Abstract
Early identification of subjects running an increased risk of contracting COPD enables focus on individual preventive measures. The slope of the alveolar plateau of the single-breath nitrogen washout test (N2-slope) is a sensitive measure of small-airway dysfunction. However, its role remains unexplored in predicting hospital admission or death related to COPD, i.e. incident COPD events, in relation to the presence of various respiratory symptoms.
A random population sample of 625 men, aged 50 (n=218) or 60 years (n=407), was followed for 38 years for incident COPD events. At baseline, a questionnaire on respiratory symptoms and smoking habits was collected, spirometry and the single-breath nitrogen test were performed, and the N2-slope was determined. Proportional hazard regression (Cox regression) analysis was used for the prediction model.
The N2-slope improved the prediction of COPD events significantly beyond that of respiratory symptoms weighted all together and other covariates (hazard ratio 1.63, 95% CI 1.20–2.22; p<0.005), a prediction applicable to subjects without (p=0.001) and with (p<0.05) airway obstruction. Dyspnoea and wheezing were the most predictive symptoms. The combination of the N2-slope and number of respiratory symptoms notably resulted in an effective prediction of incident COPD events even in nonobstructive subjects, as evidenced by a predicted incidence of ∼70% and ∼90% for a very steep N2-slope combined with many respiratory symptoms in subject without and with airway obstruction, respectively.
The alveolar N2-slope should be considered in the critical need for further research on early diagnosis of COPD.
Short abstract
The N2 slope of the single-breath nitrogen test predicts incident COPD events as well as or better than respiratory symptoms among subjects without and with airway obstruction. Combining N2 slope and symptoms results in effective prediction. https://bit.ly/3dYJdu1
Introduction
COPD is usually diagnosed late, often when the symptomatic burden increases or when the disease exacerbates, often leading to hospital admission. Because it is a progressive disease, there is increasing interest in the early identification of COPD to enable therapeutic counteraction of disease progress [1]. Respiratory symptoms predict hospital admission and mortality due to COPD, even in subjects who have not yet developed airflow obstruction [2, 3].
Symptomatic current or former smokers with preserved pulmonary function were shown to have exacerbations, activity limitation and evidence of greater airway-wall thickening on computerised tomography scan, and to use a range of respiratory medications, although they did not meet the current criteria for COPD [4]. Likewise, in a study of 97 955 individuals, Çolak et al. [5] found that chronic respiratory symptoms significantly predicted hospital admissions and death due to COPD among subjects with normal spirometry. Importantly, however, 99% of individuals with normal spirometry and respiratory symptoms were not admitted to hospital during the median follow-up time of 8.8 years. Thus, although respiratory symptoms are strong predictors of COPD events and commonly precede spirometric abnormalities, more sensitive techniques are needed for early identification of subjects at risk of COPD-related events later in life.
COPD develops at an early stage in small airways (i.e. airways with an internal diameter <2 mm) [6–9]. Therefore, diagnosis of small-airway dysfunction is an important step in the anticipation of risk of development of COPD. However, the small airways can be obstructed long before ordinary spirometry measures become abnormal or respiratory symptoms arise [10].
The alveolar slope of the single-breath nitrogen washout test (the N2-slope) is one promising alternative to other diagnostic tests of small airway dysfunction, as studies on excised lungs have shown that structural changes in small airways are related to abnormal N2-slopes [11, 12]. Furthermore, the N2-slope is generated in the very peripheral airways [13]. In addition, we have shown previously that combining the N2-slope with forced expiratory volume in 1 s (FEV1) considerably improved the prediction of COPD events later in life compared to either test alone [14].
The aim of the present study was to test the predictive power of the N2-slope in combination with various respiratory symptoms on hospital admissions and deaths related to COPD (incident COPD events) in a random population sample of middle-aged men with long-term follow-up.
Methods
Study population
The Study of Men Born in 1913 and 1923 is based on a systematic general population sample of 50-year old men (n=226) and 60-year old men (n=787) living in Gothenburg, Sweden, in 1973. The present study population is based on a subset of the systematic sample (n=628) [15]. Oral informed consent, which was the standard procedure at the time, was obtained from all study subjects. The study was first approved by the research ethics committees in Gothenburg and Uppsala, Sweden, and later by the national research ethics board.
The lung function tests, respiratory symptom and smoking habit investigations were performed at the study baseline. Three subjects had a COPD event before the study baseline in 1973, leaving 625 subjects as the study population, 218 men aged 50 years and 407 men aged 60 years. There were missing data on spirometry, N2-slope, smoking history or respiratory symptoms for 34 subjects (table 1).
