Abstract
Purpose
The impact of IUGR on holistic growth of an infant is established however, limited evidence has been reported regarding its implication on eruption of deciduous dentition.
Aim
Comparative evaluation of eruption of deciduous teeth among infants born after low risk pregnancy and infants diagnosed with Intra Uterine Growth Restriction (IUGR)
Methods
The cross-sectional study included 110 neonates recruited at birth using stratified random sampling based on inclusion and exclusion criteria. Neonates diagnosed without IUGR were allocated to Group I (n = 55) and those diagnosed with IUGR were allocated to Group II (n = 55). Perinatal case history was obtained followed by intraoral examination at birth, 6 months and monthly up to 1 year or till first evidence of teeth eruption.
Results
The first evidence of eruption of deciduous teeth was found delayed in Group II (p = 0.0001). The mean gestational age at delivery, estimated fetal weight, frequency of NICU admission, birth weight and infant weight at 6 months was found statistically higher (p = 0.001) in Group I.
Conclusion
First evidence of deciduous teeth eruption was found delayed among IUGR infants therefore, IUGR along with prematurity, LBW, LSCS delivery, NICU admission can be considered as risk factor for delayed eruption.
Keywords: Intra uterine growth restriction, Gestational age, Primary dentition, Low birth weight
1. Introduction
Oral cavity is a mirror for body as oral manifestations is the most significant or the initial of any systemic disease. Also oral cavity serves as a window for prompt detection of signs and symptoms because of its easy accessibility for visual investigation, and examination by palpation. The development of dentition is often influenced by systemic conditions. By observing the chronology of deciduous teeth suspicion of pathology can be made. One such condition of concern is Intra Uterine Growth Restriction (IUGR). However there is no baseline data which shows the impact of IUGR on dentition and also whether delayed eruption can be used as a risk predictor of IUGR.
The incidence of IUGR is six times higher in developing countries compared to that in developed countries, and this incidence can be further high in lower and middle-income countries, as many infants are born in home with no birth records.1,2 IUGR is the outcome of materno-placental, fetal, or genetic factors or due to a combination of variety of factors. Maternal factors such as age, inter-pregnancy interval maternal health, behavioral habits, and maternal infection influence the fetal growth and could be etiological factors for IUGR.1,3 The impact of IUGR on physical and mental development has been established however its association with dental development and dentition has yet not established in literature.
According to American Dental Association, primary mandibular anterior erupts first as early as six months of age after birth. Most children have a full set of primary teeth by the time they are 3 years old.10 The child's jaws continue to grow, making room for the permanent (adult) teeth that will begin to erupt at about age 6 years. Primary teeth begin to shed between ages 6 and 7 years. This process continues until about age 12 years.2 It is speculated that systemic disorders can alter the chronology of tooth eruption which demands early diagnosis and interception from a pediatric dentist.
The goal of antenatal monitoring is early detection of IUGR, so that antenatal management can be optimized for better neonatal outcome. Close monitoring will lead to changes in the time of delivery or management, but still there is controversy over the appropriate type and timing of antenatal monitoring.3 In the present study evaluation of eruption of deciduous teeth among infants born after low risk pregnancy was compared with infants diagnosed with IUGR.
2. Materials and methodology
The cross-sectional study was conducted in the Department of Pedodontics and Preventive Dentistry, Maulana Azad Institute of Dental Sciences, New Delhi and Department of Obstetrics and Gynaecology, Lok Nayak Hospital, New Delhi, after obtaining prior approval from the Ethical Committee of the M.A.I.D.S. Permission for conducting the study was obtained from Department of Obstetrics and Gynaecology, Lok Nayak Hospital, New Delhi.
3. Sample selection
A total sample of 110 neonates was recruited at birth from the antenatal and postnatal ward of Department of Obstetrics and Gynaecology, Lok Nayak Hospital, New Delhi. The sample population was divided equally into two groups of 55 neonates each by stratified random sampling based on inclusion and exclusion criteria. The sample size was calculated based on convenience and availability of sample in the ward. The inclusion criteria for Group I included neonates presented without IUGR and for Group II neonates diagnosed with IUGR antenatally. Parents willing to participate in the study and attending dental OPD for follow ups were included in the study. The neonates with history of meconium stained liquor, congenital infections and malformations or infants with sign of rickets at 6 months of age or before were excluded from the study.
