Abstract
Chronic inflammation is a hallmark of atherosclerosis and macrophages play a central role in controlling inflammation at all stages of atherosclerosis. In atherosclerosis, macrophages and monocyte-derived macrophages are continuously exposed to cholesterol, oxidized lipids, cell debris, cytokines, and chemokines. Not only do these stimuli induce a specific macrophage phenotype, but they also interact extensively, leading to macrophage heterogeneity in atherosclerotic plaques. Herein, we review the diverse phenotypes of macrophages, the mechanisms underlying macrophage activation, and the contributions of macrophages to atherosclerosis in this context. We also summarize recent studies on foamy macrophages and monocyte-derived macrophages in plaque during disease progression. We provide a comprehensive overview of transcriptional, epigenetic, and metabolic reprogramming of macrophages and discuss the emerging concepts of targeting cytokines and macrophages to modulate atherosclerosis.
Keywords: Atherosclerosis, Macrophage activation, Cytokines, Innate immune memory
INTRODUCTION
Atherosclerosis is a pronounced chronic inflammatory disease. Cholesterol, lipids, and cellular debris accumulate on the walls of blood vessels over time, causing inflammation. Anatomically, a chronic inflammatory response persists in the arterial intima, which is the layer located on top of the smooth muscle cell (SMC)-rich medial layer, and the outer layer, which is known as the adventitia. The persistent inflammatory response increases the volume of the intima, forming atherosclerotic plaques covered with a fibrous cap, leading to narrowing of the blood vessels.1,2,3,4 The progression of disease can eventually result in sudden rupture of atherosclerotic plaques and thrombosis. In turn, thrombosis can give rise to ischemia and consequent myocardial infarction, which is one of the leading causes of death.5 Although drugs that target atherosclerotic risk factors are effective at lowering cholesterol and are widely used to provide optimal treatment,6 recent studies have shown that targeting the inflammatory component of atherosclerosis, such as immune cells and pro-inflammatory cytokines, can effectively decrease atherosclerosis.7,8
As one of many types of immune cells, macrophages have been shown to play a key role in atherosclerotic lesion formation and progression.1,2,3,4 A macrophage is an essential player of the innate immune system that engulf and responds to cellular debris, pathogens, and danger signals. These cells are found in virtually all tissues and patrol for potential threats. Besides phagocytosis, they mediate inflammatory response by releasing pro- and anti-inflammatory cytokines and also help initiate adaptive immunity by recruiting other immune cells such as lymphocytes. Macrophages can adopt different functional programs and change their activation states in response to the various signals from their microenvironment. From this point of view, the macrophage activation spectrum is considered to be extremely wide,1,2,3,4 involving a complex functional response to a plethora of different signals. Thus, dysregulation of macrophage activation has been associated with many human diseases such as atherosclerosis, rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE).
In atherosclerosis, the activation states of macrophages are very dynamic because the microenvironment changes significantly during the disease progression. Macrophages can be exposed and stimulated to various cytokines, oxidized lipids, cholesterol crystals, dying cells, and hypoxia in atherosclerosis. After activation, macrophages undergo robust activation transitions, which can mediate inflammatory responses. Of note, macrophages have been shown to contribute to the initiation, growth and rupture of arterial plaques, and they exhibit phenotype rewiring, which leads to heterogeneous activation states among plaque macrophages. In this review, we will focus on the diversity of macrophage phenotypes and the underlying mechanism of heterogeneity in atherosclerosis. The activation states and phenotypes of macrophages will be summarized in terms of transcriptional, metabolic, and epigenetic regulation, and the balance of monocytes and macrophages according to disease progression will be described. Finally, we will discuss a new strategy for treating atherosclerosis by targeting cytokines produced by macrophages.
ACTIVATION STATES OF MACROPHAGES
Activation of macrophages plays a vital role in tissue homeostasis, disease pathogenesis, and inflammatory responses, and dysregulated activation of macrophages is associated with many human inflammatory diseases, including atherosclerosis, autoimmune diseases, and metabolic disorders.9,10,11,12 In the traditional, simplified classification, macrophage phenotypes are divided into 2 groups (M1 and M2), determined based on cytokine-induced in vitro conditions.10 Macrophages that promote inflammation are called M1 macrophages (or M[LPS+IFNγ]), and are important in host defense and the secretion of pro-inflammatory cytokines. M2 macrophages (or M[IL4+IL13]) are described as decreasing inflammation and aiding in tissue repair.13 Experimentally, selective markers are often used to explain macrophage polarization into these 2 groups. For example, CD206, Arg1, CD301, and Relmα (M2 markers) are induced in response to interleukin (IL)-4 stimulation, whereas CD11c and inducible nitric oxide synthase (M1 markers) are increased in M(LPS+IFNγ) macrophages in mice. The traditional classification of M1 and M2 macrophages is particularly important in terms of their function in understanding the inflammatory response, but in fact, these 2 active states only represent the extremes of the active states of macrophages (Fig. 1).10 Indeed, macrophages have remarkable plasticity and show a spectrum of activation; they can switch and reprogram their functional phenotype in response to various cues and consequently exhibit distinct functions essential to tissue homeostasis and inflammation. However, the diversity and function of activation states of macrophages still remain to be fully characterized in vivo because there is considerable diversity in gene expression patterns between macrophages in different tissues.
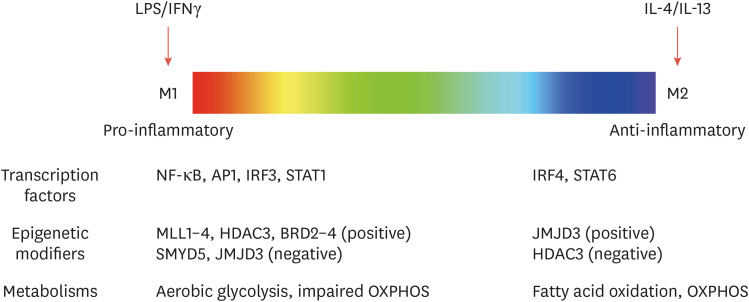
Fig. 1. The spectrum of macrophage activation and its regulators. The color spectrum indicates different activation states of macrophages, showing a linear scale of 2 macrophage designations, M1 and M2. Transcriptional, epigenetic, and metabolic factors that regulate macrophage activation are indicated.
