Abstract
Introduction
Osteosarcoma of the maxilla is recorded as the least common of all bone malignancies. It exhibits a clinical behavior and natural history distinct from their counterparts of the trunk and extremities. Transformation from a chronic pyogenic abscess of the maxilla is even more unusual.
Case summary
A 70 year old lady presented to our hospital with a hard, fixed and tender bony swelling in her left cheek. She had initially presented to a different hospital with a similar presentation which was excised after imaging and post excision was found to be a chronic pyogenic abscess. The swelling reappeared within one year and on re-excision was found to be a low grade paraosteal osteosarcoma of the hard palate. CECT and PET-CT work-up at our hospital showed a left maxillary sinus growth with prominent neck lymph nodes along with mediastinal lymphadenopathy and pulmonary metastasis. Final histopathology revealed ulcerated stratified squamous epithelium mucosa overlying a lesion suggestive of osteosarcoma.
Discussion
Complete surgical excision with negative margins continues to be the mainstay of treatment, but osteosarcomas of maxillofacial region pose difficulties in obtaining tumour free margins because of their complex anatomy around the cranium. Surgery may be complemented by radiotherapy with or without chemotherapy. Small size of the tumour and low-grade histology have been assumed to reflect a better prognosis.
Conclusion
Osteosarcoma of maxillofacial region has variable appearance clinically as well as radiologically posing a diagnostic challenge for clinicians. Any chronic abscess or recurrent cheek swelling thus necessitates further suspicion and requires a full work-up to rule out this high risk malignancy.
Keywords: Osteosarcoma, Maxilla, Chronic, Pyogenic abscess, Case report
Highlights
-
•
Osteosarcoma of the maxilla is recorded as the least common of all bone malignancies with transformation from a chronic pyogenic abscess of the maxilla being even more unusual.
-
•
Complete surgical excision with negative margins is the mainstay of treatment for better prognosis complemented with or without adjuvant radiotherapy.
-
•
Any chronic abscess or recurrent cheek swelling necessitates further suspicion and requires a full work-up to rule out this high risk malignancy.
1. Introduction
Osteosarcoma of the maxilla is reported as the least common of all bone malignancies. Transformation from a chronic pyogenic abscess of the maxilla is even more unusual. Osteosarcomas of the head and neck region are rare exhibiting a clinical behavior and natural history distinct from their counterparts of the trunk and extremities [1], [2]. It is seen mostly in the third and fourth decade (i.e. a decade later than mean age of osteosarcoma incidence in long bones) with male predominance [3]. The literature mentions the mandible as a more likely location than the maxilla in Head & neck region. Swelling is the dominant complaint in osteosarcoma of jaw bones. Other features such as tooth mobility, and paraesthesia may be present with pain, fever, or weight loss [4]. The sites of these tumours impose difficulty for resection and reconstruction than long bone tumours. Osteosarcoma of the jaw is challenging in both diagnosis and management due to the high incidence of inconclusive histopathology reports, absence of specific radiological features and the difficulty in proper resection due to proximity to the vital structures [5], [6]. We herein present a case which was initially diagnosed as chronic pyogenic abscess of maxilla but later transformed into osteosarcoma of the maxilla. Osteosarcoma shows high metastatic rate and can be very aggressive. This case has been reported in line with the SCARE criteria [7].
2. Case summary
A 70 year old lady of Indian ethnicity presented to our hospital with a hard, fixed, tender and painful bony swelling with destruction of the left maxilla, orbital floor and nasal cavity [Fig. 1]. She had initially presented to a different hospital in 2018 with a swelling in her cheek for 6 months which was insidious in onset and gradually progressive. The swelling was associated with intermittent dull aching pain. On examination, she was found to have an ulcero- proliferative lesion in the left upper alveolus of the oral cavity extending from the incisor to left upper premolar tooth. No palpable cervical lymph nodes were present. On investigations, CT scan of face revealed a well-defined dense lobulated sclerotic lesion involving the left upper premolar and 1st molar upper alveolar process and bony protuberance along the gingival region. Biopsy showed inflammatory exudates with reactive new bone formation in left maxillary region suggestive of a chronic abscess. Wide local excision of the hard palatal mass and upper alveolectomy was carried out. Intra-operatively, an ulcero-proliferative lesion in the left upper alveolus of the oral cavity was found extending from incisor to left upper molar, medially extending just short of palate in midline and laterally till gingiva. Maxillary sinus was inspected via oro-antral fistula with endoscope and it was free of any tumour [Fig. 2A]. Biopsy of the surgical specimen was found to be a chronic pyogenic abscess of the maxilla involving the hard palate with marked nodular new bone formation. Post-Operative period was uneventful.