TABLE 1.
Characteristics of the study population
| All subjects | No incident COPD events # | Incident COPD events # | p-value for no COPD – COPD difference | ||||
| Subjects n | 615 | 561 | 54 | ||||
| Person-years of observation | 13 553 | 12 717 | 836 | ||||
| Age at baseline (years) | 625 | 56.8±4.8 | 571 | 56.8±4.7 | 54 | 56.4±4.9 | 0.53 |
| BMI (kg·m−2) | 625 | 25.6±3.3 | 571 | 25.6±3.2 | 54 | 25.2±3.5 | 0.34 |
| FEV1 (% pred) ¶ | 615 | 88.7±16.4 | 561 | 90.2±15.0 | 54 | 72.2±20.9 | <0.0001 |
| FEV1/VC (% pred) ¶ | 615 | 99.6±11.4 | 561 | 100.0±9.8 | 54 | 87.0±17.8 | <0.0001 |
| N2-slope (% N2/L) + | 604 | 2.0±1.5 | 552 | 1.9±1.4 | 52 | 3.1±2.1 | <0.0001 |
| N2-slope (% pred) + | 604 | 162±118 | 552 | 154±110 | 52 | 253±155 | <0.0001 |
| Smoking habits | 625 | <0.0001 | |||||
| Never-smoker (%) | 125 | 20.0 | 121 | 21.2 | 4 | 7.4 | |
| Ex-smoker (%) | 194 | 31.0 | 181 | 31.4 | 13 | 24.1 | |
| Current smoker (%) | 306 | 49.0 | 269 | 47.1 | 37 | 68.5 | |
| Non-productive cough (%) § | 149 | 24.1 | 124 | 21.9 | 25 | 47.2 | <0.0001 |
| Productive cough (%) § | 139 | 22.5 | 115 | 20.3 | 24 | 45.3 | <0.0001 |
| Wheezing (%) § | 219 | 35.4 | 186 | 32.9 | 33 | 62.3 | <0.0001 |
| Dyspnoea (%) § | 160 | 26.0 | 131 | 23.3 | 29 | 54.7 | <0.0001 |
| Total symptom score ƒ | 619 | 2.0±2.6 | 556 | 1.8±2.4 | 53 | 4.4±3.2 | <0.0001 |
Data are presented as n or mean±sd, unless otherwise stated. Continuous variable differences were tested using the Mann–Whitney U-test; discrete variables were tested using the Chi-squared test. BMI: body mass index; FEV1: forced expiratory volume in 1 s; VC: vital capacity; N2-slope: alveolar slope of the single-breath nitrogen test. #: first admission to hospital or death related to COPD; ¶: according to Hedenström et al. [16]; +: according to Sixt et al. [17]; §: percentage of subjects reporting any of the symptoms in each symptom group; ƒ: mean of all the reported symptoms.
Lung function tests
Each subject performed two satisfactory slow vital capacity (VC) manoeuvres and three satisfactory forced vital capacity manoeuvres. The largest VC of the slow and forced manoeuvres and the largest FEV1 were used in the analyses. The FEV1/VC ratio was calculated from the highest values. 10 subjects failed to perform adequate manoeuvres and were excluded in the relevant analyses. Predicted normal values (% pred) and z-scores were computed according to Hedenström et al. [16] including predictions for VC. Airflow obstruction was defined by FEV1/VC less than lower limit of normal, i.e. z-score <−1.645.
The N2-slope was obtained by the single-breath N2 test and was calculated as the increase of the nitrogen concentration from the point where 825 mL (body temperature and pressure, saturated) had been expired from total lung capacity until the beginning of phase IV (closing point), divided by the corresponding expired volume. Two satisfactory tracings were attempted and the mean N2-slope was recorded. 21 subjects failed to perform at least one adequate N2 test (table 1). Predicted normal values were calculated according to Sixt et al. [17]. Details regarding the lung function tests have been described elsewhere [15, 18]. Quintiles of percentage of predicted normal values were calculated.
Respiratory symptoms
Symptom prevalence was measured using a translation of a questionnaire approved by the British Medical Research Council committee on the aetiology of chronic bronchitis [19]. The questionnaire comprised 12 items, including cough with or without expectoration, wheeze or squeaks and dyspnoea (for wording see supplementary table S1). For each of the 12 symptoms, subjects reported presence or absence of the symptom. Seven subjects did not respond to any of the symptom questions. The total number of reported symptoms (range 0–12) was recorded as a total symptom score. The analyses also included grouped symptoms: nonproductive cough (questions 1–3), productive cough (questions 4–6), wheezing (questions 7–10) and dyspnoea (questions 11–12). Furthermore, five symptom groups were defined according to number of reported symptoms: no symptoms, 1–2 symptoms, 3–4 symptoms, 5–6 symptoms and 7–12 symptoms.
