Highlights
-
•
Ultrasound is proved to induce the formation of acetaldehyde in model wine system.
-
•
The yield of acetaldehyde could be optimized by ultrasound parameters.
-
•
The mechanism is related to the acoustic cavitation and the initiated chain reactions.
-
•
The results contributed to understand the wine coloration promoted by ultrasound.
Keywords: Ultrasound, Model wine solution, Acetaldehyde, 1-Hydroxylethyl radical, Formation, Coloration
Abstract
In order to explore the effects of ultrasound on the formation of acetaldehyde and its mechanism in model wine solutions, ultrasound conditions and free radicals were investigated by response surface methodology and electron paramagnetic resonance spectroscopy (EPR), respectively. The results indicate that ultrasound does induce the production of acetaldehyde with the maximum amount under the conditions of ultrasound power density 0.2 W/cm2, 48 min and 32 °C. The hydroxyl radicals and the 1-hydroxyethyl free radicals are the main initiator and precursor for acetaldehyde, respectively. Furthermore, the stronger the 1-hydroxyethyl free radicals captured by EPR, the lower the formation of acetaldehyde. In addition, the content of Fe2+and ethanol also exerted a certain influence on the acetaldehyde formation. In conclusion, ultrasound does promote the production of acetaldehyde in the model wine solutions, which is beneficial for well understanding the mechanism of ultrasound in modifying the wine color and accelerating ageing.
1. Introduction
Although the oak barrel ageing is the conventional and extensively accepted method to obtain the wine with high quality, yet several disadvantages such as time-consuming, high cost, labor-intensive, etc. cannot be ignored [1]. In order to overcome these shortcomings, many emerging technologies have been tried about the accelerated wine ageing to replace the traditionally natural ageing in recent years. Ultrasound as a novel technology, with its relatively low-cost and eco-friendly, has attracted extensive attentions in wine-making [2], [3], [4], mainly attributed to the free radicals from the collapse of small bubbles created by the acoustic cavitation of the locally instant high pressures and temperatures [5], [6], [7]. With the involvement of free radicals in the locally high energy zones, certain chemical reaction rates would be accelerated among the compounds occurring in wine such as phenols, resulting in the variations of wine characteristics [8]. To be specific, appropriate ultrasound treatment could contribute to the increase of phenolic substances in wine, and to the accelerated wine ageing by promoting the polymerization and co-polymerization of tannins and anthocyanins as occur during natural wine ageing [2], [9], [10]. And the polymerization is usually related to variations of wine color and mediated by the aldehydes such as acetaldehyde and glyoxylic acid occurring in wine.
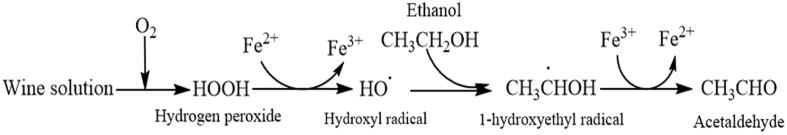
Acetaldehyde is one of the most important carbonyl compounds formed in wine brewing process, which accounts for more than 90% of the total aldehyde contents (4–212 mg/L) in wine [11], [12], and plays an essential role in the wine color formation during ageing being the key intermediate for some chemical reactions of wine coloration [13], since the coloration of wine is mainly attributed to the co-pigmentation between the catechols (or tannins) and anthocyanins. Especially, in the presence of anthocyanins and phenols such as the flavan-3-ols, hydroxycinnamic acids and hydroxycinnamoyltartaric acids, acetaldehyde could be as the ethyl-bridge for the indirect polymerization between the anthocyanins and phenols by reacting at the carbon-8 of the anthocyanins, resulting in the color change of wine [14], [15], [16], [17]. In addition, the indirect interactions could also be bridge-mediated by the acetaldehyde among the flavan-3-ols in wine [18]. Regarding the origins, acetaldehyde is a naturally occurring metabolite from the pyruvate conversion by the pyruvate decarboxylase enzymes [19], [20]. Except for the enzymatic reaction, the Fenton reaction, the non-enzymatic oxidation is also regarded as a key reaction to form the acetaldehyde in wines [21]. Elias et al. reported that the hydroxyl radicals generated from the Fenton reaction would attack the ethanol in wine and produce the 1-hydroxylethyl radicals, finally resulting in the formation of acetaldehyde in wines (Scheme 1) [21], [22]. That is to say, the generation of acetaldehyde is slow and complicated in the natural ageing process of red wine, which determines the wine coloration to a certain extent.
Scheme 1.
The oxidation mechanism initiated by Fenton reaction to produce acetaldehyde in wine.
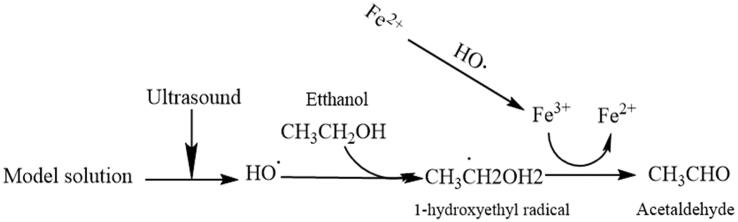
Ultrasound has been proved to significantly accelerate the wine coloration in our previous study, and it could be attributed to the accelerated bridging polymerization between flavan-3-ols by the intervention of the added acetaldehyde and glyoxylic acid as confirmed in a model wine solution [23]. In addition, the 1-hydroxyethyl radicals were also firstly captured by our group in model wine under the ultrasound irradiation [23], [24]. In the meantime, a pathway (Scheme 2) about the generation of acetaldehyde from the further reaction of the 1-hydroxyethyl radicals under the ultrasound treatment is speculated by our group [21]. To the best of our knowledge, no literature is available about this issue. Therefore, the aim of this research is to investigate the formation and its mechanism about the acetaldehyde mediated by ultrasound, and to optimize the influencing factors, so as to well-understand the mechanism of wine coloration accelerated by ultrasound.
Scheme 2.
Oxidation mechanism of acetaldehyde formation induced by ultrasonic treatment in model wine solution.
2. Materials and methods
2.1. Chemicals and reagents
The 2,4-Dinitrophenylhydrazine (DNPH) was purchased from the Zhanyun Chemical Reagent Co. Ltd. (Shanghai, China). Acetaldehyde-2, 4-Dinitrophenylhydrazone (Acetaldehyde-DNPH) was purchased from the Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). All the alcohol, ferrous sulfate heptahydrate, formic acid, perchloric acid and sulfuric acid were bought from the Tianli Chemical Reagent Co., Ltd. (Tianjin, China). 5,5-Dimethyl-1-Pyrroline N-Oxide (DMPO) was purchased from the Sigma-Aldrich Co., Ltd. (St. Louis, USA). HPLC-grade acetonitrile was purchased from the Fisher Scientific Co., Ltd. (USA). Mannitol was purchased from Shengao Chemical Reagent Co., Ltd. (Tianjin, China). The water was prepared using a Millipore Milli-Q purification system.