Fig. 1.
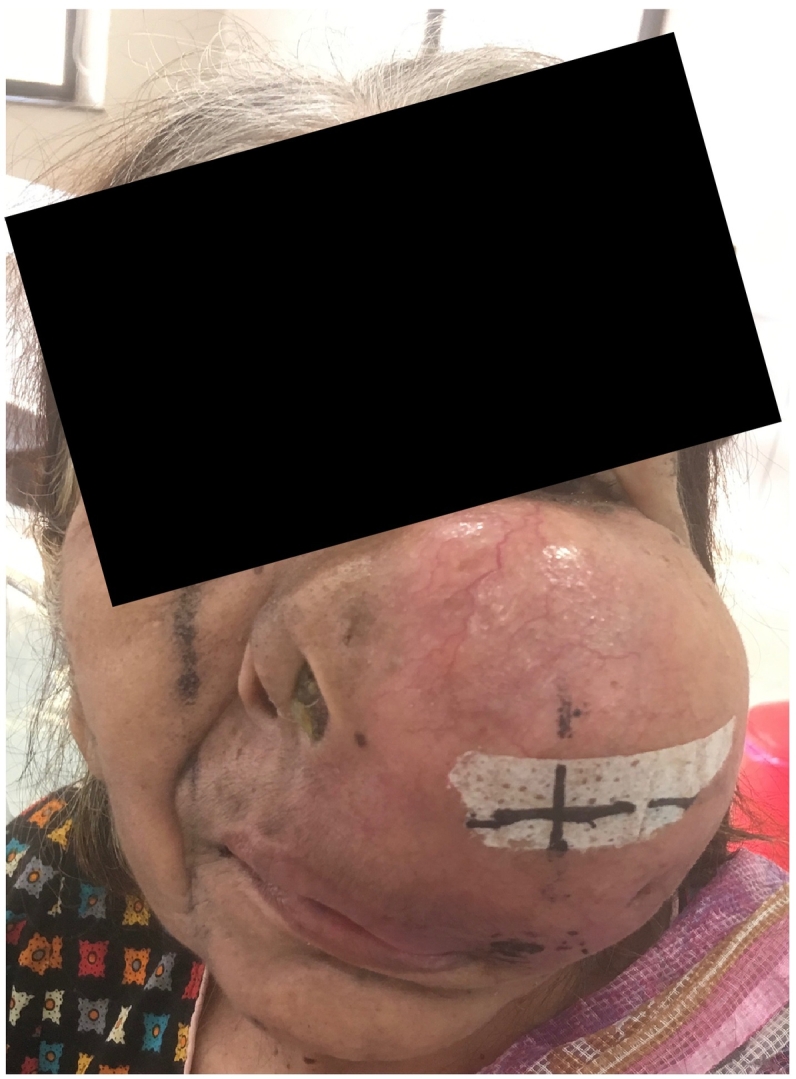
Patient presenting with hard, fixed, tender and painful bony swelling with destruction of the left maxilla, orbital floor and nasal cavity.
Fig. 2.
A- Previous CT scan of face and neck after excision of the chronic pyogenic abscess.
B- Current CT scans of face and neck showing mixed dense lesion in the left maxillary sinus with prominent lymph nodes on left side of neck.