Smoking habits
Smoking habits were categorised as follows: 1) never-smoker; 2) ex-smoker for >6 months; 3) currently smoking 1–14 g·day−1; 4) currently smoking 15–24 g·day−1; or 5) currently smoking >24 g·day−1. One cigarette was considered equivalent to 1 g of tobacco, one cheroot to 2 g of tobacco and one cigar to 5 g of tobacco. Pipe smokers were classified according to the number of grams of tobacco smoked per day. The smoking variable was used as adjustment variable.
Outcome measure
According to law, when Swedish residents, whether Swedish citizens or not, are discharged from hospital, discharge information including date of admission and discharge and discharge diagnoses must be entered in the nationwide Hospital Discharge Register. When Swedish residents, whether citizens or not, die anywhere in the world, date of death, underlying cause of death and all contributing causes of death are entered in the National Cause of Death Register. The study population data file was updated with data on hospital discharges and death with the National Hospital Discharge Register and the National Cause of Death Register from 1 January 1973 until 31 December 2011.
An incident COPD event was measured either as a first hospital admission with a diagnosis of COPD or related diagnoses [14]: n=18 (8.3%) among 50-year-olds and n=29 (7.1%) among 60-year-olds. Deaths from or with COPD for subjects with no previous hospital admission for the disease prior to 31 December 2011: n=3 (1.4%) among 50-year-olds and n=4 (1.0%) among 60-year-olds; altogether 47 (7.5%) hospital admissions and seven (1.1%) deaths.
Statistical considerations
Data were analysed using SAS Software version 9.3 (SAS Institute, Cary, NC, USA). Overall, <1% of the data used in these analyses were missing. Simple differences between groups were tested with the Mann–Whitney U-test for continuous data and the Chi-squared test for categorical data. FEV1 (% pred) and N2-slope (% pred) data were analysed as quintiles.
No statistical power analysis, i.e. the probability (β) to find a statistical association between variables if it exists, given the size of the study population, was performed when the study was planned, since no similar study was available. A post hoc power analysis based on data used in this article was performed, according to which >80% power (minimum requirement) would be obtained with 100 subjects. Based on the actual study population size (n=625), the statistical power would be >99.9%.
In the analyses of the effects of exposure on outcome, follow-up time was measured as number of days from baseline to outcome or end of follow-up. Hazard ratios (HR) for FEV1 (% pred) and N2-slope (% pred) on a first COPD event were computed with the SAS “Lifetest” procedure. The hazard rates of the groups with FEV1 (% pred) and the N2-slope (% pred) above or below mean value, respectively, were approximately proportional across time, allowing proportional hazards regression (Cox regression) analysis.
The FEV1 (% pred) and the N2-slope (% pred) measures were closely correlated (r=0.66), indicating that they might be measures of the same underlying process and thereby cause collinearity. Possible collinearity was measured with the SAS “Reg” procedure, but none was found, indicating that the two measures were affected by different underlying processes (e.g. large or small airways).
The influence of FEV1 quintiles and N2-slope quintiles on incident COPD events, adjusted for age at baseline and smoking habit score was tested with proportional hazards regression analysis. Censoring events included a first COPD event; death from COPD with no previous event; death from other causes; or no event at the end of follow-up, whichever came first, thereby adjusting for competing risk. The degree of potentiation between total symptom score and N2-slope quintiles was determined by comparing Wald's Chi-squared test for a variable based on summing the two variables (additive potentiation) and one based on multiplying the two variables (multiplicative potentiation), of which additive potentiation was significant, but not multiplicative. Figures 1–3 were compiled based on data from the proportional hazards regression model. All analyses were two-tailed and p<0.05 was considered significant. Wald's Chi-squared test was used as measure of exposure variable impact on outcome, since all exposure variables get one degree of freedom in Wald's Chi-squared test, irrespective of how they are graded, while the size of the hazard ratio is strongly dependent of grading.
FIGURE 1.
