Abstract
Objective
We investigated the effects of statin-ezetimibe combination therapy compared with statin-only treatment on the hazard of incident type 2 diabetes (T2D), myocardial infarction (MI), and stroke among adults with impaired fasting glucose (IFG) in a real-world setting.
Methods
The Korean National Health Insurance Service datasets from 2002 to 2017 were used for this propensity-matched nationwide cohort study. Among 56,633 IFG patients without baseline cardiovascular disease (CVD) and/or T2D who initiated statin therapy with or without ezetimibe, 1,155 with statin-ezetimibe combination therapy were matched based on a propensity score at a 1:5 ratio with 5,775 patients who received statin monotherapy. The hazards of T2D, MI, and stroke were compared between these treatment groups.
Results
The incidence rate per 1,000 person-years was 19.62 (statin monotherapy group) and 21.02 (combined treatment group) for T2D, 1.53 (statin monotherapy group) and 1.70 (combined treatment group) for MI, and 1.99 (statin monotherapy group) and 2.06 (combined treatment group) for stroke. The hazards of T2D, MI, and stroke were not significantly different between the statin monotherapy group and the statin-ezetimibe combination therapy group.
Conclusion
The combination of ezetimibe in addition to statin treatment was not associated with a significantly different risk of T2D and CVDs compared with statin monotherapy in Korean adults with IFG.
Keywords: Statin, Ezetimibe, Prediabetic state, Type 2 diabetes, Cardiovascular diseases
INTRODUCTION
Statins have been widely used for their beneficial effect on the prevention of cardiovascular diseases (CVDs).1,2 However, statin treatment has been reported to be associated with an increased risk of incident type 2 diabetes (T2D).1,2,3 Conversely, studies of experimental animal models and several small human studies have reported that ezetimibe may ameliorate metabolic markers such as hepatic steatosis and insulin resistance.4 Ezetimibe inhibits the transport protein Niemann Pick C1 like 1, thereby reducing the absorption of cholesterol from the intestine.4,5 It is used as an alternative lipid-lowering agent for statin-intolerant patients and is frequently used in combination with statins.5
According to the Diabetes Fact Sheets based on the Korea National Health and Nutrition Examination Survey from 2016 to 2018,6 26.9% of Korean adults aged ≥30 years had impaired fasting glucose (IFG) in 2018. In the Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial,1 the risk of new-onset T2D was increased by rosuvastatin treatment only in specific individuals with major risk factors for developing T2D, including IFG. Therefore, it is an important public health issue to establish a lipid control strategy that can manage cardiovascular risk while minimizing the risk of T2D incidence among IFG patients, who have a higher risk of incident T2D in association with statin therapy and account for more than a quarter of adults aged ≥30 years in Korea.6 Statin-ezetimibe combination therapy may be considered as a potential strategy, but it needs to be verified in comparison with statin monotherapy.
However, limited studies have compared the risk of new-onset T2D and CVDs between statin monotherapy and statin-ezetimibe combination therapy groups in a large population with IFG. In particular, insufficient studies have verified the effects of statin-ezetimibe combination therapy on cardiovascular risk and glucose metabolism. Therefore, we compared the hazards of incident T2D, myocardial infarction (MI), and stroke between treatment regimens (statin monotherapy versus statin-ezetimibe combination therapy) among adults with IFG in a real-world setting using the Korean National Health Insurance Service (KNHIS) database.
MATERIALS AND METHODS
1. Data sources
We analyzed the KNHIS datasets of claims and preventive health examinations from January 2002 to December 2017 for this propensity-matched nationwide cohort study. Previous reports have described details on this database.7,8,9 The KNHIS is a single insurer operated by the Korean government and covers all Korean residents. The KNHIS claims datasets contain anonymous identification numbers, demographics, monthly household income level, primary and secondary diagnoses classified according to the International Classification of Diseases-10th Revision (ICD-10), prescriptions, procedures, and dates of hospital visits and hospitalizations of all residents of Korea. The KNHIS actively operates a national health screening program and recommends standardized health examinations at least every 2 years under this program. These examinations are conducted only at hospitals certified by the KNHIS. The health examination results, including demographic information; smoking history; alcohol intake; physical activity; anthropometric measurements, such as waist circumference (WC), height, and weight; blood pressure (BP); and laboratory results, including fasting plasma glucose (FPG), lipid profiles, and estimated glomerular filtration rate (eGFR), are collected in the preventive health examination datasets, which form the largest-scale, nationwide cohort database with laboratory data in Korea.8,10 The Institutional Review Board (IRB) of Korea University approved this study (IRB file number: 2019GR0219). The IRB granted an informed consent exemption because the KNHIS provided the researchers with only anonymous, de-identified data.