2.2. Model solutions preparation
Model wine solutions were prepared with the ethanol solution of 12% (v/v), then adding ferrous sulfate heptahydrate to make the Fe2+ concentration to 12 mg/L. In order to investigate the different effects of ethanol and Fe2+ on the reduction of acetaldehyde, model solutions were prepared with different contents of ethanol (4, 24, 40, 54, 84, 100%, v/v) and Fe2+ (4, 8, 12, 16, 20 mg/L). In addition, the mannitol as a radical scavenger was dissolved into the model solution to make its content of 50 g/L so as to investigate the influence of free radicals on the generation of acetaldehyde. All the model solutions were eventually adjusted to the pH of 3.6 ± 0.1 using the 0.01 M hydrochloric acid, and the total volume was 250 mL [21].
2.3. Ultrasound equipment
All ultrasound treatments were carried out by the multi-ultrasound cleaner (SB-500 DTY, Ningbo Scientz Biotechnology Co. Ltd., Ningbo City, Zhejiang province, China), and the ultrasound works at a variable power output from 150 to 450 W with the frequencies of 25, 28, 40 and 59 kHz. The water temperature was controlled by circulating the cooling water during ultrasound treatment. Each treatment was conducted in triplicate.
2.4. Determination of acetaldehyde by HPLC
The DNPH derivatization method was employed to determine the acetaldehyde in the model wine solution system according to the literature [25], [26]. To be specific, the DNPH solutions were freshly prepared by dissolving 10 mg of the recrystallized DNPH in the 50 mL of acetonitrile, then acidifying with 2 mL of 70% perchloric acid.
The derivatization of acetaldehyde was conducted before the determination, and the 100 μL aliquot of the model solution was mixed with 240 μL of DNPH reagent and 40 μL of 25% H2SO4 in a 1.5 mL centrifugal tube. Thereafter, the tightly capped tube with solution was heated at 60 °C for 40 min in a water bath to accelerate the formation of the acetaldehyde-DNPH derivative. After being cooled to room temperature, 480 μL of acetonitrile/water solution (60:40) was added to the samples. The model solutions were filtered through the 0.45 μm PTFE membrane filter, then the acetaldehyde-DNPH derivatives were separated using a C18-AR-Ⅱ LiChrospher®column (4.6 mm × 250 mm, 5 µm particle size; Merck, Whitehouse Station, NJ, USA). Finally, the acetaldehyde-DNPH derivative was directly quantified using the HPLC (Dalian Elite Analytical Instrument Co. Ltd., Dalian China) at 365 nm by calculating against the external standard curve prepared with acetaldehyde-DNPH standard at the conditions of injection volume of 20 µL and 0.8 mL/min of isocratic elution with acetonitrile/water (85:15) including 0.1% formic acid, and the column temperature was 30 °C.
2.5. Response surface optimization of ultrasound parameters
To investigate the influences of ultrasound power, temperature and time on the production of acetaldehyde, the model sample solutions, consisting of 12% (v/v) ethanol, 12 mg/L of ferrous sulfate heptahydrate and pH of 3.6 ± 0.1, were treated by ultrasound with different parameters combinations, and a three-factor Box-Behnken design (BBD) was employed using the Design-Expert software of version 8.0.6 (Statease Inc., Minneapolis, USA) with the concentration of acetaldehyde as the response value and the ultrasound parameters as the independent variables, such as ultrasound power (150 to 350 W, A), temperature (30 to 70 °C, B) and exposure time (10 to 50 min, C), with the frequency fixed at 59 kHz, and the 17 groups of experiments were listed as in Table 1, and each experiment was measured in triplicate. And all the parameters were selected from preliminary results of one factor experiments (data in Supplementary file).
Table 1.
Box-Behnken design and observed responses*
| No | Run | Power |
Time |
Temperature |
Acetaldehyde concentration |
|---|---|---|---|---|---|
| (A)/W | (B)/min | (C)/°C | (Y)/mg/L | ||
| 13 | 1 | 250 | 30 | 50 | 0.63 |
| 17 | 2 | 250 | 30 | 50 | 0.63 |
| 6 | 3 | 350 | 30 | 30 | 0.60 |
| 5 | 4 | 150 | 30 | 30 | 0.44 |
| 14 | 5 | 250 | 30 | 50 | 0.63 |
| 10 | 6 | 250 | 50 | 30 | 0.67 |
| 7 | 7 | 150 | 30 | 70 | 0.58 |
| 12 | 8 | 250 | 50 | 70 | 0.61 |
| 4 | 9 | 350 | 50 | 50 | 0.66 |
| 1 | 10 | 150 | 10 | 50 | 0.45 |
| 16 | 11 | 250 | 30 | 50 | 0.63 |
| 15 | 12 | 250 | 30 | 50 | 0.63 |
| 3 | 13 | 150 | 50 | 50 | 0.51 |
| 8 | 14 | 350 | 30 | 70 | 0.57 |
| 11 | 15 | 250 | 10 | 70 | 0.60 |
| 9 | 16 | 250 | 10 | 30 | 0.45 |
| 2 | 17 | 350 | 10 | 50 | 0.53 |
* Average value of triplicate experiments.
2.6. Free radicals determination by EPR spin trapping
The DMPO (500 mM) was respectively dissolved into 1 mL of the following model solutions (pH 3.6 ± 0.1): 12% (v/v) ethanol solution with different contents of ferrous sulfate heptahydrate (4, 8, 12, 16, 20 mg/L); different concentrations of ethanol (4, 24, 54, 84, 100 % v/v) with the same content of ferrous sulfate heptahydrate (12 mg/L); 12% (v/v) ethanol solution with 12 mg/L ferrous sulfate heptahydrate and mannitol (50 mg/L). All the model solutions were ultrasonically treated at the conditions of 59 kHz, 300 W, 48 min and 32° C so as to investigate the formation of free radicals mediated by ultrasound.
After ultrasound treatment 0.5 h, EPR was used to detect the DMPO - free radical spin adducts in model solutions as described in literature [24]. To be specific, EPR spectra were immediately recorded at room temperature on a Bruker eScan R spectrometer (Bruker, Rheinstetten, Germany) operating in X-band. Sweep width was set at the 120 G, the microwave power was set at 20 mW, and the modulation frequency and the amplitude were set at 100 kHz and 1 G, respectively.
The receiver gain was set to 4.48 × 103, and the conversion and sweep times were set at 30 ms and 60 s, respectively. Total number of the scans was 10 for each sample. The intensity was quantified by adding the maximum and minimum values of the central doublet.
2.7. Statistical analysis
The analysis of variance (ANOVA) was conducted by using the SPSS 16.0 statistics software (SPSS Inc., Chicago, IL, USA). And the related data and graphs were processed by the software of Microsoft Office Excel (2019) and Origin (2018). All experiments were conducted in triplicate.