LPS, lipopolysaccharide; IFNγ, interferon gamma; IL, interleukin; NF-κB, nuclear factor κB; AP1, activator protein 1; IRF3, interferon regulatory factor 3; STAT, signal transducer and activator of transcription; MLL, mixed-lineage leukemia; HDAC3, histone deacetylase 3; BRD, bromodomain-containing protein; SMYD5, SET and MYND domain-containing protein 5; Jmjd3, Jumonji domain-containing protein D3; OXPHOS, oxidative phosphorylation.
Activation states and the distinct functions of macrophages are affected by transcriptional, metabolic, and epigenetic reprogramming, and are modulated by transcription factors, metabolites, and epigenetic enzymes (important aspects of transcriptional regulation for macrophage activation have been described extensively elsewhere.13,14). For instance, interferon gamma (IFNγ) and IL-4 exert a clear antagonistic effect on macrophage activation, mediated by signal transducer and activator of transcription (STAT) 1 and STAT6, respectively. Recent evidence has also demonstrated that the signaling pathways and transcription factors that are important for different macrophage activation states induce metabolic and epigenetic changes.13,14,15,16 Lipopolysaccharide (LPS)/IFNγ stimulation has been shown to induce extensive metabolic reprogramming of macrophages and dendritic cells. Increased glucose uptake in M(LPS/IFNγ) macrophages enhances aerobic glycolysis and impairs oxidative phosphorylation (OXPHOS).17,18 In inflammatory macrophages such as M(LPS+IFNγ), pyruvate from the glycolytic pathway is converted to lactate, while tricarboxylic acid (TCA) cycle intermediates accumulate, resulting in impaired OXPHOS with effects on the inflammatory response and macrophage function.18,19 In contrast, M(IL-4) macrophages show a sharp increase in fatty acid oxidation and OXPHOS, which are associated with the anti-inflammatory response and tissue repair.19 CD36-mediated triglyceride uptake and subsequent lipolysis through lysosomal lipase can increase OXPHOS to maintain M(IL-4) macrophages. However, it seems that the transition toward glycolysis or OXPHOS cannot predict the activation status of macrophages because M(IL-4) macrophages also depend on glucose uptake to increase OXPHOS.19 For example, inhibition of the glycolysis pathway inhibited IL-4-induced gene expression,20 whereas inhibition of carnitine palmitoyltransferase, a key enzyme required for fatty acid import, did not change the activation state of M(IL-4) macrophages.21,22
In addition to metabolic rewiring, epigenetically conferred transcriptional changes provide the molecular basis for macrophage polarization with distinct functional states and for reprogramming of macrophages upon subsequent environmental cues.9,10,23 Notably, activation states of macrophages have been shown to be associated with epigenetic enzymes such as histone modifiers. Expression of mixed-lineage leukemia (MLL), a histone methyltransferase, is required for M1 gene expression and the formation of a de novo enhancer in M(IFNγ+LPS) macrophages.24 H4K20me3, which is carried out by SET and MYND domain-containing protein 5 (SMYD5), limits the expression of LPS target genes in macrophages25 and 2 histone modifiers, Jumonji domain-containing protein D3 (Jmjd3) and histone deacetylase 3 (HDAC3), have been shown to modulate the activation state of macrophages. Expression of LPS-induced genes is significantly decreased in Jmjd3-deficient macrophages.26,27 Of interest, Jmjd3 is also increased by IL-4 treatment28 and was shown to be important for M2 responses in response to helminth infections through IRF4.29 Similarly, macrophages lacking HDAC3 exhibit an M2-like phenotype and are hyperresponsive to IL-4.30 It has been shown that HDAC3 is required to activate hundreds of STAT1-dependent, inflammatory genes in M1 macrophages, which have been shown to rely on defective IFN-β responses in macrophages.31 Collectively, these metabolic and epigenetic regulations are reprogrammed by various stimuli, which allow macrophages to respond appropriately to environmental cues and function accordingly.
MACROPHAGE PHENOTYPES IN ATHEROSCLEROSIS
In atherosclerosis, chronic inflammation can lead to aberrant activation of macrophages, and various triggers cause functional heterogeneity of macrophages that combine the features of pro-inflammatory and anti-inflammatory phenotypes.3,32 Furthermore, different types of macrophages in plaques are organized in atherosclerotic vessels33 and affect lesion stability and the clinical outcome.3 The microenvironment around atherosclerotic vessels is influenced by hyperlipidemia, pro-inflammatory cytokines, cholesterol crystals, hypoxia, oxidative stress, and danger-associated molecular patterns derived from cell death, which macrophages detect. Hyperglycemia, which accelerates atherosclerosis, is also an important factor affecting macrophage activation, as shown in people with diabetes. Together, a variety of stimuli in atherosclerosis create a complex microenvironment in atherosclerotic vessels that ultimately influences disease progression and plaque stability.4 These microenvironmental signals drive the transcriptional, metabolic, and epigenetic reprogramming of macrophages and perpetuate macrophage inflammation (Fig. 2). Thus, there is considerable interest in using the plasticity of macrophage activation to rebalance the inflammatory response and protect tissue homeostasis in atherosclerosis.