4. Procedure
The procedure of the study was explained followed by obtaining informed consent and detailed perinatal case history was taken at birth. Based on ultrasonographic assessment of estimated fetal weight of the neonates, the neonates were allocated to Group I and II. Neonates with EFW greater than 10th percentile of its gestational age were allotted to Group I and those less than 10th percentile of its gestational age were allocated to Group II (Nicolaides KH et al., 2018).4 Intraoral examination was performed at birth followed by recording gestational age and birth weight.
5. Follow UP
Intraoral examination was carried out at 6 months of age followed by monthly examination up to 1 year of age or till first evidence of teeth eruption, to record the first evidence of eruption of deciduous teeth followed by comparative evaluation between both the groups.
6. Statistical analysis
Data analysis was performed using the Statistical Package for Social Science version 21.0 (SPSS Inc. Chicago [IL], USA). Descriptive statistics that included mean, median, standard deviation and percentages were calculated for each of the variables. Statistical analysis was performed using chi-square test and student't’ test. Significance for all statistical tests was predetermined at a probability value of 0.05 or less.
7. RESULTS
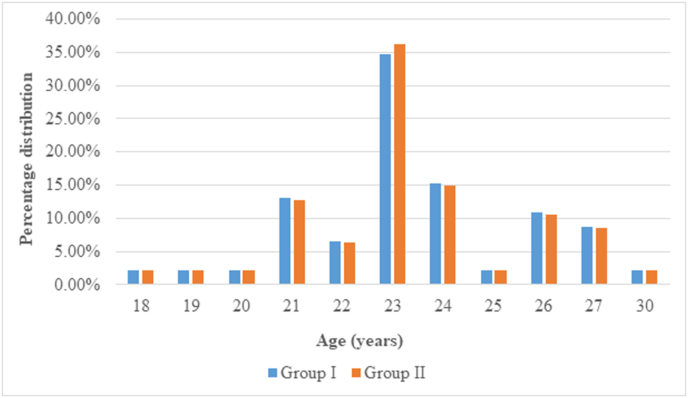
A total of 110 neonates were recruited at birth with 55 neonates each allocated to Group I and Group II. There were reported dropouts of 9 cases in Group I and 8 cases in Group II, with the net total sample size of 93 with 46 neonates in Group I and 47 in Group II. In the present study, the maternal age range was found between 18 and 30 years in Group I and Group II as shown in Fig. 1. The mean maternal age in Group I was found 23.43 ± 23 years and 23.43 ± 2.272 in Group II, with median age in both groups being 23 years and difference being statically insignificant (p > 0.05).
Fig. 1.
Percentage distribution of neonates in Group I and Group II according to maternal age.
Estimated fetal weight (EFW) recorded ultrasonographically during third trimester of pregnancy was noted for allocation of neonates in the respective groups. Neonates with EFW greater than 10th percentile of their respective gestational age were allocated to Group I and less than 10th percentile of gestational age were allocated to Group II (as per the Fetal Medicine Foundation fetal and neonatal population weight charts).12 There was no case of ≤1000g and 73.91% neonates with EFW ≥2501g in Group I as shown in Table 1. In Group II, 25.53% of neonates had EFW ≥2501g with the difference being statistically significant (p < 0.05) as shown on Table 1.
Table 1.
Ultra sonographic assessment of EFW of Group I and Group II.
| ESTIMATED FETAL WEIGHT (IN GRAMS) | GROUP I |
GROUP II |
TOTAL |
|||
|---|---|---|---|---|---|---|
| (N) | (%) | (N) | (%) | (N) | (%) | |
| ≤1000 | No case | No case | 4 | 8.51 | 4 | 4.31 |
| 1001–1500 | 1 | 2.17 | 8 | 17.02 | 9 | 9.68 |
| 1501–2500 | 11 | 23.92 | 23 | 48.94 | 34 | 36.56 |
| ≥2501 | 34 | 73.91 | 12 | 25.53 | 46 | 49.46 |
| Total | 46 | 100 | 47 | 100 | 93 | 100 |
The distribution of neonates on the basis of gestational age at birth in Group I and Group II was done using Battaglia and Lubchenco (1960) classification. It was found that 55.32% neonates in Group II and 10.86% in Group I were early preterm (<34 weeks) and 23.4% in Group II and 45.65% In Group I were full term (37–41weeks) at birth as shown in Table 2. The mean difference between both the groups was calculated using student ‘t’ test with the difference being statistically significant (p < 0.05).
