Abstract
Purpose
Real-world evidence on the impact of forced expiratory volume in one second (FEV1) and exacerbations on health-related quality of life (HRQoL) in patients with chronic obstructive pulmonary disease (COPD) is sparse especially with regard to GOLD ABCD groups. This study investigates how changes in FEV1 and exacerbations affect generic and disease-specific HRQoL in COPD patients over one year.
Methods
Using German claims data and survey data, we classified 3016 COPD patients and analyzed their health status by GOLD groups AB and CD. HRQoL was measured with the disease-specific COPD assessment test (CAT) and the visual analog scale (VAS) from the generic Euro-Qol 5D-5L. We applied change score models to assess associations between changes in FEV1 (≥100 mL decrease/no change/≥100 mL increase) or the development of severe exacerbations with change in HRQoL.
Results
FEV1 decrease was associated with a significant but not minimal important difference (MID) deterioration in disease-specific HRQoL (mean change [95% CI]: CAT +0.74 [0.15 to 1.33]), while no significant change was observed in the generic VAS. Experiencing at least one severe exacerbation also had a significant impact on CAT deterioration (+1.58 [0.52 to 2.64]), but again not on VAS. Here, GOLD groups AB showed not only a statistically but also a clinically relevant MID deterioration in CAT (+2.1 [0.88 to 3.32]). These particular patient groups were further characterized by a higher probability of being male, having a higher mMRC and Charlson index, and a lower probability of having higher FEV1 or BMI values.
Conclusion
FEV1 decline and the occurrence of ≥1 severe exacerbation are significantly associated with overall deterioration in disease-specific HRQoL. Preventing severe exacerbations particularly in patients without previous severe exacerbations (ABCD groups A and B) may help to stabilize the key patient-reported outcome HRQoL.
Keywords: COPD, health-related quality of life, longitudinal study, real-world evidence, forced expiratory volume in 1 second, exacerbation
Introduction
Chronic obstructive pulmonary disease (COPD) is a serious and usually progressive disease characterized by not fully reversible airflow obstruction and respiratory symptoms, such as breathlessness, cough, and sputum production.1 In 2019, COPD was the third leading cause of death worldwide and claimed more than 3 million lives.2 In the future, the burden of COPD is predicted to increase because of continued exposure to risk factors, such as smoking, and an aging population.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) suggests characterizing COPD patients by airflow limitation and clinical presentation: first, GOLD grades I–IV, which are based on the lung function measure forced expiratory volume in one second (FEV1); and second, ABCD groups that reflect a combination of exacerbation history and symptom burden. This highlights the importance of the FEV1 and the exacerbation history as two markers of the disease.
Exacerbations can be attributed to patient-reported outcomes (PROMs).4,5 One of the most important PROMs is the patients’ health-related quality of life (HRQoL). The HRQoL reflects the patient’s perspective of their disease and is a key outcome in the German disease management programs (DMPs).6 Therefore, HRQoL measures are meaningful instruments, as they cover the subjective impact of the disease on daily life and the severity of symptoms.7
In COPD, HRQoL decreases with increasing severity of airflow limitation (GOLD I–IV8,9) and also with higher symptom burden, and is thus worse in groups B and D.10,11 In COPD patients, FEV1 usually shows a downward trajectory,12–14 which is steeper than the regular annual lung function decline in the healthy population (17.7–46.4 mL/year)15 especially in early disease stages.14 This decline in FEV1 in COPD is associated with a decline in patients’ HRQoL.16–18 On the other hand, exacerbations also negatively affect HRQoL.19–22 Nonetheless, the longitudinal association between these two clinical factors and HRQoL is not fully understood,23–25 especially with regard to GOLD ABCD groups. Although the relationship between clinical factors and HRQoL has often been analyzed for GOLD I–IV, there are few published reports regarding ABCD groups, even though classification by ABCD groups is more closely associated with HRQoL.10
Therefore, the aim of this study is to analyze the impact of changes in FEV1 and severe exacerbations on generic and disease-specific HRQoL in COPD patients within one year with regard to ABCD groups, based on a large real-world dataset from a German statutory health insurance fund and survey data.
Methods
Data
Our real-world dataset combines pseudonymized health insurance claims data with survey data. The claims data were provided by the AOK Bavaria, a regional statutory health insurance (SHI) fund with almost 4.5 million policyholders and a 40.5% share in this regional SHI market during conduct of the study.26 We only included data from patients participating in the structured DMP for COPD. Incorporating DMP data made clinical factors such as, eg, FEV1 or BMI, which are not documented routinely within claims data, available.