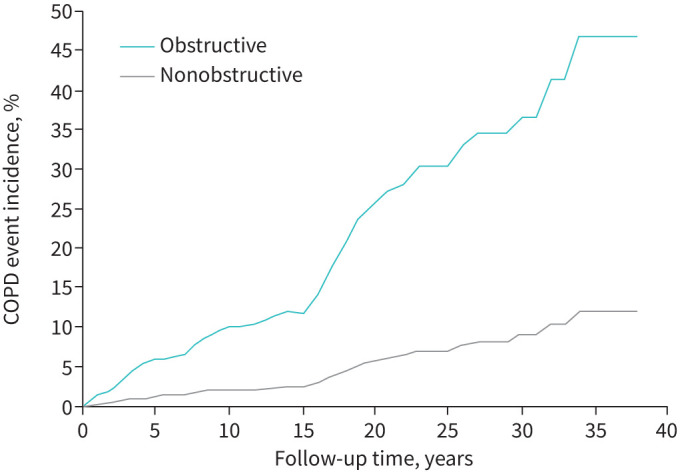
Cumulative first COPD event incidence across 38 years of follow-up among men who were nonobstructive and obstructive at baseline.
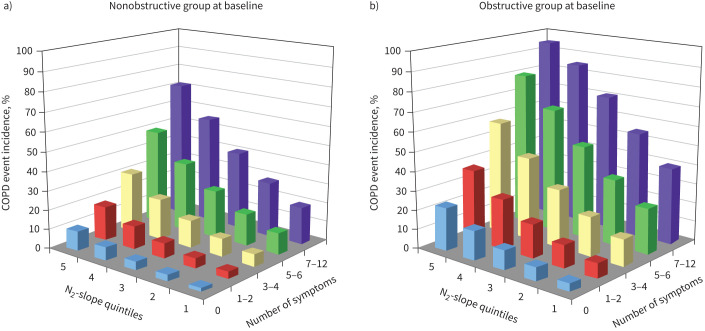
FIGURE 3.
Incidence rate of first admission to hospital or death related to COPD (COPD event incidence) during 38 years of follow-up in a) nonobstructive and b) obstructive men based on N2-slope quintiles and number of reported symptoms. Number of subjects reporting symptoms among nonobstructive subjects were: 0 n=232, 1–2 n=168, 3–4 n=68, 5–6 n=36, 7–12 n=28. The corresponding numbers among obstructive subjects were: 0 n=22, 1–2 n=19, 3–4 n=16, 5–6 n=10, 7–12 n=20.
Results
Table 1 presents the characteristics of the study population at baseline and subdivided according to incident COPD events. Lung function variables and symptom group prevalences differed significantly between subjects with no COPD events and subjects with COPD events during the follow-up.
Table 2 presents prediction of incident COPD events by total symptom score, FEV1 quintiles, N2-slope quintiles, age and smoking habit score in proportional hazards regression analysis. Total symptom score had the highest impact on the prediction, followed by N2-slope quintiles and FEV1 quintiles. Thus, total symptom score, N2-slope and FEV1 were independently and significantly related to the incidence of incident COPD events after adjustment for smoking habit score and age.
TABLE 2.
Multivariate proportional hazards regression of total symptom score, forced expiratory volume in 1 s (FEV1), N2-slope, attained age and smoking habit score on admission to hospital or death related to COPD
| Parameter estimate | Wald's Chi-squared # | p-value | Hazard ratio (95% CI) | |
| Total symptom score ¶ | 0.20 | 20.7 | <0.0001 | 1.22 (1.12–1.33) |
| FEV1 (quintiles)+ | −0.35 | 5.6 | <0.05 | 0.71 (0.53–0.94) |
| N2-slope (quintiles)§ | 0.49 | 9.5 | <0.005 | 1.63 (1.20–2.22) |
| Attained ageƒ | 0.02 | 0.5 | ns | 1.02 (0.96–1.08) |
| Smoking habit score## | 0.03 | 0.1 | ns | 1.04 (0.79–1.37) |
ns: nonsignificant. #: expresses impact of exposures on outcome with one degree of freedom for all variables; ¶: sum of yes-responses from all 12 symptoms; +: FEV1 in percentage of predicted normal according to Hedenström et al. [16] in quintiles; §: alveolar slope of the single-breath nitrogen test in percentage of predicted normal according to Sixt et al. [17] in quintiles; ƒ: age at each year of follow-up; ##: 1=never-smoker, 2=ex-smoker since >6 months, 3=currently smoking 1–14 g·day−1, 4=smoking 15–24 g·day−1, 5=smoking >24 g·day−1.
Each of the 12 respiratory symptoms was highly significantly associated with incident COPD events (supplementary table S1). Wheeze or squeaks at any time, unconnected with a common cold, had the strongest association to outcome.