3. Results and discussion
3.1. Identification and determination for the acetaldehyde in the model wine solutions mediated by ultrasound
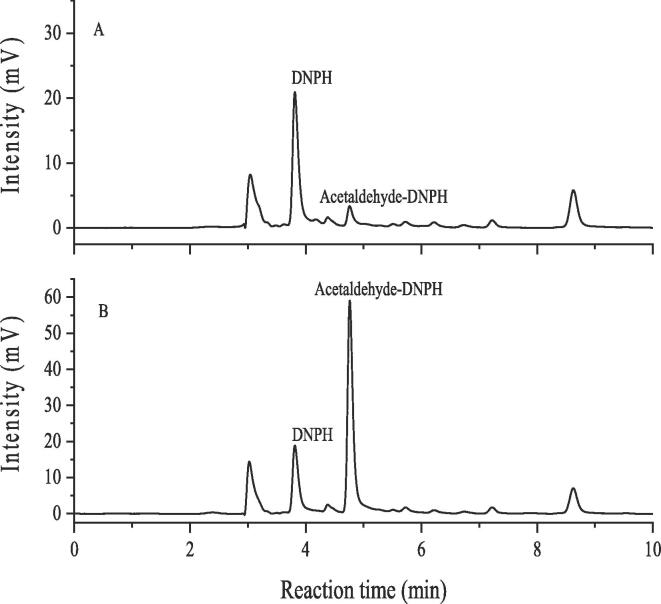
Generally, the reagent of DNPH could constantly react with the aldehyde-based compounds, and the formed products are as the derivative of aldehyde-DNPH (acetaldehyde-DNPH), which can be detected by HPLC [25], [26]. As shown in Fig. 1, the derivative of acetaldehyde was detected by HPLC in the model solutions after being treated by ultrasound irradiation. Compared with the peak intensities of acetaldehyde-DNPH in model wine solution, the peak intensity of acetaldehyde-DNPH was significantly increased in the model wine solution with the acetaldehyde-DNPH standard added as the internal compound, which further confirmed the formation of acetaldehyde-DNPH in the ultrasonically treated model wine solution. That is to say, ultrasound definitely mediated the generation of acetaldehyde, and the reason might be attributed to the acoustic cavitation of ultrasound, which firstly decomposed the molecules of water to form the hydroxyl radicals, then attacking the ethanol molecules to produce the 1-hydroxylethyl radical, finally resulting in the production of acetaldehyde [7], [24]. Considering the important role of the acetaldehyde in the formation of red wine color such as the co-pigmentation of catechols (or tannins) and anthocyanins, and the polymerization between flavanols, the results might explain the reason why ultrasound could accelerate the wine color variation to a certain extent [23], [27]. However, its specific mechanism should be further investigated.
Fig. 1.
Chromatogram for the acetaldehyde in the model solutions treated by ultrasound (A) model solution; (B) model solution with acetaldehyde-DNPH added.
3.2. Effect of free radical scavenger (mannitol) on the acetaldehyde formation in model solutions treated by ultrasound
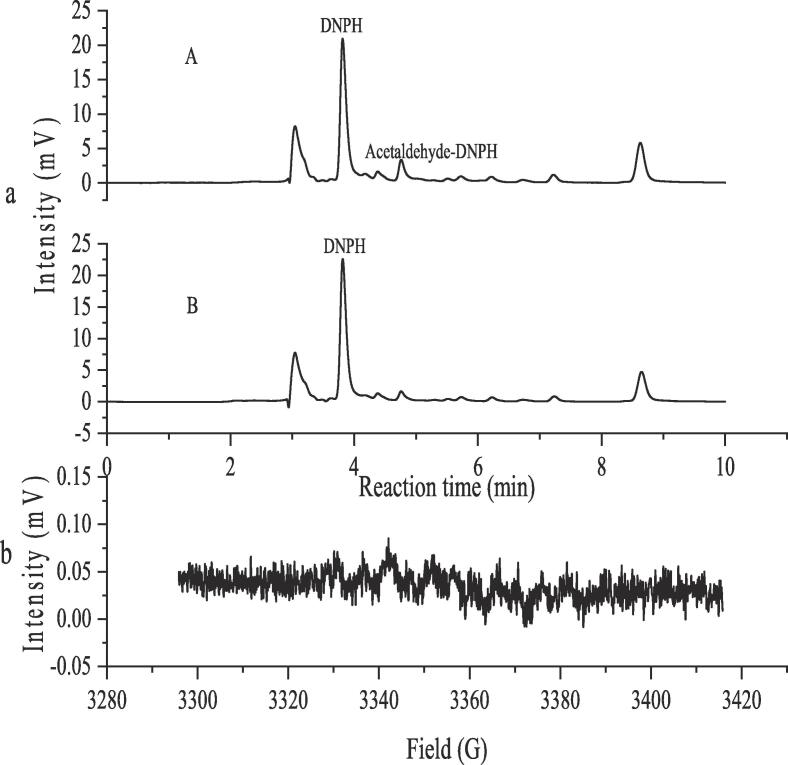
As above-discussed, the free radicals triggered by ultrasound might greatly contribute to the production of acetaldehyde in model wine solution. As an evidence, the mannitol, an effective scavenger of free radicals, was therefore employed so as to investigate the role of free radicals in the production of acetaldehyde, since it can immediately react with the hydroxyl radicals produced from the acoustic cavitation, resulting in no generation of 1-hydroxylethyl radicals from attacking the ethanol by the hydroxyl radicals [28]. Fig. 2a demonstrated the chromatograms of the acetaldehyde-DNPH derivative in model wine solutions with mannitol-free (A) and added (B), and the results indicate that the peak intensity of the acetaldehyde-DNPH derivative significantly decreased in the presence of mannitol, suggesting that the production of acetaldehyde was inhibited by the addition of mannitol during ultrasound irradiation. Fig. 2b illustrated the free radical spectra by EPR, and no free radical species can be identified, i.e. the mannitol added in the model wine solution quickly scavenged the hydroxyl radicals from the acoustic cavitation during ultrasound irradiation, resulting in no free radicals being captured. And, in combination with the results in Fig. 2a, it can be definitely concluded that the acetaldehyde is mainly produced according to the proposed pathway in the Scheme 2. In other words, ultrasound indeed triggers the formation of the acetaldehyde in model wine solutions, and it is mainly attributed to the free radicals produced by acoustic cavitation..
Fig. 2.
Influence of mannitol on the formation of acetaldehyde mediated by ultrasound in model wine solutions (a) chromatogram of acetaldehyde-DNPH derivative: A mannitol-free; B mannitol-added (50 g/L) (b) Free radicals spectra by EPR.
