Abstract
Background
The incidence of incisional hernia after major abdominal surgery via a midline laparotomy is 20–41 per cent with short-term follow-up, and over 50 per cent in those surviving an abdominal catastrophe. Abdominal wall reconstruction (AWR) requires complex operations, often involving mesh resection, management of scarred skin, fistula takedown, component separation or flap reconstruction. Patients tend to have more complex conditions, with multiple co-morbidities predisposing them to a vicious cycle of complications and, subsequently, hernia recurrence. Currently there appears to be variance in perioperative practice and minimal guidance globally. The aim of this Delphi consensus was to provide a clear benchmark of care for the preoperative assessment and perioperative optimization of patients undergoing AWR.
Methods
The Delphi method was used to achieve consensus from invited experts in the field of AWR. Thirty-two hernia surgeons from recognized hernia societies globally took part. The process included two rounds of anonymous web-based voting with response analysis and formal feedback, concluding with a live round of voting followed by discussion at an international conference. Consensus for a strong recommendation was achieved with 80 per cent agreement, and a weak recommendation with 75 per cent agreement.
Results
Consensus was obtained on 52 statements including surgical assessment, preoperative assessment, perioperative optimization, multidisciplinary team and decision-making, and quality-of-life assessment. Forty-six achieved over 80 per cent agreement; 14 statements achieved over 95 per cent agreement.
Conclusion
Clear consensus recommendations from a global group of experts in the AWR field are presented in this study. These should be used as a baseline for surgeons and centres managing abdominal wall hernias and performing complex AWR.
TOC summary: The Delphi method was used to provide consensus statements for preoperative assessment and perioperative optimization of patients with complex abdominal wall hernias. Clear recommendations are provided as a baseline for surgeons and centres managing complex abdominal wall hernias.
Introduction
Abdominal wall reconstruction (AWR) is a rapidly developing area of subspecialization. Despite the increasing use of laparoscopy and robotics in surgery, the number of patients developing a ventral hernia and undergoing AWR is growing1. The incidence of abdominal wall incisional hernia (AWH) after major abdominal surgery via a midline laparotomy is 20–41 per cent with short-term follow-up2–4, and over 50 per cent among those surviving an abdominal catastrophe5,6. Ventral hernia repair is one of the most commonly performed operations worldwide, with over 450 000 undertaken in the UK and USA, including over 7000 incisional hernia repairs in the UK alone, each year7,8. The demand for AWR in patients with increasingly complex conditions is rising.
AWR is a major operation that can require extensive adhesiolysis, mesh resection, management of atrophic or scarred skin, fistula takedown, component separation or flap reconstruction, which demands a multiprofessional approach9 with experienced, specially trained surgeons. These operations also tend to be more complex owing to the patients’ increased rates of co-morbidities and other risk factors, which predisposes them to a vicious cycle of complications and, subsequently, hernia recurrence10,11. These adverse surgical outcomes have a heavy impact on cost, quality of life (QoL), and hospital resources, and, if the hernia repair fails, it will need to be repeated.
As a rapidly growing subspeciality caring for patients with increasingly complex needs, AWR surgeons look after an almost entirely elective patient cohort. This allows time for thorough preoperative planning, physiological and psychological optimization of the patient, and decision-making regarding technical aspects of the operation. This is often best suited to multidisciplinary team (MDT) input9. Currently there appears to be modifiable variance in perioperative practice and minimal guidance globally, specifically on the assessment, optimization, and decision-making processes for these patients.
The objective of this study was to obtain a consensus from a panel of world-leading experts in the field of AWR to provide a clear benchmark of care for the preoperative assessment and perioperative optimization of patients undergoing AWR.
Methods
The Delphi method is an established and recognized approach for determining consensus through iterative rounds, or iterations, of anonymous voting, feedback, and finally an open discussion at an internationally recognized meeting12. Anonymous voting ensures there are no undue external pressures on voting. Circulation of feedback after each round of voting prevents dominant individuals from influencing the process, and allows reassessment and deliberation of responses from all panellists so as not to revise earlier answers in light of the replies of other members of the panel.
The Delphi method consists of five phases: expert panel participant selection, discussion of research priorities, leading to questionnaire development and planning for online distribution, and iteration 1 of online questionnaire (round 1), iteration 2 of online questionnaire following controlled structured feedback (round 2), and a final questionnaire iteration (round 3), with face-to-face discussion (AWR Europe, February 2020, London, UK).