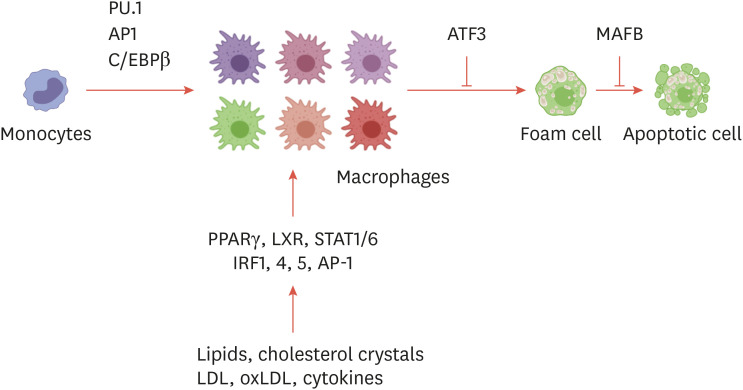
Fig. 2. Transcription factors that regulate the macrophage phenotype in atherosclerosis. Transcription factors that regulate macrophage differentiation, activation, and the formation of foam cells are indicated.
PU.1, purified anti-SPI1; AP1, activator protein 1; C/EBPβ, CCAAT/enhancer binding protein β; ATF3, activating transcription factor 3; MAFB, MAF BZIP transcription factor B; PPARγ, peroxisome proliferator-activated receptor γ; LXR, liver X receptor; STAT, signal transducer and activator of transcription; IRF, interferon regulatory factor; LDL, low-density lipoprotein; oxLDL, oxidized low-density lipoprotein.
Recent studies have shown that advanced plaques of Ldlr −/− mice consist of heterogeneous populations of macrophages, including CD86high M1-like macrophages (≤40%), CD206+ M2-like macrophages (≤20%), and M(ox) macrophages (≤30%). Although macrophages of various active states co-exist in atherosclerosis, it was demonstrated that pro-inflammatory macrophages (M1-like) are preferentially located in the plaque shoulder, the prone area of unstable rupture of the plaque.33 In general, plaque macrophages have been thought to be responsible for causing inflammation and destroying tissues.34 Pro-inflammatory macrophages produce high levels of tumor necrosis factor (TNF), IL-1β, IL-6 and many chemokines such as C-C motif chemokine ligand (CCL) 235 and these pro-inflammatory cytokines can contribute to the progression of atherosclerosis and the stability of plaques. For example, the development of plaque and inflammation was suppressed in TNF-deficient apolipoprotein E (ApoE) knockout (KO) mice,36,37 whereas treatment of anti-TNF antibody enhanced the plaque burden with decreased levels of IL-5 and CCL5.38 The administration of IL-6 exacerbates plaque lesions in ApoE models, and IL-6 messenger RNA (mRNA) and protein levels were increased in CD68+ plaque macrophages.39,40,41 In contrast, M2-like, anti-inflammatory macrophages in atherosclerotic plaques contribute to disease and plaque regression. Treatment of IL-13, which is an M2 polarizing factor, hampered atherosclerosis progression in Ldlr KO mice42 and Stat6-deficient macrophages were associated with impaired plaque regression in a mouse regression model.43,44 In humans, M2-like macrophages are found near highly vascularized zones and calcified areas, and these macrophages participate in angiogenesis.45 Another phenotype directly associated with atherosclerotic plaques is M(ox) macrophages, which can be polarized in vitro in response to oxidized phospholipids.46 Oxidized phospholipids or oxidized low-density lipoproteins (oxLDLs) are recognized by toll-like receptors (TLRs) and CD36 of macrophages, leading to inflammatory responses and chemokine expression.47,48,49,50 These M(ox) macrophages highly express NRF2-dependent genes, such as Hmox1 and Srxn1, and show glutathione-related phenotypes. Importantly, M(ox) macrophages have a weaker phagocytic function than typical M1-like or M2-like macrophages, which could potentially contribute to the development of advanced plaque. In addition to activated macrophages, foam cells are the major driver of plaque formation in atherosclerosis.4 Cholesterol is an important molecule in cellular components such as cell membranes and is a precursor to various biological metabolites.51 As cholesterol and triglycerides are retained in the artery wall, tissue macrophages try to get rid of the excess inflammatory lipids and store those in cytoplasmic lipid droplets.52 The imbalance of intracellular cholesterol hemostasis by modified inflammatory lipids transforms macrophages or monocytes into foamy macrophages.14,53 The ability of these foam cells to move decreases; as a result, they persist in the artery wall, causing chronic inflammation. For example, cholesterol crystals within macrophages are formed early in atherosclerotic plaques and activate the inflammasome and production of IL-1β.50 Long-term defects in cholesterol efflux and cholesterol esterification by the endoplasmic reticulum can also lead to macrophage death in plaques.