3.3. Optimization of ultrasound parameters on the acetaldehyde production
The response surface optimization was conducted by using the Design-Expert software of version 8.0.6 about the ultrasound parameters on the formation of acetaldehyde, and results are presented in Tables 1 and 2. The ANOVA analysis indicates that the quadratic polynomial models could adequately represent the experimental data, since all the predicted response models are found to be significant (p < 0.001 or 0.05), either the linear parameters, one quadratic parameter or all the interaction parameters, demonstrating that the ultrasound power, temperature and time are the major contributing factors influencing the formation of acetaldehyde. In addition, the coefficient of determination (R2) of the model is 0.9860, suggesting that the model could fully reflect the true relationship between the selected parameters.
To investigate the ultrasound conditions on the production of acetaldehyde, the quadratic regression model, consisting of the coded level of ultrasound power (A), time (B) and temperate (C) with the total concentrations of acetaldehyde as the response (Y), is established as follows:
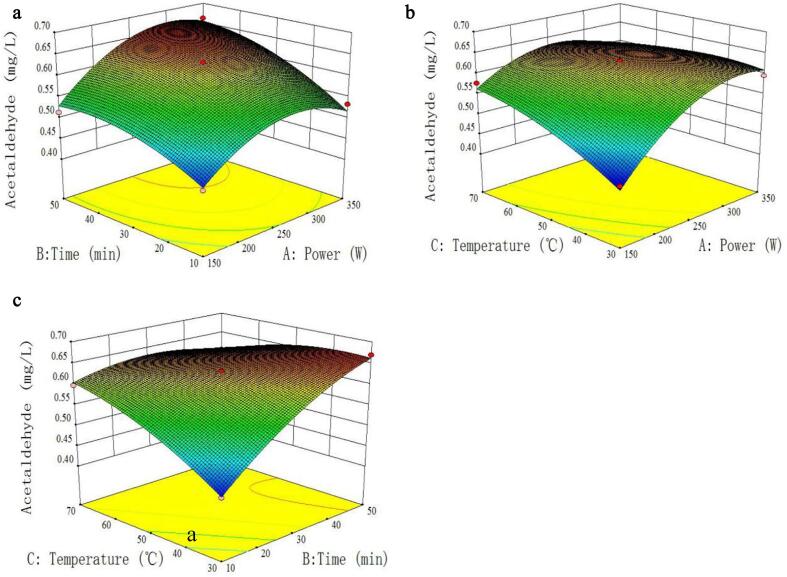
The 3D diagrams of the response surface can intuitively reflect the influence of the interaction between various factors on the response values. Fig. 3a demonstrates the interaction of ultrasound power (A) and time (B) on the formation of acetaldehyde with temperature (C) fixed at 50 °C. With the increase of ultrasound power, the contents of acetaldehyde firstly increased significantly, thereafter decreased slightly. Generally, the bubble rupture from cavitation would become more violent with the ultrasound power increased, which results in the greater sonochemical effect [29]. In term of the effect of ultrasound time, the contents of acetaldehyde increased significantly, followed by a slight decrease at a designated ultrasound power. When the ultrasound power was fixed, the plot of the acetaldehyde content firstly presented an obviously increasing trend and followed by a decrease with the increase of ultrasound time. In general, the trend is similar about the effect of ultrasound power and time on the generation of acetaldehyde, suggesting that there are some interactions between ultrasound power and time on the acetaldehyde formation.
Fig. 3.
Response surface plots of the concentration of acetaldehyde affected by ultrasound power, time and temperature.
The interaction between the ultrasound power (A) and temperature (C) is shown in Fig. 3b with the ultrasound time fixed at 30 min, and the results indicate that the concentrations of acetaldehyde increased with the increase of ultrasound power and temperature. In general, the effect of ultrasound power on the acetaldehyde contents presented an upward trend firstly, then showed a gradually downward trend. Especially with the further increasing of ultrasound temperature, the downward trend became more obvious, indicating that there is an interaction between ultrasound power and temperature. In the meantime, the similar effect of ultrasound temperature was also observed on the contents of acetaldehyde. Generally, the higher the temperature is, the more vapors there will be inside the cavity, then the extra steam buffers the implosion of the cavity and reduces the implosion temperature [7]. Therefore, with all the different chemical reactions, the sonochemical reactions decrease in rate with the rising of temperature, which might explain the reason of the variation about the concentrations of acetaldehyde during ultrasound irradiation.
Fig. 3c indicates the contents of acetaldehyde influenced by the ultrasound time (B) and temperature (C) with the ultrasound power fixed at 250 W, and both the ultrasound time and temperature showed a quadratic effect on the acetaldehyde concentration, which is consistent with the results in Table 2. That is to say, the production of acetaldehyde firstly obtained a maximum value, and followed by a decrease with the further increase of the ultrasound temperature or time.
Table 2.
The quadratic polynomial model of estimated regression coefficients and the ANOVA analysis results of the response surface optimized experiments.
| Source | Sum of Squares | DF | Mean Square | F Value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 0.08796 | 9 | 0.009773 | 54.91217 | <0.0001 | *** |
| A-Power | 0.0176 | 1 | 0.0176 | 98.8882 | <0.0001 | *** |
| B-Time | 0.022548 | 1 | 0.022548 | 126.6906 | <0.0001 | *** |
| C-Temperature | 0.004643 | 1 | 0.004643 | 26.08869 | 0.0014 | ** |
| A^2 | 0.016452 | 1 | 0.016452 | 92.43525 | <0.0001 | *** |
| B^2 | 0.003546 | 1 | 0.003546 | 19.92546 | 0.0029 | ** |
| C^2 | 0.00209 | 1 | 0.00209 | 11.74114 | 0.0110 | * |
| AB | 0.001 | 1 | 0.001 | 5.619796 | 0.0496 | * |
| AC | 0.006474 | 1 | 0.006474 | 36.37432 | 0.0005 | ** |
| BC | 0.011663 | 1 | 0.011663 | 65.52803 | <0.0001 | *** |
| Residual | 0.001246 | 7 | 0.000178 | |||
| Lack of Fit | 0.001246 | 3 | 0.000415 | Not significant | ||
| Pure Error | 0 | 4 | 0 | |||
| Cor. Total | 0.089205 | 16 | ||||
| R-Squared | 0.986034 | |||||
| Adj. R-Squared | 0.968077 | |||||
| Pred R-Squared | 0.776541 | |||||
| Adeq. Precision | 22.25447 |
*Indicated significant difference (P < 0.05), **indicated highly significant difference (P < 0.01), ***indicated extremely significant difference (P < 0.0001).
According to the above-established formula, the optimal conditions were obtained at the ultrasound power of 300 W, time of 48 min and temperature of 32 °C. To recheck the experiments performed under the optimized conditions, the practical experiments were conducted and the obtained values of 0.65 ± 0.08 mg/L (n = 3) were compared with the predicated result of 0.63 mg/L. There is no significant differences between the predicated result and the practice value (p greater than 0.05), indicating that the response model is adequate to reflect the expected optimization and the RSM model is effective.
