Abstract
Introduction
Incidentally found congenital pulmonary airway malformations (CPAM) in older children are extremely rare and have traditionally been managed with minimally invasive versus open lobectomy of the affected lobe.
Presentation of case
In this report, we present a 11-year-old male who presented with a recurrent spontaneous pneumothorax and was found to have a large symptomatic CPAM confined to a single segment of the right lower lobe. The patient was successfully treated with thoracoscopic segmentectomy without any residual disease seen on follow up imaging.
Discussion
Minimally invasive thoracoscopic approach has many advantages over open approach including better pain control, reduced hospital length of stay, and decreased intraoperative blood loss. With increasing use of minimally invasive approaches, lung-sparing surgery has demonstrated to be a viable and an attractive option for definitive resection of CPAM, without compromising resection margins and/or future lung function.
Conclusion
This report demonstrates that minimally invasive lung-sparing surgical treatment of a large CPAM is feasible in older children.
Keywords: Segmentectomy, CPAM, Thoracoscopy, Case report
Abbreviations: CPAM, congenital pulmonary airway malformations; CCAM, congenital cystic adenomatoid malformation; VATS, video-assisted thoracoscopic surgery; PPB, pleuropulmonary blastoma; CXR, chest X-ray; ICS, intercostal space; Fr, French; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; CT, computerized tomography
Highlights
-
•
Older children with CPAM can present with a spontaneous pneumothorax.
-
•
CPAM confined to a segment of a single lobe should be managed with segmentectomy.
-
•
VATS segmentectomy for CPAM in older children can be successful.
1. Introduction
Congenital pulmonary airway malformations (CPAM), previously known as congenital cystic adenomatoid malformations (CCAM), are very rare lung anomalies with an incidence rate of 1/35000 births. This condition more commonly affect males, and is frequently diagnosed antenatally on routine ultrasound (US) [1]. CPAM lesions can be both asymptomatic and symptomatic. Majority are asymptomatic; however, if the child become symptomatic, they typically include recurrent infections and/or respiratory distress [2].
Surgery remains the cornerstone of treatment in both symptomatic and asymptomatic CPAM cases, due to patients being at an increased risk of recurrent infections and/or malignant transformation [1]. Resection of these lesions have traditionally been performed through an open approach; however, video-assisted thoracoscopic surgical (VATS) approach has now gained more popularity amongst pediatric surgeons [1]. Current controversy remains on whether to remove the entire lobe or, perhaps, to use a lung-sparing approach: segmentectomy. In some series, a non-anatomic resection of small lesions has been the predominant experience [3]. In this case report, we describe an older child with a previously undiagnosed large CPAM, who underwent anatomic thoracoscopic segmentectomy at an academic tertiary care center. This report has been organized in line with the SCARE guidelines [4].
2. Presentation of case
A 11-year-old male, without any significant medical or surgical history, presented with one-day history of right-sided chest pain that woke him up. The patient did not have any relevant drug or family history. Due to progressive worsening of pain, in addition to increased work of breathing, the child was brought in to the emergency room, where a large right-sided spontaneous tension pneumothorax was noted on the chest x-ray (CXR) (Fig. 1A).
Fig. 1.
CXR demonstrating a spontaneous right-sided pneumothorax A (left) at the initial presentation and B (right) during the recurrent presentation.
An 8 French (Fr) pigtail chest tube was inserted in the emergency room. Post-placement CXR demonstrated a decreased right-sided pneumothorax without midline shift. Serial CXRs were obtained until his pneumothorax resolved. Due to a prolonged air leak, his chest tube was not removed until hospital day four, and his post-pull CXR was without any evidence of a pneumothorax or underlying CPAM.
The patient represented two days after discharge with recurrent symptoms, including dyspnea and right-sided chest pain. A CXR demonstrated a recurrent tension pneumothorax with mediastinal shift (Fig. 1B). A 14 Fr chest tube was inserted at this time with demonstration of lung re-expansion. Due to the recurrent nature of the “spontaneous” pneumothorax, VATS blebectomy and pleurodesis was recommended. Upon initial thoracoscopic inspection, a series of multiple large bullae in the right lower lobe (RLL), with collapse of right upper lobe (RUL) and right middle lobe (RML), were revealed. A clinical diagnosis of CPAM was made and the procedure was aborted to map out the extent of the disease radiographically.