Table 2.
Distribution of gestational age (fetal maturity at birth) in Group I and Group II.
| GESTATIONAL AGE (in weeks) | GROUP I |
GROUP II |
TOTAL |
|||
|---|---|---|---|---|---|---|
| (N) | (%) | (N) | (%) | (N) | (%) | |
| EARLY PRE TERM (<34) | 5 | 10.86 | 26 | 55.32 | 31 | 33.33 |
| LATE PRE TERM (34–36 + 6) | 12 | 26.08 | 9 | 19.15 | 21 | 22.58 |
| FULL TERM (37–41) | 21 | 45.65 | 11 | 23.40 | 32 | 34.4 |
| POST TERM (≥42) | 8 | 17.4 | 1 | 2.13 | 9 | 9.67 |
| TOTAL | 46 | 100 | 47 | 100 | 93 | 100 |
Neonatal outcomes were recorded which included variables such as onset of labor, mode of delivery, neonate gender, APGAR score and Neonatal Intensive Care Unit (NICU) admission as shown in Table 3. Spontaneous labor was recorded among 88.23% of preterm neonates in Group I and 73.52% preterm neonates in Group II while, 11.76% of preterm neonates in Group I and 26.47% in Group II had induced onset of labor. 85.71% of term neonates in Group I and 81.81% in Group II had spontaneous onset of labor while, 14.28% term neonates in Group I and 18.18% in Group II had induced labor. The chi-square test was performed and statistically insignificant difference was found (p > 0.05).
Table 3.
Neonatal outcomes in Group I and Group II.
| ONSET OF LABOR(Preterm) | GROUP I |
GROUP II |
Chi-square test |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | χ2 | P value | |
| SPONTANEOUS | 15 | 88.23 | 25 | 73.52 | 1.448 | 0.228 |
| INDUCED | 2 | 11.76 | 9 | 26.47 | ||
| ONSET OF LABOR(Term) | ||||||
| SPONTANEOUS | 18 | 85.71 | 9 | 81.81 | 0.083 | 0.773 |
| INDUCED | 3 | 14.28 | 2 | 18.18 | ||
| MODE OF DELIVERY | ||||||
| LSCS | 7 | 15.22 | 17 | 36.2 | 5.709 | 0.02 |
| NVD | 39 | 88.78 | 30 | 63.8 | ||
| NEONATE GENDER | ||||||
| MALE | 27 | 58.7 | 28 | 59.6 | 0.0001 | 1.000 |
| FEMALE | 19 | 41.3 | 19 | 40.4 | ||
| APGAR SCORE | ||||||
| ≤6 | No case | No case | 2 | 4.3 | 0.489 | 0.484 |
| 7–10 | 46 | 100 | 45 | 95.7 | ||
| NICU ADMISSION | ||||||
| YES | 6 | 13.3 | 27 | 57.4 | 17.578 | 0.0001 |
| NO | 39 | 86.7 | 20 | 42.6 | ||
15.22% neonates in Group I and 36.2% neonates in Group II undergone lower segment caesarean section (LSCS) delivery. Among 46 neonates in Group I, 58.7% were males and 41.3% were females whereas among 47 neonates in Group II, 59.6% were males and 40.4% were females. The difference in both groups on the basis of neonate gender was found statistically insignificant (p > 0.05).
The APGAR status of neonates after 5 min of delivery was recorded. No case of concerning and moderately abnormal APGAR status was reported in Group I with all neonates presented with reassuring APGAR status. In Group II, no case of concerning status was found with 4.3% neonates having moderately abnormal and 95.7% having reassuring status. The difference in both groups was found statistically insignificant (p > 0.05). 13.3% of neonates in Group I and 57.4% in Group II required NICU admission with statistically significant difference (p < 0.05) as shown in Table 3.