The patient again presented with a recurrent swelling at the same site one year later which on local examination revealed an ulcero-proliferative growth in the left hard palate not crossing the midline. CT neck showed a well-defined bony dense lesion involving the left premolar and 1st molar alveolar process with bony protuberance along the left gingival region. Pre-operative biopsy of the left side of hard palate showed epithelial hyperplasia with focal intra- epithelial inflammation and no malignancy. Features were suggestive of a benign osseous, osteocartilaginous or infectious lesion including sclerosing osteomyelitis. Total re-excision of the mass was done. Final histopathology and biopsy of the operative specimen was consistent with low grade paraosteal osteosarcoma of the left sided hard palate. Two months later patient noticed a tingling sensation of the left cheek associated with episodes of shooting pain in the same region and after another month there was a small recurrence of the swelling at the same site. CT scan of face showed a well-defined soft tissue density lesion with osteoid matrix within the medial wall and palatine process of the left maxilla.
Patient's performance score was ECOG = 4 with inability to eat and breathe properly and the peripheral vision was affected on the left side. CT of face and neck showed a large 7.2 cm × 10.1 cm × 7.1 cm heterogenous mixed dense lesion in the left maxillary sinus with prominent lymph nodes on left side of neck [Figs. 2B, 4]. CT thorax showed few mediastinal lymphadenopathy and calcified nodular lesions in both lungs [Fig. 3]. Whole body PET-CT scan revealed FDG avid soft tissue density mass with osteoid matrix epicentre in left maxillary sinus with lung metastasis. Histopathology revealed ulcerated mucosa with stratified squamous epithelium overlying a lesion suggestive of osteosarcoma. Immuno-histochemistry was negative for Vimentin and S100. Her haemoglobin was low (6.5 g/dL) and she underwent resuscitation and blood transfusion followed by symptomatic care. Serum alkaline phosphatase levels were high. She subsequently underwent feeding gastrostomy and was discharged in a hemodynamically stable condition with an advice of follow up with palliative care department. Patient presented to us for further care. Case was reviewed by the Multi-disciplinary tumour board and patient was offered palliative chemotherapy with Doxorubicin, Gemcitabine, Pazopanib and Celecoxib regimen.
Fig. 4.

CT scan of face and neck with 3D reconstruction showing extension to the orbit, destruction of the septum with extension to the opposite side and involvement of the skull base can also be noted.
Fig. 3.
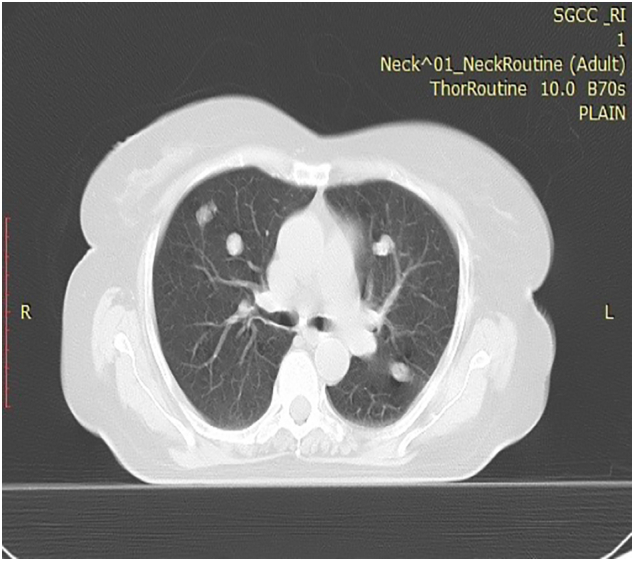
HRCT thorax showing few mediastinal lymphadenopathy and calcified nodular lesions in both lungs.
3. Discussion
Osteosarcomas are highly malignant and rare bone tumours most commonly affecting the long bones. Craniofacial osteosarcomas account for only 1% of all head and neck malignancies [8], [9]. In general, osteosarcoma of the jaws tends to occur later in life than that of the long bones [10]. Osteosarcoma is an osteoid-producing tumour where the identification of anaplastic stromal cells producing osteoid aids in the histological diagnosis. They can be further classified based on their cellular differentiation as osteoblastic, chondroblastic and fibroblastic variants [2]. The exact etiology of osteosarcomas is still largely unknown; however, some predisposing factors are implicated in its development. Although rare, transformation from a chronic pyogenic abscess or chronic osteomyelitis is possible as seen in our case. Others may include prior exposure to radiation, pre-existing Paget's disease of bone, fibrous dysplasia, multiple, trauma or myositis [11].