Historically, foam cells have been considered to correspond to pro-inflammatory states of macrophages. For instance, foam cells exhibited a decreased migratory capacity and promoted the production of pro-inflammatory cytokines.54,55 However, recent studies using single-cell RNA sequencing (scRNA-seq) of immune cells from atherosclerotic aorta illustrated that foam cells in plaques failed to express pro-inflammatory genes for IL-1β or other cytokines, but instead, CCR2+ non-foamy macrophages showed dramatic expression of pro-inflammatory cytokines or chemokines.53,56 These studies suggest that non-foamy macrophages are more similar to recently recruited monocytes, and the role of monocytes and monocyte-derived macrophages should be considered in order to understand local inflammation, especially at plaque shoulder regions. In concurrence with these scRNA-seq data, it has been shown that lipid-overloaded foam cells exhibit distinct transcription profiles representing an anti-inflammatory phenotype. The activation of peroxisome proliferator-activated receptor γ (PPARγ) and liver X receptor (LXR) transcription factor-dependent pathways increased a subset of genes related to lipid-handling proteins such as the ATP binding cassette lipid transporter (Fig. 2).56,57 In addition to PPARγ and LXR, several transcription factors have been associated with foam cell formation and maintenance during atherosclerosis development. MAF BZIP transcription factor B (MAFB), which is associated with macrophage differentiation and polarization, has been shown to regulate the development of foam cells. In the early stage of atherosclerosis in mice, MAFB promoted plaque development through inhibition of foam cell apoptosis.58 In contrast, MAFB-deficient mice had larger necrotic cores in advanced plaques, suggesting that MAFB stabilizes atherosclerotic plaques in late stages of disease development.59 Another example is the transcription factor Kruppel-like factor 4 (KLF4) in the regulation of cholesterol 25-hydroxylase (Ch25h) expression.60 Increased expression of Ch25h mRNA in mouse macrophages activated LXR expression in a KLF4-dependent manner60 and inhibited the NLRP3 inflammasome and IL-1β. Thus, activation of the KLF4-dependent pathway has yielded anti-inflammatory and atheroprotective effects in a mouse model. Activating transcription factor 3 (ATF3) also plays an important role in foam cell formation and disease progression in mice.61 ATF3 inhibited Ch25h expression and regulated lipid droplet formation in macrophages. Thus, ATF3 deficiency enhanced the formation of 25-hydroxycholesterol and caused foam cell formation.61 The anti-inflammatory function of high-density lipoprotein (HDL) has consistently been found to be mediated by HDL-induced ATF3 expression, leading to suppression of inflammatory cytokine expression in macrophages.62
INNATE IMMUNE MEMORY OF MACROPHAGES: PRIMING EFFECT
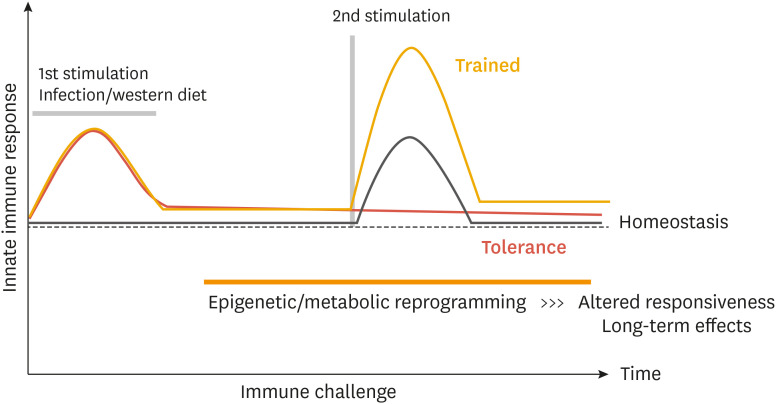
As described above, macrophages and immune cells in the atherosclerotic microenvironment are continuously exposed to various stimuli such as cholesterol, lipoproteins, dying cells, cytokines, hypoxia, and oxidative stress. Macrophages or monocytes exposed to chronic or repeated stimuli in vivo can reprogram their activation states to mount a response based on “innate immune memory” to subsequent stimulation.13,14,16 Over the last decade, the mechanism of innate immune memory has been well established in the context of infections by pathogens. There are 2 representative arms of innate immune memory, referred to as trained immunity and tolerance, which are mediated by transcriptional, epigenetic, and metabolic reprogramming (Fig. 3). These innate immune memory mechanisms allow innate immune cells to adapt to previous environmental factors and respond more appropriately to the next stimulus. For example, pre-exposure to β-glucan or Bacillus Calmette-Guérin can train and prime macrophages, which display an enhanced defensive response against unrelated infections.13,14,16 In contrast, macrophages or monocytes can be tolerized by pre-exposure to TLR agonists or TNF. Tolerized macrophages are not able to produce pro-inflammatory cytokines in response to subsequent stimuli and prevent excessive inflammatory responses.13,14,16 Therefore, innate immune memory can affect the overall immunological status in a clinically relevant manner.
Fig. 3. Innate immune memory: trained versus tolerance. A proposed model of trained immunity and tolerance is depicted in the graph.
Sterile inflammatory mediators can also establish innate immune memory. In atherosclerosis, both hypercholesterolemia and oxLDL have been shown to induce innate immune training.63 Human macrophages pretreated with oxLDL and restimulated with LPS or Pam3Cys produced increased pro-inflammatory cytokines including TNF, IL-6, IL-8, and CCL2.63 Interestingly, a methylation inhibitor reversed this training effect, suggesting that reprogramming of the chromatin landscape is required for the induction of the oxLDL priming effect. Another study demonstrated that human monocytes pretreated with oxLDL and restimulated with LPS displayed increased inflammatory responses, and this effect was abrogated by co-treatment with a recombinant IL-1R antagonist.64 Hypercholesterolemia is also a sterile driver of innate immune training in mice.64 In Ldlr –/– mice fed a western diet for 4 weeks, monocytes exhibited enhanced expression of pro-inflammatory cytokines in response to TLR agonists ex vivo. Consistently, the serum inflammatory cytokines in mice fed a western diet were higher than in mice fed a chow diet.64 Myelopoiesis was also enhanced in mice fed a western diet, and RNA-seq data showed that genes related to cholesterol-biosynthesis pathways were downregulated, whereas the expression of pro-inflammatory genes was enhanced. Interestingly, when mice fed a western diet were switched back to a normal diet for 4 weeks, systemic inflammation decreased, but the primed response of monocytes to ex vivo TLR stimulation was not normalized. This study revealed that the western diet trained myeloid progenitor GMP population in an IL-1β dependent manner through reprogramming at the transcriptional and epigenetic levels.64 In addition, clinical studies have shown that circulating monocytes in patients with atherosclerosis also have enhanced inflammatory signatures associated with epigenetic and metabolic remodeling.65,66,67 For example, monocytes isolated from patients with established atherosclerosis induce a stronger inflammatory response to TLR agonists, such as LPS and Pam3Cys, than monocytes from healthy patients. Increased production of IL-6 and IL-1β in monocytes isolated from the atherosclerosis patients was observed, with higher expression of glycolytic, TCA cycle, and pentose phosphate pathway genes, indicating that monocyte priming occurred in the disease microenvironment through metabolic reprogramming. Taken together, the emerging concept of innate immune memory adds a new layer of macrophage regulation and offers a new understanding of chronic inflammation and atherosclerosis.
