Abstract
Purpose
Sweptsource optical coherence tomography (SS-OCT) permits cross-sectional observation of surface/subsurface characteristics of enamel including early carious lesions (ECL) or remineralization non-invasively.This study aimed to visually compare the cross-sectional remineralizing efficacy of various agents on ICDAS-II scores-1&2 by using SS-OCT and histology.
Methods
Baseline SS-OCT (grey-scale/false-colour) and histology was performed on the randomly selected two samples with scores-1&2. Four remineralizing agents [fluoride-varnish (FV), CPP-ACP, nanohydroxy-paste (NHP) and silver-diamine-fluoride (SDF)]were evaluated for 2-or 6-weeks post-remineralization using SS-OCT and histology.
Results
Score-1&2 baseline SS-OCT images showed a linear-shaped demineralization with dentino-enamel junction (DEJ) visible; and bowl-shaped demineralization with DEJ invisible respectively. Remineralizing agents were assessed on the basis of their ability to remineralize the surface, subsurface and made visualize the DEJ in score-2. SS-OCT showed an outer growth layer in post-remineralization score-1, 2-weeks samples with FV and NHP. All the agents showed progressive subsurface remineralization in 6 weeks. Active lesions showed rapid uptake of minerals on surface. Subsurface mineralization in pigmented score-2 matched sound enamel with NHP and SDF. Surface remineralization was comparable in FV and SDF followed by NHP. SDF demonstrated deeper subsurface remineralization followed by NHP and CPP-ACP.
Conclusion
SS-OCT images correlated to histology. SS-OCT could monitor surface/subsurface in-situde/remineralization activity non-invasively.
Keywords: Early carious lesions, Enamel, ICDAS-II, Remineralization, Smooth surface caries, SS-OCT
1. Introduction
Detection of early carious lesions (ECLs) followed by their monitoring in an effort to arrest or remineralize them is of utmost importance to modern dentistry. Current technology available for in-vivouse makes it difficult to measure a complex disease like dental caries. Among many popular traditional methods for detecting enamel and dentin caries(visual, visual-tactile, radiograph, electrical conductance, FOTI, DIFOTI), a most suitable method has evolved having good reliability is International caries detection and assessment system (ICDAS-II). It measures surface changes and potential histological depth by relying on surface characteristics.1,2 Nevertheless, Abramset al3 correlated caries lesion depth of the canary system, DIAGNOdent and ICDAS-II in smooth and occlusal surfaces of posterior teeth and found canary system had a higher correlation with lesion depth. Vaswani et al1 found reduced lesion depth in pigmented ICDAS-II score-2 while tomographic evaluation using Polarization Sensitive Optical Coherence Tomography (PS-OCT). Thus, the cross-sectional lesion activity may not always relate with the optical changes on enamel surface. Moreover, hidden carious lesions are difficult to detect visually, which may not correlate with ICDAS scorings.4 Recent advances in diagnostic methods based on light-induced or laser-stimulated fluorescence and electric conductivity do not provide cross-sectional images of dental structure and their effectiveness to track minimal changes in enamel subsurface have limited sensitivity.5 Therefore, the newer tools that could monitor in-situ de/remineralizationnon-invasively, prospectively and determine the need for intervention is under research priority.
Optical Coherence Tomography (OCT)is such a non-invasive, cross-sectional imaging system that has recently gained popularity in the diagnostic field.6 It provides a real-time characterization of ECLs. Initial studies with PS-OCT found it excellent for evaluating surface and subsurface relative mineral volume changes of enamel.1, 6, 7 Vaswani et al1 showed that even black and white tomographic images had comparable sensitivity with ICDAS-II when observers were trained to interpret. Little less sensitivity may be because of grey-scale images of PS-OCT. Nonetheless, imaging with PS-OCT is time consuming. Later Swept source optical coherence tomography (SS-OCT) work on ECL showed promising results with real-time imaging of the lesions.8, 9, 10 The potential of this non-polarizing sensitive OCT system to provide subsurface de/remineralization characteristics of the lesion is yet to be explored. Moreover, researchers have found false-colour OCT images to be more useful for interpreting the de/remineralizing processin-situ.6,7
SS-OCT is a variant of OCT, wherein the light source is a tunable laser that sweeps the wavelength over a certain range. The high acquisition speed of SS-OCT, providing near real-time video rate imaging while improving the overall signal-to-noise ratio of the acquired images, has made clinical applications of OCT more feasible.6 Ibusuki et al8 observed white spot lesions (WSL) using SS-OCT and found that the SS-OCT images of WSL corresponded to that of conventional Light Microscope(LM). Mandurah et al9 observed increased reflectivity from demineralized enamel while decreased reflectivity of the remineralized lesion. Nakagawa et al10 defined accuracy of SS-OCT in detecting and estimating the depth of smooth surface enamel ECLsby comparing the images with that of Confocal Laser Scanning Microscope (CLSM).
With the newer cross-sectional imaging technique, now comes the role of remineralizing agents for ECLs. Amongst the plethora of remineralizing agents, fluoride is the pioneer and well-accepted remineralizing agent. Nevertheless rapid deposition of fluorapatite crystals forming a firm surface layer, not only believed to resist further subsurface remineralization11 but also provide a false positive visual assessment of cross-sectional remineralization.On the other hand, the same layer may act as a reservoir of minerals to remineralize subsurface in due course of time.12 Newer remineralizing agents, eg.Casein Phosphopeptide Amorphous Calcium Phosphate (CPP-ACP), Nanohydroxyapatite paste (NHP) and Silver Diamine Fluoride (SDF) are claimed to be more potent one than the other. CPPs has been claimed to increase subsurface remineralization compared to fluoride alone.11,13 Nano-sized particles of NHP having similarity to the apatite crystal of tooth enamel in morphology, crystal structure and crystallinity14claimed to have potential to remineralize artificial carious lesions following addition to toothpastes, mouthwashes etc. Ex-vivo studies15 demonstrated that SDF increased the microhardness and mineral density of the caries lesions for having highest available fluoride. However, studies conducted so far were mainly topographical with uncertainty about the subsurface remineralization. An ideal remineralizing agent should be able to remineralizethelesion three dimensionally and not just the surface. Histological studies that can provide information about the subsurface remineralizing properties are not feasible in in-vivo conditions, hampering the possibility of progressive studies.
This study aimed therefore to visually compare thecross-sectionalremineralizing efficacy of Fluoride Varnish (FV), CPP-ACP, NHP and SDF in ICDAS-II scores-1&2 for smooth surface caries with the help of SS-OCT images and to visually correlate it with stereomicroscopic histologic images, the ‘gold standard’. Null hypothesis was that no difference is expected among remineralizing agents and with ‘gold standard’ on early carious lesions.
2. Materials and methodology
The Institutional Ethics Committee of Modern Dental College and Research Centre, India(MDC/131/2017/5), gave prior clearance to the study. Permission to work on SS-OCT was obtained from the School of Medical Science and Technology, Indian Institute of Technology, Kharagpur (WB, India).