3.4. Other factors affecting the acetaldehyde formation in model wine solutions treated by ultrasound
The Fenton reaction, as one of the important oxidation–reduction reactions in wine, is regarded as the main contributor to the formation of acetaldehyde, which comes from the oxidized ethanol by the hydroxyl radicals from the metal-catalyzed reduction of trace oxygen in wines [21]. That is to say, under the natural ageing conditions, the oxygen, ethanol, and metal ions in wine play an extremely important role in the formation of acetaldehyde, respectively. While in the ultrasonically-treated model wine solution, little oxygen would exist in the solutions due to the degassing effect of ultrasound, so the Fenton reaction would not occur to produce hydroxyl radicals and initiate the chain reactions, resulting in the formation of 1-hydroxyethyl radicals and acetaldehyde in theory. However, it was unexpectedly found that the 1-hydroxyethyl radicals and acetaldehyde were all detected in the ultrasonically-treated model wine solutions [24], and these highlight us that the Fenton reaction is no longer the main contributor for the production of 1-hydroxyethyl radicals and acetaldehyde. Considering the sonochemical effect, the formation pathway about 1-hydroxyethyl radicals and acetaldehyde in model wine solutions was proposed in Scheme 2 under ultrasound irradiation, and some experiments were further conducted to investigate the factors influencing the generation of acetaldehyde in the model solutions exposed to ultrasound irradiation such as the intensity of 1-hydroxyethyl radical, the concentration of ferrous ion and the content of ethanol.
3.4.1. Relationship between the intensity of 1-hydroxyethyl radicals and the amount of acetaldehyde in ultrasonically-treated model solutions
DMPO is a type of spin trapping agent with high efficiency in capturing free radicals such as oxygen radicals and hydroxyl radicals, and the adducts such as DMPO-OH produced by the reaction have the distinctive EPR patterns [22], which can be captured by the EPR equipment [24]. With regard to the hydroxyl radicals in aqueous solutions, the captured EPR spectrum of the DMPO-OH consists of four peaks, and its hyperfine coupling constant is aN = aH = 14.9 G, which is very identical to the reports [22], [30]. However, in the solutions containing ethanol, ethanol is a compound that would easily undergo the hydrogen abstraction reaction by hydroxyl radicals, when it occurs, the corresponding carbon center free radicals can be formed immediately, and the DMPO captured pattern would also change accordingly. As a result, the EPR spectrum of the DMPO-CH(OH)CH3 adduct would become 6 peaks with equal intensity, and the hyperfine coupling constant would change into aN = 15.6 G, aH = 22.5 G [22], [30].
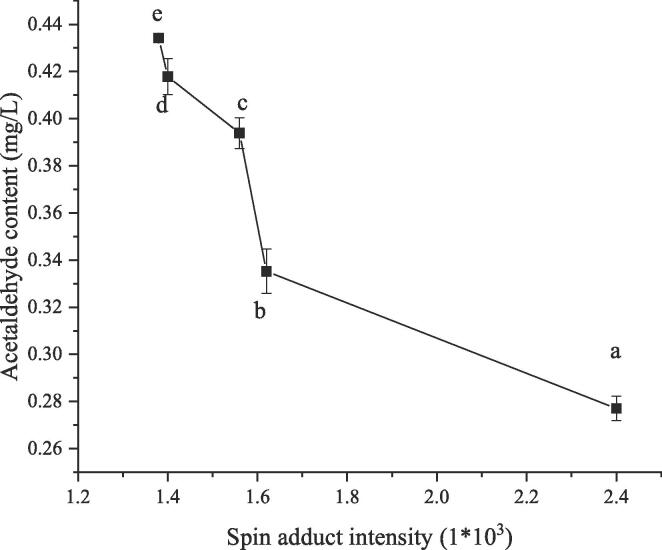
The 1-hydroxyethyl radical is considered to be the major free radicals occurring in wine, mainly derived from the oxidation of ethanol mediated by hydroxyl free radical [21]. Generally, the hydrogen attached to the C-1 carbon of ethanol is the preferred position to be captured by the hydroxyl radical, since the production yield of the 1- hydroxyethyl radical is as high as 85% [31]. Although the 2-hydroxyethyl radical can also be generated from the C-2 carbon during ethanol oxidation, yet it is considered as the minor species compared with 1- hydroxyethyl radical [31]. Under aerobic conditions, the 1-hydroxyethyl radicals would react with oxygen at the diffusion-controlled rates, eventually forming the acetaldehyde. As a comparison, the 1-hydroxyethyl radical could also be reduced to generate the acetaldehyde by the abstracting of hydroxyl radical from the hydrogen atom of ethanol at ultrasonic conditions. Furthermore, the amount of the produced acetaldehyde is negatively correlated to the intensity of 1-hydroxyethyl radicals as shown in Fig. 4. To be specific, the concentration of acetaldehyde in model wine solutions follows a downward trend with the increasing of the strength of the DMPO-CH(OH)CH3 free radical spin adducts, i.e. the 1-hydroxyethyl radicals positively participated in the formation of the acetaldehyde under ultrasound irradiation.
Fig. 4.
Relationship between the intensity of 1-hydroxyethyl radicals adduct and the acetaldehyde content.