To obtain generic and disease-specific HRQoL information, a two-wave survey was conducted in 49662 DMP participants. Wave 1 took place in November 2017, with 14754 (29.7%) responders. Wave 2, conducted in November 2018, only addressed responders from wave 1 and yielded 9232 (62.6%) responders. Additionally, the survey addressed breathlessness measured by the modified British Medical Research Council (mMRC)27 and sociodemographic data.
Disease Severity and HRQoL Assessment
Lung function was assessed as FEV1 liters, because FEV1% predicted (FEV1%pred.) values were not uniformly available for all patients on account of documentation issues (see limitations). Nonetheless, wherever possible, we additionally calculated GOLD grades I–IV based on FEV1%pred. with reference values taken from the Global Lung Initiative1 to perform a sensitivity analysis. FEV1 values represent an annual average, whenever several measurements per patient were available within one year. Exacerbations were defined as moderate when COPD symptoms worsened and required a doctor’s visit or as severe when symptoms worsened and required a hospital stay, both measured by the respective utilization documented in claims data.28 We assessed disease severity by GOLD ABCD groups, starting from the least severe group A. The groups A–D reflect a combination of exacerbation history and COPD symptoms. More severe groups C and D are classified by ≥2 moderate or at least one severe exacerbation occurring. Symptoms can be assessed (among others) by COPD assessment test (CAT)29 or by mMRC.27 More severe groups B and D are classified by mMRC ≥2. We decided to use the mMRC for classification, because we used CAT as an outcome measure and several literature sources suggested other cut-off points than the GOLD standard (≤10) to define ABCD groups using the CAT.30,31
Further, we measured disease-specific HRQoL via CAT29 and generic HRQoL via Euro-Qol 5D (EQ-5D-5L).32,33 The CAT is scaled from 40 to 0 and comprises eight dimensions with six answer levels in each dimension (0 = best state to 5 = worst state). Thus, a CAT of 40 describes the worst possible HRQoL state and 0 the best possible HRQoL state. The eight CAT dimensions include disease assessments such as cough, sputum, sleep, and energy. The minimal clinically important difference for the CAT has been determined to be a minimum 2.0 point change.34,35 The decision for the CAT as disease-specific measure was made in line with the results of a proceeding pilot study. This pilot study unveiled that the CAT outperformed two other disease-specific questionnaires (St. George’s Respiratory Questionnaire (SGRQ), Clinical COPD Questionnaire (CCQ)) regarding response rates, validly answered questions (eg, non-missing items), explanatory power, and ceiling/floor effects. Furthermore, given its shortness, the CAT was deemed more easily intelligible and hence applicable for a HRQoL self-reporting by COPD patients.36
The EQ-5D-5L consists of two parts. First, a valuation section with five dimensions (mobility, self-care, activity, pain, anxiety/depression) and, second, the visual analog scale (VAS). The VAS is a scale on which patients can value their current health status between 0 (worst state) and 100 (best state). The EQ-5D-5L has been validated for use in COPD patients.33 Several estimates exist for minimal clinically important difference for the VAS in COPD patients.33,37 A frequently used value for the minimal important difference (MID) is a 6.9 point change.33 For our analysis, we used only the VAS because it was found to differentiate better between COPD grades than the EQ-5D index,9 as it is easily comprehensible for participants to display their generic HRQoL and we do not address resource allocation issues requiring population preferences.
Assessment of Covariates
All models included the following covariates: age, sex, exacerbation history, comorbidities (claims data based), smoking status, BMI, lung function (DMP data based), as well as education, breathlessness, and HRQoL baseline (survey data based). Reference categories were female, non-smokers, and basic education. Comorbidities were identified based on the Charlson index.38 Further information about the index conditions can be found elsewhere.28 Breathlessness was interrogated by mMRC scale, which indicates dyspnea on an ascending scale from 0 to 4.27
Statistical Analysis
We calculated means and standard deviations (SD) for continuous variables or counts and percentages for categorical variables. For bivariate comparisons of baseline characteristics between HRQoL MID-Deterioration/No-MID-Deterioration groups within our study population, we used Chi-squares for nominal variables, Wilcoxon–Mann–Whitney-tests for ordinal variables, and t-tests for continuous variables. Based on claims data, we contrasted survey-responders and non-responders regarding main characteristics to detect a potential non-response bias.