The study followed the ‘Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0)’
And ‘The APOSTEL recommendations for reporting quantitative optical coherence tomography studies'publication guidelines.16,17 ‘Checklist for Reporting In-vitro Studies (CRIS)’ guidelines were followed for sample distribution.18
2.1. Sample preparation
Fifty primary teeth were collected from the Department of Pediatric Dentistry at our institute by CST and DSS.Sample size was determined so that all the 16 subgroups has atleast two samples for SS-OCT scanning and thus any one can be randomly picked up for histology. More than required teeth were stored for the sake of randomization while group making and also for the fear of damage.The selection was performed based on visual criteria to obtain sound teeth with smooth surface ECLs. Teeth collected were those indicated for extraction (over-retained, orthodontic purposes) and naturally exfoliated primary teeth. Grossly decayed teeth, teeth with developmental anomalies, previously restored teeth, teeth with discolouration not due to caries were excluded.Samples were cleaned using an ultrasonic scalerand were stored frozen (-4 °C) in zip-lock polyethylene packets without any medium till the time of experimental procedures to minimize any surface activities similar to our previous studies.1,19
2.2. ICDAS-II scoring of samples
Samples were defrosted and were examined for ICDAS-II scores by one trained examiner (CST), who was the one among independent examinersin previous research.1 The smooth surfaces of the teeth were assessed using ICDAS-II criteria with the aid of a three-in-one air syringe in day light. First, the teeth were assessed moist and then dried for 5 s. The sites were recorded as ICDAS-II score-0 (score-0) for sound; ICDAS-II score-1(score-1) for first visible sign of non-cavitated lesion, seen only when a tooth is dry; ICDAS-II score-2(score-2) for visible non-cavitated lesion, seen when a tooth is wet and dry. High-resolution photographs were taken under the wet and dry condition from investigation sites under standardized conditions with a digital camera (Nikon D-60 with lens 0–55 mm) for record purpose.Out of 50 samples with ECLs, 28 teeth were detected as ICDAS-II score-1; and 22 as ICDAS-II score-2.All the teeth were marked with specific codes.Samples were further kept frozen in different zip-lock packs labeled according to their scores without any medium. Coding of teeth and labelling of packs was done by CSS only, while other authors were kept blinded.
2.3. SS-OCT system
The SS-OCT (OCM1300SS, Thorlabs Incorporation, and Newton, New Jersey) used in the current study incorporates a high-speed frequency-swept external cavity laser (1325-nm central wavelength) with 3-dB spectral bandwidth (BW) (>100 nm) and an average output power of 10 mW. It comprises of a Michel interferometer and a built-in Mach-Zehnder interferometer (MZI, Thorlabs INT-MZI-1300), which provides a frequency clock of the laser. A camera that is integrated in the probe provides live video imaging during SS-OCT data acquisition. The 1325 nm central wavelength and 6 mm coherence length of the swept laser source enables deep image infiltration, up to 3 mm, optical resolution of is 25 μm transversally and 12 μm axially in air (7–8 μm in tissues with a refractive index around 1.5). Briefly, the laser beam scans the object in X and Z dimensions. Collected backscattered light is returned to the system, digitized in a time scale, and analyzed in the Fourier domain to form a depth-resolving scan (A-scan) at each point. A serial set of A-scans along a certain section creates a cross-sectional B-scan, from which a high resolution, a 2-dimensional C-scan image can be obtained by converting B-scan raw data into a grey-scale image. The system also has an option for false-colouring the grey-scale image in green-yellow-red-blue range.
2.4. Standardization of method
A pilot study was performed to standardize the mode of transportation of samples to the laboratory, the imaging techniques and image interpretation.Ten frozen samples (five in each score), apart from the main study, in thezip-lock packets were wrapped in an ice-gel pack and packed in a thermocol box for transport to allow gradual increase in temperature till it reached laboratory. Samples were removed from the packet and cleaned withwater.SS-OCT scanning of all the samples was performed by DSS, CST and AM under the guidance of DS, an expertise in SS-OCT.To reduce back scattering, small amount of glycerin was spread flat on the specimen surface withmicrobrush. Specimens were mounted on sticky wax to stabilizeso that surface of each specimen was parallel to the probe plane. The scanning probe connected to the SS-OCT system was set at a distance from the specimen surface, with the scanning beam oriented about 900to the sample surface(Fig. 1). Scans were acquired with the linear laser beam oriented occluso-cervically ormesio-distally (longitudinal or transverse) to have the idea of maximum depth of the lesions. The SS-OCT had a scanning pulsed laser with imaging width of 10 mm and maximum imaging depth of 3 mm.Maximum depth of ECL was found in mid of lesion in both the planes tried. For standardization of imaging pattern, we decided to scan ECL longitudinally in the direction from mesial to distal on buccal/lingual surfaces; or buccal to lingual on proximal surfaces to represent better visual correlation with histologiccross-sectional images.Samples were then cross cut in the similar plane of SS-OCT scan in the middle of ECL using carborundum disc (0.3 mm thick, D.F·S m.i., Germany) and fine polished using diamond paste (15 μm Diamond Paste, GmbH, Struers, Germany) (CST, SS). It was then observed in stereomicroscope (10X Magnification, Lawrence and Mayo, Bangalore, India) under 10X magnification. The tomographic SS-OCT and histological images were thoroughly studied visually and compared.
Fig. 1.
Scanning of samples with SS-OCT.
ECLs wereassessed by DSS, CST, DS on the basis of three observable landmarks: surface, subsurface and dentino-enamel junction (DEJ).Surface is the outer perimeter of enamel, lining the carious and sound enamel. Subsurface is the area extending from surface to the DEJ(line separating enamel from dentin).
2.5. Randomization into subgroups and remineralization of ECLs
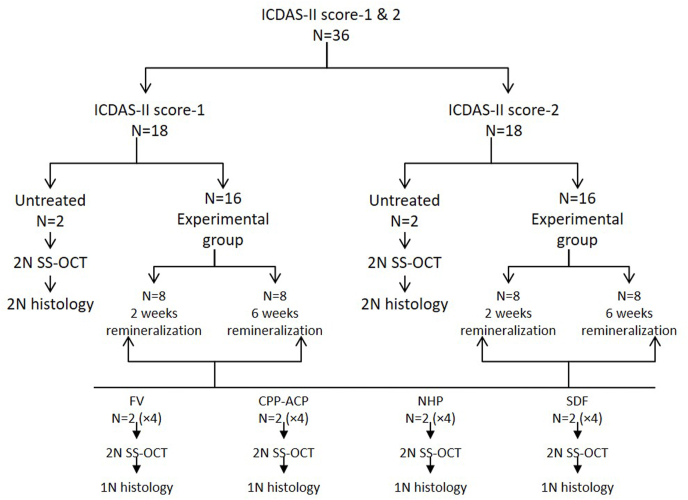
Samples for groups and subgroups were selected by lottery method. Twenty-eight and 22 chits for score-1 and 2 respectively were prepared with hidden codes, same as on teeth. An independent researcher present in the laboratory picked up 18 chits from each of groups. Out of selected 18 chits/group, 2 chits/group were selected for SS-OCT scanning and histology. Out of remaining 16 chits/group, two chits were randomly picked up four times for four subgroups representing experimental remineralization regimen followed by SS-OCT scan. Out of these 2 chits/subgroups, one was picked up for histology. Following randomization, codes were revealed and noted down on the paper according to group distribution (Fig. 2).While performing experiment, samples with same codes were taken. Samples were defrosted overnight and six-week remineralization regime was started first, while two-week regime was started after four weeks. All the teeth were further refrigerated and transported to laboratory as before.