Presenting signs and symptoms of craniofacial osteosarcomas primarily include regional swelling. Pain and paraesthesia, change in tooth position, loose tooth or change in fit of dental prosthesis are other possible presenting symptoms. Many of these signs and symptoms are non-specific and can be produced by a number of different developmental, infectious, benign neoplastic lesions or malignancies. Thus an osteosarcoma of maxillofacial region often goes undiagnosed for a significant period of time [4], [5]. The diagnosis of these tumours is sometimes difficult also due to their rarity [6].
.Diagnostic modalities include radiological imaging followed by biopsy and histopathological diagnosis. The combination of both CT and MRI improves the diagnostic accuracy for patients suffering from osteosarcoma [12]. Osteosarcomas of the Head and neck region primarily exhibit osteolysis or osteoblastic destruction with an irregular tumour margin on CT imaging. The mixed and sclerotic radiological pattern in the head and neck region is highly suggestive of osteosarcoma, with differential diagnoses of metastasis, lymphoma, and chondrosarcoma. The primary features are local or patchy high-density shadows in the medullary cavity with varying degrees of bone destruction and matrix mineralization [13]. The CT findings of osteosarcoma of maxilla vary from predominantly radiolucent, poorly delineated lesions to dense radiopaque masses with sunburst appearance. Extension to the orbit, destruction of the septum with extension to the opposite side and involvement of the skull base can also be noted [Fig. 4], [8], [14]. MRI is widely accepted as the imaging method of choice for the evaluation of the extent of primary lesions and their relationship with anatomic structures. MRI depicts soft tissues and bone marrow infiltration (medulla) better than CT imaging, showing cortical destruction and expansive masses [13]. Similar findings were present in our case. In addition a PET CT scan was done to rule out distant metastases in the presence of such extensive recurrent tumour.
Biopsy of the lesion may be diagnostic with histopathological features consistent with osteosarcoma. However, variants including osteoblastic and chondroblastic osteosarcomas as well as heterogenous appearances may make histopathological diagnosis difficult [6]. Immuno-histochemistry helps in differentiating chondroblastic osteosarcoma from chondrosarcoma as it is positive for Vimentin, Epithelial Membrane antigen, S100 and rarely positive for Cytokeratin whereas chondrosarcoma is positive only for Vimentin and S100. In chondroblastic osteosarcoma, the presence of osteoid distinguishes it from chondrosarcoma. Raised serum alkaline phosphatase in osteosarcoma also distinguishes it from chondrosarcoma [15], [16].
The management of head and neck osteosarcomas comprises of a multi-disciplinary approach. The treatment of choice in oral osteosarcomas is surgical resection [17], [18]. Complete surgical excision with negative margins continues to be the mainstay of treatment, but osteosarcomas of maxillofacial region pose difficulties in obtaining tumour-free margins because of their complex anatomy and close proximity to the cranium. Surgery may be complemented by radiotherapy with or without chemotherapy. Adjuvant postoperative radiotherapy is indicated for those with close or positive margins. The use of chemotherapy before and after surgery promotes local control by size reduction [19]. The role of induction or adjuvant chemotherapy remains uncertain and debatable with no prospective studies having examined these strategies [6], [20]. Currently, doxorubicin, cisplatin, methotrexate with Leukovorin and Ifosfamide are considered the most active agents against osteosarcoma. The optimum time for commencing chemotherapy is within 21 days of surgery. Overall, prognosis in OS is 25%-50% 5 year survival rate [21].