We also evaluated linear change in HRQoL over one year for all 3016 patients in our study population based on t-tests. To investigate the association between clinical changes and changes in HRQoL, we used two different statistical approaches.
First, we applied generalized additive models (GAM) to evaluate the association between HRQoL and FEV1. GAMs are non-parametric regression models that are able to portray non-linear relationships between a dependent variable (change in HRQoL) and an independent variable (change in FEV1 liter) using a smoothing function while adjusting for covariates.39 The variance inflation factor was below any meaningful threshold (<2) and thus did not indicate any multicollinearity issues.40 Second, we ran change score models based on ordinary least squares (OLS) linear regression to evaluate the relationship between (1) clinical changes in FEV1 or (2) the occurrence of severe exacerbations and change in HRQoL. We stratified the analyses by GOLD groups AB and CD because of the small number of patients in single groups.
In a first step, we regressed the HRQoL mean change between baseline and one-year follow-up on three categories of FEV1 change (increase/decrease/no change). The categories were defined as a FEV1 ≥100mL increase, a FEV1 ≥100mL decrease, and no change (in between). This 100mL change cut-off in FEV1 is considered to represent the MID.41 As we assumed that the change in FEV1 depends on the lung function’s baseline,41 we included an interaction term to account for different relative milliliter declines, eg, lower decline values in more severe GOLD stages14 resulting from a lower initial FEV1.
Subsequently, we regressed the change in severe exacerbation history on the HRQoL mean change. Therefore, we built a subgroup for patients without severe exacerbations in the year before wave 1 but at least one severe exacerbation between wave 1 and wave 2. Earlier exacerbation history preceding the year before wave 1 was not taken into account. To characterize these patients at risk, we conducted a logistic regression with this particular subgroup as binary outcome.
Both GAMs and change score models were stratified by GOLD ABCD group and adjusted for age, sex, education, smoking status, moderate/severe exacerbations, Charlson Comorbidity index, BMI, FEV1, mMRC, and HRQoL baseline.
To check the robustness of our results, we performed a sensitivity analysis for all models containing a core group. Our core group comprised only patients with plausible mMRC and GOLD combinations in wave 2. Therefore, patients with combinations of GOLD grade I and mMRC of 3 or 4 were excluded (N=65), as well as GOLD IV patients with a mMRC of 0 or 1 (N=25). This core group was built to rule out possible data issues resulting from a FEV1 measure change in the DMP, which are discussed further in the limitations.
For all analyses, we used the SAS software (SAS Institute Inc., Cary, NC, USA, Version 9.4) and considered p-values <0.05 as statistically significant.
Results
Study Population
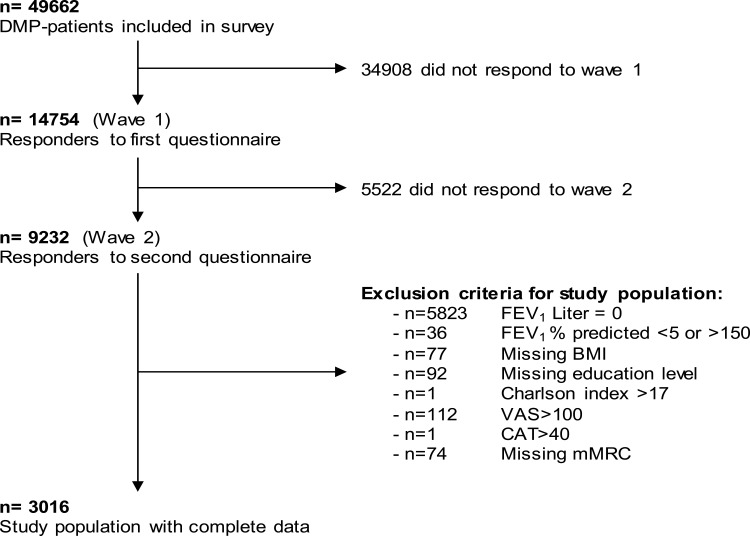
From 14754 initial participants, 9232 individuals took part in both waves; 3016 of these had complete information for FEV1, Charlson index, mMRC, BMI, education status, and HRQoL (VAS and CAT) and were thus included in the analysis. Reasons for exclusion are summarized in Figure 1. The characteristics of responders and the study population can be seen in Additional File 1. In general, responders presented a population at lower risk, with higher FEV1, and less severe exacerbations.