Fig. 2.
Distribution of samples.
The composition of remineralizing agents and method of application is summarized in Table 1.
Table 1.
Composition and method of application of remineralizing agents.
| Brands | Composition | Application |
|---|---|---|
| Flor opal Varnish (FV) Ulrtadent product.inc USA |
Sodium Fluoride 5% | Thin film of varnish was applied on to the dried lesion site with the help of microsponge and left undisturbed for 2 or 6 weeks; later removed with the help of scalpel followed by rubbing with acetone and washed with deionised water for 30 s. |
| GC Tooth Mousse (CPP-ACP) GC corporation, Tokyo, Japan |
Casein Phosphopeptide, Amorphous CalciumPhosphate |
Paste was applied onto the dried lesion site with help of an applicator tip; washing the lesion with deionised water; followed by applying the paste each day for 2 or 6 weeks. |
| Apagard M Plus Sangi.Co.Ltd, Tokyo, Japan |
Medical Hydroxyl Apatite Macrogol 400(PEG 400) | Applied onto the dried lesion site with friction with the help of a micro brush; washing the lesion with deionised water; followed by applying the paste each day for 2 or 6 weeks. |
| Fagamin(SDF) Tedequin SRL, Argentina |
Silver diamine fluoride 38% Silver 24%–28% Fluoride 5%–6% Available fluoride 44,800 ppm |
Applied to dried lesion site using manual applicator soaked in solution; washing the lesion with deionised water was followed by applying the liquid each day for 2 or 6 weeks. |
2.6. Post-remineralizationscanning of samples with SS-OCT
Remineralized samples from one labeled zip-lock packet were scannedwith SS-OCT at a time. Images were saved both ingrey-scale and false-colour. Histologic sectioning and observations were done in the similar manner, described before.
3. Results
Fig. 3 (A-C) illustrates baseline while Fig. 4, Fig. 5, Fig. 6, Fig. 7 the post-remineralization cross-sectional images of SS-OCT (grey-ii; coloured-iii) and histology (iv) to visually compare. The broken red line on the tooth surface represents the plane parallel to which SS-OCT scanning was done and histological sectioning was done along that line (Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7,i).
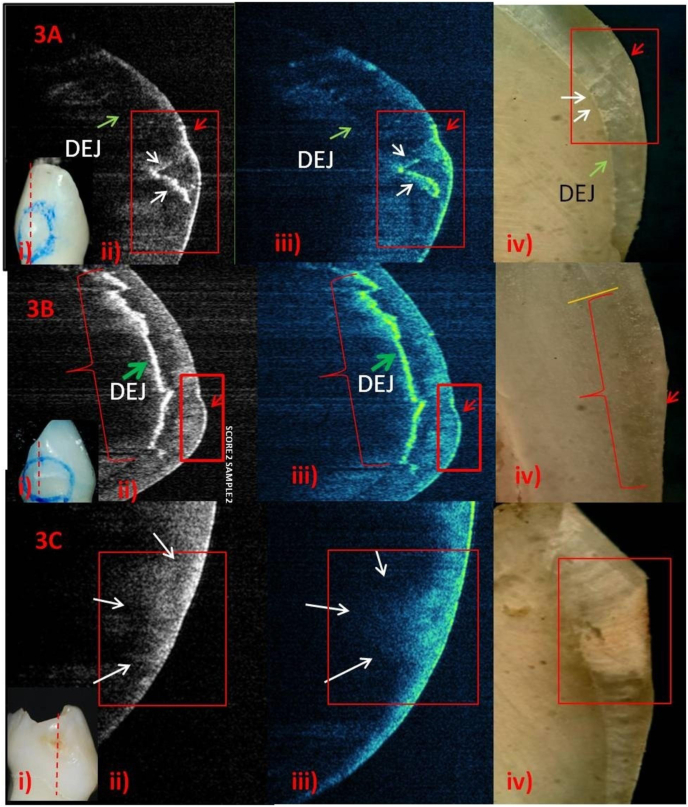
Fig. 3.
Baseline Observation
A-i) score-1.ii) & iv) Linear shapedlesion-limited to outer third of enamel (red arrow). Clearly demarcated lesion boundary from sound enamel (deep blue). Additional finding: developmental defect projecting from DEJ towards the surface (white arrows). Both the findings, correlating with the SS-OCT (red arrow). B-i-iv) Score-1 (red box) with DEJ involvement as an additional finding (green arrow and red braces), correlating to histology -DEJ is not sharp below yellow line (red braces). C-i) score-2.ii) & iii) Deep bowl-shapedlesiontapering towards the DEJ (white arrows). DEJ is not visible.iv) Bowl shaped chalky white lesion (red box) up to DEJ, correlating with the SS-OCT.
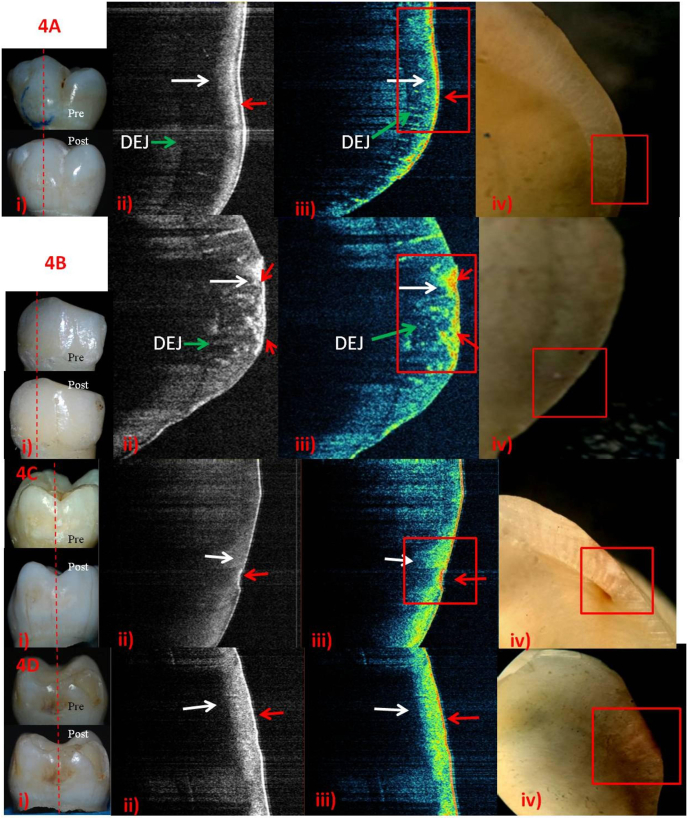
Fig. 4.