Table 3 shows the predictive power on incident COPD events of the four symptom groups and of a fifth group with total symptom score, each in combination with N2-slope, age and smoking habit score, and subdivided according to men without and with airways obstruction. The N2-slope was a stronger predictor than nonproductive cough and productive cough among men without and with airways obstruction. Wheezing, dyspnoea, total symptom score and the N2-slope were strong predictors in both groups. Age and smoking habit score were not significant in any of the predictions when N2-slope and symptoms were included and the study population was subdivided according to spirometry results.
TABLE 3.
Results of proportional hazards regression analyses of five symptom groups on incident COPD events during follow up of subjects with normal and subjects with reduced forced expiratory volume in 1 s (FEV1)/vital capacity (VC).
| Nonobstructive # | Obstructive ¶ | |||
| Wald's Chi-squared + | p-value | Wald's Chi-squared + | p-value | |
| Subjects n | 544 | 71 | ||
| Nonproductive cough § | 3.68 | 0.054 | 1.02 | 0.31 |
| N2-slope quintilesƒ | 10.90 | 0.001 | 6.33 | <0.05 |
| Attained age## | 0.13 | 0.72 | 0.91 | 0.34 |
| Smoking habit score¶¶ | 0.29 | 0.59 | 0.42 | 0.52 |
| Productive cough § | 3.86 | <0.05 | 2.51 | 0.11 |
| N2-slope quintilesƒ | 11.71 | 0.0006 | 7.26 | 0.0071 |
| Attained age## | 0.13 | 0.72 | 0.89 | 0.35 |
| Smoking habit score¶¶ | 0.38 | 0.54 | 0.51 | 0.48 |
| Wheezing § | 9.65 | 0.0019 | 5.98 | 0.0145 |
| N2-slope quintilesƒ | 11.86 | 0.0006 | 5.91 | 0.0150 |
| Attained age## | 0.002 | 0.97 | 1.28 | 0.26 |
| Smoking habit score¶¶ | 0.41 | 0.52 | 0.17 | 0.68 |
| Dyspnoea § | 14.51 | 0.0001 | 5.94 | 0.0148 |
| N2-slope quintilesƒ | 10.28 | 0.0013 | 7.58 | 0.0059 |
| Attained age## | 0.003 | 0.96 | 0.08 | 0.78 |
| Smoking habit score¶¶ | 0.62 | 0.43 | 0.30 | 0.58 |
| Total symptom score ++ | 14.82 | <0.0001 | 6.58 | 0.0103 |
| N2-slope quintilesƒ | 10.26 | 0.0014 | 6.35 | 0.0117 |
| Attained age## | 0.06 | 0.81 | 1.66 | 0.20 |
| Smoking habit score¶¶ | 0.06 | 0.81 | 0.34 | 0.56 |
p<0.05 was considered significant. #: FEV1/VC ≥lower limit of normal (LLN) according to Hedenström et al. [16]; ¶: FEV1/VC <LLN according to Hedenström et al. [16]; +: expresses impact of exposures on outcome; §: represented by the sum of the questions involved; ƒ: alveolar slope of the single-breath nitrogen test in percentage of predicted normal according to Sixt et al. [17] in quintiles; ##: 50- or 60-year-old men in 1973; ¶¶: comprises five categories (never-smoker, ex-smoker, currently smoking 1–14 g·day−1, 15–24 g·day−1 and >24 g·day−1); ++: sum of yes-responses from all 12 symptoms.
Figure 1 shows cumulative first COPD event incidence across 38 years of follow-up among men who had no airflow limitation at baseline and those who did. Those who had no airflow limitation at baseline had a total 12.2% cumulative COPD event incidence while the corresponding incidence among those who had airflow limitation was 46.7%.
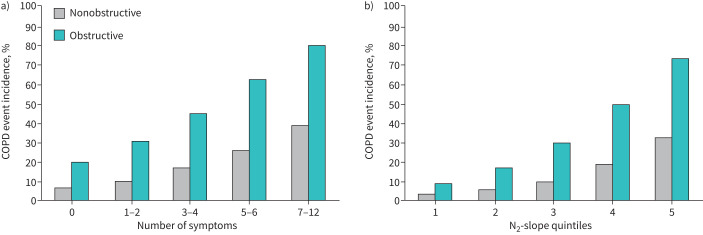
Figure 2a shows that the incidence of incident COPD events (COPD event incidence) during 38 years of follow-up increased with number of respiratory symptoms among subjects without and with airflow obstruction. Figure 2b shows that the COPD event incidence increased with N2-slope quintile among subjects without and with airflow obstruction.