The distribution of neonatal birth weight based on classification by WHO statistical information systems (2011) was done as shown in Table 4. It was found that neonates in Group I had birth weight >10th percentile of its gestational age and in Group II < 10th percentile of gestational age. There was no case of ≤1000g in Group I and 8.51% cases in Group II. 73.91% neonates in Group I and 14.89% in Group II had birth weight ≥2501g. The mean birth weight in Group I was found significantly higher in comparison to Group II (p > 0.05) as shown in Table 5.
Table 4.
Distribution of neonatal Birth weight in Group I and Group II.
| NEONATAL WEIGHT AT BIRTH (IN GRAMS) | GROUP I |
GROUP II |
TOTAL |
|||
|---|---|---|---|---|---|---|
| (N) | (%) | (N) | (%) | (N) | (%) | |
| ≤1000 | No case | No case | 4 | 8.51 | 4 | 4.30 |
| ≥1001-<1500 | 1 | 2.17 | 8 | 17.02 | 9 | 9.68 |
| ≥1501-<2500 | 11 | 23.91 | 28 | 59.57 | 39 | 41.93 |
| ≥2501 | 34 | 73.91 | 7 | 14.89 | 41 | 44.09 |
| Total | 46 | 100 | 47 | 100 | 93 | 100 |
Table 5.
Statistical analysis of variables in Group I and Group II.
| Group I |
Group II |
Mean difference |
Std. error difference |
T- value |
P value |
|||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | |||||
| GA (in weeks) | 36.25 ± 3.32 | 36.20 | 33.53 ± 3.75 | 33.40 | 2.71 | 0.752 | 3.612 | 0.001 |
| EFW (in grams) | 2865.37 ± 547.28 | 3000 | 1927.72 ± 647.18 | 1850 | −937.650 | 124.413 | −7.537 | 0.0001 |
| BW (in grams) | 2817.89 ± 527.79 | 2925 | 1868.468 ± 618.65 | 1780 | −949.43 | 119.363 | −7.954 | 0.0001 |
| ERUPTION OF DECIDUOUS TEETH (in months) | 6.9 ± 0.37 | 7.00 | 12.88 ± 1.16 | 12.80 | −5.977 | 0.180 | −33.18 | 0.0001 |
| Infant Weight at 6 months(in grams) | 6717 ± 583.59 | 7000 | 4370 ± 822.87 | 4300 | 2346.92 | 148.217 | 15.83 | 0.0001 |
The gravidity of mothers was recorded and it was found that 67.4% mothers in Group I and 57.4% in Group II were primigravida with no statistically significant difference (p > 0.05) as shown in Table 6.
Table 6.
Gravidity in group I and group II.
| Gravidity |
Groups |
Chi square test |
||||
|---|---|---|---|---|---|---|
| N | % | N | & | χ2 | P value | |
| Primigravida | 31 | 67.4 | 27 | 57.4 | 1.318 | 0.517 |
| Multigravida | 9 | 19.6 | 10 | 21.3 | ||
| Grand multipara | 6 | 13 | 10 | 21.3 | ||
The first evidence of eruption of deciduous teeth was recorded at 6 months, followed by monthly examination up to 1 year of age or till the first evidence is noted. It was found that the first tooth erupted in both the groups was primary mandibular central incisor with mean age of eruption in Group I being 6.9 ± 0.37 months and in Group II being 12.88 ± 1.16 months. On evaluating the results using student't’ test statistically significant difference was found (p < 0.05) as shown in Table 5.
8. Discussion
The mean maternal age was found to be 23.43 ± 23 years in Group I and 23.43 ± 2.272 years in Group II, with the difference being statistically insignificant (p = 1.000, p > 0.005). There was no reported case of <18 or >30 years with similar distribution of mothers on the basis of age in both the groups. Similar results were reported by Rocha et al.,5 with mean maternal age being 25.1 ± 5.5 years with no statistically significant difference between IUGR and AGA groups (P > 0.05). Ashwini et al.6 reported mean maternal age of 20.2 ± 2.857 years in IUGR group. The results were in contrast to Muhammad et al.,7 reporting mean maternal age of 22.9 ± 4.5 years for IUGR neonates and 26.8 ± 4.8 for AGA neonates with difference being statistically significant (p = 0.001).