MONOCYTE-DERIVED MACROPHAGES IN ATHEROSCLEROSIS
In humans and mice, monocytes can differentiate into macrophages and myeloid lineage dendritic cells. Monocytes compose 2%–10% of all immune cells in the human body and play multiple roles in immune function. Monocytes are derived from the bone marrow and spleen and then released into the circulation for surveillance.68 In the event of infection or tissue damage, monocytes are recruited into the damaged tissue, where they regulate the inflammatory responses and tissue homeostasis. It has also been shown that recruited monocytes are the major precursor of the tissue macrophage population in certain pathogenic conditions. There are at least 3 subsets of monocytes in human blood based on their surface markers: classic CD14++CD16− monocytes, non-classic CD14+CD16++ monocytes, and intermediate CD14++CD16+ monocytes.68 Under normal conditions, CD14++CD16− monocytes are involved in recruitment into tissues and contribute to the maintenance of tissue macrophage populations. In contrast, non-classic CD14+CD16++ monocytes are less recruited to tissues in a CCR5-dependent manner.69 Intermediate monocytes are specifically enriched in the bone marrow and express high levels of surface receptors related to reparative processes. In mice, monocytes can be divided into 2 subpopulations: inflammatory Ly6Chigh, CCR2pos, CX3CR1low monocytes, which are comparable to human classical monocytes, and resident Ly6Clow, CCR2neg, CX3CR1high monocytes, which are equivalent to human non-classical monocytes.7,68,70
In atherosclerosis, monocytes have been associated with plaque macrophages and disease progression due to their ability to trigger pro-inflammatory or anti-inflammatory macrophage states, and the levels of monocytes in the blood showed a strong correlation with the progression of atherosclerosis.7 Importantly, their activation states of monocytes are determined by local environmental cues and cytokine availability. Various inflammatory stimuli accelerate the production of bone marrow monocytes and the outflow of blood monocytes to the inflamed area. For example, in atherosclerosis, the modified lipoproteins give rise to the recruitment of monocytes into the subendothelial space.7 The monocytes then differentiate into macrophages capable of removing accumulated lipoproteins, a process that is initially thought to be a beneficial response. However, the sustained process ultimately leads to the accumulation of cholesterol-laden foam cells that contribute to plaque formation. In addition to modified lipoproteins, the local production of chemokines promotes the migration of monocytes from blood to tissue.7,68,70 For instance, CCL2 is highly expressed in atherosclerotic plaques and promotes the recruitment of CCR2+ monocytes. Other chemokines such as CCL5, CX3CL1, and CXCL12 promote the development and recruitment of bone marrow and blood monocytes.70 Once monocytes arrive at the inflamed tissues, monocyte-derived macrophages require colony-stimulating factor 1 (CSF-1) for their survival and maintenance.71,72 Recently, it has been shown that CSF-1 derived from local smooth muscle and endothelial cells supports plaque macrophage survival.73 In addition, rapid adaption and differentiation of monocytes in plaques requires CSF-1 receptor-mediated signaling in aortic stromal cells and macrophages.71,72 Among the various subsets of monocytes, CD14+CD16++ monocytes are associated with maintaining the vasculature, cell survival, and localization within plaques through CX3CR1/CX3CL1 interactions in atherosclerosis.69,74,75 In mice, CD14+CD16++ monocyte-deficiency in NR4A1 KO mice switched the activation states of macrophages toward an inflammatory phenotype and exacerbated endothelial dysfunction and atherosclerosis.76,77,78 In addition to the recruitment of monocytes to maintain and replenish the plaque foam cell pool, it can also be expanded by macrophage self-proliferation.79 However, the relative balance in atherosclerosis between macrophage proliferation and recruitment from blood is still somewhat unclear. While many tissue macrophages are replenished by circulating LyC6high monocytes during homeostasis, some tissue-resident macrophages such as microglia can exist independently of circulating monocytes. In general, Ly6Clow monocytes have less potential to differentiate into tissue-resident macrophages.80 In atherosclerosis, newly formed lesions mostly recruit monocytes from the circulation, but more advanced niches rely mainly on local macrophage proliferation, and the contribution of new monocyte recruitment is less substantial.79 Although the proliferating macrophages in advanced lesions initially derive from monocytes recruited from the blood, it is now clear that the balance between local macrophage proliferation and recruitment from blood is crucial for disease progression. This hypothesis is supported by the mouse regression model, in which Cre-mediated inhibition of liver lipoprotein production or ApoE KO aortic transplantation into wild-type mice can reset physiological blood LDL levels.43,81,82,83,84 In the setting of disease regression, the number of plaque macrophages decreases, and the remaining macrophages exhibit less inflammatory and more reparative phenotypes. After aortic transplantation, in regression lesions, most less inflammatory and reparative macrophages came from the blood circulation, indicating the recruitment of new monocytes into the arterial wall, even in the context of plaque regression.43 In the regression model, Ly6Chigh monocytes are newly recruited in a CCR2-dependent manner and differentiated into reparative and less inflammatory macrophages through a STAT6 regulated differentiation program. Therefore, knowing exactly the difference between monocytes and monocyte-derived macrophages and tissue-resident macrophages at the site of local inflammation is necessary to understand the formation of foam cells and plaques and the progression of atherosclerosis.