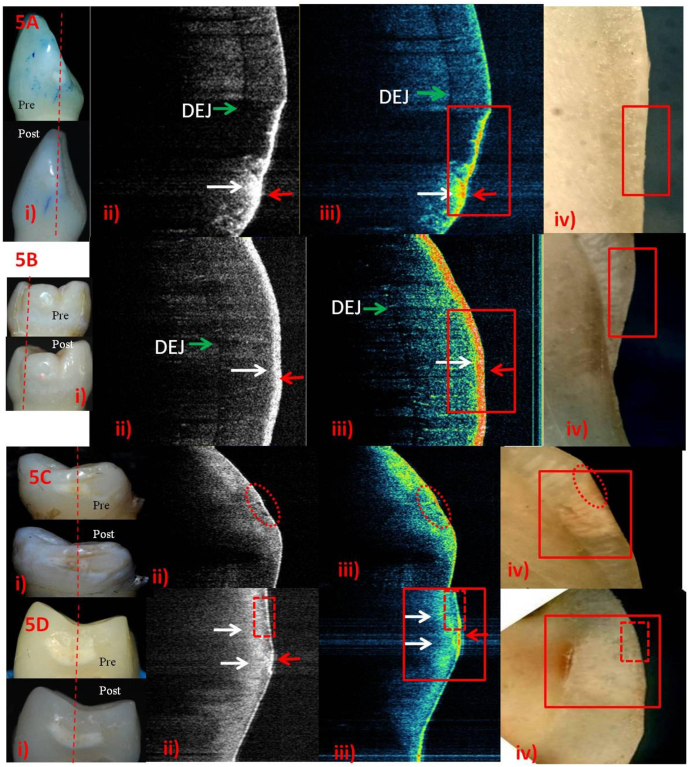
Post-remineralization ICDAS-II score-1&2 using FVA-i) score-1. ii) &iii) Rapid remineralization (Red arrow) above surface. Outer third of subsurface -initial remineralization (white arrow).iv) No identifiable remineralization.B-i) score-1. Roughened surface - active lesion.ii) & iii) Yellow interspersed with thicker red on the surface and in outer half of subsurface -rapid surface remineralization (red arrow). iv) Roughened surface but no identifiable remineralization.C-i) score-2. ii) & iii) Rapid surface remineralization (red arrow) and initial remineralization (white arrow)respectively.iv) Score-2 involving DEJ. D-i) Pigmented score-2. ii) & iii) Bright-white and line of red on the surface (red arrow). Dense, thicker band on the outer half of subsurface -increased remineralization with time (white arrow). Band like remineralization of subsurface - reduced depth of pigmented ECL. DEJ, visible as a faint line.iv) Brown colour on surface and chalky white lesion - healed or no priorly involved DEJ.
Fig. 5.
Post-remineralization ICDAS-II score-1&2 using CPP-ACP
A-i) score-1, rough surface. ii) & iii) Outer third of subsurface (white arrow) - initial remineralization. Active lesion (red arrow) -rapid surface mineralization. iv) Intact surface but no identifiable remineralization.B-i) score-1. ii) & iii) Band in the outer half of subsurface; band of red interspersed with yellow (between white and red arrows)- dense subsurface remineralization at 6 weeks. iv) Outer subsurface as smooth with chalky white enamel in inner two third.C-i) score-2 (both white and pigmented areas) – no marked improvement. ii) & iii) Outer half of enamel -initial subsurface remineralization. Reduced depth of demineralized subsurface & pigmented surface, has minimal surface remineralization (red arrow) with almost healed subsurface (white arrow). DEJ became visible.iv)Small area of smooth subsurfacecorrelate to the area with white and red arrows in SS-OCT. Chalky white area around smooth area up to DEJ is observed.D-i) score-2. ii) & iii) Majorly blue on the surface depicting mature phase of remineralization. Yellow with red, site of active lesion (red arrow). Blue coloured subsurface (red box) -mature phase; beneath this, yellow -remineralizing front of the lesion (white arrows). iv) Chalky white lesion involving DEJ. Activity observed in SS-OCT is not identifiable.
Fig. 6.
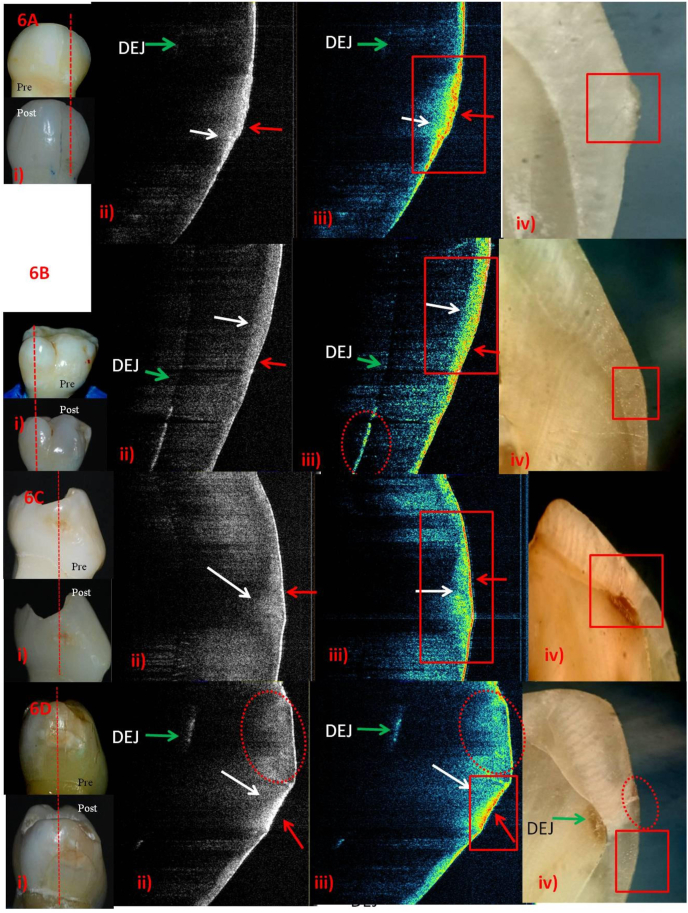
Post-remineralization ICDAS-II score-1&2 using NHP.
A-i) score-1.ii) & iii) Rapid mineralization (red arrow). Outer third of subsurface - initial remineralization (white arrow). DEJ became faintly visible. iv) Elevated surface correlating SS-OCT.B-i) score-1.ii) & iii) Red interspersed with blue and yellow on surface - initial to mature remineralizing phase (red arrow). Yellow interspersed with red in outer third of subsurface (white arrow). Accidental finding of DEJ involvement with apparently normal surface (broken red circle).iv) No demineralized ECL, correlating SS-OCT.C-i) score-2.ii) & iii) Layer of red - rapid remineralization of surface(red arrow). Bowl shaped less dense white (grey scale) and blue & yellow in the outer half of enamel-initial to mature phase of subsurface remineralization (white arrow). iv) Chalky white ECL involving DEJ. D-i) score-2.ii) & iii) Surface -mature phase of remineralization (red arrow). Less dense white and blue with yellow in the outer half of subsurface - initial to mature phase of remineralization (white arrow). Accidental finding of DEJ involvement underneath normal surface (green arrow).iv) Chalky white inner subsurface underneath smooth outer third with DEJ involvement correlate SS-OCT.