2. Patient selection and propensity score (PS) matching
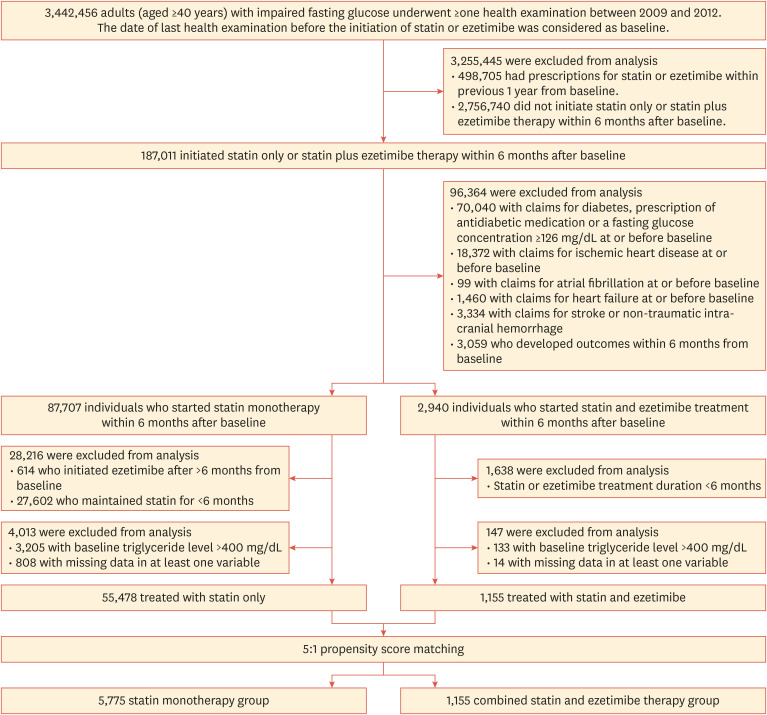
We selected adults (≥40 years) with IFG (FPG 100–125 mg/dL) who met the following criteria: (1) underwent at least 1 health examination between 2009 and 2012, (2) had never been prescribed statin or ezetimibe within 1 year prior to baseline, and (3) initiated statin-only (statin monotherapy group) or statin with ezetimibe therapy (statin-ezetimibe combination therapy group) within 6 months after baseline. The time point of the health examination between 2009 and 2012 was considered as the baseline. We excluded individuals who had, at or before baseline, claims for ischemic heart disease (ICD-10 codes I20–25), stroke (ICD-10 codes I63–64), non-traumatic intracranial hemorrhage (ICD-10 codes I60–62), heart failure (ICD-10 code I50), and/or atrial fibrillation (ICD-10 codes I48.0–4 or I48.9); and those with claims for diabetes mellitus (ICD-10 codes E10–14), prescription of an antidiabetic medication, or a FPG concentration ≥126 mg/dL at or before baseline. Furthermore, we excluded individuals who developed the outcomes (T2D, MI, and/or stroke) within 6 months from baseline; those who maintained statin therapy for <6 months; those in the statin-ezetimibe combination therapy group who maintained ezetimibe for <6 months; those who initiated ezetimibe after >6 months from baseline; those with baseline triglyceride level >400 mg/dL; and those with missing data for at least 1 variable (Fig. 1).
Fig. 1. Flow diagram of participant selection.