SMC-DERIVED MACROPHAGES IN ATHEROSCLEROSIS
One of the most striking discoveries in recent studies is that not all macrophage foam cells are derived from macrophages or monocyte-derived macrophages. Although many studies have highlighted the formation of foam cells from monocyte-derived macrophages of atherosclerotic lesion formation, in fact, many foam cells are also derived from intima SMCs.85 Recent studies demonstrated that SMCs have the capacity to convert and transdifferentiate into macrophage-like cells that make up the foam cells present in plaque.86,87,88 Foam cells with at least enough features to identify the features of SMCs share relatively non-inflammatory gene expression profiles with more classical macrophage foam cells.56 In response to platelet-derived growth factor beta (PDGFβ) signaling, SMCs can lose their contractile phenotype and transdifferentiate into cells characterized by extracellular matrix production and a reparative function that heals and stabilizes the artery wall.89,90,91 These SMCs thicken and stabilize the fibrous cap in atherosclerotic lesions. As the disease progresses, SMCs become one of the first cell types to take up and retain lipoproteins.92 Then, SMCs transdifferentiate into plaque foam cells when stimulated by microenvironmental signals and stimuli in plaques such as oxidized lipids, TGF-β and other cytokines.93,94,95 Cholesterol itself has been shown to promote transdifferentiation of mouse SMCs into cells that induce high expression of macrophage markers CD68, ABCA1 and galectin 3.87 Interestingly, there are more foam cells that are not macrophages than have been thought. Co-staining with SMC and foam cell-specific markers illustrated that about 50% of foam cells in human atherosclerotic lesions are SMC-derived cells.96 Nevertheless, it remains unclear to what extent the origin and proportion of foam cells are derived from SMCs in terms of expression phenotype and trajectory, but lineage-tracing experiments in mice further suggested a better understanding of the underlying mechanism of transdifferentiation. For example, it is known that purified anti-SPI1, a major macrophage transcription factor, binds to the KLF4 promoter in response to PDGF signals in SMCs. Similar to the function of STAT6, KLF4 increases ZC3H12A expression, which inhibits nuclear factor κB function and activates the CCAAT/enhancer binding protein β and PPARγ pathways to drive SMC transdifferentiation and anti-inflammatory phenotypes.88,97,98 SMC-derived foam cells develop the above-mentioned macrophage markers and accumulate cholesterol and lipoproteins, but these SMC-derived foam cells are not considered bona fide macrophages because they do not have a phagocytic or efferocytotic capacity.99 In the meantime, it was difficult to isolate these cells from their monocyte-derived counterparts in experiments due to the gradual loss of SMC markers in SMC-derived foam cells,100 and this limitation was a challenge for research on SMC-derived foam cells. However, recent single-cell experimental techniques and lineage tracking methods are expected to reveal more about SMC-derived macrophages.
TARGETING INFLAMMATORY CYTOKINES IN ATHEROSCLEROSIS
Whether inflammatory or anti-inflammatory, macrophages in atherosclerosis are the major producers of cytokines, and these cytokines are closely associated with all stages of atherosclerosis. It has been shown that therapeutically targeting major cytokines such as TNF-α, IL-1β, and IL-17 is effective in a wide variety of inflammatory diseases including RA, SLE, and Crohn's disease. In atherosclerosis, medicines targeting lipid and cholesterol management have been the primary options so far, but new evidence has recently emerged that targeting cytokines that coordinate inflammation can relieve atherosclerosis.
The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS),101 the first test of anti-inflammatory treatment of atherosclerosis, was a clinical trial targeting therapeutically soluble IL-1β in patients. Canakinumab, which neutralizes IL-1β, was administered to patients with high-risk cardiovascular disease with a history of myocardial infarction and elevated high-sensitivity C reactive protein (hsCRP, >2 mg/L). IL-1β is secreted by myeloid cells such as macrophages and monocytes, as well as by arterial SMCs and endothelial cells.102,103,104 In atherosclerosis, the uptake of excessive oxLDL via CD36 produces intracellular cholesterol crystals that destabilize lysosomes and activate the inflammasome complex, leading to IL-1β secretion.48,50 Thus, NLRP3-inflammasome activation has been associated with numerous dysfunctions of plaque macrophages, including inflammation and impaired metabolism.105 The CANTOS trial demonstrated that therapeutically targeting IL-1β effectively decreased levels of IL-6 (an inflammatory cytokine) and hsCRP. A dose of 150 mg exhibited a remarkable benefit in the primary endpoint examining recurrence of cardiovascular events between 3 and 4 years later. However, there was no difference in the overall mortality rate because the patients who received treatment had a high incidence of fatal infections. Notably, there was no change in cholesterol levels, which underscored the importance of inflammatory cytokines in atherosclerosis and suggested a potential cytokine-targeting approach for the treatment of atherosclerosis. In contrast to the implications of the CANTOS results, neutralizing IL-1β antibodies in mice at advanced atherosclerotic states reduced SMC remodeling and fibrous cap formation and eventually led to elevated plaque vulnerability.106 Taken together, these results imply that IL-1β may have multiple deleterious or beneficial effects dependent on the stage of atherosclerosis, and more detailed mechanisms should be explored for clinical applications. Besides targeting IL-1β, anti-inflammatory approaches are being attempted to target inflammatory cytokines such as TNF-α and IL-6, which are well known to be associated with the pathological mechanisms of atherosclerosis. However, the limited success of IL-1β blockade and unexplored questions of these pathways and mechanisms have presented both potential possibilities and disappointments. Broadening our understanding of tissue-specific macrophage and monocyte differentiation programs will enable targeted treatments specific to atherosclerosis without perturbing a wide range of inflammatory pathways.
CONCLUSION
In this review, we described recent evidence and emerging concepts in the basic understanding of macrophages in atherosclerosis. Atherosclerosis is a chronic inflammation-driven disease, and macrophages play an important role in controlling inflammation in all stages of atherosclerosis. In atherosclerosis, macrophages respond to dyslipidemia, cytokines, dying cells, and hypoxia and exhibit heterogeneity in their activation state depending on the type of stimulus they receive. The heterogeneity of this activation state includes foam cells and plaque macrophages, along with typical inflammatory and anti-inflammatory macrophages. In the field of basic biology, surprising results have been found over the past decade, but there are still many shortcomings in understanding the pathogenesis of atherosclerosis. In this regard, it is necessary to understand more about the metabolic and epigenetic regulation of macrophages and the heterogeneity and plasticity of macrophages across multiple stages of atherosclerosis. Emerging concepts about metabolic and epigenetic regulation, such as innate immune memory, will greatly aid in a better understanding of foam cell formation and advanced or fragile plaques. Distinct effector responses, such as phagocytosis, cytokine production, and cholesterol modulation, depending on the macrophage phenotype should be further studied. The CANTOS trial positively suggested that inflammation can be targeted in atherosclerosis to reduce clinically important cardiovascular disease. Besides targeting inflammatory cytokines, using the plasticity of macrophages to rebalance dysregulated inflammatory responses would be a promising therapeutic target for atherosclerosis because it would enable more tissue-specific and disease-specific modulation. Flexible but tight regulation of macrophages by interfering with epigenetic and metabolic factors will form the basis for interventions to rebalance inflammation and macrophage activity. In particular, characterizing the chromatin landscape of pathogenic cells such as plaque macrophages can help pinpoint the targeting of macrophage-type specific gene expression programs. Furthermore, since the complex environment within atherosclerotic plaques in vivo simultaneously affects macrophages and monocyte-derived macrophages, investigating the effects of signaling crosstalk on inflammatory responses can help us to understand macrophage activation in plaques and to develop targeted therapeutic approaches.