The first evidence of eruption was noted as initial cusp emergence on the dental arch. In Group I, the mean first evidence of tooth eruption was 6.9 ± 0.37 months and median was 7 months in all the cases. No case in Group I showed variation in the timing of incision from the average results. In Group II, the mean first evidence of tooth eruption was 12.88 ± 1.16 and median was 12.80 months with statistically significant difference (p = 0.0001, p < 0.05). The results depicted delayed deciduous teeth eruption in Group II as an outcome of IUGR. It was found that the first evidence of tooth eruption in both the groups was of mandibular central incisor. In Group I, the eruption of mandibular lateral incisor was also noted with the mean of 8.3 ± 032 months and median of 9 months. However, in Group II, eruption of deciduous teeth was not noted beyond 13 months and only first evidence of deciduous teeth was noted for comparison due to longer follow up duration.
The mean EFW in Group I was 2865.37 ± 547.28 and 1927.72 ± 647 in Group II, with the difference being statistically significant (p = 0.0001, p < 0.05). Similar results were reported by Chauhan et al.,8 in which significant difference of EFW was found between SGA (n = 99) and AGA (n = 70) groups (p = 0.007). The association of EFW with delayed deciduous teeth eruption was reported in the present study however, limited literature is available.
The mean gestational age (GA) to assess fetal maturity at birth in Group I was 36.25 ± 3.32 and 33.53 ± 3.75 in Group II, with statistically significant difference in both the groups (p = 0.001, p < 0.05). It was concluded that prematurity was prevalent in Group II and associated with delayed deciduous teeth eruption. Similar results were reported by Ntani et al.9 stating that babies of longer gestation and with larger size at birth had their first tooth earlier, had more teeth at one year, and were more likely to have >16 teeth at age two years. Ramos et al.10 concluded that preterm infants show a delay in the time of eruption of the first deciduous tooth when compared to full-term infants, with the difference being statistically significant (p = 0.004).
The results in the present study were similar to Seow et al.11 and Viscardi et al.12 which noted that 60% of prematurely born infants had delayed teeth eruption. It was suggested that nutritional factors and other complications related to preterm birth such as infants who need sustained mechanic ventilation contribute to delay the eruption of the first teeth. Paulsson et al.13 reported delay in dental development and eruption of preterm children regarding the chronological age. This was in contrast to Fadavi et al.14 who reported no significant correlation between the time of intubation and the delayed eruption. Andrade et al.15 also reported no delay in the chronology of eruption of deciduous teeth among high-risk preterm infants.
In the present study no association of onset of labor with delayed deciduous teeth eruption was found (p = 0.773). The results were in contrast to a study conducted by Rocha et al.13 in which among 50 singleton IUGR neonates, 78% required preterm induction whereas 22% patients went in to spontaneous labor. Manandhar et al.16 among 60 IUGR patients reported with 13.33% spontaneous labor and induction among 46.66% patients. The results were contrasting to a study conducted by Shavit et al.17 among 669 IUGR patients, in which it was found that 499 had spontaneous labor and 170 had induced labor.
The association of mode of delivery with delayed deciduous teeth eruption was studied and found statistically insignificant (p = 0.02, p < 0.05). According to Rocha et al.,13 the frequency of LSCS delivery in IUGR group was 92% and 25% in AGA group. Similar results were reported by Kreko et al.18 with 78% of IUGR neonates and 50% AGA neonates undergoing LSCS delivery. Stewart et al.19 reported with, 45.2% of IUGR neonates and 35% of non-IUGR neonates undergoing LSCS delivery. This could be explained as most of the growth restricted fetuses with impaired placental function poorly withstand the stress of labor so the chances for operative delivery is much more frequent among IUGR fetuses.
It was found that neonate gender was not significantly associated with IUGR (p = 1.000, p > 0.05) implying that no specific gender has specific propensity for IUGR. Similar findings were reported by Muhammad et al.15 in which, male neonates in IUGR group were 67% and 65.5% in AGA group. According to Stewart et al.,27 50.7% IUGR neonates and 52.2% of non-IUGR neonates were males. The results were in contrast to a study conducted by Lohaugen et al.20 which showed unequal distribution of neonate gender among non-IUGR and IUGR groups. The results in the present study depicted no correlation of neonate gender with IUGR with no association with delayed deciduous teeth eruption.