PS matching (1:5) was conducted for those treated with statin-ezetimibe combination therapy and those treated with statin monotherapy. The PS was obtained from a multiple logistic regression model that included age, sex, WC, body mass index (BMI), systolic BP, FPG, smoking status (current, former, or never smoker), alcohol consumption, regular exercise, low-income status, presence of chronic kidney disease (CKD), use of antithrombotic agents, anticoagulants, and antihypertensive agents (including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, and beta-blockers), and baseline low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, triglyceride levels. A caliper was set for nearest-neighbor matching within the first 4 to 8 digits; for instance, 2 participants with PSs of 0.12345678 and 0.12347123 matched on the first 4 digits (0.1234). Overall, we selected 1,155 patients from the statin-ezetimibe combination therapy group and 5,775 from the statin monotherapy group.
3. Outcomes and follow-up
The outcomes of interest were incident T2D, MI, and stroke during follow-up. T2D was determined as ≥1 claim per year for the prescription of antidiabetic medications under ICD-10 codes E11–14 or an FPG level ≥126 mg/dL after excluding individuals with claims under the ICD-10 code E10 referring to previous studies.8,11,12,13,14 MI was defined as ≥1 claim under ICD-10 codes I21 or I22 during hospitalization or ≥2 claims under those codes, and stroke was defined as the presence of ICD-10 codes I63 or I64 during hospitalization with claims for brain imaging (magnetic resonance imaging or computed tomography) according to previous reports.8,15,16 The study population was followed up from baseline (the date of the health examination between 2009 and 2012) until the date of death, occurrence of the outcome, or December 31, 2017, whichever came first.
4. Measurements and definitions
We considered low-income status as being covered by the Medical Aid program for the lowest-income population or being part of the lowest 20% of the population registered in the National Health Insurance (NHI) based on monthly household income. For the universal coverage of all Korean residents, the KNHIS operates 2 programs: NHI (encompassing approximately 97% of the population) and Medical Aid (covering the remaining 3% of the population).8,9,10 Questionnaires were used to obtain information on smoking history, alcohol consumption, and regular exercise. An average alcohol intake ≥1 g/day was defined as alcohol consumption, and individuals with an average alcohol intake <1 g/day were considered as nondrinkers.17,18 An average alcohol ingestion of ≥30 g/day was classified as heavy alcohol consumption.17,18 Regular exercise was regarded as high-intensity physical activity (accompanied by extreme shortness of breath for >20 minutes per session, ≥3 days per week) and/or moderate-intensity physical activity (leading to substantial shortness of breath for >30 minutes per session, ≥5 days per week).10,17 BMI was obtained from body weight in kilograms divided by the height in meters squared (kg/m2). Venous samples for blood tests, including FPG and lipid profiles, were obtained after an overnight fast. The presence of CKD was defined as an eGFR <60 mL/min/1.73 m2, referring to previous studies.11,19
5. Statistical analyses
Continuous variables with normal distributions are presented as means±standard deviations, while those with non-normal distributions are shown as geometric means (95% confidence intervals) and categorical variables are expressed as numbers (percentages). Absolute standardized differences were used to assess the baseline covariate balance between the 2 treatment groups. The incidence rates of outcomes were calculated from the number of incident cases divided by the total follow-up duration (person-years). We used a stratified Cox proportional hazards regression model for matched data to assess the relationship between the treatment groups and outcome incidence. In the matched sample, all absolute standardized differences in the baseline characteristics between the 2 treatment groups were <0.1. We used SAS version 9.3 (SAS Institute, Cary, NC, USA) for all statistical analyses. Two-sided p-values <0.05 were considered to indicate statistical significance.
RESULTS
1. Baseline characteristics and the study population
The flow of participants through the study is summarized in Fig. 1. After PS matching, 1,155 patients from the statin-ezetimibe combination therapy group and 5,775 from the statin monotherapy group were selected. The baseline covariates of the statin-ezetimibe combination therapy group and statin monotherapy group were well balanced after propensity-weighted matching (Table 1). After PS matching, the mean age was 55.46 and 55.69 years for individuals in the statin monotherapy group and those in the statin-ezetimibe combination therapy group, respectively. Among the selected participants of both groups after PS matching, more than 40% were male, and approximately 16% were current smokers. The mean BMI was 24.95 kg/m2 in the statin monotherapy group and 24.86 kg/m2 in the statin-ezetimibe combination therapy group. The mean LDL-C level was approximately 168 mg/dL in both treatment groups.