FIGURE 2.
a) Incidence of first admission to hospital or death related to COPD (COPD event incidence) in nonobstructive and obstructive subjects according to symptom groups where the numbers along the horizontal axis represent number of reported respiratory symptoms. b) The same incidence of admission to hospital or death related to COPD according to alveolar N2-slope quintiles. Number of subjects reporting symptoms among nonobstructive subjects were: 0 n=232, 1–2 n=168, 3–4 n=68, 5–6 n=36, 7–12 n=28. The corresponding numbers among obstructive subjects were: 0 n=22, 1–2 n=19, 3–4 n=16, 5–6 n=10, 7–12 n=20.
Figure 3a shows that in nonobstructive subjects, COPD event incidence is almost zero for a very flat N2-slope and absence of symptoms, and ∼70% in case of a very steep N2-slope and many symptoms; for obstructive subjects, for a similar combination, a higher level of COPD event incidence is shown, reaching >90% (figure 3b).
Discussion
The present results showed that the N2-slope significantly and independently improved the prediction of hospital admission and mortality related to COPD beyond the predictive value of respiratory symptoms, age and smoking habits in middle-aged men with and without airway obstruction.
The present results are in line with the predictive strength of respiratory symptoms that has been reported repeatedly [2, 4, 5, 20–27], as well as the predictive power of mucus hypersecretion when controlled for age, smoking status and FEV1 [3, 20, 24]. In the present analysis, dyspnoea and wheezing were stronger predictors of incident COPD events than was cough with expectoration. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations [28], wheezing is not specifically included in the diagnostic criteria. Wheezing is also a core symptom in chronic asthma, but nevertheless, wheezing is strongly associated with hospital admission and death related to COPD, why this symptom may be considered in the grading of COPD severity in future GOLD recommendations.
The predictive ability of the N2-slope beyond that of respiratory symptoms, and smoking habits among subjects with normal spirometry, is an important finding in our efforts to predict subjects at risk of future COPD complications. Small-airway abnormalities precede the development of emphysema and overt COPD [8, 29, 30]. As the presence of respiratory symptoms strongly predicts COPD outcomes, there is a high probability that these symptoms relate to early abnormalities in small airways. Consequently, adequate measures of small airway function might further fine-tune the early prediction of COPD outcomes. However, there is little consensus regarding the prognostic value of so-called small-airway tests [31, 32]. Nevertheless, Oxhoj et al. [15] found that the N2-slope was abnormally steep among some smokers with normal spirometry, presumably indicating sensitivity to smoking-induced changes in small airways. In a 7-year follow-up study of 460 men, Olofsson et al. [33] found that a steep N2-slope at the start of the study predicted a future increased rate of FEV1 decline, probably predicting a greater risk of developing COPD. Furthermore, the N2-slope was found to predict COPD events beyond that of spirometry [14] and has been shown to be associated to morphological changes in small airways [11, 12]. Thus, it is reasonable that the N2-slope is associated with small airway obstruction, at least when spirometry is normal or accounted for.
The present results clearly show the strong predictive power of the N2-slope, which might be worth considering regarding the identification of pre-COPD among younger subjects as asked for by Han et al. [1] for early identification of subjects at risk for overt COPD and complications. But it is worth pointing out that it is the combination of respiratory symptoms and the N2-slope that results in high incidence of incident COPD events.
It is valuable to compare the present results with that of the extensive study by Çolak et al. [5] because of its strength and generalisability. Both studies are based on random population samples from Nordic cities, i.e. Copenhagen and Gothenburg, and both report on respiratory symptoms and airway obstruction and hospital admission because of exacerbations or respiratory death. The proportion of subjects with airway obstruction is also similar, ∼14% and ∼12% in Copenhagen and Gothenburg, respectively. The overall prevalence of respiratory symptoms in the Gothenburg study was ∼59%, whereas the Copenhagen study reported 39%. The questionnaire in the Gothenburg study contains more questions regarding nonproductive cough, productive cough and wheezing, but fewer regarding dyspnoea as compared to the Copenhagen study, which may account for different proportions of subjects identified as symptomatic.
In the Copenhagen study, 1.4% of nonobstructive subjects with respiratory symptoms, but without airway obstruction, experienced admission to hospital because of exacerbations or respiratory death as compared to ∼10% in the present study. Presumably, this is due to the roughly 4.4 times longer follow-up in the Gothenburg study and a nonlinear effect of follow-up time. Thus, the similarities and explainable dissimilarities between the studies strengthens the generalisability of the present results.