Figure 1.
Overview of the study population.
Abbreviations: DMP, disease management program; FEV1, forced expiratory volume in 1 second; BMI, body mass index; VAS, visual analog scale; CAT, COPD Assessment Test; mMRC, Modified Medical Research Council Questionnaire.
Table 1 shows the baseline characteristics of the entire study population and contrasts the participants with and without a MID-relevant deterioration in HRQoL. In the total sample, 59.8% were male, mean age was 68.9 years, and mean FEV1 was 1.9 liters. Regarding HRQoL, mean VAS score was 58.6 (SD ±19.9) and mean CAT score was 19.2 (±7.7). Around one in four patients experienced at least one moderate and around one in twenty patients at least one severe exacerbation within the baseline year. Patients with a clinically relevant VAS deterioration were older, had worse lung function with higher breathlessness, and reported better HRQoL at baseline (p-values ranging between 0.03 and <0.0001). Patients with clinically relevant CAT deterioration had a lower burden of breathlessness and a better baseline HRQoL (p-values between 0.03 and <0.0001). Moreover, we found a moderate linear relationship between VAS and CAT (Pearson correlation –0.61; Spearman correlation –0.62).
Table 1.
Baseline Characteristics of Participants Who Experienced a Minimal Important Difference Deterioration in VAS (≥-6.9) and CAT (≥+2)
| Total Sample | No VAS MID-Deterioration | VAS MID-Deterioration | No CAT MID-Deterioration | CAT MID-Deterioration | ||||
|---|---|---|---|---|---|---|---|---|
| n | 3016 | 2044 | 972 | p-value | 1759 | 1257 | p-value | |
| Male | 1804 (59.81%) | 1221 (59.74%) | 583 (59.98%) | 0.8985 | 1047 (59.52%) | 757 (60.22%) | 0.6989 | |
| Age, yrs.a | 68.85 (±9.49) | 68.52 (±9.53) | 69.55 (±9.36) | 0.0051 | 68.72 (±9.45) | 69.04 (±9.54) | 0.3501 | |
| Smoking statusa | Never smoker | 1651 (54.74%) | 1147 (56.12%) | 504 (51.85%) | 0.0850 | 981 (55.77%) | 670 (53.3%) | 0.1317 |
| Current smoker | 1014 (33.62%) | 664 (32.49%) | 350 (36.01%) | 566 (32.18%) | 448 (35.64%) | |||
| Former smoker | 351 (11.64%) | 233 (11.4%) | 118 (12.14%) | 212 (12.05%) | 139 (11.06%) | |||
| Educationa | Basic (9 yrs.) | 2451 (81.27%) | 1656 (81.02%) | 795 (81.79%) | 0.2022 | 1414 (80.39%) | 1037 (82.5%) | 0.1603 |
| Secondary (10 yrs.) | 340 (11.27%) | 233 (11.4%) | 107 (11.01%) | 216 (12.28%) | 124 (9.86%) | |||
| Higher (12–13 yrs.) | 83 (2.75%) | 61 (2.98%) | 22 (2.26%) | 52 (2.96%) | 31 (2.47%) | |||
| University | 49 (1.62%) | 38 (1.86%) | 11 (1.13%) | 29 (1.65%) | 20 (1.59%) | |||
| None | 93 (3.08%) | 56 (2.74%) | 37 (3.81%) | 48 (2.73%) | 45 (3.58%) | |||
| BMIa | 29.18 (±6.02) | 29.3 (±6.07) | 28.93 (±5.92) | 0.1144 | 29.25 (±5.94) | 29.08 (±6.14) | 0.4341 | |
| Exacerbationsa | Moderate | 0.84 (±1.93) | 0.8 (±1.88) | 0.92 (±2.04) | 0.1272 | 0.81 (±1.91) | 0.88 (±1.97) | 0.2897 |
| Severe | 0.07 (±0.39) | 0.07 (±0.38) | 0.08 (±0.4) | 0.6313 | 0.08 (±0.45) | 0.07 (±0.29) | 0.2940 | |
| FEV1 (Liter)a | 1.88 (±0.75) | 1.91 (±0.75) | 1.83 (±0.76) | 0.0060 | 1.88 (±0.74) | 1.88 (±0.76) | 0.7869 | |
| Charlson indexa | 3.59 (±2.69) | 3.52 (±2.67) | 3.72 (±2.73) | 0.0567 | 3.57 (±2.66) | 3.61 (±2.73) | 0.6279 | |