Table 1. Baseline characteristics of participants.
| Characteristics | Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|---|
| Statin monotherapy group (n=55,478) | Statin-ezetimibe combination therapy group (n=1,155) | ASD | Statin monotherapy group (n=5,775) | Statin-ezetimibe combination therapy group (n=1,155) | ASD | ||
| Age (yr) | 56.22±8.72 | 55.69±8.48 | 0.0616 | 55.46±8.68 | 55.69±8.48 | 0.0268 | |
| Men | 21,535 (38.82) | 482 (41.73) | 0.0592 | 2,447 (42.37) | 482 (41.73) | 0.0130 | |
| Low-income status | 10,360 (18.67) | 194 (16.80) | 0.0490 | 988 (17.11) | 194 (16.80) | 0.0083 | |
| Non-smoker | 38,427 (69.27) | 771 (66.75) | 0.0540 | 3,835 (66.41) | 771 (66.75) | 0.0072 | |
| Ex-smoker | 8,472 (15.27) | 200 (17.32) | 0.0555 | 1,026 (17.77) | 200 (17.32) | 0.0118 | |
| Current-smoker | 8,579 (15.46) | 184 (15.93) | 0.0129 | 914 (15.83) | 184 (15.93) | 0.0027 | |
| Nondrinker | 34,217 (61.68) | 714 (61.82) | 0.0029 | 3,550 (61.47) | 714 (61.82) | 0.0072 | |
| Non-heavy alcohol consumer | 17,321 (31.22) | 348 (30.13) | 0.0236 | 1,762 (30.51) | 348 (30.13) | 0.0083 | |
| Heavy alcohol consumer | 3,940 (7.10) | 93 (8.05) | 0.0359 | 463 (8.02) | 93 (8.05) | 0.0011 | |
| Regular exercise | 11,642 (20.98) | 235 (20.35) | 0.0156 | 1,148 (19.88) | 235 (20.35) | 0.0117 | |
| Chronic kidney disease | 4,290 (7.73) | 88 (7.62) | 0.0041 | 457 (7.91) | 88 (7.62) | 0.0108 | |
| Concurrent drug treatment | |||||||
| Aspirin | 5,475 (9.87) | 99 (8.57) | 0.0449 | 475 (8.23) | 99 (8.57) | 0.0123 | |
| P2Y12 inhibitor | 200 (0.36) | 2 (0.17) | 0.0370 | 11 (0.19) | 2 (0.17) | 0.0047 | |
| Warfarin | 32 (0.06) | 3 (0.26) | 0.0501 | 9 (0.16) | 3 (0.26) | 0.0218 | |
| NOAC | 4 (0.01) | 0 (0.00) | - | - | |||
| ACE inhibitor | 1,042 (1.88) | 14 (1.21) | 0.0543 | 60 (1.04) | 14 (1.21) | 0.0161 | |
| ARB | 9,365 (16.88) | 211 (18.27) | 0.0365 | 1,040 (18.01) | 211 (18.27) | 0.0067 | |
| Beta blocker | 5,594 (10.08) | 108 (9.35) | 0.0247 | 526 (9.11) | 108 (9.35) | 0.0083 | |
| Calcium channel blocker | 11,515 (20.76) | 209 (18.1) | 0.0673 | 1,033 (17.89) | 209 (18.10) | 0.0055 | |
| Waist circumference (cm) | 82.87±9.60 | 82.93±8.37 | 0.0067 | 83.07±8.33 | 82.93±8.37 | 0.0168 | |
| Body mass index (kg/m2) | 24.82±3.02 | 24.86±2.94 | 0.0134 | 24.95±3.08 | 24.86±2.94 | 0.0299 | |
| Systolic blood pressure (mmHg) | 130.42±17.26 | 129.88±17.08 | 0.0314 | 130.38±17.49 | 129.88±17.08 | 0.0289 | |
| Fasting glucose (mg/dL) | 107.59±6.37 | 107.28±6.25 | 0.0491 | 107.39±6.33 | 107.28±6.25 | 0.0175 | |
| Triglycerides (mg/dL) | 144.03 (143.45–144.60) | 151.41 (147.19–155.75) | 0.1031 | 152.93 (151.01–154.88) | 151.41 (147.19–155.75) | 0.0204 | |
| LDL-C (mg/dL) | 167.59±50.58 | 168.28±37.97 | 0.0154 | 167.62±58.54 | 168.28±37.97 | 0.0134 | |
| HDL-C (mg/dL) | 58.02±23.07 | 56.16±14.04 | 0.0974 | 56.14±14.76 | 56.16±14.04 | 0.0014 | |
Values are presented as number (%), mean±standard deviation, or geometric mean (95% confidence interval).
