?
Results: Exercise resulted in significant increases in measured aerosol concentration in proportion to exercise intensity, especially at or above 50% of predicted heart rate reserve and tracked closely with cumulative ventilation during the exercise test protocol.
Interpretation: Vigorous aerobic exercise activity generates significant quantities of aerosols that may create a high risk for airborne viral transmission. Results can guide recommendations for safety of exercise testing and other indoor exercise activities.
Acknowledgments
Author contributions: The guarantor for all content of this manuscript is T. Allison. P. Sajgalik, lead author, had full access to all data in the study and contributed to the study design, data collection and interpretation, and writing of manuscript. A. Garzona-Navas had full access to all data and contributed to data collection and interpretation and writing of manuscript. I. Csécs contributed literature search and a review of manuscript. J. Askew contributed to the study design and review of manuscript. F. Lopez-Jimenez contributed to the study design and review of manuscript. A. Niven contributed to data collection and interpretation. B. Johnson provided laboratory space and equipment and contributed substantially to study design, data collection, and review of manuscript. T. Allison, corresponding author, had full access to all data and contributed to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors acknowledge the contribution of the Department of Engineering at Mayo Clinic, Rochester for their technical support and advice in developing the aerosol laboratory. Contributing individuals include Thomas G. Halvorsen, Steven W. Steele, Matthew E. Hainy, and Susheil Uthamaraj; also and Lukas S. Johnson, Facilities. We also acknowledge technical and administrative contributions from members of the Extreme Physiology Laboratory under the direction of Bruce D. Johnson, PhD, including Alex R. Carlson, Chul-Ho Kim, PhD, Brad Cierzan, Jessi Johnston, and Briana L. Ziegler. We thank Anwesh Babu Poosala, MBBS, for valuable assistance with literature review. Finally, we acknowledge the statistical advice of Kent R. Bailey, PhD, Mayo Clinic Department of Health Sciences Research, and critical comments on final presentation of data by Ray W. Squires, PhD, Department of Cardiovascular Medicine.
Footnotes
FUNDING/SUPPORT: Support for this work was provided by the Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN as part of the effort to ensure patient and staff safety in the face of COVID-19 concerns.
Supplementary Data
References
- 1.Health WHO, Programme E, Panel EA, et al Transmission of SARS-CoV-2 : implications for infection prevention precautions. 2020;(July):1-10. Accessed March 19, 2021. https://www.who.int/news room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Moulson N., Bewick D., Selway T. Cardiac rehabilitation during the COVID-19 era: guidance on implementing virtual care. Can J Cardiol. 2020;36(8):1317–1321. doi: 10.1016/j.cjca.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faghy M.A., Sylvester K.P., Cooper B.G., Hull J.H. Cardiopulmonary exercise testing in the COVID-19 endemic phase. Br J Anaesth. 2020;125(4):447–449. doi: 10.1016/j.bja.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinds WiC. 2nd edition. Wiley-Interscience; 1999. Aerosol technology: properties, behavior, and measurement of airborne particles. [Google Scholar]
- 6.Bake B., Larsson P., Ljungkvist G., Lungström E. Exhaled particles and small airways. Respir Res. 2019;20:8. doi: 10.1186/s12931-019-0970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabian P., McDevitt J.J., DeHaan W.H. Influenza virus in human exhaled breath: An observational study. PLoS One. 2008;3(7) doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu C., Nielsen P., Liu L., Jensen R.L., Gong G. Human exhalation characterization with the aid of schlieren imaging technique. Build Environ. 2017;112:190–199. doi: 10.1016/j.buildenv.2016.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C., Nielsen P., Liu L., Jensen R.L., Gong G. Measuring the exhaled breath of a manikin and human subjects. Indoor Air. 2015;25(2):188–197. doi: 10.1111/ina.12129. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Feng G., Bi Y., Cai, Zhang Z., Cao G. Distribution of droplet aerosols generated by mouth coughing and nose breathing in an air-conditioned room. Sustainable Cities and Society. 2019;51:101721. [Google Scholar]
- 11.Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19(1):101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 13.Lendacki F.R., Teran R.A., Gretsch S., Fricchione M.J., Kerins J.L. COVID-19 outbreak among attendees of an exercise facility-Chicago, IL, August-September 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):321–325. doi: 10.15585/mmwr.mm7009e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groves L.M., Usagawa L., Elm J. Community transmission of SARS-CoV-2 at three fitness facilities-Hawaii, June-July 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):316–320. doi: 10.15585/mmwr.mm7009e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang S., Han S.H., Rhee J.Y. Cluster of coronavirus disease associated with fitness dance classes, South Korea. Emerg Infect Dis. 2020;26(8):1917–1920. doi: 10.3201/eid2608.200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atrubin D., Wiese M., Bohinc B. An outbreak of COVID-19 associated with a recreational hockey game-Florida, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(41):1492–1493. doi: 10.15585/mmwr.mm6941a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel M., Kloppenburg B., Woerle S., Sjoblom S., Danyluk G. Notes from the field: SARS-CoV-2 transmission associated with high school football team members - Florida, September-October 2020. MMWR Morb Mortal Wkly Rep. 2021;70(11):402–404. doi: 10.15585/mmwr.mm7011a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourouiba L., Dehandschoewercker E., Bush J.W.M. Violent expiratory events: on coughing and sneezing. J Fluid Mech. 2014;745:537–563. [Google Scholar]