It is worth underlining that the majority of subjects with respiratory symptoms have no airflow obstruction and do not develop incident COPD events. Thus, the mechanisms involved in the sensation of respiratory symptoms are usually unknown. Another important circumstance to consider is that the main underlying cause of COPD has gone down tremendously. In 1973 the majority of men (54%) were smokers. According to the World Health Organization, Sweden was the first country with <20% smokers.
One limitation of the present study was the limited sample size. However, the number of person-years of observation was substantial and provided the study with >99.9% statistical power. Another limitation is that only middle-aged men were included, which limits the generalisability of the results. Moreover, there was only one measurement of respiratory symptoms, lung function and smoking habits in the present study, and only severe COPD events were evaluated. Strengths of the study include its high statistical power, the strictly-controlled N2 test performance and evaluation, and the clear definition of the N2-slope.
In conclusion, the N2-slope is independently predictive of incident COPD events beyond respiratory symptoms in middle-aged men, and importantly also in men with normal spirometry, in agreement with several studies demonstrating that structural and functional abnormalities of the lungs and airways in subjects with normal spirometry precede clinical COPD. The combination of the N2-slope and respiratory symptoms results in an effective prediction. The N2-slope should be considered in the critical need for further research on early diagnosis of COPD.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00383-2021.SUPPLEMENT (106.1KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: Björn Bake and Kurt Svärdsudd are the guarantors for the content of the manuscript, including data and analysis. Jan Olofson, Björn Bake, Bengt Bergman, Lowie E.G.W. Vanfleteren and Kurt Svärdsudd designed and performed the study, analysed data and wrote the manuscript.
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: J. Olofson has nothing to disclose.
Conflict of interest: B. Bake has nothing to disclose.
Conflict of interest: B. Bergman has nothing to disclose.
Conflict of interest: L.E.G.W. Vanfleteren reports grants and personal fees from AstraZeneca, and personal fees from Novartis, GSK, Chiesi, Menarini, Pulmonx, Resmed, Boehringer, Verona Pharma and AGA Linde, outside the submitted work.
Conflict of interest: K. Svärdsudd has nothing to disclose.
Support statement: This work was supported by grants from the Swedish Medical Research Council, the Swedish Heart and Lung Foundation, Sahlgrenska University Hospital and the Swedish state under the agreement between the Swedish government and the county councils relating to the economic support of research and education under the ALF agreement (grant number ALFGBG-721351). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Han MK, Agusti A, Celli BR, et al. From GOLD 0 to pre-COPD. Am J Respir Crit Care Med 2021; 203: 414–423. doi: 10.1164/rccm.202008-3328PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestbo J, Rasmussen FV. Respiratory symptoms and FEV1 as predictors of hospitalization and medication in the following 12 years due to respiratory disease. Eur Respir J 1989; 2: 710–715. [PubMed] [Google Scholar]
- 3.Vestbo J, Knudsen KM, Rasmussen FV. The value of mucus hypersecretion as a predictor of mortality and hospitalization. An 11-year register based follow-up study of a random population sample of 876 men. Respir Med 1989; 83: 207–211. doi: 10.1016/S0954-6111(89)80033-2 [DOI] [PubMed] [Google Scholar]
- 4.Woodruff PG, Barr RG, Bleecker E, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med 2016; 374: 1811–1821. doi: 10.1056/NEJMoa1505971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Çolak Y, Nordestgaard BG, Vestbo J, et al. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur Respir J 2019; 54: 1900734. doi: 10.1183/13993003.00734-2019 [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968; 278: 1355–1360. doi: 10.1056/NEJM196806202782501 [DOI] [PubMed] [Google Scholar]
- 7.Niewoehner DE, Kleinerman J. Morphologic basis of pulmonary resistance in the human lung and effects of aging. J Appl Physiol 1974; 36: 412–418. doi: 10.1152/jappl.1974.36.4.412 [DOI] [PubMed] [Google Scholar]
- 8.Hogg JC, Paré PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev 2017; 97: 529–552. doi: 10.1152/physrev.00025.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 2015; 385: 899–909. doi: 10.1016/S0140-6736(14)60446-3 [DOI] [PubMed] [Google Scholar]