Fig. 7.
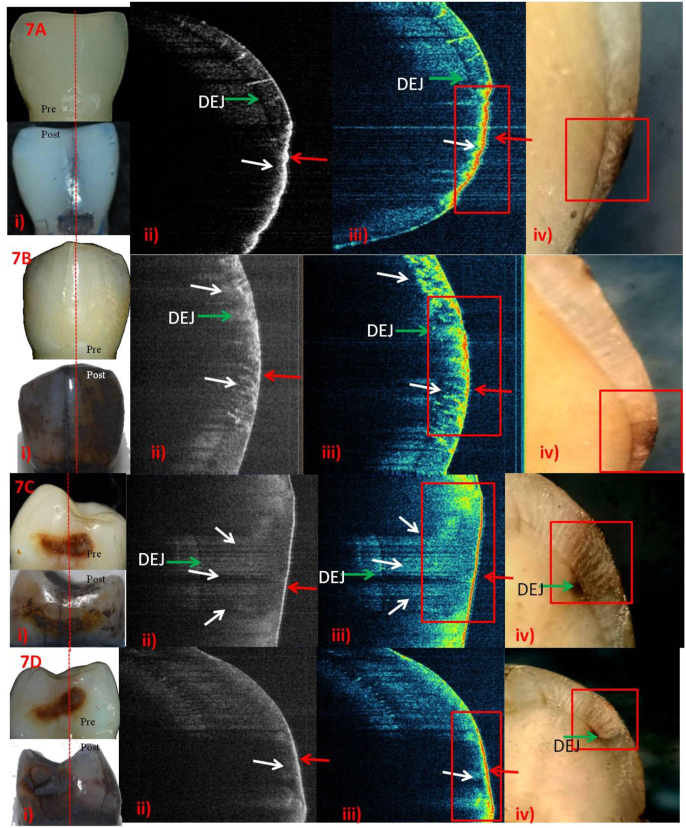
Post-remineralization ICDAS-II score-1&2 using SDF.
A-i) score-1.ii) & iii) Rapid surface remineralization on active lesion (red arrow). Outer third of enamel-initial phase of subsurface remineralization (white arrow). Additionally, mineral uptake, seen as yellow in the cracks of enamel.iv) Black staining on the surface and subsurface.B-i) score-1.ii) & iii) Red interspersed with yellow on the surface - initial phase of surface remineralization (red arrow). Dense yellow interspersed with red in the subsurface -almost full depth remineralization with time (white arrows). iv) Stained full thickness enamel up to DEJ.C-i) score-2.ii) & iii) Rapid surface remineralization (red arrow). Dense-yellow in the outer half of enamel, near the red line -initial phase of subsurface remineralization (white arrow). iv) Black stained lesion and DEJ including part of dentine.D-i) score-2.ii) & iii) Rapid surface remineralization (red arrow). Outer half of enamel - initial phase of subsurface remineralization (white arrow). iv) Black stained lesion and DEJ including part of dentine.
Fig- 3: ECLs imagesin grey-scale showed area of high reflectivity as bright-white and low reflectivity as dark area in sound enamel (score-0). Surface of ECL was brighter than sound enamel. The subsurface of enamel having ECLhas increased depth of bright-white compared to that of in sound enamel. Converting same grey-scale image to the false-colour ones, bright-white area corresponded to yellowish-green in ECL and transparent deep-blue in neighboring sound enamel. Score-1 demonstrated linear but curved lesion front, limited to outer third of enamel with clear cut lesion boundary from sound subsurface. DEJ was clear and sharp in sound enamel and in one score-1 sample (Fig. 3 A, ii-iv) but not in another one, where DEJ was shown as involved (Fig. 3B, ii-iv).Score-2 showed increased lesion depth (bowl shape) as a tapering bright area through subsurface, making the estimation of extent of lesion difficult and disappearance of DEJ underneath (Fig. 3C, ii-iv). Histology images correlated well with SS-OCT images except DEJ involvement was visible in score-2 in histology but not in SS-OCT; and surface activity was evident in SS-OCT but not in histology.
Fig. 4, Fig. 5, Fig. 6, Fig. 7: The A&B (score-1); and C&D (score-2) rows represent 2- and 6-weekspost-remineralization images respectively. Digital photographs of ICDAS-II surface examinationpre- and post-remineralization, grey-scale and false-colour SS-OCT images followed by histology images are shown in columns i, ii, iii and iv respectively.
The tomographic SS-OCT images displayed some additional in-situ findings as well i.e. developmental demineralization and hidden DEJ involvement (Fig. 3A&B; 6B&D).
Different stages of remineralization could be observed in false-colour SS-OCT images compared to grey-scale ones. On the basis of colours, there was better superficialprecipitation of minerals in FV (Fig. 4A,ii& iii) and SDF (Fig. 7A,C&D, ii & iii)and little lesser in NHP (Fig. 6).Active lesions, visually as rough surface, depicted more uptake of minerals in SS-OCT (Fig. 4B,C; 5A,D; 6A; 7A,D). NHP showed overgrowth of apatite crystals as elevated surface on active lesion. NHPand SDF showed mineralizationof almost entire subsurface (Fig. 6&7,D) while CPP-ACP in the outerhalf (Fig. 5 A-D). SDF penetrationuptoDEJwasvisible as black in histology (Fig. 7A-D, iv).
4. Discussion
This work was performed on primary teeth as caries evolution in primary teeth is faster than in permanent teethnecessitatingnon-invasive progressive evaluation.20 A system with axial resolution of the order of 10 μm would be advantageous to promote early detection and monitor the process of remineralization.This research was performed on natural ECLs making it a unique study. The freshly extracted teeth were stored frozen to prevent any further surface activities to rule out any effect of storing solution on enamel surface, owing to difference in the ionizing product of enamel and storing solution.1, 19 Because of the high resolution of SS-OCT it was important to fix the de/remineralizing activity in enamel.
The sound enamel was almost transparent in SS-OCT images while demineralization increased signal attenuation, as dissolution of minerals lead to increased porosity and hence increased scattering at this microinterfaces.6 Glycerin, applied on the enamel surfacepreserved surface hydration more effectively compared to our previous study1 and thereby improving the efficiency of SS-OCT to determine minimal changes in relative mineral volume on surface as well as subsurface. Glycerin was found to be the best medium by Jones21 as well. Shimada et al22 and Fried et al23also used themedium with intermediate refractive index to reduce strong reflection from the surface.