ASD, absolute standardized difference; NOAC, new oral anticoagulant; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
2. Hazard of incident T2D
There were 800 incidents of T2D during a mean follow-up of 7.06±1.77 years in the statin monotherapy group, while 162 cases of T2D developed during a mean follow-up of 6.67±1.75 years in the statin-ezetimibe combination therapy group. The incidence rate and hazard of T2D were compared between the 2 treatment groups (Table 2). The hazard of T2D was not significantly different between the statin monotherapy group and the statin-ezetimibe combination therapy group.
Table 2. Hazards of myocardial infarction, stroke, and type 2 diabetes incidence according to the treatment group.
| Treatment groups | Event | Follow-up duration (person-years) | Incidence rate (per 1,000 person-years) | HR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Myocardial infarction | ||||||
| Statin monotherapy group (n=5,775) | 66 | 43,242.69 | 1.52627 | 1.000 (ref.) | 0.5984 | |
| Statin-ezetimibe combination therapy group (n=1,155) | 14 | 8,248.83 | 1.69721 | 1.168 (0.656–2.081) | ||
| Stroke | ||||||
| Statin monotherapy group (n=5,775) | 86 | 43,165.13 | 1.99235 | 1.000 (ref.) | 0.8024 | |
| Statin-ezetimibe combination therapy group (n=1,155) | 17 | 8,236.02 | 2.06410 | 1.069 (0.635–1.799) | ||
| Type 2 diabetes | ||||||
| Statin monotherapy group (n=5,775) | 800 | 40,768.66 | 19.6229 | 1.000 (ref.) | 0.3170 | |
| Statin-ezetimibe combination therapy group (n=1,155) | 162 | 7708.62 | 21.0154 | 1.090 (0.921–1.291) | ||
HR, hazard ratio; CI, confidence interval.
3. Hazard of incident CVDs
In the statin monotherapy group, 66 cases of incident MI were observed during a mean follow-up of 7.49±1.28 years, and 86 stroke cases occurred during a mean follow-up of 7.47±1.30 years. In the statin-ezetimibe combination therapy group, there were 14 incidents of MI during a mean follow-up of 7.14±1.22 years and 17 incidents of stroke during a mean follow-up of 7.13±1.25 years. No significant difference was observed between the 2 treatment groups with respect to the hazards of MI and stroke during follow-up (Table 2).
DISCUSSION
In this propensity-weighted nationwide cohort study, the combination of ezetimibe in addition to statin treatment was not associated with a significantly different risk of T2D and CVDs compared with statin monotherapy in Korean adults with IFG. The baseline characteristics of both treatment groups, including cardiovascular risk factors and metabolic syndrome parameters such as smoking status, regular exercise, WC, BMI, systolic BP, fasting glucose, and LDL-C levels, were well balanced through PS matching.
The results of the current study suggest that statin-ezetimibe combination therapy is as effective as statin monotherapy in terms of preventing MI and stroke in IFG patients. Although our study indicated only the noninferiority of statin-ezetimibe combination therapy compared to statin-only treatment among individuals with IFG, the addition of ezetimibe to a certain dose of statin has been demonstrated to improve cardiovascular outcomes in adults with recent acute coronary syndrome.20 In the IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), the combination of simvastatin (40 mg) with ezetimibe compared with simvastatin (40 mg) and placebo resulted in an incremental reduction in LDL-C levels and improved cardiovascular outcomes among adults (aged ≥50 years) who had experienced acute coronary syndrome within the preceding 10 days,20 and the benefit of adding ezetimibe on cardiovascular outcomes in IMPROVE-IT was particularly pronounced in patients with diabetes.20,21 A meta-analysis of 7 randomized clinical trials (RCTs)22 also showed that compared with statins alone, statin-ezetimibe combination therapy reduced the risk of major adverse cardiovascular events (MACEs), and the benefit of combination therapy in terms of reduction in MACEs was greater in patients with diabetes than in those without diabetes.