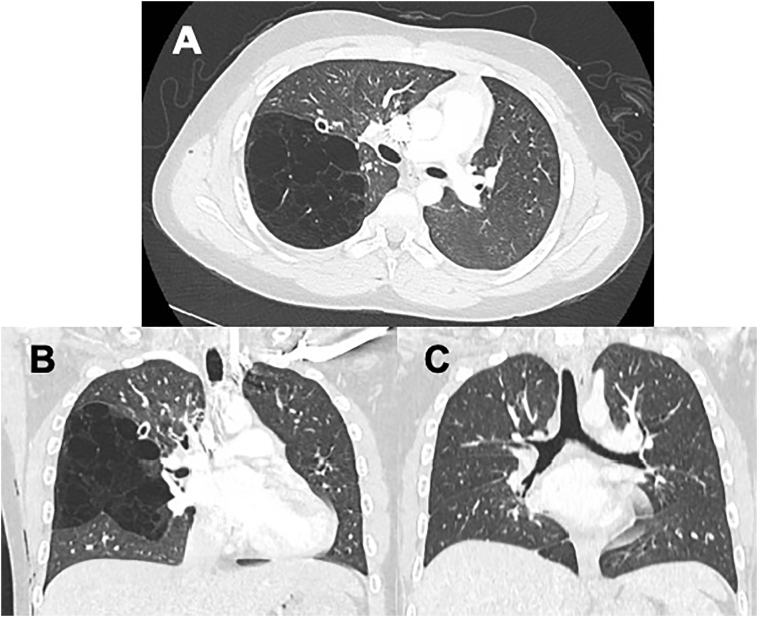
Computerized tomography (CT) scan revealed a large CPAM occupying the superior segment of RLL (Fig. 2A and B).
Fig. 2.
Cystic disease of RLL. A (Top). Axial cross-sectional chest imaging demonstrating cystic disease confined to the right lower lobe. B (Bottom left). Coronal section demonstrating cystic disease of the superior segment of RLL. C (Bottom right). Follow up CT scan after resection of CPAM demonstrating no residual disease.
The decision was made to perform a lung-sparing resection via thoracoscopic approach to preserve suspected normal lung tissue. The patient was taken to the operating room and was positioned in left lateral decubitus with double-lumen endotracheal tube to assist in single-lung ventilation. Two 5-mm trocars were placed through the sixth intercostal space (ICS) in the anterior axillary line and the eighth ICS along the midclavicular line. A 12-mm trocar was placed through the fourth ICS in mid clavicular line as well.
Diagnostic thoracoscopy confirmed the CPAM location, and an accessory fissure was noted connecting to the major fissure. The large cysts of the CPAM were decompressed with multiple applications of the 5-mm vessel sealing device. The fissure was approached and opened with multiple applications of the vessel sealing device. A clamp was passed lateral to the interlobar artery towards the posterior hilum and a vessel loop was used to keep the tract open. A powered 45 mm stapler with a blue load was advanced into position, and the fissure was transected. The inferior pulmonary ligament was opened, and the inferior pulmonary vein was dissected, allowing visualization of the superior segmental branch. The superior segment of the right lower lobe was then lifted, confirming that there were no definite superior segmental bronchial or arterial branches identified. This was later re-confirmed by histopathological examination. At this point, the superior segmental draining vein was divided with a vascular load of the stapler described above.
Two-lung ventilation was re-established, and the right lung was reinflated under direct visualization. This confirmed ventilation of not only the right upper and middle lobes, but also of all basilar segments of the right lower lobe. Close inspection also noted that there was no additional cystic lung disease. The specimen was placed into an endo-catch bag and the entire bag was removed through the 12-mm trocar site. The procedure was performed by an experienced attending pediatric surgeon. A chest tube was placed through the 12-mm trocar site and was removed on postoperative day three. A follow up CT scan of the chest, performed on an outpatient basis, was done 45 days after surgery and did not demonstrate any residual disease (Fig. 2C).
Gross features of the resected specimen demonstrated a 16x7x1.5 cm portion of the lung without a clear primary bronchus. After removal of the staple line, a few small structures were presented suggestive of either bronchi or vessels. The largest cyst measured 14 × 7.5 cm. The outer wall of this cyst consisted of fibrotic thickened pleura. The parenchyma around the main cyst revealed multiple smaller cystic structures ranging from 0.1 to 0.8 cm (Fig. 3A).