The APGAR status depicted no statistically significant difference between both the groups (p = 0.484, p > 0.05) considering that lower APGAR score cannot be considered as risk predictor of IUGR and delayed teeth eruption. Similar results were reported by Zubair et al.21 in which, 1% of normal neonates and 9% IUGR neonates had concerning APGAR status. Rocha et al.13 reported, 1% IUGR neonates had APGAR score <7 at 5 min and none in AGA group. Stewart et al.27 reported, 4.9% of IUGR neonates and 2.7% non-IUGR neonates had APAGR score <7 at 5 min.
13.3% of neonates in Group I and 57.4% in Group II, required NICU admission at delivery with difference being statistically significant (p = 0.0001, p < 0.05). It was concluded that NICU admissions of neonates at birth with timing of deciduous tooth eruption can be correlated. Similar results were reported by Rocha et al.,13 with mean NICU admission in IUGR group being 5.92 ± 2.5 days and for AGA group being 1.28 days. Kreko et al.26 reported, the mean length of NICU stay of IUGR neonates was 9.45 days and of AGA neonates was 5.5 days. Chourasia et al.22 reported that 52.2% of neonates in IUGR group and 13.84% neonates on AGA group required NICU admission. This was in contrast to a study conducted by Stewart et al.27 with 2.1% of IUGR neonates and 2.9% of non-IUGR neonates requiring extensive resuscitation.
The mean birth weight in Group I was 2817.89 ± 527.79 g and in Group II, 1868.468 ± 618.65 g with difference being statistically significant (p = 0.0001, p < 0.05). It was concluded that LBW can be used a potential predictor for delayed emergence of primary teeth. Similar results were reported by Viscardi et al.23 with LBW or GA less than 37 weeks with greater likelihood of delayed eruption of the first tooth, even with chronological age adjusted for prematurity. It was reported that, in healthy premature infants, the first tooth erupts at the appropriate chronological age, but the eruption may be delayed in children who required prolonged mechanical ventilation or received inadequate neonatal nutrition. Similar results were reported by Rezende et al.24 in which children with LBW had higher occurrence of delayed eruption as compared to children with birth weight greater than 2,500g.
Grivu et al.25 and Billewicz et al.26 reported that children with high birth-weight and birth length had earlier dental eruption up to 20 months of age. The study concluded that birth weight influenced the number of teeth emerged however it was less significant than height. Holman et al.27 reported that birth weight has widely been used as a marker of intrauterine nutritional environment, thus, children with nutritional deficiencies displayed delayed primary teeth emergence. This was in contrast to a study conducted by Haddad et al.28 concluding that LBW children may have as many teeth as those with a normal birth-weight or even more, as long as they catch up with their growth, and thus, growing the same height or even taller, for their age, than normal birth-weight children. Um et al.29 reported no statistically significant correlation between the timing of eruption of the deciduous teeth and birth weight.
In the present study, gravidity (order of birth) was assessed in the perinatal history. The chi-square test result depicted no statistically significant difference based on gravidity between the two groups (p = 0.517, p > 0.05). This was similar to study conducted by Fikree et al.,30 in which primipara women had 23.3% incidence of IUGR as opposed to only 12.9% in control women (OR 2.3). This was in contrast to a study conducted by Manandhar et al.,31 in which 75% of IUGR cases were multigravida and 15% were primigravida. Muhammad et al.,7 reported that 46.5% of AGA mothers were multigravida and 50.5% IUGR mothers were primigravida. In the present study it was found that gravidity does not have statistical association with timing of deciduous teeth eruption.
It was found that the mean weight at 6 months in Group I was, 6717 ± 583.59, which was significantly higher than Group II, which was 4370 ± 822.87 (p = 0.0001, p < 0.05). The results showed that the catch up growth of IUGR infants was less till 6 months as compared to infants born after low risk pregnancy. It was concluded that infants in Group II had poor neonatal outcome as compared to Group I which is related to delayed emergence of primary teeth in Group II. The scarcity of similar studies in the literature makes comparisons with present results difficult. More studies in the area of tooth eruption among IUGR infants should be conducted, and results should be used to establish preventive measures and health promotion programs to give children a better quality of life.