.Prognostic factors affecting the survival outcomes has been noted as adverse for tumours more than 6 cm in size, age greater than 60 years, a non-mandibular tumour location, osteoblastic histological type, advanced disease stage, non-surgical initial therapy and a positive margin of resection. Small size of the tumour and low-grade histology have been assumed to reflect a better prognosis. Among all the factors, surgical margin status seemed to have the most vital impact on the survival outcomes [22]. In the literature, soft tissue extensions are found to be an adverse prognostic factor [23].
Local recurrences predominate in osteosarcoma of the head and neck with a reported incidence of 17–70% compared with 5–7% in extremity osteosarcoma. On the contrary, distant metastases are observed less often than with the more common osteosarcomas arising in the long bones [2], [18]. In our patient distant metastases to the lungs were found.
4. Conclusion
Osteosarcoma of maxillofacial region has variable appearances clinically as well as radiologically posing a diagnostic challenge for clinicians. Due to the life-threatening nature of osteosarcoma of the jaw bones, particularly the maxilla, any chronic abscess or recurrent cheek swelling therefore necessitates further suspicion and requires a full work-up to rule out this high risk malignancy. The optimal treatment is surgery which entails a wide excision with microscopically negative margins. Adjuvant external beam radiation therapy should be considered for patients with close or positive margins and other adverse prognostic factors. The role of neo-adjuvant chemotherapy is ill-defined and is evolving.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sources of funding
None.
Ethical approval
Exempted from Institutional Ethics Committee of Saroj Gupta Cancer Centre and Research Institute, Kolkata, India.
Informed consent from patient
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Dr Arnab Gupta- study concept
Dr Baijaeek Sain- study design, data collection, analysis and Interpretation
Dr Radha Raman Mondal-analysis and interpretation
Dr Samir Bhattacharyya- conceptualisation
Dr Saradindu Ghosh- Conceptualisation
Dr Aruni Ghose- review of literature
Research registration
None.
Guarantor
Dr. Arnab Gupta, Dr. Baijaeek Sain, Dr. Saradindu Ghosh.
Declaration of competing interest
None.
Acknowledgments
Acknowledgement
Dept. of Surgical Oncology, Saroj Gupta Cancer Centre and Research Institute, Kolkata, India.
References
- 1.Nissanka E., Amaratunge E., Tilakaratne W. Clinicopathological analysis of osteosarcoma of jaw bones. Oral Dis. 2007;13:82–87. doi: 10.1111/j.1601-0825.2006.01251.x. [DOI] [PubMed] [Google Scholar]
- 2.Krishnamurthy A., Palaniappan R. Osteosarcomas of the head and neck region: a case series with a review of literature. J. Maxillofac. Oral Surg. 2018;17(1):38–43. doi: 10.1007/s12663-017-1017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Institute Bethesda, MD. SEER cancer stat facts: bone and joint cancer. 2017. http://seer.cancer.gov/statfacts/html/bones.html
- 4.Jasnau S., Meyer U., Potratz J., Jundt G., Kevric M., Joos U.K. Craniofacial osteosarcoma experience of the cooperative German–Austrian–Swiss osteosarcoma study group. Oral Oncol. 2008;44:286–294. doi: 10.1016/j.oraloncology.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Marx R.E., Stern D. Oral & Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Quintessence, Illinois. 2003. Malignant neoplasms of bone; pp. 799–828. [Google Scholar]
- 6.Rosenthal M.A., Mougos S., Wiesenfeld D. High-grade maxillofacial osteosarcoma: evolving strategies for a curable cancer. Oral Oncol. 2003;39:402–404. doi: 10.1016/s1368-8375(02)00147-1. [DOI] [PubMed] [Google Scholar]