In contrast to statins, which have been reported to increase the risk of new-onset T2D,1,2,3 previous studies4,23,24 have suggested possible beneficial effects of ezetimibe on insulin resistance and glycemia. In a mouse model, ezetimibe was demonstrated to stimulate intestinal glucagon-like peptide-1 secretion, which was closely related to improved glycemia.25 Nakamura et al.24 reported that ezetimibe reduced postprandial triglyceride and insulin levels in patients with metabolic syndrome, suggesting potential beneficial effects of ezetimibe on insulin resistance. A meta-analysis including 16 RCTs23 indicated no significant change in fasting glucose and glycated hemoglobin in ezetimibe with low-dose statin therapy compared with high-dose statin treatment. However, according to their subgroup analysis, compared with high-dose statin treatment, ezetimibe plus low-dose statin therapy for >3 months led to a significant decrease in fasting glucose.23 However, in our study, although the duration of statin or statin with ezetimibe treatment was at least 6 months, in comparison with statin-only therapy, statin-ezetimibe combination therapy did not change the hazard of incident T2D, demonstrating a neutral effect. Likewise, in a retrospective study including 877 individuals treated for dyslipidemia,5 high-intensity statin therapy was associated with a higher risk of new-onset diabetes, mostly in individuals with prediabetes, whereas adding ezetimibe to statin treatment showed a neutral effect on glucose metabolism.
Since we compared the risk of outcomes between 2 treatment regimens (statin monotherapy versus statin-ezetimibe combination therapy) through a real-world observational study, not through an RCT, the findings should be interpreted cautiously. Considering that a large-scale observational study can be affected by measured or unmeasured confounders including baseline characteristics and their changes, in addition to the effects of the treatment group, we prepared several strategies to address this issue in the design of the study. These strategies included washout of individuals who had been prescribed a statin or ezetimibe within 1 year prior to baseline, the inclusion of only individuals who newly initiated medications (a statin only or a statin with ezetimibe) within 6 months after baseline, exclusion of those who maintained medications (a statin only or a statin with ezetimibe) for <6 months, PS matching between the 2 treatment groups for diverse variables such as age, sex, social history, anthropometric measures, laboratory results, concomitantly administered drugs, and the presence of CKD. Despite these steps, the effects of other factors, including but not restricted to the medication dose, changes in the prescription patterns of various medications, and characteristics of individual patients that cannot be fully adjusted through PS matching but can affect the prescription patterns of clinicians, cannot be completely excluded.
In addition to the points mentioned in the previous paragraph, other limitations of the current study should be acknowledged. First, because of the observational nature of our study, the ability of the findings to elucidate causal relationships is inevitably limited. However, to minimize the potential reverse-causality effect, we excluded individuals with claims for ischemic heart disease and/or stroke, those with claims for diabetes, prescription of antidiabetic medication, or a FPG level ≥126 mg/dL at or before baseline, as well as those with outcome incidence within 6 months from baseline. Second, since the study population was restricted to Korean adults only, our findings should be extrapolated cautiously to populations with other ethnicities. Third, the dose, intensity, and type of individual statins were not reflected, although the effect of statins on CVD or T2D incidence may vary according to those factors. Fourth, the use of medications (including statin and ezetimibe) was determined based on prescription data, which might not perfectly correspond to the actual intake of drugs in each patient. However, prescription records have been widely used to assess the effect of medications in real-world studies,26,27,28 and previous reports described good correlations between prescription and actual exposure to medications.26,29,30 Despite these limitations, our study has several strengths, including a large sample size, use of the KNHIS database (a nationwide cohort database of all Korean residents managed by the Korean government), and PS matching for diverse factors.