- 19.Helgeson S.A., Lee A.S., Patel N.M., Taylor B.J., Lim K.G., Niven A.S. Cardiopulmonary exercise and the risk of aerosol generation while wearing a surgical mask. Chest. 2021;159(4):1567–1569. doi: 10.1016/j.chest.2020.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klompas M., Baker M.A., Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324(5):441–442. doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- 21.Nardell E.A., Nathavitharana R.R. Airborne spread of SARS-CoV-2 and a potential role for air disinfection. JAMA. 2020;324(2):141–142. doi: 10.1001/jama.2020.7603. [DOI] [PubMed] [Google Scholar]
- 22.Zayas G., Chiang M.C., Wong E. Cough aerosol in healthy participants: Fundamental knowledge to optimize droplet-spread infectious respiratory disease management. BMC Pulm Med. 2012;12 doi: 10.1186/1471-2466-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han Z.Y., Weng W.G., Huang Q.Y. Characterizations of particle size distribution of the droplets exhaled by sneeze. J R Soc Interface. 2013;10(88):20130560. doi: 10.1098/rsif.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inouye S. SARS transmission: language and droplet production. Lancet. 2003;363(9378):170. doi: 10.1016/S0140-6736(03)13874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hui D.S., Chow B.K., Chu L. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0050845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson G.R., Morawska L., Ristovski Z.D. Modality of human expired aerosol size distributions. J Aerosol Sci. 2011;42:839–851. [Google Scholar]
- 27.Hu H., Li T., Chen X. The concentration distribution of exposures to particulate air pollution on different road sections. Transportation Research Procedia. 2017;25:3343–3353. [Google Scholar]
- 28.Mao H.L., He H.-Di. Distribution characteristics of particulate matter pollution in Shanghai Huangpu River ferry. J Dalian Marit Museum. 2019;45(2):113–122. [Google Scholar]
- 29.Wallace L.A., Wheeler A.J., Kearney J. Validation of continuous particle monitors for personal, indoor, and outdoor exposures. J Expo Sci Environ Epidemiol. 2011;21(1):49–64. doi: 10.1038/jes.2010.15. [DOI] [PubMed] [Google Scholar]
- 30.Wardoyo A.Y.P., Budianto A., Abdurrouf Filtration of submicron particles from motorcycle emission using a DC low electrostatic filter. Int J Appl Eng Res. 2017;12(8):1725–1728. [Google Scholar]
- 31.Wardoyo A.Y.P., Juswono U.P., Noor J.A.E. A study of the correlation between ultrafine particle emissions in motorcycle smoke and mice erythrocyte damages. Exp Toxicol Pathol. 2017;69(8):649–655. doi: 10.1016/j.etp.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Fluke 985. Airborne particle counter users manual. Fluke Corporation; 2012. [Google Scholar]
- 33.TSI . TSI Inc; 2012. P-trak Ultrafine Particle Counter Model 8525 operation manual. [Google Scholar]
- 34.Liu Y., Chen Y., Guo M. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 35.Loudon R.G., Roberts R.M. Droplet expulsion from the respiratory tract. Am Rev Respir Dis. 1967;95(3):435–442. doi: 10.1164/arrd.1967.95.3.435. [DOI] [PubMed] [Google Scholar]
- 36.Papineni R.S., Rosenthal F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10(2):105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 37.Johnson B.D., Weisman I.M., Zeballos R.J., Beck K.C. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest. 1999;116(2):488–503. doi: 10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- 38.Doggett N., Chow C.W., Mubareka S. Characterization of experimental and clinical bioaerosol generation during potential aerosol-generating procedures. Chest. 2020;158(6):2467–2473. doi: 10.1016/j.chest.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woolard E.W., Einstein A., Furth R., Cowper A.D. Investigations on the theory of the Brownian movement. Am Math Mon. 1928;35(6):318–320. [Google Scholar]
- 40.Einstein A. The elementary theory of the Brownian motion. Zeit für Elektrochemie. 1908;14:235–239. [Google Scholar]
- 41.Galvani A.P., May R.M. Epidemiology: dimensions of superspreading. Nature. 2005;438(7066):293–295. doi: 10.1038/438293a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asadi S., Wexler A.S., Cappa C.D., Barreda S., Bouvier N.M., Ristenpart W.D. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep. 2019;9(1):2348. doi: 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. Accessed March 19, 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 44.Jackson T., Deibert D., Wyatt G. Classification of aerosol-generating procedures: a rapid systematic review. BMJ Open Respir Res. 2020;7(1) doi: 10.1136/bmjresp-2020-000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hui D.S., Chan M.T., Chow B. Aerosol dispersion during various respiratory therapies: a risk assessment model of nosocomial infection to health care workers. Hong Kong Med J. 2014;20(suppl4):9–13. [PubMed] [Google Scholar]
- 46.Brown J., Gregson F.K.A., Shrimpton A. A quantitative evaluation of aerosol generation during tracheal intubation and extubation. Anaesthesia. 2021;76(2):174–181. doi: 10.1111/anae.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao C.Y.H., Wan M.P., Morawska L. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J Aerosol Sci. 2009;40(2):122–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.