Phase 1 proceeded with a list of panellists drawn up by three of the authors. Final inclusion was determined by a systematic search of their academic record in the field, prominence in a recognized hernia or surgical society, and geographical location. Panellists from the UK, USA, Europe, Africa, and Australasia were invited via e-mail to take part. Panel members included hernia surgeons from the British Hernia Society, European Hernia Society (EHS), Italian Society of Hernia and Abdominal Wall Surgery, American Association of Plastic Surgeons, Americas Hernia Society (AHS), Hernia interest Group of the South African Society of Endoscopic Surgeons, Asian and Pacific Hernia Society, and faculty for the international conference AWR Europe. Attempts were made to ensure diversity of race and sex within the panel. No invitation to participate was declined.
Phase 2 started with questionnaire development, during which the lead researchers drafted a long list of key elements for research priorities. Identification of key elements involved literature review, expert opinion, and review of currently available guidelines for ventral hernia repair12–16. Five broad domains were identified: surgical assessment, preoperative assessment, perioperative optimization, role of the MDT and multidisciplinary decision-making, and QoL assessment.
A final list of research priorities in the five domains was drawn up by the steering group to focus on areas of greatest importance. The questionnaires comprised a compilation of individual statements in these five domains that the panellists were asked to vote on. Voting was in the form of a Likert scale from 1 to 5, representing strongly disagree to strongly agree respectively for all statements. Abstaining from votes was not considered as agreement to the statement. At the end of each section, panellists were able to leave additional comments that would be analysed and, when deemed important by the consensus steering group, incorporated into the next iteration.
The focus of this research was prioritization of patient assessment and optimization for surgery. Therefore, although patient participation and involvement can prove invaluable in determining assessment of patient-reported outcomes, it was not considered appropriate in this setting.
Questionnaires were published and distributed by the lead author using the online platform Typeform© (www.typeform.com). Typeform© is an online service that provides software for surveys and questionnaires. Panellists were able to vote on answers anonymously. Panellists were individually e-mailed a link to the questionnaire, and asked to provide written consent and maintain anonymity until voting was concluded. Panellists were also asked to consent to Committee on Publication Ethics17 criteria, thereby authenticating co-authorship.
Two weeks was given for panellists to complete each questionnaire iteration for rounds 1 and 2. In between rounds the results were collated and distributed via e-mail. It was anticipated that three rounds would be required to achieve consensus. Round 3 was conducted using the online polling platform Slido (www.sli.do), during a live discussion session at AWR Europe 2020. This allowed real-time results to be displayed and focused discussion to occur, facilitated by chaired authors.
Outcome measures
Consensus was considered to have been achieved if there was over 75 per cent ‘agree’ or ‘strongly agree’ responses in the online questionnaire iteration or at the live discussion session. Subsequent recommendations on practice were felt to be strong recommendations where there was greater than 80 per cent agreement, and weak recommendations where there was 75–79 per cent agreement. These cut-off values have been used in previously published literature18.
Statements achieving consensus were removed from future iterations. Statements for which consensus was nearly achieved (60–74 per cent ‘agree’ or ‘strongly agree’) were modified using additional feedback comments from responding panellists, moved into the next iteration, and, if required, to the final discussion meeting. Statements clearly lacking any consensus were removed from future iterations.
Definitions
Hernia definitions have been used heterogeneously throughout the literature and between different institutions19. For this study, the following definitions were used to aid consensus agreement: hernia—a musculofascial defect associated with protrusion of intra-abdominal viscera as described by the EHS20; AWH—any abdominal wall gap with or without a bulge in the area of a postoperative scar perceptible or palpable by clinical examination or imaging21; complex abdominal wall hernia—any abdominal wall hernia complicated by any negative influencing factors including large defect size (greater than 10 cm), previous repair, previous mesh, infection, and patient co-morbidities22; and complex AWR—ventral hernia repair, primary or incisional, whereby fascial closure and hernia repair is complicated by large hernia size, need for component separation, need for adhesiolysis or need for flap reconstruction23,24.
Results
Thirty-two experts were approached and all those invited agreed to participate. The response rate to all three iterations of questionnaires was 100 per cent. The expert panel consisted of 14 representatives from the UK, eight from Europe, eight from the USA, one from Africa, and one from Australia. Voting and discussion was completed at a final face-to-face meeting held on 7 February 2020. Panel members not available for the discussion meeting were able to contribute electronically.
A flow diagram of the process can be seen in Fig. 1. Following rounds 1 and 2 of questionnaire iterations, 47 recommendation points achieved consensus in the five domains. Five remaining statements and contributor feedback from iterations 1 and 2 were analysed thoroughly, and the statements were revised. The final consensus discussion meeting included these statements for further discussion. Following round 3 and the consensus discussion meeting, all five points achieved consensus for strong recommendation (greater than 80 per cent agreement).