Excellent visual correlation was found between SS-OCT and histology images of ECLs except that the surface activity was not visible in histology and DEJ was not visible in deeper lesions in SS-OCT.This correlation is in general agreement with previous studiesby Ibusuki et al8 Nakagawa et al10 and Shimada et al22 The linear but curved lesion front of score-1suggested its progression towards DEJ along the direction of enamel rods which correlated well to the chalky white area in histology (Fig. 3A, B). Ibusukiet al8 observed ECLs with typical bowl or linear appearance with SS-OCT.We found all thescore-1 had linear shaped lesions with DEJ visible while deeper lesions i.e.score-2(Fig. 3C), a tapering bright area was seen through subsurface, making the estimation of extent of lesion difficult. This observation was comparable to that of Nakagawa et al10 that the depth of superficial enamel caries could be easily discriminated in SS-OCT, but not so in deep caries. Nonetheless, thedeeplesions converging towards DEJ, were similar to the ‘bowl shaped ECL’ of Ibusukiet al8 and ‘V shaped ECL’ of Vaswani et al1 DEJ was not observed in score-2 images because of the increased scattering of light from increased depth ofdemineralized zone. Histology confirmed the bowl pattern of lesion involving DEJ.Some additional in-situ findings (Fig. 3A&B; 6B&D) inconclusive otherwise by both visual ICDAS-II and histology proves the potencyof SS-OCT to modify the treatment strategy.
By comparingremineralization efficacy of FV, a gold standard, to recently devised CPP-ACP, NHPandSDF, this study becomes unique as to our best of knowledge there are no studies that compares tomographic infiltration of these agentswith SS-OCT according to ICDAS-II criteria and validating it with histology. Despite various compositions each material was found capable of remineralizing the ECLs. Grey-scale or false-colour images of samples display different shades of grey/colours according to different reflectivity of back-scattered light, which in turn is associated with increased or decreased porosity within the crystal lattice of enamel. Nonetheless, future researches of A-scans would be necessary to know about colour difference as per density of remineralization. Because diminished visibility of DEJ was found correlated with increased depth of demineralization, an effective remineralizing agent would be that makes DEJ reappear post-remineralization. This progressive tomographic monitoring was only possible with non-invasive technique such as SS-OCT.Previous quantitative and qualitative researches on remineralization helped to discern the colour codes in SS-OCT images.11, 14, 24 The researcheswith surface hardness,24 scanning electron microscope (SEM), OCT and CLSM7, 8, 10 concluded that FV causes rapid remineralizationon the surface while less effective towards subsurface. On comparing ourgrey-scale SS-OCT images to that of previous studies and to our false-colour images, we concluded that red dots lining the surface, yellow on the subsurface and deep blue immediately below the surface defined the densely mineralized, theinitial mineralizing and thematurephasesofremineralization respectively (Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7).
In general, possible mechanism could be that the surface of ECLgot repaired to an almost similar mineral density as that of sound enamel. Yellow colour or reduced intensity on the subsurface denoteddecrease in overall pore volume along with increased mineral content in the lesion body but yet to reach up to original mineral content and crystal organization. This observation is consistent with previous caries remineralization studies24, 25, 26, 27 that in a remineralizingcarious lesion, deeper porosities accumulate mineral to a limited extent than the surface zone. Infact, it is suggested that the successful remineralization of surface decreases the continued diffusion of ions into the deeper region of lesion.
FV showed excellent potential to remineralize the surface. Score-1(Fig. 4A) post-remineralization2 weeks showed an additional outer growth layer andinfiltration of minerals into subsurface was minimal. Jones et al7 had similar results, andidentifiedouter growth layer had mineral volume close to that of sound enamel with highly crystalline phase of apatite. Score-1 at 6 weeks (Fig. 4B)haddisappearance ofouter layer with increased yellow in the subsurface, depictingsubsurfaceinfiltrationof minerals. Probably the outer growth layer at 2 weeks as seen in ours and others’ studies worked like a reservoir of minerals12for supplying minerals to the body of lesion, making this layer disappear in 6 weeks in SS-OCT. The longer duration of our study made it possible to observe this phenomenon.Score-2 (Fig. 4C)post-remineralizationshowedincreased uptake of minerals within the body of lesion owing to increased porosity in score-2. Score-2 samples of 6 weeks (Fig. 4D) showed increased contrast with dense subsurface yellow more than 2 weeks owing to more infiltration of minerals with time.
Surface remineralizing efficacy of FV is in accord with the previous studies byVyavhareet al,24 Babu et al11 and Choksi et al25 who while experimenting on artificial caries found rapid deposition of fluorapatite crystals forming a firm layer which although is more resistant to further demineralization but also prevents furtherinfiltration of calcium and phosphate ions required to rebuild the lesion depth. Present study supported this mechanism post-remineralization 2 weeks samples for scores-1&2 as the subsurface remineralization with FV was comparatively minimal. Choksi et al25 using confocal microscope found no difference in remineralization at 20, 40 days of remineralization using FV, but the current study showed increasedremineralizationin 6 weeks (42 days).Nevertheless, remineralization did not reach up to normal level. Our findings suggest that topographic criteria of assessing de/remineralization may not be conclusive as related to cross-sectional mineral volume of ECL.
In case of CPP-ACP, score-1 at 2 weeks (Fig. 5A) showedbetter subsurface than surface remineralizationwhich was just reverse of FV probably because CPP-ACP could penetratethedepth of ECL.Score-1samples at 6 weeks (Fig. 5B)showed better subsurface remineralizationcompared to 2 weeks samples, with a consistent thick band of red in outer third of ECL. However, subsurface remineralizing efficacy was tapering towards the DEJ. The score-2 samples at 2 weeks (Fig. 5C)demonstrated similar subsurface remineralization as in score-1eventhough DEJ was not visible.Six weeks samples (Fig. 5B)showed that the surface immediately below the remineralizing surface had similar contrast as that of sound enamel. Vyavhareet al24 also observed no outer layer deposition on topographic SEM examination post-remineralization 12 days. Nonappearance of DEJ at 6 weeks but yellow in subsurface shows remineralizing process was continuing yet has not reached up to that of sound enamel. Either some more time is needed or CPP-ACP is not able to penetrate full thickness of score-2 lesion.Theconsistent or steady subsurface remineralization was similar to theresultsby Babu et al11 probably because CPP-ACP stabilized calcium and phosphate in a metastable form thus facilitated high concentration of ions to diffuse into ECLs.