Footnotes
Funding: None.
Conflict of Interest: The author has no conflicts of interest to declare.
References
- 1.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 3.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AY, Leiter LA. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Curr Opin Cardiol. 2006;21:400–404. doi: 10.1097/01.hco.0000231412.15049.fb. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity. 2017;47:621–634. doi: 10.1016/j.immuni.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 9.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glass CK, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol. 2016;17:26–33. doi: 10.1038/ni.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuznetsova T, Prange KH, Glass CK, de Winther MP. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Nat Rev Cardiol. 2020;17:216–228. doi: 10.1038/s41569-019-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 16.Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19:526–537. doi: 10.1038/s41590-018-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21:65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Reports. 2016;17:684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Tan Z, Xie N, Cui H, Moellering DR, Abraham E, Thannickal VJ, et al. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol. 2015;194:6082–6089. doi: 10.4049/jimmunol.1402469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nomura M, Liu J, Rovira II, Gonzalez-Hurtado E, Lee J, Wolfgang MJ, et al. Fatty acid oxidation in macrophage polarization. Nat Immunol. 2016;17:216–217. doi: 10.1038/ni.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Kittan NA, Allen RM, Dhaliwal A, Cavassani KA, Schaller M, Gallagher KA, et al. Cytokine induced phenotypic and epigenetic signatures are key to establishing specific macrophage phenotypes. PLoS One. 2013;8:e78045. doi: 10.1371/journal.pone.0078045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaikkonen MU, Spann NJ, Heinz S, Romanoski CE, Allison KA, Stender JD, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. 2013;51:310–325. doi: 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Santa F, Narang V, Yap ZH, Tusi BK, Burgold T, Austenaa L, et al. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 2009;28:3341–3352. doi: 10.1038/emboj.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruidenier L, Chung CW, Cheng Z, Liddle J, Che K, Joberty G, et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488:404–408. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244–3254. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 30.Mullican SE, Gaddis CA, Alenghat T, Nair MG, Giacomin PR, Everett LJ, et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25:2480–2488. doi: 10.1101/gad.175950.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Barozzi I, Termanini A, Prosperini E, Recchiuti A, Dalli J, et al. Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages. Proc Natl Acad Sci U S A. 2012;109:E2865–E2874. doi: 10.1073/pnas.1121131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, et al. Single-Cell RNA-Seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122:1661–1674. doi: 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- 33.Stöger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17:34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- 35.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 36.Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 37.Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, et al. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180:11–17. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Oberoi R, Vlacil AK, Schuett J, Schösser F, Schuett H, Tietge UJ, et al. Anti-tumor necrosis factor-α therapy increases plaque burden in a mouse model of experimental atherosclerosis. Atherosclerosis. 2018;277:80–89. doi: 10.1016/j.atherosclerosis.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Seino Y, Ikeda U, Ikeda M, Yamamoto K, Misawa Y, Hasegawa T, et al. Interleukin 6 gene transcripts are expressed in human atherosclerotic lesions. Cytokine. 1994;6:87–91. doi: 10.1016/1043-4666(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 40.Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 41.Sukovich DA, Kauser K, Shirley FD, DelVecchio V, Halks-Miller M, Rubanyi GM. Expression of interleukin-6 in atherosclerotic lesions of male ApoE-knockout mice: inhibition by 17beta-estradiol. Arterioscler Thromb Vasc Biol. 1998;18:1498–1505. doi: 10.1161/01.atv.18.9.1498. [DOI] [PubMed] [Google Scholar]
- 42.Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J, et al. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest. 2017;127:2904–2915. doi: 10.1172/JCI75005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin JD, Nishi H, Poles J, Niu X, Mccauley C, Rahman K, et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4:e124574. doi: 10.1172/jci.insight.124574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo L, Akahori H, Harari E, Smith SL, Polavarapu R, Karmali V, et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest. 2018;128:1106–1124. doi: 10.1172/JCI93025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 50.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cyster JG, Dang EV, Reboldi A, Yi T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol. 2014;14:731–743. doi: 10.1038/nri3755. [DOI] [PubMed] [Google Scholar]
- 52.Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN. Mechanisms of foam cell formation in atherosclerosis. J Mol Med (Berl) 2017;95:1153–1165. doi: 10.1007/s00109-017-1575-8. [DOI] [PubMed] [Google Scholar]
- 53.Williams JW, Huang LH, Randolph GJ. Cytokine circuits in cardiovascular disease. Immunity. 2019;50:941–954. doi: 10.1016/j.immuni.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ Res. 2018;123:1127–1142. doi: 10.1161/CIRCRESAHA.118.312804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamada M, Nakamura M, Tran MT, Moriguchi T, Hong C, Ohsumi T, et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat Commun. 2014;5:3147. doi: 10.1038/ncomms4147. [DOI] [PubMed] [Google Scholar]