Fig. 1.
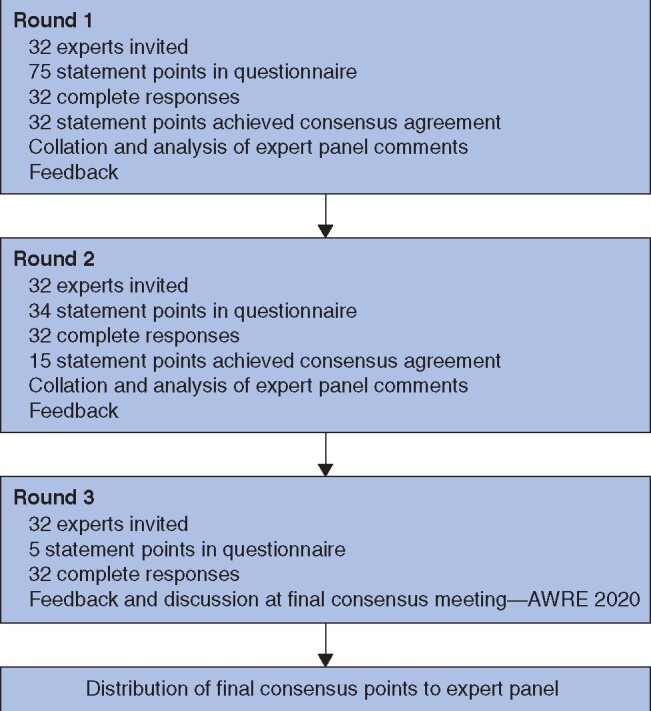
Flow diagram
AWRE, Abdominal Wall Repair Europe. AWRE – Abdominal Wall Reconstruction Europe 2020.
Statement recommendations
Surgical assessment
Structured preoperative surgical assessment allows standardization of operative recommendations and procedures, and enables comparison of outcomes. Variation in practice between surgeons and centres is common. It was agreed that patients with AWH requiring complex surgery should be managed in, or referred to, centres or surgeons that regularly perform AWR. The nine strong recommendations and two weak recommendations are shown in Table 1.
Table 1.
Consensus statements: surgical assessment
|
Strong recommendations (>80% agreement) Patients with AWH are best managed by referral to a specialist AWH surgeon (91.2%) Consideration for AWR must include assessment of: – Previous abdominal surgery/trauma (100%) – Previous AWH repair or AWR including mesh location (100%) – Previous or current abdominal wall infection (100%) – Previous mesh explantation (97%) Current or previous gastrointestinal tract opening (stoma/enterotomy) (84%) All patients with AWH must have relevant cross-sectional imaging (88%) All patients must have BMI recorded (97%) Risk stratification scores (e.g., CeDAR/VHWG) provide a useful adjunct to decision-making and should be used when discussing surgery with patients (100%) |
|
Weak recommendations (75-79% agreement) Consideration for AWR should include clinical assessment of: – Functional status (75%) – Exercise tolerance (75%) |
Percentage agreement shown in parentheses. AWH, abdominal wall incisional hernia; AWR, abdominal wall repair; CeDAR, Carolinas Equation for Determining Associated Risks; VHWG, Ventral Hernia Working Group.
Preoperative assessment
Patients requiring AWR often have multiple co-morbidities with extensive polypharmacy. It was agreed that a structured systematic framework for preoperative assessment is helpful to allow appropriate preoperative optimization. Table 2 shows the 11 strong recommendations.
Table 2.
Consensus statements: preoperative assessment
|
Strong recommendations (>80% agreement) Consideration for AWR must include assessment of co-morbidities, specifically: – Antiplatelet/anticoagulant use (91%) – Cancer status (100%) – Ischaemic heart disease (97%) – Glycaemic control (97%) – Steroid use (97%) All patients with diabetes must have HbA1c testing before surgery (82%) All patients must have ASA grade recorded (88%) Consideration for AWR must include recording of: – Alcohol excess/dependence (93%) – Smoking status (97%) – Nutritional status (97%) All patients with AWH considered for surgery must attend a formal anaesthetic preoperative assessment (88%) |
Percentage agreement shown in parentheses. AWR, abdominal wall repair; Hb, haemoglobin; AWH, abdominal wall incisional hernia.