Fig. 3.
Examination of the resected specimen. A (Left) Gross examination of the resected specimen. External Surface of the removed lung showing a large subpleural cyst (arrow). B (Right) Histopathological examination of the resected specimen demonstrated typical bronchial-like cysts of adenomatoid malformation, lined by bronchial type epithelium (stars). Evaluation of the staple line demonstrated two sets of small bronchi and arteries. Large cysts were also present, not include in this image.
Microscopic examination of the resected specimen revealed a large cyst lined by a thick fibrous membrane. Deeper lung tissue showed multiple small cysts lined by bronchial epithelium, in addition to underlying smooth muscle. There was no evidence of primitive mesenchymal cells, suggestive of pleuropulmonary blastoma. Evaluation of the tissue beneath the staple line did not demonstrate any evidence of a cystic lesion, but did show two sets of small bronchi and arteries (Fig. 3B).
3. Discussion
In the absence of any traumatic events, pneumothoraces are classified as either primary or secondary [5]. Primary spontaneous pneumothoraces (PSP) occur in the adolescent age-group, predominantly males, without any underlying lung diseases. Secondary spontaneous pneumothoraces (SSP) usually occur as a sequela of pre-existing lung disease, including cystic fibrosis and interstitial lung disease [5]. There are currently no universally applied guidelines for management of spontaneous pneumothoraces in children in the United States. Based on the British Thoracic Society guidelines, the management of PSP depend primarily on whether or not the patient is symptomatic and on the size of the pneumothorax. If the size of the pneumothorax is >2 cm with associated shortness of breath, aspiration with a 16- or 18-Gauge needle is recommended [6]. If a decrease in size of the pneumothorax is observed in addition to resolution of symptoms, discharge should be considered. However, if the pneumothorax is persistent despite aspiration, patient is hemodynamically unstable at presentation or there are bilateral pneumothoraces, then insertion of a chest tube is recommended. American College of Chest Physicians (ACCP) practice guidelines similarly recommend observation in the emergency department for clinically stable patients with small pneumothoraces, whereas for those with large pneumothoraces, patients are recommended to have a chest tube or pigtail catheter placed followed by hospitalization [7]. Surgical management should be considered in patients with recurrent pneumothoraces or in cases with persistent air leak (beyond 4 day) [6]. Historically, most surgeons opted to order CT scans prior to operative intervention. However, a study by Lauturi et al. in 2011, demonstrated that chest CT scans were not sensitive in the identification of pleural blebs (sensitivity 36%), which diminished the rationale for its use. This practice has largely been abandoned, with now only about a quarter of pediatric surgeons routinely obtaining one [8].
Despite its rarity, CPAM still accounts for 95% of the congenital cystic lung lesions. They are commonly located in one of the lower lobes of the lungs in 75% of cases, with bilateral and multifocal disease being extremely rare [9]. While the majority of CPAM's are asymptomatic at birth, approximately 25% will develop symptoms during infancy at a median age of 7.5 months. These symptoms mainly consist of pulmonary infection related complications such fever, dyspnea and respiratory distress [2]. Occasionally, CPAM's can be diagnosed later in life as an incidental finding (18.5%), in the setting of a pneumothorax (14.8%), hemoptysis (14.8%) and/or dyspnea (3.7%) [9]. Our patient presented with a right-sided spontaneous tension pneumothorax initially thought to be a result of a primary spontaneous pneumothorax.