- 7.for the SCARE Group. Agha R.A., Franchi T., Sohrabi C., Mathew G. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Prasad K., Dexith J., Lalitha R.M. Maxillary osteosarcoma masquerading as chondromyxoid fibroma: report of a case. J. Maxillofac. Oral Surg. 2015;14(Suppl 1):87–92. doi: 10.1007/s12663-012-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiele O.C., Frier K., Bacon C., Egerer G., Hofele C.M. Interdisciplinary combined treatment of craniofacial osteosarcoma with neoadjuvant and adjuvant chemotherapy and excision of the tumour: a retrospective study. Br. J. Oral Maxillofac. Surg. 2008;46:533–536. doi: 10.1016/j.bjoms.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Godoy R.L.M.R., Garcia A.M., Taylor A.M., Salazar J.D.G. Well-differentiated intraosseous osteosarcoma of the jaws: experience of two cases from the Institute Nacional de Cancerologia, Mexico. Oral Oncol. 1999;35:530–533. doi: 10.1016/s1368-8375(99)00005-6. [DOI] [PubMed] [Google Scholar]
- 11.Dickens P., Wei W.I., Sham J.S.T. Osteosarcoma of the maxilla in Hong Kong. Chinese postirradiation for nasopharyngeal carcinoma. Cancer. 1990;66:1924–1926. doi: 10.1002/1097-0142(19901101)66:9<1924::aid-cncr2820660912>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang S., Shi H., Yu Q. Osteosarcoma of the jaws: demographic and CT imaging features. Dentomaxillofac. Radiol. 2012;41:37–42. doi: 10.1259/dmfr/86834844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo Z., Chen W., Shen X. Head and neck osteosarcoma: CT and MR imaging features. Dentomaxillofac. Radiol. 2020;49(2) doi: 10.1259/dmfr.20190202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panda N.K., Jain A., Reddy C.E.E. Osteosarcoma and chondrosarcoma of the maxilla. Br. J. Oral Maxillofac. Surg. 2003;41:329–333. doi: 10.1016/s0266-4356(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa T., Hirose T., Kudo E., Hiwawa K., Usui M., Ishii S. Lmmunophenotypic heterogeneity in osteosarcomas. Hum. Pathol. 1991;22(6):583–590. doi: 10.1016/0046-8177(91)90236-i. [DOI] [PubMed] [Google Scholar]
- 16.Bielack S., Carrle D., Casali P.G. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009;20(Suppl 4):137–139. doi: 10.1093/annonc/mdp154. [DOI] [PubMed] [Google Scholar]
- 17.Caron A.S., Hajdu S.I., Strong E.W. Osteogenic sarcoma of the facial and cranial bones. Am. J. Surg. 1971;122:719–725. doi: 10.1016/0002-9610(71)90434-x. [DOI] [PubMed] [Google Scholar]
- 18.Kassir R.R., Rassekh C.H., Kinsella J.B., Segas J., Carrau R.L., Hokanson J.A. Osteosarcoma of the head and neck: meta-analysis of nonrandomized studies. Laryngoscope. 1997;107:56–61. doi: 10.1097/00005537-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Smeele B.L.E., Kostense P.J., Van Der Waal I., Snow G.B. Effect of chemotherapy on survival of craniofacial osteosarcoma: a systematic review of 201 patients. J. Clin. Oncol. 1997;15(1):363–367. doi: 10.1200/JCO.1997.15.1.363. [DOI] [PubMed] [Google Scholar]
- 20.Mendenhall W.M., Fernandes R., Werning J.W., Vaysberg M., Malyapa R.S., Mendenhall N.P. Head and neck osteosarcoma. Am. J. Otolaryngol. 2011;32(6):597–600. doi: 10.1016/j.amjoto.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Mamachan P., Dang V., Bharadwaj N.S., DeSilva N., Kant P. Chondroblastic osteosarcoma-a case report and review of literature. Clin. Case Rep. 2019;8(11):2097–2102. doi: 10.1002/ccr3.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y., Shen Q., Gokavarapu S., Lin C., Yahiya Cao W., Chauhan S., Liu Z., Ji T., Tian Z. Osteosarcoma of head and neck: a retrospective study on prognostic factors from a single institute database. Oral Oncol. 2016;58:1–7. doi: 10.1016/j.oraloncology.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Granados-Garcia M., Luna-Ortiz K., Castillo-Oliva H.A. Free osseous and soft tissue surgical margins as prognostic factors in mandibular osteosarcoma. Oral Oncol. 2006;42:172–176. doi: 10.1016/j.oraloncology.2005.06.027. [DOI] [PubMed] [Google Scholar]