Perioperative optimization
Preoperative optimization of patients was considered vital in reducing perioperative morbidity, duration of hospital stay, and requirement for further intervention. The seven strong recommendations and one weak recommendation are shown in Table 3.
Table 3.
Consensus statements: perioperative optimization
|
Strong recommendations (>80% agreement) Patients with AWH with a high BMI (>35 kg/m2 as a minimum) should be encouraged to lose weight before surgery (94%) Patients with AWH with a high BMI (>35 kg/m2 as a minimum) who were encouraged to lose weight but have failed to do so should be offered referral to a weight loss service (including dietetics) (91%) Patients with malnutrition should be offered referral to a dietetics service (91%) Patients with poor exercise tolerance should be offered specialist prehabilitation/physiotherapy (94%) Where appropriate all patients must be offered: – Specialist diabetes advice (91%) – Smoking cessation advice (88%) Centres managing AWH should have the facility to offer botulinum toxin injection to the lateral musculature of the abdominal wall as a perioperative adjunct to AWR (87%) |
|
Weak recommendations (75% agreement) All patients, where appropriate, should have formal referral to alcohol liaison services (76%) |
Percentage agreement shown in parentheses. AWH, abdominal wall incisional hernia; AWR, abdominal wall repair.
Role of multidisciplinary team and decision-making
Multiprofessional and multidisciplinary decision-making is important in complex surgery18. It was agreed that patients should be managed in, or referred to, centres that routinely offer assessment of AWR with access to an MDT. Table 4 shows the 19 strong recommendations and two weak recommendations.
Table 4.
Consensus statements: role of multidisciplinary team and decision-making
|
Strong recommendations (> 80% agreement) Surgeons managing patients with AWH should have access to an MDT (local or virtual) (87%) Running an MDT meeting on a regular basis provides structure for innovation in AWR—an opportunity for surgical and clinical development and good clinical governance (87.1%) Running an MDT meeting on a regular basis provides a stratified structure for documentation of decision making—protecting surgeons and patients (81.2%) AWH MDT must include: – An experienced gastrointestinal surgeon (91%) – An experienced AWR surgeon—plastic surgeon/general surgeon (97%) – An expert in radiology (94%) – Dietary service/access to a dietary service (94%) – Prehabilitation or physiotherapy service/access to a prehabilitation or dietary service (94%) AWH MDT discussion must include hernia characteristics: – Hernia size (85%) – Hernia location (88%) – Loss of domain (94%) – Skin integrity (91%) AWH MDT discussion must include operative technique: – Requirement for mesh (82%) – Type of mesh to be used (80%) – Requirement for component separation (82%) – Need for reconstructive surgery, i.e., flap reconstruction (91%) – Need for concurrent procedure, i.e., stoma reversal/adhesiolysis (94%) – Need for botulinum toxin (87%) Critical care/intensive care beds must be available if needed for all patients with AWH (97%) |
|
Weak recommendations (75-79% agreement) AWH MDT discussion must include operative technique: – Required dissection planes (75%) – Relevant muscle bulk and integrity (79%) |
Percentage agreement shown in parentheses. AWH, abdominal wall incisional hernia; MDT, multidisciplinary team; AWR, abdominal wall repair.
Quality of life
QoL is an important factor in AWR but, despite a number of tools in use, it remains understudied25. Although AWR is performed primarily for improvement in QoL, measurement and assessment of this aspect is often overlooked and not discussed with the patient. It was agreed that it should be included in the assessment of all patients with AWH, but that further research is required into methodologies and approach. Following three rounds of voting, there was only one weak recommendation: QoL should be assessed in all patients with AWH (75 per cent agreement).
Discussion
Using the Delphi method, 52 key statements for the assessment and perioperative optimization of patients with AWH have been agreed through consensus by a panel of international experts in AWR. These statements are relevant to all surgeons undertaking AWR and will provide a benchmark of care for this complex cohort. There is adaptability, and there will be variability within the statements owing to differences in local resources and expertise. However, the key themes are generalizable globally; they can be, and are recommended to be, applied to essentially any centre undertaking or planning to undertake AWR.
Guidance in the literature for hernia repair of all kinds remains varied and heterogeneous. This is epitomized in complex AWR. Hernia work has previously been a ‘bit part’ of many general surgeon’s practice, often as a secondary interest. Fortunately, complex AWR is increasingly being recognized as a subspeciality in its own right. Patients often have inherently complex conditions with hernia and patient-related factors that are recognized to increase the complexity of AWH surgery. Known factors that negatively influence outcome and increase complexity, and should be addressed in all patients include: large hernia defect (over 10 cm), obesity, previous hernia surgery, previous mesh use, gastrointestinal (GI) tract opening (stoma or fistula), increasing age, and co-morbidities (including diabetes, smoking, and anticoagulant use)26. The panel of experts have strongly agreed that these should all be assessed and addressed before undertaking major surgery.