- 10.Mead J. The lung's “quiet zone”. N Engl J Med 1970; 282: 1318–1319. doi: 10.1056/NEJM197006042822311 [DOI] [PubMed] [Google Scholar]
- 11.Cosio M, Ghezzo H, Hogg JC, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1978; 298: 1277–1281. doi: 10.1056/NEJM197806082982303 [DOI] [PubMed] [Google Scholar]
- 12.Petty TL, Silvers GW, Stanford RE, et al. Small airway pathology is related to increased closing capacity and abnormal slope of phase III in excised human lungs. Am Rev Respir Dis 1980; 121: 449–456. doi: 10.1164/arrd.1980.121.3.449 [DOI] [PubMed] [Google Scholar]
- 13.Paiva M, Engel LA. The anatomical basis for the sloping N2 plateau. Respir Physiol 1981; 44: 325–337. doi: 10.1016/0034-5687(81)90027-X [DOI] [PubMed] [Google Scholar]
- 14.Olofson J, Bake B, Bergman B, et al. Prediction of COPD and related events improves by combining spirometry and the single breath nitrogen test. COPD 2018; 15: 424–431. doi: 10.1080/15412555.2018.1538330 [DOI] [PubMed] [Google Scholar]
- 15.Oxhoj H, Bake B, Wilhelmsen L. Ability of spirometry, flow–volume curves and the nitrogen closing volume test to detect smokers. A population study. Scand J Respir Dis 1977; 58: 80–96. [PubMed] [Google Scholar]
- 16.Hedenström H, Malmberg P, Fridriksson HV. Reference values for lung function test in men: regression equations with smoking variables. Ups J Med Sci 1986; 91: 299–310. doi: 10.3109/03009738609178670 [DOI] [PubMed] [Google Scholar]
- 17.Sixt R, Bake B, Oxhöj H. The single-breath N2-test and spirometry in healthy non-smoking males. Eur J Respir Dis 1984; 65: 296–304. [PubMed] [Google Scholar]
- 18.Oxhøj H, Bake B. Measurement of closing volume with the single breath nitrogen method. Scand J Respir Dis 1974; 55: 320–331. [PubMed] [Google Scholar]
- 19.Chronic Bronchitis in Great Britain: national survey carried out by the Respiratory Diseases Study Group of the College of General Practitioners. Br Med J 1961; 2: 973–979. doi: 10.1136/bmj.2.5258.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996; 153: 1530–1535. doi: 10.1164/ajrccm.153.5.8630597 [DOI] [PubMed] [Google Scholar]
- 21.Ekberg-Aronsson M, Pehrsson K, Nilsson JA, et al. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res 2005; 6: 98. doi: 10.1186/1465-9921-6-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frostad A, Søyseth V, Andersen A, et al. Respiratory symptoms as predictors of all-cause mortality in an urban community: a 30-year follow-up. J Intern Med 2006; 259: 520–529. doi: 10.1111/j.1365-2796.2006.01631.x [DOI] [PubMed] [Google Scholar]
- 23.Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med 2006; 100: 115–122. doi: 10.1016/j.rmed.2005.03.035 [DOI] [PubMed] [Google Scholar]
- 24.de Marco R, Accordini S, Cerveri I, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med 2007; 175: 32–39. doi: 10.1164/rccm.200603-381OC [DOI] [PubMed] [Google Scholar]
- 25.Voll-Aanerud M, Eagan TM, Wentzel-Larsen T, et al. Changes in respiratory symptoms and health-related quality of life. Chest 2007; 131: 1890–1897. doi: 10.1378/chest.06-2629 [DOI] [PubMed] [Google Scholar]
- 26.Bridevaux PO, Gerbase MW, Probst-Hensch NM, et al. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax 2008; 63: 768–774. doi: 10.1136/thx.2007.093724 [DOI] [PubMed] [Google Scholar]
- 27.Lowe KE, Regan EA, Anzueto A, et al. COPDGene® 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis 2019; 6: 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Pocket guide to COPD. A Guide for Diagnosis, Management, and Prevention. 2020. Available from: http://goldcopd.org
- 29.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011; 365: 1567–1575. doi: 10.1056/NEJMoa1106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koo HK, Vasilescu DM, Booth S, et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir Med 2018; 6: 591–602. doi: 10.1016/S2213-2600(18)30196-6 [DOI] [PubMed] [Google Scholar]
- 31.Verbanck S. Physiological measurement of the small airways. Respiration 2012; 84: 177–188. doi: 10.1159/000341742 [DOI] [PubMed] [Google Scholar]
- 32.Gove K, Wilkinson T, Jack S, et al. Systematic review of evidence for relationships between physiological and CT indices of small airways and clinical outcomes in COPD. Respir Med 2018; 139: 117–125. doi: 10.1016/j.rmed.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 33.Olofsson J, Bake B, Svärdsudd K, et al. The single breath N2-test predicts the rate of decline in FEV1. The Study of Men Born in 1913 and 1923. Eur J Respir Dis 1986; 69: 46–56. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00383-2021.SUPPLEMENT (106.1KB, pdf)