In conclusion, this PS-matched nationwide cohort study demonstrated a neutral effect of statin-ezetimibe combination therapy compared with statin-only treatment in terms of incident T2D and CVD risk in adults with IFG. Ezetimibe may be safely added to statin treatment for patients with IFG, especially those with less tolerance to the dose escalation of statins. Further studies including RCTs would be required to confirm the influence of statin-ezetimibe combination therapy in comparison with statin monotherapy.
Acknowledgements
This work was performed using the database from the Korean National Health Insurance Service (KNHIS). The National Health Information Database constructed by the KNHIS (NHIS-2020-4-133) was used, and the results do not necessarily represent the opinion of the KNHIS.
Footnotes
Funding: This study was supported by the 2019 big data analysis grant from the Korean Society of Lipid and Atherosclerosis. The funding agency had no role in the design, collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Yoo HJ.
- Formal analysis: Kim B, Han K.
- Funding acquisition: Yoo HJ.
- Methodology: Lee YB, Han K, Hong SH, Yoo HJ.
- Resources: Roh E.
- Supervision: Choi KM, Baik SH.
- Validation: Kim JA.
- Writing - original draft: Lee YB.
- Writing - review & editing: Yoo HJ.
References
- 1.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajpathak SN. Intensive statin therapy, compared with moderate dose, increases risk of new onset diabetes but decreases risk of cardiovascular events. Evid Based Med. 2012;17:55–56. doi: 10.1136/ebm.2011.100198. [DOI] [PubMed] [Google Scholar]
- 3.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 4.Zafrir B, Jain M. Lipid-lowering therapies, glucose control and incident diabetes: evidence, mechanisms and clinical implications. Cardiovasc Drugs Ther. 2014;28:361–377. doi: 10.1007/s10557-014-6534-9. [DOI] [PubMed] [Google Scholar]
- 5.Barkas F, Elisaf M, Liberopoulos E, Klouras E, Liamis G, Rizos EC. Statin therapy with or without ezetimibe and the progression to diabetes. J Clin Lipidol. 2016;10:306–313. doi: 10.1016/j.jacl.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Jung CH, Son JW, Kang S, Kim WJ, Kim HS, Kim HS, et al. Diabetes fact sheets in Korea, 2020: an appraisal of current status. Diabetes Metab J. 2021;45:1–10. doi: 10.4093/dmj.2020.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seong SC, Kim YY, Khang YH, Park JH, Kang HJ, Lee H, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YB, Han K, Kim B, Lee SE, Jun JE, Ahn J, et al. Risk of early mortality and cardiovascular disease in type 1 diabetes: a comparison with type 2 diabetes, a nationwide study. Cardiovasc Diabetol. 2019;18:157. doi: 10.1186/s12933-019-0953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YH, Han K, Ko SH, Ko KS, Lee KU Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data analytic process of a nationwide population-based study using National Health Information Database established by National Health Insurance Service. Diabetes Metab J. 2016;40:79–82. doi: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YB, Kim DH, Kim SM, Kim NH, Choi KM, Baik SH, et al. Hospitalization for heart failure incidence according to the transition in metabolic health and obesity status: a nationwide population-based study. Cardiovasc Diabetol. 2020;19:77. doi: 10.1186/s12933-020-01051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YB, Han K, Kim B, Jun JE, Lee SE, Ahn J, et al. Risk of end-stage renal disease from chronic kidney disease defined by decreased glomerular filtration rate in type 1 diabetes: a comparison with type 2 diabetes and the effect of metabolic syndrome. Diabetes Metab Res Rev. 2019;35:e3197. doi: 10.1002/dmrr.3197. [DOI] [PubMed] [Google Scholar]