The vast majority of patients diagnosed with CPAM undergo surgical resection to avoid the risk of associated infectious complication and the potential risk of malignant transformation. The risk of malignancy and even association with CPAM is not clearly defined. Malignancies potentially associated with CPAM were eluded by Laberge et al. These include bronchioloalveolar carcinoma, pleuropulmonary blastoma (PPB), and rhabdomyosarcoma [10]. Priest et al. by utilizing the pleuropulmonary blastoma (PPB) registry, later demonstrated that the majority of PPB's were associated with pulmonary cysts (66%) and 29% were purely cystic [11]. In this series, some risk factors that were more likely to be associated with a PPB, were the development of a pneumothorax, bilateral lung cysts, multifocality or a family history of a similar lesion. However, this review showed that these entities are not only difficult to distinguish radiologically, but they also have histologically overlapping elements. Priest et al. also proposed that PPB may be a separate histological entity, which is not associated with an underlying CPAM [11]. Others have proposed that CPAM type 4 can evolve into a PPB type 1 by acquiring a DICER-1 mutation [12]. Given the uncertainty of a potentially underlying malignant lesion when evaluating a child with a cystic lung lesion, current efforts have been focused on distinguishing CPAM from PPB both clinically and radiographically. Factors favoring the diagnosis of CPAM are prenatal detection, presence of systemic feeding vessels on imaging, asymptomatic nature, and hyperinflated lungs. PPB is associated with multisegmental or bilateral involvement [13]. Despite this, the ability to differentiate these two entities remains challenging. In one study, PPB was found in 4% of patients who were initially thought to have an asymptomatic CPAM [14]. The American Pediatric surgical Association (APSA) outcomes and evidence-based practice committee performed a systematic review and concluded that in any radiographically identified CPAM lesion, there may be a 4% risk of PPB and an unidentified risk of late malignant degeneration into other epithelial and mesenchymal malignancies [15]. This systematic review also addressed the question of surgical approach. Amongst the 29 manuscripts reviewed, 9 manuscripts included patients who underwent lung sparing resections. These included formal anatomical segmentectomy (as performed in this case), wedge resection and other variations of non-anatomic resections. None of the patients during the study follow up period experienced a malignancy. It is difficult to draw any definitive conclusions from this study as these malignancies tend to present in the 2nd - 3rd decade of life.
The extent of surgical resection in CPAM has always been a controversial topic. Surgical approaches include lung-sparing resections (LSR) (wedge resection and segmentectomy), lobectomy or more extensive resections, with the goal of removing all grossly diseased lung [16]. Segmentectomy offers superior pulmonary function preservation compared to lobectomy, especially if patients require subsequent lung resections [17]. Traditionally, formal lobectomy has been the mainstay of treatment because of the concern for inadequate resection margins, potentially leaving abnormal tissue behind with resultant infection and/or malignant transformation later in life [18]. A systematic review done by Downward et al. evaluated 29 studies, where LSR (both segmental and non-anatomic resection) outcomes were compared to lobectomy in patients who were asymptomatic and with a single lobe lesion. They discovered that patients who underwent LSR had a 9% reoperation rate due to residual disease, bronchopleural fistula or persistent air leak. This very high complication rate, however, may be related to grouping of non-anatomical resections with formal segmentectal resections. No malignancies were noted during follow-up. However, follow up duration was variable [15]. Despite these numbers, formal segmentectomy has more recently gained traction for CPAM, because it was demonstrated not to carry a higher risk of residual disease or recurrence of disease if patients were appropriately selected (small asymptomatic lesions) [19]. Our patient had a large CPAM which was managed by a formal segmentectomy, which to our knowledge, has never been described before. Prospective long-term data is needed to clearly identify patients who would benefit from LSR versus lobectomy, especially in patients with large CPAM's.
If feasible, thoracoscopic approach has several advantages over the traditional open approach: less intraoperative blood loss, less postoperative complications, less pain, shorter duration of chest tube insertion, and shorter length of hospital stay [20]. Fascetti-Leon et al. reported a mean chest tube insertion of 2.8 days in patients who underwent thoracoscopic approach, which matches our experience [19]. Our patient was discharged on postoperative day three, which is consistent with published data.
4. Conclusion
In summary, with appropriate patient selection, a thoracoscopic segmentectomy can be considered a reasonable option for both symptomatic and asymptomatic large CPAM's confined to a segment of a lung lobe, compared to traditional thoracoscopic or open lobectomy.
Consent
Assent was obtained as the patient is a ward of the state. Legal guardian consent could not be obtained, and we made sure to not include any identifying information.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical approval
This is exempt from ethical approval.
Sources of funding
None.
Research registration
N/A.
Guarantor
Faraz A. Khan.