In case of NHP,(Fig. 6) outer growth layer was observed asanelevated cluster of red and yellow dots on active site.In general, NHP showed surfacered line in false-colour, similar to FV, but only as bright-white in grey-scale, dissimilar to FV at 2 weeksscore-1samples(Fig. 6A).Najibfrad et al28 found NHP inducing a consistent enamel caries remineralization by precipitating homogeneous synthetic hydroxyapatite nano-crystals on the surfaces of enamel which is chemically bond to natural enamel crystals. The un-observable outer layer in grey-scale may be because the layer chemically bounded to natural enamel crystals. Interestingly, the hardness and elastic modulus of the restored enamel was found similar to those of natural ones.Similar were the findings by Vyavhareet al24 in their SEM and microhardness study. Bhusari and Sharma19 inanatomic force microscope study found rapid deposition and growth of flat or needle shaped hydroxyapatite on the exposed ‘anisotropic’ apatite crystal template on bleached enamel surface. The elevated surface in SS-OCT/histology is probably the growth of needle like hydroxyapatite crystal on the applied nanohydroxyapatite templateat active ECL site. Nevertheless, outer growth layer was missingin score-1sample at 6 weeks (Fig. 6B), yet the subsurface remineralization was more than 2 weeks sample. Najibfrad et al28 also observed that the progressive transfer of hydroxyapatite nano-crystals from the new apatite coating to the lesion maintaining high concentration gradient of calcium and phosphate ions in the subsurface, facilitating remineralization over time. Our study supports their finding. Aziznezhadet al29 in his remineralization study compared resin infiltrant, FV and NHP to assess surface hardness.The surface remineralizationof NHP was lesser than fluoride but somewhat equivalent to CPP-ACP. On the other hand, Vyavhareet al24 found almost equal surface hardness of enamel remineralizedwith NHP and 1000 ppm fluoride and that of CPP-ACP was lesser than former two, very similar to our observations.NHP penetrates the enamel pores and acts as a template in precipitation and continuously attracts a large amount of calcium and phosphate from remineralization solution to fill the vacant positions of apatite crystalspromoting crystal growth and integrity.14
In case of SDF,(Fig. 7), score-1 at 2 weeks the surface remineralization was superior to 6 weeks probably because of the active lesion, as demonstrated by the roughened surface visually which took up intense black stain onto the lesion(Fig. 7A). Also, at 2 weeks probably because of the reduced enamel thickness at the cervical region. The subsurface mineralization increased with time, evident till DEJThe subsurface mineralization was better than FV or CPP-ACP in score-1as yellow dots infiltrated till the DEJ(Fig. 7B). Histology confirmed it as black staining of whole ECL. SDF, score-2 at 2 weeks (Fig. 7C) showed surface remineralizationwith blue subsurface in pigmented ECL. At 6 weeks, surface remineralization was similar to that of 2 weeks (Fig. 7, C &D) with blue subsurface in pigmented ECL. Blue subsurface depictedmineralvolumeand crystallinity of hydroxyapatitecomparable to that of sound enamel. As pigmented ECL has reduced depth of ECL,1 SDF could remineralize this already mineralized lesion to the level of sound enamel, while in nearby subsurface area, initial stage of remineralization was evident as yellow (Fig. 7C). A recent review30 hypothesized that silver in SDF may diffuse into the hydroxyapatite crystal and produce silver-containing hydroxyapatite. Yu et al27 in micro-CT assessment found that SDF showed stronger remineralizing effects than NaF, similar to our study. Theformation of insoluble AgCl and metallic silver was confirmedvia the X-Ray diffraction. This phenomenon was evident in histology as black staining of the entire ECLupto and including DEJ both in score-1 & 2 (Fig. 7, A-D).
Most of the OCT studies used artificial caries with a polished flat surface which have a band like appearance of de/remineralized enamel. In a quest to observe surface and subsurface activity of natural ECLs we tried our best to fix the activity of natural lesion owing to high resolution of SS-OCT. There was increased infiltration of minerals from surface to subsurface from 2 to 6 weeks.Ouroverall results correlate with the study by Mandurah et al9 where they found significant correlation with cross-sectional nano-hardness and optical depth of remineralization depicted by SS-OCT images which increased with time but not up to the level of sound enamel. They also noticed that superficial (10μ) layer was harder when fluoride was added in remineralizing agent. Their result confirms our observations that red in false-color images denoted highly mineralized zone duringremineralization, probably mimicking natural hydroxyapatite chemically. Subsurface remineralization increased with loss of outer growth layer, which probably worked as reservoir of minerals. We can say thatonce ECL occurs, there is a need of regular remineralization probably as samples at 6 weeks started showing colour of sound enamel immediately below surface.NHP and SDF both showed blue subsurface under pigmented surface together with faint reappearance of DEJ reconfirms that pigmented ECLs acquired mineral volume and crystal lattice to that of sound enamel (Fig. 6D, 7C). Remineralizing activity in SS-OCT demonstrated pigmented ECL had shallow bowl-shaped lesion, the finding similar to our previous study.1Yet more studies will confirm the observation.
On the basis of our observations a combination of CPP-ACP followed by fluoride can be an efficient regimen for remineralization of shallow ECLs(score-1)to ascertain the bio-availibilty of calcium and phosphate ions on surface and subsurface. While for deep ECLs (score-2) NHP and SDF would be better for effective deeper subsurface remineralization.It will be interesting to monitor ECLs in-vivo with SS-OCT to assess the time required for full depth remineralizationupto the mineral level of sound enamel.Lack of established algorithm for observations and developing software to quantify tomographic mineral volume content and high cost of SS-OCT are the limitations for generalizability of work. However, recently in-vivo clinical study has been conducted by Ibusiki et al8 on buccal WSLs using an experimental probe of SS-OCT and found clear in-depth images of these natural WSLs that would not only help observing the internal structure of WSLs, but also enable quantitative assessment of WSL depth and recommended such data could be considered in the clinical management of WSLs. Shimada et al22 in another in vivo study, revealed hidden proximal carious lesions through occlusal surface of posterior teeth using SS‐OCT and bitewing radiographs, among these SS-OCT was found to be a more reliable and accurate method than bitewing radiographs for the detection and estimation of the depth of proximal lesions in the clinical environment. The collective outcome of various in vitro and in vivo studies bring hope for the feasibility of commercial OCT probe in near future. Non-ionizing real-time tomographic imaging of vital tissues, and broad application of OCT in dentistry will prove to be most sustainable tool to non-invasively monitor any normal or pathologic vital tissues in clinics.
5. Conclusion
Within the limitation of the study, it can be said that although ICDAS-II criteria is effective for visual ECL assessment, but it may not always correlate with sub-surface demineralization and/or DEJ involvement especially in ICDAS-II score 2.
Surface remineralization with FV and SDF was found comparable, followed by NHP and CPP-ACP at 2 and 6 weeks. Deepest subsurface remineralization was seen with SDF followed by NHP and CPP-ACP. Subsurface remineralization with NHP and SDF in pigmented ECL, was almost equivalent to that of sound enamel. However, progressive remineralization was found from 2 to 6 weeks with all the agents tested. The results proved SS-OCT as an efficient tool to assess surface/subsurface de/remineralization activity in ECLs effectively.
Acknowledgement
Our sincere gratitude to Dr. Jyotirmoy Chatterjee, Professor, Medical Imaging and Theragnostic, School of Medical Science & Technology, Indian Institute of Technology, Kharagpur, West Bengal, India for permitting to work in his laboratory. We are thankful to AyanGope, JRF in the same laboratory for his assistance during SS-OCT work in the laboratory. We are especially grateful to Dr. B.T. Amaechi for his great support as an expert while preparing the manuscript.
References
- 1.Vaswani S., Sharma D.S., Mishra S., Sharma S. Histologic validation of ICDAS‐II and polarization sensitive optical coherence tomography to detect smooth surface early carious lesions. Int J Pediatr Dent. 2019;29(2):193–202. doi: 10.1111/ipd.12440. [DOI] [PubMed] [Google Scholar]
- 2.Ismail A.I., Sohn W., Tellez M. The International Caries Detection and Assessment System (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol. 2007;35:170–178. doi: 10.1111/j.1600-0528.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Abrams S.H., Sivagurunathan K.S., Silvertown J.D. Correlation with caries lesion depth of the canary system, DIAGNOdent and ICDAS II. Open Dent J. 2017;11:679–689. doi: 10.2174/1874210601711010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trevisan TC, Andrade MC, Presoto CD, Oliveira OB, Andrade MF, BortolattoJF.Hidden caries. A critical review. Sci J Dent2015;2:33-36.