The surgery can be time- and resource-consuming, and technically difficult with prolonged postoperative stay, often in ICU. Adverse outcomes have a lasting impact on patients’ physical and mental health, which often leads to hernia recurrence, prolonged healing, and impaired QoL and ability to return to work25, leading to wider-reaching socioeconomic implications. A US readmission database study27 reported that around one-fifth of patients undergoing ventral hernia repair are readmitted within 1 year, and 35 per cent of these require reoperation. Reducing the risks of surgery for each patient has to be central in any decision-making. A patient-centred approach with medical and surgical planning and care tailored to the specific patient each time is essential to ensure good outcomes.
Complex AWHs should be managed by specialist surgeons in specialist centres. Currently, there is no mandatory requirement for surgeons to contribute to a national registry or record outcomes of any sort for AWH surgery. The ACCESS project launched by the EHS28 has made drives for accreditation of complex AWH surgeons and centres, and has set out criteria for this. This includes suggestions regarding minimum operation numbers, outcome recording and reporting, access to services such as MDTs, and appropriate postoperative care. However, complex AWR is currently being performed without any clear guidance or monitoring. The importance of the need for common reporting sets29, and for monitoring and reporting of surgical outcomes in cancer, has long been established through the National Bowel Cancer Audit in the UK30 and the Consortium for Optimizing the Treatment of Rectal Cancer in the USA31. This must become commonplace for complex hernia surgery. While this is awaited, the present study provides clear guidance for units and surgeons caring for these patients. Centres offering AWR now have a clear framework and mandate to focus on improving and standardizing their service. Clear definitive guidance on preoperative assessment, decision-making, and perioperative optimization can improve patient care, reduce adverse outcomes and hernia repair failure, and also guide policymakers in the allocation of evermore constrained health and social care resources.
Several key areas for prioritization of research in the coming years were highlighted from the Delphi process. Concerns were raised in the individual comments or at the discussion meeting around a number of points that demonstrate a diversity of care for one of the most common surgical problems in the world.
A strong recommendation was made that all patients with AWH being considered for AWR have relevant cross-sectional imaging as part of surgical assessment. However, there were concerns around the timing of this imaging. Opinions ranged from 3 months before surgery up to 1 year, with no clear majority for preference. There were a number of reasons for the difference in opinion: first, cost and, second, exposure to ionizing radiation inherent in CT. Potentially unnecessary imaging should be avoided, especially when many experts felt that the overall anatomy of an abdominal wall hernia is unlikely to change significantly in less than a year. However, over longer periods, body composition, including levels of fat, muscle bulk, and muscle integrity, may change, as can the hernia volume, size of the defect (owing to muscular distraction), and intra-abdominal volume32.
A strong recommendation was made that all patients with AWH should have their BMI measured and recorded. It was also strongly recommended that all patients with a high BMI should be encouraged to lose weight. The optimum BMI for AWR remains unclear, and no consensus could be achieved on a minimum BMI that patients should be expected to reach before surgery. Although it was agreed, as is the case with other types of major surgery33, that patients of lower weight are likely to have better outcomes, the effect of body composition, and particularly sarcopenia, on outcomes in AWR is not well understood34. Asking all patients to lose weight may result in a reduction of not just fat but also muscle bulk, which may adversely affect surgical outcomes. There are ethical considerations too, with many experts feeling that it is difficult to refuse surgery based solely on BMI. Some patients may start with an incredibly high BMI, and lose significant weight, but never be able to achieve an arbitrary target value. Patients with a high BMI who have favourable anatomy, significant symptoms, and poor QoL may see more value from surgery than a patient with a lower BMI.
A weak recommendation was made that QoL should be assessed in all patients. Discussion and conflict arose among the panellists regarding when and how this should be done. There are a number of tools available for QoL assessment, but at present none has become accepted universally35. Although it was agreed to be an important factor in decision-making, how and when to undertake QoL assessment remains unclear.