Credit authorship contribution statement
SSP, EPT, AR, FAK provided patient care and contributed to manuscript concept and development. SSP contributed to the data collection, analysis, and interpretation. SSP, MAS contributed to writing the manuscript. SSP, EPT, MAS, AH, AR, FAK contributed to critical reviewing and editing of the manuscript and its revisions.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgements
None.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Leblanc C. Congenital pulmonary airway malformations: state-of-the-art review for pediatrician's use. Eur. J. Pediatr. 2017;176(12):1559–1571. doi: 10.1007/s00431-017-3032-7. [DOI] [PubMed] [Google Scholar]
- 2.Kantor N., Wayne C., Nasr A. Symptom development in originally asymptomatic CPAM diagnosed prenatally: a systematic review. Pediatr. Surg. Int. 2018;34(6):613–620. doi: 10.1007/s00383-018-4264-y. [DOI] [PubMed] [Google Scholar]
- 3.Johnson S.M. Thoracoscopic segmentectomy for treatment of congenital lung malformations. J. Pediatr. Surg. 2011;46(12):2265–2269. doi: 10.1016/j.jpedsurg.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Agha R.A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Sahn S.A., Heffner J.E. Spontaneous pneumothorax. N. Engl. J. Med. 2000;342(12):868–874. doi: 10.1056/NEJM200003233421207. [DOI] [PubMed] [Google Scholar]
- 6.MacDuff A. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65 Suppl 2:ii18–ii31. doi: 10.1136/thx.2010.136986. [DOI] [PubMed] [Google Scholar]
- 7.Baumann M.H. Management of spontaneous pneumothorax: an american College of Chest Physicians Delphi consensus statement. Chest. 2001;119(2):590–602. doi: 10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- 8.Laituri C.A. The utility of computed tomography in the management of patients with spontaneous pneumothorax. J. Pediatr. Surg. 2011;46(8):1523–1525. doi: 10.1016/j.jpedsurg.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Herrero Y. Cystic adenomatoid malformation of the lung presenting in adulthood. Ann. Thorac. Surg. 2005;79(1):326–329. doi: 10.1016/S0003-4975(03)01655-2. [DOI] [PubMed] [Google Scholar]
- 10.Laberge J.M., Puligandla P., Flageole H. Asymptomatic congenital lung malformations. Semin. Pediatr. Surg. 2005;14(1):16–33. doi: 10.1053/j.sempedsurg.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Priest J.R. Pulmonary cysts in early childhood and the risk of malignancy. Pediatr. Pulmonol. 2009;44(1):14–30. doi: 10.1002/ppul.20917. [DOI] [PubMed] [Google Scholar]
- 12.Brcic L. Pleuropulmonary blastoma type I might arise in congenital pulmonary airway malformation type 4 by acquiring a dicer 1 mutation. Virchows Arch. 2020;477(3):375–382. doi: 10.1007/s00428-020-02789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinberg A. Can congenital pulmonary airway malformation be distinguished from type I pleuropulmonary blastoma based on clinical and radiological features? J. Pediatr. Surg. 2016;51(1):33–37. doi: 10.1016/j.jpedsurg.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasr A. Is congenital cystic adenomatoid malformation a premalignant lesion for pleuropulmonary blastoma? J. Pediatr. Surg. 2010;45(6):1086–1089. doi: 10.1016/j.jpedsurg.2010.02.067. [DOI] [PubMed] [Google Scholar]
- 15.Downard C.D. Treatment of congenital pulmonary airway malformations: a systematic review from the APSA outcomes and evidence based practice committee. Pediatr. Surg. Int. 2017;33(9):939–953. doi: 10.1007/s00383-017-4098-z. [DOI] [PubMed] [Google Scholar]
- 16.Knight S. Current management of pleuropulmonary blastoma: a surgical perspective. Children (Basel) 2019;6(8) doi: 10.3390/children6080086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito H. Pulmonary function after lobectomy versus segmentectomy in patients with stage I non-small cell lung cancer. World J. Surg. 2014;38(8):2025–2031. doi: 10.1007/s00268-014-2521-3. [DOI] [PubMed] [Google Scholar]
- 18.Muller C.O. Is radical lobectomy required in congenital cystic adenomatoid malformation? J. Pediatr. Surg. 2012;47(4):642–645. doi: 10.1016/j.jpedsurg.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Fascetti-Leon F. Sparing-lung surgery for the treatment of congenital lung malformations. J. Pediatr. Surg. 2013;48(7):1476–1480. doi: 10.1016/j.jpedsurg.2013.02.098. [DOI] [PubMed] [Google Scholar]
- 20.Xie J., Wu Y., Wu C. Is thoracoscopy superior to thoracotomy in the treatment of congenital lung malformations? An updated meta-analysis. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620980267. 1753466620980267. [DOI] [PMC free article] [PubMed] [Google Scholar]