9. CONCLUSION
The present study highlighted the importance of early diagnosis of IUGR and possible risk factors associated with IUGR with significant impact on dentition. Delayed deciduous teeth eruption was found delayed among IUGR neonates hence, IUGR along with associated variables such as premature delivery, LSCS delivery, NICU admission and LBW can be considered as risk predictors for delayed dentition. The study highlights the need of further studies in order to evaluate and report possible changes in the pattern of regular eruption of the primary dentition in IUGR fetuses and neonates. There is a need to study the calcification of deciduous teeth in intrauterine period especially in FGR neonates in order to improve the dental outcome.
Author contributions
Aditi Garg: Data Collection, Manuscript Writing. Gyanendra Kumar: Conceived The Idea And Supervision. Mridula Goswami: Manuscript Checking And Analyzing With Co-Supervision. Devender Kumar: Analyzing And Interpretation Of Data With Co-Supervision. Devendra Mishra: Analyzing And Interpretation Of Data With Co-Supervision.
Declaration of competing interest
The authors declare no conflict of interest and no funding source.
References
- 1.Lee P.A., Chernausek S.D., Hokken-Koelega A.C.S., Czernichow P. International small for gestational age advisory board. International small for gestational age advisory board consensus development conference statement: management of short children born small for gestational age, april 24-october 1, 2001. Pediatrics. 2003;111(6):1253–1261. doi: 10.1542/peds.111.6.1253. [DOI] [PubMed] [Google Scholar]
- 2.American Dental Association JADA. 2005;(136):16–19. [Google Scholar]
- 3.GRIT Study Group A randomized trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG. 2003;110(1):27–32. doi: 10.1046/j.1471-0528.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 4.Nicolaides K.H., Wright D., Syngelaki A., Wright A., Akolekar R. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obstet Gynecol. 2018;52(1):44–51. doi: 10.1002/uog.19073. [DOI] [PubMed] [Google Scholar]
- 5.Rocha O.C., Bittar R.E., Zugaib M. Neonatal outcomes of late-preterm birth associated or not with intrauterine growth restriction. Obstet Gynecol Int. 2010:231842. doi: 10.1155/2010/231842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashwani N., Rekha N.A., Babu M.S., Kumar C.S., Pratap O.T. Maternal risk factors associated with intrauterine growth restriction: hospital based study. Int J Med Res Rev. 2016;4(12):2125–2129. [Google Scholar]
- 7.Muhammad T., Khattak A.A., Rehman S. Maternal factors associated with intrauterine growth restriction. J Ayub Med Coll Abbottabad. 2010;22(4):64–69. [PubMed] [Google Scholar]
- 8.Chauhan S.P., Cole J., Sanderson M., Magann E.F., Scardo J.A. Suspicion of intrauterine growth restriction: use of abdominal circumference alone or estimated fetal weight below 10% J. Matern.-Fetal Neonatal Med. 2006;19(9):557–562. doi: 10.1080/14767050600798267. [DOI] [PubMed] [Google Scholar]
- 9.Ntani G., Day P.F., Baird J. Maternal and early life factors of tooth emergence patterns and number of teeth at 1 and 2 years of age. J Dev Orig Health Dis. 2015;6(4):299–307. doi: 10.1017/S2040174415001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos S.R., Gugisch R.C., Fraiz F.C. The influence of gestational age and birth weight of the newborn on tooth eruption. J Appl Oral Sci. 2006;14(4):228–232. doi: 10.1590/S1678-77572006000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seow W.K., Humphrys C., Mahanonda R., Tudehope D.I. Dental eruption in low birth-weight prematurely born children: a controlled study. Pediatr Dent. 1988;10(1):39–42. [PubMed] [Google Scholar]
- 12.Viscardi R.M., Romberg E., Abrams R.G. Delayed primary tooth eruption in premature infants: relationship to neonatal factors. Pediatr Dent. 1994;16(1):23–28. [PubMed] [Google Scholar]