A strong recommendation was made that all patients with AWH should be managed in a centre with access to an MDT. Strong recommendations were also made regarding the members of this team. Certain aspects of this were debated, such as the need for an AWH surgeon and an experienced GI surgeon, as some surgeons will be experienced in both areas. However, this may not be the case and, with increasing complexity owing to stoma, fistula, GI cancer, and inflammatory bowel disease, expert input into discussion and management was considered important for improving outcome. The recommendations made here are considered a minimum standard for the MDT. It is clear from the literature that patients with complex cancer have better outcomes when managed by an MDT36,37. It was emphasized that an MDT is not just ‘a meeting’ but a way of working and managing patients. The majority of panellists were clear that single-handed care of these patients is not likely to result in good or sustainable outcomes, and that all centres offering AWR in patients with complex conditions should at least have access to the basic MDT framework.
Collaborators
Members of the AWR Europe Collaborative: A. C. de Beaux (NHS Lothian, Edinburgh, UK); M. A. Boermeester (Amsterdam UMC, AMC, the Netherlands); H. Bougard (New Somerset Hospital, Cape Town, RSA); C. E. Butler (MD Anderson Cancer Centre, Houston, TX, USA); S. Chintapatla (York Teaching Hospital, York, UK); P. Chitsabesan (York Teaching Hospital, York, UK); D. Cuccurullo (Monaldi Hospital, Naples, Italy); I. R. Daniels (Royal Exeter and Devon Hospitals, Exeter, UK); D. van Dellen (Manchester University NHS Foundation Trust, Manchester, UK); G. A. Dumanian (Northwestern University Feinberg Hospital School of Medicine, Chicago, Illinois, USA); B. East (Motol University Hospital, 1st and 2nd Medical Faculty of Charles University, Prague, Czech Republic); D. T. Efron (Johns Hopkins University School of Medicine, Baltimore, Maryland, USA); H. Friis-Andersen (Horsens Regional Hospital, Denmark); B. T. Heniford (Gastrointestinal and Minimally Invasive Surgery, Carolinas Medical Center, Charlotte, North Carolina, USA); N. A. Henriksen (Zealand University Hospital, Denmark); L.S. Horgan (Northumbrian Healthcare NHS Foundation Trust, Newcastle, UK); N. Ibrahim (Macquerie University Hospital and Hernia Institute Australia, Sydney, Australia); J. E. Janis (Ohio State University Wexner Medical Centre, Columbus, Ohio, USA); A. Montgomery (Department of Surgery, Skåne University Hospital SUS, Malmö, Sweden); M. Y. Nahabedian (National Centre for Plastic Surgery, McLean, Virginia, USA); Y. Novitsky (Colombia University Medical Centre, New York, New York, USA); S. G. Parker (University College Hospital, London, UK); G. H. van Ramshorst Ghent University Hospital, Ghent, Belgium); Y. Renard (Robert Debre University Hospital, University of Reims Champagne-Ardenne, Reims Cedex, France); D. A. Ross (Guys and St Thomas' NHS Foundation Trust, London, UK); D. L. Sanders (North Devon District Hospital, Barnstaple, UK); D. A. J. Slade (Salford Royal NHS Foundation Trust, Manchester, UK); S. G. Talbot Brigham Women and Children's Hospital, Boston, Massachusetts, USA); J. J. Torkington (University hospital of Wales, Cardiff, UK); O. Warren (Chelsea and Westminster Hospital, London, UK); J. Warusavitarne (St Marks Hospital, London, UK); A. C. J. Windsor (HCA Healthcare, London, UK).
Acknowledgements
The authors acknowledge Abdominal Wall Reconstruction Europe 2020, London, UK.
Disclosure. The authors declare no conflict of interest.
Contributor Information
AWR Europe Collaborative:
A de Beaux, M Boermeester, H Bougard, C Butler, S Chintapatla, P Chitsabesan, D Cuccurullo, I Daniels, D van Dellen, G Dumanian, B East, D Efron, H Friis-Andersen, B T Heniford, N Henriksen, L Horgan, N Ibrahim, J Janis, A Montgomery, M Nahabedian, Y Nowitsky, S Parker, G van Ramshorst, Y Renard, D Ross, D Sanders, D Slade, S Talbot, J Torkington, O Warren, J Warusaviturane, and A Windsor
Members of the AWR Europe Collaborative and contributors to this study and are listed under the heading Collaborators.