3.4.2. Effects of iron (Ⅱ) ions on the production of acetaldehyde in the model solutions exposed to ultrasound
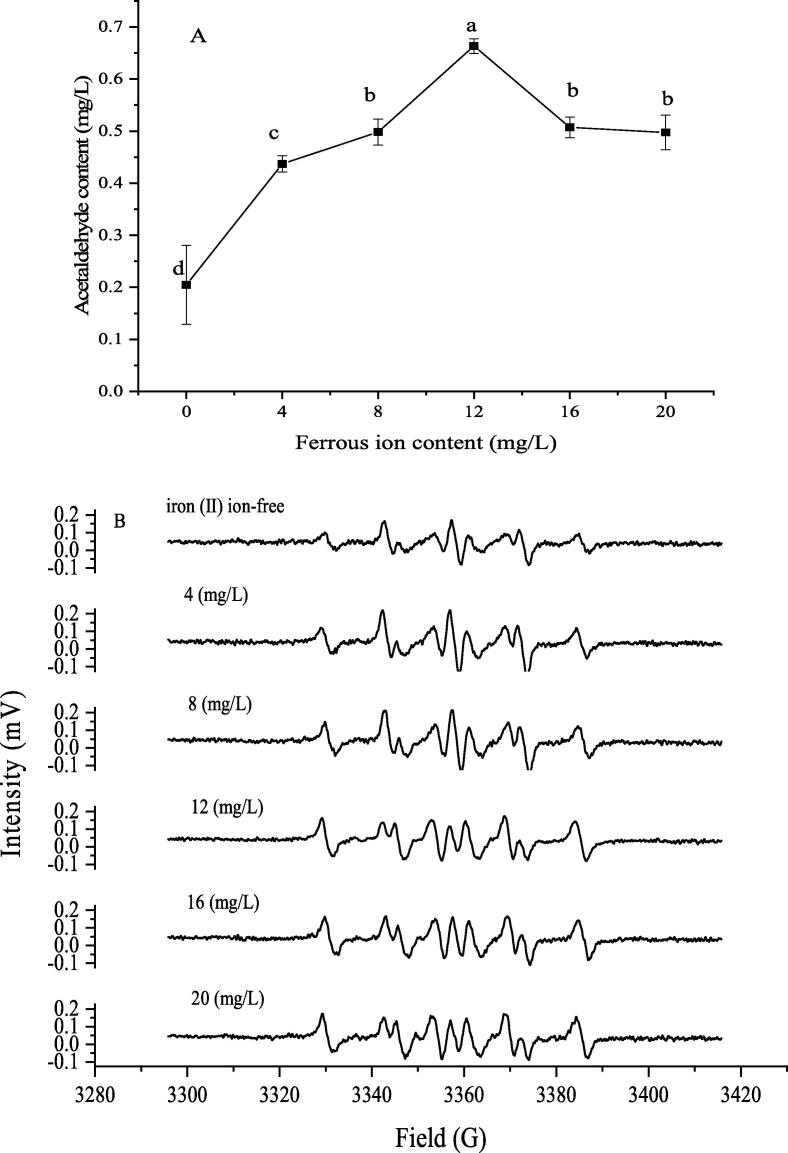
Ferrous ion, as a kind of transition metal ions, exerts an extremely impact on wine, especially to the Fenton reaction during natural ageing. As a whole, the concentrations of acetaldehyde in ultrasonically-treated model solutions firstly presented a rapid rising trend from the ferrous ion content of 0 to 12 mg/L, and followed by a decrease with its further increasing than 12 mg/L (Fig. 5A), while there is no statistical significance of these results, i.e., the iron (Ⅱ) ion content only has a certain effect on the production of acetaldehyde, rather than a decisive role, since the iron (Ⅱ) ions might be oxidized to ferric ion under the hydroxyl radicals mediated by ultrasound in the model wine solutions [31] and the acetaldehyde was also detected in the ultrasonically-treated model solutions without adding ferrous ion. As a whole, the appropriate amounts of ferrous ions could promote the formation of acetaldehyde, and excessive amounts might inhibit its formation. In the meantime, the DMPO-CH(OH)CH3 adduct was also detected in model solutions even without addition of Fe2+ as in Fig. 5B, suggesting that the 1-hydroxyethyl radicals were produced in the model solutions by ultrasound irradiation. Furthermore, the intensity of the 1-hydroxyethyl free radicals was almost unchanged with the increase of the iron (Ⅱ) ion contents. In the natural oxidation process, the iron (III) ion obtained from the oxidation of ferrous ions could oxidize 1-hydroxyethyl radical to acetaldehyde [21]. Under ultrasound conditions, ferrous ions might be oxidized by the hydroxyl radicals from the acoustic cavitation to iron (III) ions in model solutions, then it would reduce the 1-hydroxyethyl radicals to form the acetaldehyde.
Fig. 5.
Effect of iron (Ⅱ) ion concentration on the acetaldehyde in the ultrasonically model solution (A) and 1-hydroxyethyl radicals adducts spectrum by EPR with different ethanol contents (B).
3.4.3. Effects of ethanol concentrations on the production of acetaldehyde in model solutions by ultrasonic treatment
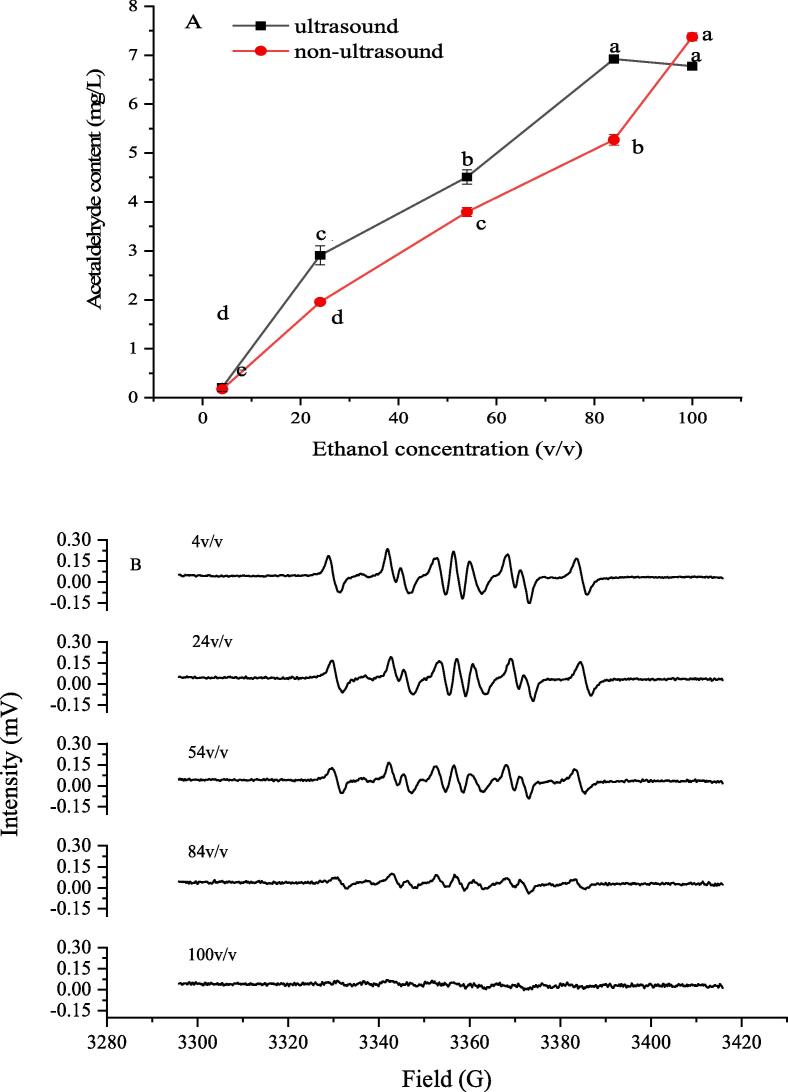
Ethanol is the second major compound following water and also the most abundant volatile compound in wine, which exhibits significantly impact on the sensory properties of wine such as color and aromatic perception [32], [33], [34]. Due to the high content and the lower oxidation–reduction potential, it is also the main target easily attacked by hydroxyl radicals in the wine and could be oxidized to acetaldehyde, i.e., the existence of ethanol plays a decisive role in the acetaldehyde formation. As shown in Fig. 6A, the production of acetaldehyde gradually increased with the increase of ethanol concentration from 4 to 100 % (v/v) in the ultrasonically-treated model wine solutions, which might be the result of the hydroxyl radicals attacking the ethanol molecules to generate acetaldehyde. Furthermore, the intensities of DMPO/1-hydroxyethyl radicals spin adducts in the ultrasonically-treated model wine solutions were significantly weakened with the increase of ethanol concentrations (Fig. 6B), suggesting that ethanol might contribute to the production of acetaldehyde. Generally, the •OH radical is an extremely potent oxidant, which can extract the hydrogen atom from other compounds (such as ethanol), resulting in formation of the 1-hydroxyethyl radicals [30]. Fig. 6A also demonstrated the promotion of ultrasound on the production of acetaldehyde, since the intensity of the DMPO-CH(OH)CH3 spin products was extremely weakened in the 100% ethanol model solution in Fig. 6B, and the content of acetaldehyde is higher than that of the lower ethanol concentration. In a word, the hydroxyl radical from ultrasonic cavitation might be the main initiator for the 1-hydroxyethyl radicals and its terminated product of acetaldehyde in model wine solution exposed to ultrasound irradiation.