- 59.Hasegawa H, Watanabe T, Kato S, Toshima T, Yokoyama M, Aida Y, et al. The role of macrophage transcription factor MafB in atherosclerotic plaque stability. Atherosclerosis. 2016;250:133–143. doi: 10.1016/j.atherosclerosis.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Li Z, Martin M, Zhang J, Huang HY, Bai L, Zhang J, et al. Krüppel-like factor 4 regulation of cholesterol-25-hydroxylase and liver x receptor mitigates atherosclerosis susceptibility. Circulation. 2017;136:1315–1330. doi: 10.1161/CIRCULATIONAHA.117.027462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gold ES, Ramsey SA, Sartain MJ, Selinummi J, Podolsky I, Rodriguez DJ, et al. ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation. J Exp Med. 2012;209:807–817. doi: 10.1084/jem.20111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Nardo D, Labzin LI, Kono H, Seki R, Schmidt SV, Beyer M, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol. 2014;15:152–160. doi: 10.1038/ni.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, Riksen NP. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol. 2014;34:1731–1738. doi: 10.1161/ATVBAHA.114.303887. [DOI] [PubMed] [Google Scholar]
- 64.Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018;172:162–175.e14. doi: 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee SJ, Thien Quach CH, Jung KH, Paik JY, Lee JH, Park JW, et al. Oxidized low-density lipoprotein stimulates macrophage 18F-FDG uptake via hypoxia-inducible factor-1α activation through Nox2-dependent reactive oxygen species generation. J Nucl Med. 2014;55:1699–1705. doi: 10.2967/jnumed.114.139428. [DOI] [PubMed] [Google Scholar]
- 66.van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134:611–624. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, et al. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. 2016;254:228–236. doi: 10.1016/j.atherosclerosis.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 68.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1412–1418. doi: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, et al. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sinha SK, Miikeda A, Fouladian Z, Mehrabian M, Edillor C, Shih D, et al. Local M-CSF (macrophage colony-stimulating factor) expression regulates macrophage proliferation and apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2021;41:220–233. doi: 10.1161/ATVBAHA.120.315255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 76.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li P, Bai Y, Zhao X, Tian T, Tang L, Ru J, et al. NR4A1 contributes to high-fat associated endothelial dysfunction by promoting CaMKII-Parkin-mitophagy pathways. Cell Stress Chaperones. 2018;23:749–761. doi: 10.1007/s12192-018-0886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schyns J, Bai Q, Ruscitti C, Radermecker C, De Schepper S, Chakarov S, et al. Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat Commun. 2019;10:3964. doi: 10.1038/s41467-019-11843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lieu HD, Withycombe SK, Walker Q, Rong JX, Walzem RL, Wong JS, et al. Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation. 2003;107:1315–1321. doi: 10.1161/01.cir.0000054781.50889.0c. [DOI] [PubMed] [Google Scholar]
- 84.Llodrá J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dubland JA, Francis GA. So Much Cholesterol: the unrecognized importance of smooth muscle cells in atherosclerotic foam cell formation. Curr Opin Lipidol. 2016;27:155–161. doi: 10.1097/MOL.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 86.Cherepanova OA, Gomez D, Shankman LS, Swiatlowska P, Williams J, Sarmento OF, et al. Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat Med. 2016;22:657–665. doi: 10.1038/nm.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A. 2003;100:13531–13536. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res. 1992;71:1525–1532. doi: 10.1161/01.res.71.6.1525. [DOI] [PubMed] [Google Scholar]
- 90.Barrett TB, Benditt EP. Platelet-derived growth factor gene expression in human atherosclerotic plaques and normal artery wall. Proc Natl Acad Sci U S A. 1988;85:2810–2814. doi: 10.1073/pnas.85.8.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 92.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma S, Yang D, Li D, Tang B, Yang Y. Oleic acid induces smooth muscle foam cell formation and enhances atherosclerotic lesion development via CD36. Lipids Health Dis. 2011;10:53. doi: 10.1186/1476-511X-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Costales P, Fuentes-Prior P, Castellano J, Revuelta-Lopez E, Corral-Rodríguez MÁ, Nasarre L, et al. K domain CR9 of low density lipoprotein (LDL) receptor-related protein 1 (LRP1) is critical for aggregated LDL-induced foam cell formation from human vascular smooth muscle cells. J Biol Chem. 2015;290:14852–14865. doi: 10.1074/jbc.M115.638361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rivera J, Walduck AK, Thomas SR, Glaros EN, Hooker EU, Guida E, et al. Accumulation of serum lipids by vascular smooth muscle cells involves a macropinocytosis-like uptake pathway and is associated with the downregulation of the ATP-binding cassette transporter A1. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:1081–1093. doi: 10.1007/s00210-013-0909-5. [DOI] [PubMed] [Google Scholar]
- 96.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–1559. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 97.Deaton RA, Gan Q, Owens GK. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H1027–H1037. doi: 10.1152/ajpheart.01230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kapoor N, Niu J, Saad Y, Kumar S, Sirakova T, Becerra E, et al. Transcription factors STAT6 and KLF4 implement macrophage polarization via the dual catalytic powers of MCPIP. J Immunol. 2015;194:6011–6023. doi: 10.4049/jimmunol.1402797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, et al. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol. 2015;35:535–546. doi: 10.1161/ATVBAHA.114.304029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 101.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 102.Libby P, Ordovas JM, Birinyi LK, Auger KR, Dinarello CA. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986;78:1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Libby P, Ordovas JM, Auger KR, Robbins AH, Birinyi LK, Dinarello CA. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986;124:179–185. [PMC free article] [PubMed] [Google Scholar]
- 104.Zhuang T, Liu J, Chen X, Zhang L, Pi J, Sun H, et al. Endothelial Foxp1 suppresses atherosclerosis via modulation of Nlrp3 inflammasome activation. Circ Res. 2019;125:590–605. doi: 10.1161/CIRCRESAHA.118.314402. [DOI] [PubMed] [Google Scholar]
- 105.Haneklaus M, O'Neill LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265:53–62. doi: 10.1111/imr.12285. [DOI] [PubMed] [Google Scholar]
- 106.Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med. 2018;24:1418–1429. doi: 10.1038/s41591-018-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]