- 5.Bader J.D., Shugar D.A., Bonito A.J. Systematic reviews of selected dental caries diagnostic and management methods. J Dent Edu. 2001;65(10):961–968. [PubMed] [Google Scholar]
- 6.Katkar R.A., Tadinada S.A., Amaechi B.T., Fried D. Optical coherence tomography. Dent Clin N Am. 2018;62(3):421–434. doi: 10.1016/j.cden.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Jones R.S., Darling C.L., Featherstone J.D., Fried D. Remineralization of in vitro dental caries assessed with polarization-sensitive optical coherence tomography. J Biomed Opt. 2006;11(1):1–9. doi: 10.1117/1.2161192. [DOI] [PubMed] [Google Scholar]
- 8.Ibusuki T., Kitasako Y., Sadr A., Shimada Y., Sumi Y., Tagami J. Observation of white spot lesions using swept source optical coherence tomography (SS-OCT): in vitro and in vivo study. Dent Materials J. 2015;34(4):545–552. doi: 10.4012/dmj.2015-058. [DOI] [PubMed] [Google Scholar]
- 9.Mandurah M., Sadr A., Shimada Y. Monitoring remineralization of enamel subsurface lesions by optical coherence tomography. J Biomed Opt. 2013;18(4):1–7. doi: 10.1117/1.JBO.18.4.046006. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa H., Sadr A., Shimada Y., Tagami J., Sumi Y. Validation of swept source optical coherence tomography (SSOCT) for the diagnosis of smooth surface caries in vitro. J Dent. 2013;41:80–89. doi: 10.1016/j.jdent.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Babu K.G., Subramaniam P., Teleti S. Remineralization potential of varnish containing casein phosphopeptides-amorphous calcium phosphate with fluoride and varnish containing only fluoride: a comparative study. Saudi J Oral Sci. 2018 1;5(1):35–40. [Google Scholar]
- 12.Kidd E., FejerskovO, editors. Dental Caries:The Disease and its Clinical Management. John Wiley and Sons; 2009. [Google Scholar]
- 13.Zhou C, Zhang D, Bai Y, Li S. Casein phosphopeptide-amorphous calcium phosphate remineralization of primary teeth early enamel lesions. J Dent2014;41:21-29. [DOI] [PubMed]
- 14.Huang S.B., Gao S.S., Yu H.Y. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Materials. 2009 5;4(3):1–5. doi: 10.1088/1748-6041/4/3/034104. [DOI] [PubMed] [Google Scholar]
- 15.Delbem A.C., Bergamaschi M., Sassaki K.T., Cunha R.F. Effect of fluoridated varnish and silver diamine fluoride solution on enamel demineralization: pH-cycling study. J Appl Oral Sci. 2006;14(2):88–92. doi: 10.1590/S1678-77572006000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogrinc G., Davies L., Goodman D., Batalden P., Davidoff F., Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016 Dec;25(12):986–992. doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T et. al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology2016;86(24):2303–2309.doi: 10.1212/WNL.0000000000002774. [DOI] [PMC free article] [PubMed]
- 18.Krithikadatta J., Gopikrishna V., Datta M. CRIS Guidelines (Checklist for Reporting In-vitro Studies): a concept note on the need for standardized guidelines for improving quality and transparency in reporting in-vitro studies in experimental dental research. J Conserv Dent. 2014;17:301–304. doi: 10.4103/0972-0707.136338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhusari C.P., Sharma D.S. Pattern of hydroxyapatite crystal growth on bleached enamel following the application of two antioxidants: an atomic force microscope study. J Clin Pediatr Dent. 2017;41(1):38–47. doi: 10.17796/1053-4628-41.1.38. [DOI] [PubMed] [Google Scholar]
- 20.Maia A.M., Fonseca D.D., Kyotoku B.B., Gomes A.S. Characterization of enamel in primary teeth by optical coherence tomography for assessment of dental caries. Int J Paediatr Dent. 2010;20(2):158–164. doi: 10.1111/j.1365-263X.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones R.S. Doctoral Dissertation, University Of California; San Francisco: 2006. Near Infrared Optical Imaging of Early Dental Caries. [Google Scholar]
- 22.Shimada Y., Nakagawa H., Sadr A. Noninvasive cross-sectional imaging of proximal caries using swept-source optical coherence tomography (SS-OCT) in vivo. J Biophotonics. 2014;7:506–513. doi: 10.1002/jbio.201200210. [DOI] [PubMed] [Google Scholar]
- 23.Fried D., Xie J., Shafi S., Featherstone J.D., Breunig T., Le C.Q. Imaging caries lesions and lesion progression with polarization-sensitive optical coherence tomography. J Biomed Opt. 2002;7:618–627. doi: 10.1117/1.1509752. [DOI] [PubMed] [Google Scholar]
- 24.Vyavhare S., Sharma D.S., Kulkarni V.K. Effect of three different pastes on remineralization of initial enamel lesion: an in vitro study. Journal of Clin Pediatr Dent. 2015;39(2):149–160. doi: 10.17796/jcpd.39.2.yn2r54nw24l03741. [DOI] [PubMed] [Google Scholar]
- 25.Chokshi K., Chokshi A., Konde S. An in vitro comparative evaluation of three remineralizing agents using confocal microscopy. J Clin Diagnos Res. 2016;10(6):1–9. doi: 10.7860/JCDR/2016/18191.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esfahani K.S., Mazaheri R., Pishevar L. Effects of treatment with various remineralizing agents on the microhardness of demineralized enamel surface. J Dent Res Dent Clin Dent Prospects. 2015;9(4):239–245. doi: 10.15171/joddd.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu O.Y., Mei M.L., Zhao I.S., Li Q.L., Lo E.C., Chu C.H. Remineralisation of enamel with silver diamine fluoride and sodium fluoride. Dent Mater J. 2018;34(12):344–352. doi: 10.1016/j.dental.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Najibfard K., Ramalingam K., Chedjieu I., Amaechi B.T. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J Clin Dent. 2011;22(5):139–143. [PubMed] [Google Scholar]
- 29.Aziznezhad M, Alaghemand H, Shahende Z et. al. Comparison of the effect of resin infiltrant, fluoride varnish and nanohydroxy apatite paste on surface hardness and streptococcus mutans adhesion to artificial enamel lesions. Electron Physician2017;9(3):3934-3942. [DOI] [PMC free article] [PubMed]
- 30.Mei M.L., Lo E.C.M., Chu C.H. Arresting dentine caries with silver diamine fluoride: what's behind it? J Dent Res. 2018;97:751–758. doi: 10.1177/0022034518774783. [DOI] [PubMed] [Google Scholar]