References
- 1. Carney MJ, Weissler JM, Fox JP, Tecce MG, Hsu JY, Fischer JP.. Trends in open abdominal surgery in the United States—observations from 9 950 759 discharges using the 2009–2013 National Inpatient Sample (NIS) datasets. Am J Surg 2017;214:287–292. [DOI] [PubMed] [Google Scholar]
- 2. Blair LJ, Cox TC, Huntington CR, Groene SA, Prasad T, Lincourt AE. et al. The effect of component separation technique on quality of life (QOL) and surgical outcomes in complex open ventral hernia repair (OVHR). Surg Endosc 2017;31:3539–3546. [DOI] [PubMed] [Google Scholar]
- 3. Deerenberg EB, Harlaar JJ, Steyerberg EW, Lont HE, van Doorn HC, Heisterkamp J. et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet 2015;386:1254–1260. [DOI] [PubMed] [Google Scholar]
- 4. Baucom RB, Ousley J, Beveridge GB, Phillips SE, Pierce RA, Holzman MD. et al. Cancer survivorship: defining the incidence of incisional hernia after resection for intra-abdominal malignancy. Ann Surg Oncol 2016;23:764–771. [DOI] [PubMed] [Google Scholar]
- 5. Moussavian MR, Schuld J, Dauer D, Justinger C, Kollmar O, Schilling MK. et al. Long term follow up for incisional hernia after severe secondary peritonitis: incidence and risk factors. Am J Surg 2010;200:229–234. [DOI] [PubMed] [Google Scholar]
- 6. Parker SG, Halligan S, Erotocritou M, Wood CPJ, Boulton RW, Plumb AAO. et al. A systematic methodological review of non-randomised interventional studies of elective ventral hernia repair: clear definitions and a standardised minimum dataset are needed. Hernia 2019;23:859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Itani KMF. New findings in ventral incisional hernia repair. JAMA 2016;316:1551–1552. [DOI] [PubMed] [Google Scholar]
- 8. Pawlak M, Tulloh B, de Beaux A.. Current trends in hernia surgery in NHS England. Ann R Coll Surg Engl 2020;102:25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muirhead LJ, Shaw AV, Kontovounisios C, Warren OJ.. Establishing a robust multidisciplinary team process in complex abdominal wall reconstruction within a colorectal surgical unit. Tech Coloproctol 2019;23:379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maloney SR, Schlosser KA, Prasad T, Kasten KR, Gersin KS, Colavita PD. et al. Twelve years of component separation technique in abdominal wall reconstruction. Surgery 2019;166:435–444. [DOI] [PubMed] [Google Scholar]
- 11. Holihan JL, Alawadi Z, Martindale RG, Roth JS, Wray CJ, Ko TC. et al. Adverse events after ventral hernia repair: the vicious cycle of complications. J Am Coll Surg 2015;221:478–485. [DOI] [PubMed] [Google Scholar]
- 12. Rowe G, Wright G.. The Delphi technique as a forecasting tool: issues and analysis. Int J Forecast 1999;15:353–375. [Google Scholar]
- 13. Cuccurullo D, Piccoli M, Agresta F, Magnone S, Corcione F, Stancanelli V. et al. Laparoscopic ventral incisional hernia repair: evidence-based guidelines of the first Italian Consensus Conference. Hernia 2013;17:557–566. [DOI] [PubMed] [Google Scholar]
- 14. Bittner R, Bain K, Bansal VK, Berrevoet F, Bingener-Casey J, Chen D. et al. Update of guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS))—Part A. Surg Endosc 2019;33:3069–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henriksen NA, Montgomery A, Kaufmann R, Berrevoet F, East B, Fischer J. et al. ; European and Americas Hernia Societies (EHS and AHS). Guidelines for treatment of umbilical and epigastric hernias from the European Hernia Society and Americas Hernia Society. Br J Surg 2020;107:171–190. [DOI] [PubMed] [Google Scholar]
- 16. Silecchia G, Campanile FC, Sanchez L, Ceccarelli G, Antinori A, Ansaloni L. et al. Laparoscopic ventral/incisional hernia repair: updated guidelines from the EAES and EHS endorsed Consensus Development Conference. Surg Endosc 2015;29:2463–2484. [DOI] [PubMed] [Google Scholar]
- 17. Committee on Publication Ethics. What constitutes authorship? COPE Discussion Document. COPE Counc 2014;1–6. https://publicationethics.org/files/Authorship_DiscussionDocument.pdf (accessed October 2020).