Fig. 6.
Effect of ethanol concentrations on the production of acetaldehyde in the ultrasonic treatment model solutions (A) and 1-hydroxyethyl radicals adducts spectrum by EPR with different ethanol contents (B).
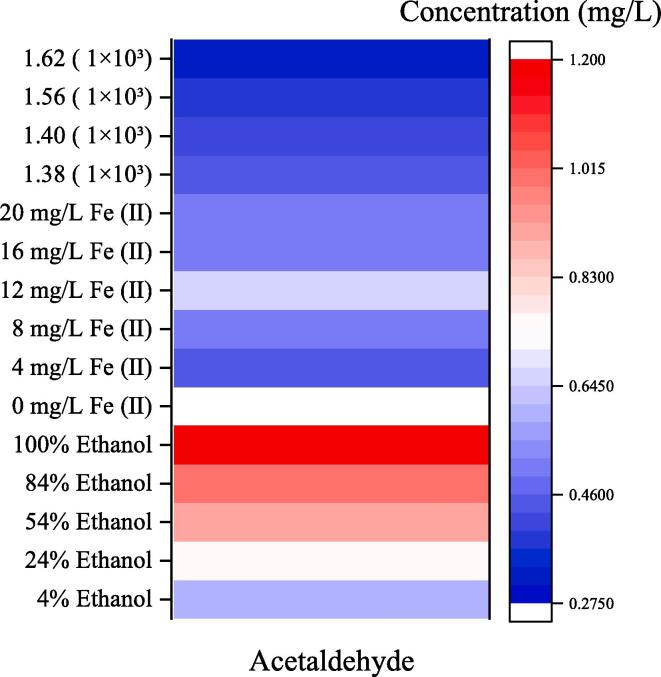
3.5. Heatmap analysis about the relationship between the above factors and the acetaldehyde production
The heatmap analysis was employed to investigate the contribution of the affecting factors 1-hydroxyethyl radicals, ferrous ion and ethanol in the ultrasonically-treated model solutions on the production of acetaldehyde. According to the color distribution in Fig. 7, the ethanol concentration had the most significant influence on the production of acetaldehyde, followed by the content of ferrous ion and 1-hydroxyethyl radical intensity. As other chemical reactions, the production of acetaldehyde presented a general upward trend with the increasing of the 1-hydroxyethyl radical intensity. It can also be found from Fig. 7 that the generation rate of acetaldehyde decreased with the increase of the intensity of DMPO-CH(OH)CH3 free radical spin adducts. To be specific, the higher the acetaldehyde contents in the model solutions, the weaker the intensity of the 1-hydroxyethyl radicals, suggesting the production of acetaldehyde in the ultrasonically-treated model wine solution major induced by acoustic cavitation.
Fig. 7.
The heatmap analysis of the 1-hydroxyethyl radical, ethanol and iron (Ⅱ) ions and the acetaldehyde in the ultrasonically-treated model wine solutions.
Ferrous ions play an extremely key role in oxidizing the ethanol molecule to acetaldehyde during the natural ageing, while in the ultrasonically-treated model solutions, the presence of ferrous ion might promote the reduction of the 1-hydroxyethyl radicals to the acetaldehyde to a certain extent, although the effect is not statistical different.
Ethanol in the model wine solutions with ultrasound treatment could be oxidized by the hydroxyl radicals from ultrasonic cavitation to the 1-hydroxyethyl free radicals, consequently resulting in the production of acetaldehyde. As shown in Fig. 7, the acetaldehyde concentrations in the ultrasonically-treated model solutions positively correlate with the ethanol content, which might be due to that the acoustic cavitation triggered hydroxyl radicals would attack more ethanol molecules, producing more acetaldehydes.
4. Conclusion
In summary, the acetaldehyde could be induced by the free radicals from ultrasound cavitation in model wine solutions, and the maximum amount could be achieved by optimizing the ultrasound parameters such as ultrasonic power, time and temperature. In addition, it was further found all the intensity of the 1-hydroxyethyl radicals, the content of ferrous ions and ethanol concentration were the affecting factors to the formation of the acetaldehyde. The mechanism might be that the water molecules were firstly dissociated by acoustic cavitation, resulting in the hydroxyl radicals, subsequently attacking the ethanol molecules to form the 1-hydroxyethyl radicals, which might be converted into the acetaldehyde under the involvement of ferrous ions and this conversion should be further confirmed.