- 12.Lee YB, Kim DH, Kim SM, Kim NH, Choi KM, Baik SH, et al. Risk of type 2 diabetes according to the cumulative exposure to metabolic syndrome or obesity: a nationwide population-based study. J Diabetes Investig. 2020;11:1583–1593. doi: 10.1111/jdi.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YB, Kim DH, Roh E, Hong SH, Kim JA, Yoo HJ, et al. Variability in estimated glomerular filtration rate and the incidence of type 2 diabetes: a nationwide population-based study. BMJ Open Diabetes Res Care. 2020;8:e001187. doi: 10.1136/bmjdrc-2020-001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noh J, Han KD, Ko SH, Ko KS, Park CY. Trends in the pervasiveness of type 2 diabetes, impaired fasting glucose and co-morbidities during an 8-year-follow-up of nationwide Korean population. Sci Rep. 2017;7:46656. doi: 10.1038/srep46656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MK, Han K, Kim HS, Park YM, Kwon HS, Yoon KH, et al. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population-based study. Eur Heart J. 2017;38:3560–3566. doi: 10.1093/eurheartj/ehx585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MK, Han K, Koh ES, Kim ES, Lee MK, Nam GE, et al. Blood pressure and development of cardiovascular disease in Koreans with type 2 diabetes mellitus. Hypertension. 2019;73:319–326. [PubMed] [Google Scholar]
- 17.Lee YB, Han K, Park S, Kim SM, Kim NH, Choi KM, et al. Gamma-glutamyl transferase variability and risk of dementia: a nationwide study. Int J Geriatr Psychiatry. 2020;35:1105–1114. doi: 10.1002/gps.5332. [DOI] [PubMed] [Google Scholar]
- 18.Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352:245–253. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- 19.Lee YB, Lee JS, Hong SH, Kim JA, Roh E, Yoo HJ, et al. Optimal blood pressure for patients with chronic kidney disease: a nationwide population-based cohort study. Sci Rep. 2021;11:1538. doi: 10.1038/s41598-021-81328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 21.Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalán R, Špinar J, et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: results from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) Circulation. 2018;137:1571–1582. doi: 10.1161/CIRCULATIONAHA.117.030950. [DOI] [PubMed] [Google Scholar]
- 22.Hong N, Lee YH, Tsujita K, Gonzalez JA, Kramer CM, Kovarnik T, et al. Comparison of the effects of ezetimibe-statin combination therapy on major adverse cardiovascular events in patients with and without diabetes: a meta-analysis. Endocrinol Metab. 2018;33:219–227. doi: 10.3803/EnM.2018.33.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Shang H, Wu J. Effect of ezetimibe on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Endocrine. 2018;60:229–239. doi: 10.1007/s12020-018-1541-4. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura A, Sato K, Kanazawa M, Kondo M, Endo H, Takahashi T, et al. Impact of decreased insulin resistance by ezetimibe on postprandial lipid profiles and endothelial functions in obese, non-diabetic-metabolic syndrome patients with coronary artery disease. Heart Vessels. 2019;34:916–925. doi: 10.1007/s00380-018-1319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang E, Kim L, Choi JM, Park SE, Rhee EJ, Lee WY, et al. Ezetimibe stimulates intestinal glucagon-like peptide 1 secretion via the MEK/ERK pathway rather than dipeptidyl peptidase 4 inhibition. Metabolism. 2015;64:633–641. doi: 10.1016/j.metabol.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Lee HS, Lee KY. Effect of statins on fasting glucose in non-diabetic individuals: nationwide population-based health examination in Korea. Cardiovasc Diabetol. 2018;17:155. doi: 10.1186/s12933-018-0799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim NH, Han KH, Choi J, Lee J, Kim SG. Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: propensity matched cohort study. BMJ. 2019;366:l5125. doi: 10.1136/bmj.l5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee M, Sun J, Han M, Cho Y, Lee JY, Nam CM, et al. Nationwide trends in pancreatitis and pancreatic cancer risk among patients with newly diagnosed type 2 diabetes receiving dipeptidyl peptidase 4 inhibitors. Diabetes Care. 2019;42:2057–2064. doi: 10.2337/dc18-2195. [DOI] [PubMed] [Google Scholar]
- 29.Lee JK, Grace KA, Foster TG, Crawley MJ, Erowele GI, Sun HJ, et al. How should we measure medication adherence in clinical trials and practice? Ther Clin Risk Manag. 2007;3:685–690. [PMC free article] [PubMed] [Google Scholar]
- 30.Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro-Breault M, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]