- 18. Bhangu A, Beynon J, Brown G, Chang G, Das P, Desai A. et al. ; Beyond TME Collaborative. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg 2013;100:E1–E33. [DOI] [PubMed] [Google Scholar]
- 19. Parker SG, Halligan S, Liang MK, Muysoms FE, Adrales GL, Boutall A. et al. International classification of abdominal wall planes (ICAP) to describe mesh insertion for ventral hernia repair. Br J Surg 2020;107:209–217. [DOI] [PubMed] [Google Scholar]
- 20. Muysoms AM, Miserez AF, Berrevoet AG, Campanelli AGG, Champault AEE, Chelala AUA et al.. Classification of primary and incisional abdominal wall hernias. Hernia 2009;13:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Korenkov M, Paul A, Sauerland S, Neugebauer E, Arndt M, Chevrel JP. et al. Classification and surgical treatment of incisional hernia: results of an experts’ meeting. Langenbecks Arch Surg 2001;386:65–73. [DOI] [PubMed] [Google Scholar]
- 22. Slater NJ, Montgomery A, Berrevoet F, Carbonell AM, Chang A, Franklin M. et al. Criteria for definition of a complex abdominal wall hernia. Hernia 2014;18:7–17. [DOI] [PubMed] [Google Scholar]
- 23. Fink C, Baumann P, Wente MN, Knebel P, Bruckner T, Ulrich A. et al. Incisional hernia rate 3 years after midline laparotomy. Br J Surg 2013;101:51–54. [DOI] [PubMed] [Google Scholar]
- 24. Stylianides N, Slade DA.. Abdominal wall reconstruction. Br J Hosp Med (Lond) 2016;77:151–156. [DOI] [PubMed] [Google Scholar]
- 25. Grove TN, Muirhead LJ, Parker SG, Brogden DRL, Mills SC, Kontovounisios C. et al. Measuring quality of life in patients with abdominal wall hernias: a systematic review of available tools. Hernia 2020;25:491– 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Köckerling F, Sheen AJ, Berrevoet F, Campanelli G, Cuccurullo D, Fortelny R. et al. The reality of general surgery training and increased complexity of abdominal wall hernia surgery. Hernia 2019;23:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rios-Diaz AJ, Cunning JR, Broach RB, Metcalfe D, Elfanagely O, Serletti JM. et al. One-year health care utilization and recurrence after incisional hernia repair in the United States: a population-based study using the nationwide readmission database. J Surg Res 2020;255:267–276. [DOI] [PubMed] [Google Scholar]
- 28. Köckerling F, Sheen AJ, Berrevoet F, Campanelli G, Cuccurullo D, Fortelny R. et al. Accreditation and certification requirements for hernia centers and surgeons: the ACCESS project. Hernia 2019;23:185–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel A, Rockall A, Guthrie A, Gleeson F, Worthy S, Grubnic S. et al. Can the completeness of radiological cancer staging reports be improved using proforma reporting? A prospective multicentre non-blinded interventional study across 21 centres in the UK. BMJ Open 2018;8:e018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Bowel Cancer Audit. Reports—National Bowel Cancer Audit. https://www.nboca.org.uk/reports/ (accessed 8 July 2020).
- 31. Dietz DW; Consortium for Optimizing Surgical Treatment of Rectal Cancer (OSTRiCh). Multidisciplinary management of rectal cancer: the OSTRICH. J Gastrointest Surg 2013;17:1863–1868. [DOI] [PubMed] [Google Scholar]
- 32. Schlosser KA, Maloney SR, Gbozah K, Prasad T, Colavita PD, Augenstein VA. et al. The impact of weight change on intra-abdominal and hernia volumes. Surgery 2020;167:876–882. [DOI] [PubMed] [Google Scholar]
- 33. Cummings A, Grimmett C, Calman L, Patel M, Permyakova NV, Winter J. et al. ; members of CREW Study Advisory Committee. Comorbidities are associated with poorer quality of life and functioning and worse symptoms in the 5 years following colorectal cancer surgery: results from the ColoREctal Well-being (CREW) cohort study. Psychooncology 2018;27:2427–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clark ST, Malietzis G, Grove TN, Jenkins JT, Windsor ACJ, Kontovounisios C. et al. The emerging role of sarcopenia as a prognostic indicator in patients undergoing abdominal wall hernia repairs: a systematic review of the literature. Hernia 2020;24:1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jensen KK, Henriksen NA, Harling H.. Standardized measurement of quality of life after incisional hernia repair: a systematic review. Am J Surg 2014;208:485–493. [DOI] [PubMed] [Google Scholar]
- 36. Morris E, Haward RA, Gilthorpe MS, Craigs C, Forman D.. The impact of the Calman–Hine report on the processes and outcomes of care for Yorkshire’s colorectal cancer patients. Br J Cancer 2006;95:979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Munro A, Brown M, Niblock P, Steele R, Carey F.. Do multidisciplinary team (MDT) processes influence survival in patients with colorectal cancer? A population-based experience. BMC Cancer 2015;15:686. [DOI] [PMC free article] [PubMed] [Google Scholar]