In conclusion, ultrasound could do promote the production of acetaldehyde in the model wine solutions, and it is conducive to well understanding the mechanism of ultrasound in modifying the wine color, since the acetaldehyde greatly contributes to the polymerization between anthocyanins and flavan-3-ols, resulting in the coloration of wine during ageing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by National Natural Science Foundation of China [No. 31972206], Key Research Development Program of Shaanxi Province, China [No.2021NY-163], the Innovation Talents of Science and Technology Serving Enterprise Project of Xi’an, Shaanxi Province, China [2020KJRC0011], and the Fundamental Research Funds for the Central Universities of China [No. GK202102009].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105757.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Tao Y., García-Martín J.F., Sun D.-W. Advances in wine ageing technologies for enhancing wine quality and accelerating wine ageing process. Crit. Rev. Food Sci. Nutr. 2013;54:817–835. doi: 10.1080/10408398.2011.609949. [DOI] [PubMed] [Google Scholar]
- 2.Clodoveo M.L., Dipalmo T., Rizzello C.G., Corbo F., Crupi P. Emerging technology to develop novel red winemaking practices: an overview. Innov. Food Sci. Emerg. Technol. 2016;38:41–56. [Google Scholar]
- 3.Knorr D., Zenker M., Heinz V., Lee D.-U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004;15(5):261–266. [Google Scholar]
- 4.García-Martín J.F. Nova Science Publishers; 2016. Applications of Ultrasound in the Beverage Industry. [Google Scholar]
- 5.Crum L.A. Comments on the evolving field of sonochemistry by a cavitation physicist. Ultrason. Sonochem. 1995;2:S147–S152. [Google Scholar]
- 6.Neuenschwander U., Neuenschwander J., Hermans I. Cavitation-induced radical-chain oxidation of valeric aldehyde. Ultrason. Sonochem. 2012;19:1011–1014. doi: 10.1016/j.ultsonch.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Suslick K.S. The chemical effect of ultrasound. Sci. Am. 1989;260:80–86. [Google Scholar]
- 8.Hemwimol S., Pavasant P., Shotipruk A. Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrason. Sonochem. 2006;13:543–548. doi: 10.1016/j.ultsonch.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Tudose-Sandu-Ville Ş., Cotea V.V., Colibaba C., Nechita B., Niculaua M., Codreanu M. By promoting the polymerization and co-polimerization of anthocyanins and tannins as the wine matures. Cercetari agronomice in Moldova. 2012;45(4):89–98. [Google Scholar]
- 10.García Martín J.F., Sun D.-W. Ultrasound and electric fields as novel techniques for assisting the wine ageing process: the state-of-the-art research. Trends Food Sci. Technol. 2013;33:40–53. [Google Scholar]
- 11.Nykanen L. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am. J. Enol. Viticult. 1986;37:84–96. [Google Scholar]
- 12.Liu S.-Q., Pilone G.J. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 2000;35:49–61. [Google Scholar]
- 13.Oliveira C.M., Ferreira A.C.S., De Freitas V., Silva A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011;44:1115–1126. [Google Scholar]
- 14.Timberlake C.F., Bridle P. Interactions between anthocyanins, phenolic compounds, and acetaldehyde and their significance in red wines. Am. J. Enol. Viticult. 1976;27:97–105. [Google Scholar]
- 15.M. Monagas, B. Bartolomé, Anthocyanins and Anthocyanin-Derived Compounds, Wine Chemistry and Biochemistry. (2009) 439–462.
- 16.Forino M., Picariello L., Lopatriello A., Moio L., Gambuti A. New insights into the chemical bases of wine color evolution and stability: the key role of acetaldehyde. Eur. Food Res. Technol. 2020;246:733–743. [Google Scholar]
- 17.Zhang X.-K., Li S.-Y., Zhao X., Pan Q.-H., Shi Y., Duan C.-Q. HPLC-MS/MS-based targeted metabolomic method for profiling of malvidin derivatives in dry red wines. Food Res. Int. 2020;134 doi: 10.1016/j.foodres.2020.109226. [DOI] [PubMed] [Google Scholar]
- 18.N. Terrier, C. Poncet-Legrand, V. Cheynier, Flavanols, Flavonols and Dihydroflavonols, Wine Chemistry and Biochemistry. (2009) 463-507.
- 19.Pronk J.T., Steensma H.Y., Dijken J.P.V. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast (Chichester, England) 1996;12:1607–1633. doi: 10.1002/(sici)1097-0061(199612)12:16<1607::aid-yea70>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Romano P., Suzzi G. Origin and production of acetoin during wine yeast fermentation. Appl. Environ. Microbiol. 1996;62:309–315. doi: 10.1128/aem.62.2.309-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias R.J., Waterhouse A.L. Controlling the Fenton reaction in wine. J. Agric. Food Chem. 2010;58:1699–1707. doi: 10.1021/jf903127r. [DOI] [PubMed] [Google Scholar]
- 22.Elias R.J., Andersen M.L., Skibsted L.H., Waterhouse A.L. Identification of free radical intermediates in oxidized wine using electron paramagnetic resonance spin trapping. J. Agric. Food Chem. 2009;57:4359–4365. doi: 10.1021/jf8035484. [DOI] [PubMed] [Google Scholar]
- 23.Fu X.-Z., Zhang Q.-A., Zhang B.-S., Liu P. Effect of ultrasound on the production of xanthylium cation pigments in a model wine. Food Chem. 2018;268:431–440. doi: 10.1016/j.foodchem.2018.06.120. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q.-A., Shen Y., Fan X.-H., Martín J.F.G., Wang X.i., Song Y. Free radical generation induced by ultrasound in red wine and model wine: An EPR spin-trapping study. Ultrason. Sonochem. 2015;27:96–101. doi: 10.1016/j.ultsonch.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Elias R.J., Laurie V.F., Ebeler S.E., Wong J.W., Waterhouse A.L. Analysis of selected carbonyl oxidation products in wine by liquid chromatography with diode array detection. Anal. Chim. Acta. 2008;626:104–110. doi: 10.1016/j.aca.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 26.Gislason N.E., Currie B.L., Waterhouse A.L. Novel antioxidant reactions of cinnamates in wine. J. Agric. Food Chem. 2011;59:6221–6226. doi: 10.1021/jf200115y. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q.-A., Wang T.-T. Effect of ultrasound irradiation on the evolution of color properties and major phenolic compounds in wine during storage. Food Chem. 2017;234:372–380. doi: 10.1016/j.foodchem.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Clark A.C., Scollary G.R. Copper(II)-mediated oxidation of (+)-catechin in a model white wine system. Aust. J. Grape Wine Res. 2002;8:186–195. [Google Scholar]
- 29.Kiani H., Sun D.-W., Delgado A., Zhang Z. Investigation of the effect of power ultrasound on the nucleation of water during freezing of agar gel samples in tubing vials. Ultrason. Sonochem. 2012;19:576–581. doi: 10.1016/j.ultsonch.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Andersen M.L., Skibsted L.H. Electron spin resonance spin trapping identification of radicals formed during aerobic forced aging of beer. J. Agric. Food Chem. 1998;46:1272–1275. [Google Scholar]
- 31.Asmus K.D., Mockel H., Henglein A. Pulse radiolytic study of site of oh radical attack on aliphatic alcohols in aqueous solution. J. Phys. Chem. 1973;77:1218–1221. [Google Scholar]
- 32.Sherman E., Greenwood D.R., Villas-Boâs S.G., Heymann H., Harbertson J.F. Impact of grape maturity and ethanol concentration on sensory properties of Washington state merlot wines. Am. J. Enol. Viticult. 2017;68:344–356. [Google Scholar]
- 33.Cretin B.N., Dubourdieu D., Marchal A. Influence of ethanol content on sweetness and bitterness perception in dry wines. LWT - Food Sci. Technol. 2018;87:61–66. [Google Scholar]
- 34.Jones P.R., Gawel R., Francis I.L., Waters E.J. The influence of interactions between major white wine components on the aroma, flavour and texture of model white wine. Food Qual. Prefer. 2008;19:596–607. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.