- 13.Paulsson L., Bondemark L., Söderfeldt B. A systematic review of the consequences of premature birth on palatal morphology, dental occlusion, tooth-crown dimensions, and tooth maturity and eruption. Angle Orthod. 2004;74:269–279. doi: 10.1043/0003-3219(2004)074<0269:ASROTC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Fadavi S., Punwani I.C., Adeni S., Vidysagar D. Eruption pattern in the primary dentition of premature low-birth-weight children. ASDC J Dent Child. 1992;59:120–122. [PubMed] [Google Scholar]
- 15.Andrade I.R., Bezerra A.C.B. Estudo longitudinal comparativo da cronologia de erupçao em crianças. JPB & Odontologia do Bebe. 1998;1(2):41–47. [Google Scholar]
- 16.Manandhar T., Prashad B., Pal M.N. Risk factors for intrauterine growth restriction and its neonatal outcome. Gynecol Obstet. 2018;8:464. [Google Scholar]
- 17.Shavit T., Ashual E., Regev R., Sadeh D., Fejgin M.D., Biron-Shental T. Is it necessary to induce labor in cases of intrauterine growth restriction at term? J Perinat Med. 2012;40(5):539–543. doi: 10.1515/jpm-2011-0189. [DOI] [PubMed] [Google Scholar]
- 18.Kreko E., Kola E., Sadikaj F., Dardha B., Tushe E. Neonatal morbidity in late preterm infants associated with intrauterine growth restriction. Open Access Maced J Med Sci. 2019;7(21):3592–3595. doi: 10.3889/oamjms.2019.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart B., Karahalios A., Pszczola R., Said J. Moderate to late preterm intrauterine growth restriction: a restrospective, observational study of the indications for delivery and outcomes in an Australian perinatal centre. Aust N Z J Obstet Gynaecol. 2018;58(3):306–314. doi: 10.1111/ajo.12721. [DOI] [PubMed] [Google Scholar]
- 20.Lohaugen G.C., Ostgard H.F., Andreassen S. Small for gestational age and intrauterine growth restriction decreases cognitive function in young adults. J Pediatr. 2013;163(2):447–453. doi: 10.1016/j.jpeds.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 21.Zubair D.S., Gour S.S. Comparison of outcome in IUGR and Normal Pregnancies- A retrospective study. Int J Med Res Rev. 2016;4(4):646–649. [Google Scholar]
- 22.Chourasia S., Agarwal J., Dudve M. Clinical assessment of intrauterine growth restriction and its correlation with fetal outcome. J Evol of Med and Den Sci. 2013;2(41):7944–7950. [Google Scholar]
- 23.Viscardi R.M., Romberg E., Abrams R.G. Delayed primary tooth eruption in premature infants: relationship to neonatal factors. Pediatr Dent. 1994;16(1):23–28. [PubMed] [Google Scholar]
- 24.Rezende K.M.P.C., Zöllner M.S.A.C., Santos M.R.N. Avaliaçao da Erupcao Dental Decídua em Bebes Considerados de Risco. PesqBrasOdontopedClin Integr. 2010;10(1):61–65. [Google Scholar]
- 25.Grivu O., Ardeleanu M., Mecher E. Les variations biorythmiquesde leruption des dents temporaires. Bull Group Int Rech Sc Stomat. 1972;15:193–206. [PubMed] [Google Scholar]
- 26.Billewicz W.Z., Thomson A.M., Baber F.M., Field C.E. The development of primary teeth in Chinese (Hong Kong) children. Hum Biol. 1973;45(2):229‐241. [PubMed] [Google Scholar]
- 27.Holman D.J., Yamaguchi K. Longitudinal analysis of deciduous tooth emergence: IV. Covariate effects in Japanese children. Am J Phys Anthropol. 2005;126(3):352–358. doi: 10.1002/ajpa.10420. [DOI] [PubMed] [Google Scholar]
- 28.Haddad A.E., Correa M.S. The relationship between the number of erupted primary teeth and the child's height and weight: a cross-sectional study. J Clin Pediatr Dent. 2005;29(4):357–362. doi: 10.17796/jcpd.29.4.jl0510371q155847. [DOI] [PubMed] [Google Scholar]
- 29.Um L., Hsu C.S., Yee R. Influence of metabolic linked early life factors on the eruption timing of the first primary tooth. Clin Oral Invest. 2016;20(8):1871–1879. doi: 10.1007/s00784-015-1670-6. [DOI] [PubMed] [Google Scholar]
- 30.Fikree F.F., Berendes H.W. Risk factors for term intrauterine growth restriction: a community-based study in Karachi. Bull World Health Organ. 1994;72:581–587. [PMC free article] [PubMed] [Google Scholar]
- 31.Manandhar T., Prashad B., Pal M.N. Risk factors for intrauterine growth restriction and its neonatal outcome. Gynecol Obstet. 2018;8:464. [Google Scholar]