| mMRCb | 1.64 (±0.99) | 1.62 (±0.99) | 1.7 (±0.99) | 0.0343 | 1.69 (±1) | 1.58 (±0.97) | 0.0060 | |
| HRQoL: VAS baselineb | 58.59 (±19.94) | 55.48 (±20.43) | 65.13 (±17.14) | <0.0001 | 57.73 (±20.04) | 59.8 (±19.75) | 0.0048 | |
| HRQoL: CAT baselineb | 19.2 (±7.7) | 19.08 (±7.76) | 19.47 (±7.56) | 0.1952 | 20.84 (±7.46) | 16.91 (±7.45) | <0.0001 | |
| GOLD grade | I (FEV1 ≥80%) | 412 (13.66%) | 288 (14.09%) | 124 (12.76%) | 0.0012 | 233 (13.25%) | 179 (14.24%) | 0.5241 |
| II (FEV1 50–79%) | 1417 (46.98%) | 992 (48.53%) | 425 (43.72%) | 827 (47.02%) | 590 (46.94%) | |||
| III (FEV1 30–49%) | 940 (31.17%) | 616 (30.14%) | 324 (33.33%) | 556 (31.61%) | 384 (30.55%) | |||
| IV (FEV1 < 30%) | 247 (8.19%) | 148 (7.24%) | 99 (10.19%) | 143 (8.13%) | 104 (8.27%) | |||
| ABCD (mMRC) | A | 1299 (43.07%) | 911 (44.57%) | 388 (39.92%) | 0.0775 | 742 (42.18%) | 557 (44.31%) | 0.0312 |
| B | 1118 (37.07%) | 746 (36.5%) | 372 (38.27%) | 680 (38.66%) | 438 (34.84%) | |||
| C | 222 (7.36%) | 146 (7.14%) | 76 (7.82%) | 113 (6.42%) | 109 (8.67%) | |||
| D | 377 (12.5%) | 241 (11.79%) | 136 (13.99%) | 224 (12.73%) | 153 (12.17%) | |||
Notes: Data are presented as mean (± SD) or n (%). Education is represented as three German school levels by years. P-values based on t-test, Chi-square-test and Wilcoxon–Mann–Whitney-test. Baseline data = Data previous 12 months beforea or fromb first questionnaire.
Abbreviations: Yrs, years; BMI, body mass index; FEV1, forced expiratory volume in 1 second; mMRC, Modified Medical Research Council Questionnaire; HRQoL, Health-related quality of life; VAS, visual analog scale; CAT, COPD assessment test.
Within the ABCD groups, the baseline distribution was 43.1% (A), 37.1% (B), 7.4% (C), 12.5% (D). Analysis group AB comprised 2417 and group CD 599 patients. Overall, the largest proportion of COPD patients (12.9%) deteriorated in terms of symptom burden from AC to BD, of which 9.5% deteriorated from A to B (Additional Files 2 and 3).
Change in FEV1, Exacerbations, and HRQoL
As visualized in Table 2, FEV1 and HRQoL decreased within one year. FEV1 declined in the entire sample (significant) and in all ABCD groups (significant for group A only), except for group D, where a significant increase was observed. All these changes did not reach the established MID of 100mL.41 Regarding the occurrence of exacerbations, changes in the one-year observation period were small. We observed a statistically significant increase in moderate exacerbations in the total sample and all ABCD subgroups, except for a non-significant decrease in group C. Regarding severe exacerbations, there was an increase for the entire sample and groups A and B, although we observed a decrease for groups C and D. These changes were statistically significant for all subgroups except for group D. The biggest increase was found in group B (+0.22(moderate)/+0.08(severe)). An overview of the distribution of exacerbation frequency can be found in Additional File 4.
Table 2.
Change in FEV1, Exacerbations and HRQoL for 3016 Patients Who Completed Both Questionnaires
| Baseline | 1-Year | 1-Year Change | 1-Year % Change | p-value | ||
|---|---|---|---|---|---|---|
| FEV1 (Liter) | Total sample | 1.88 (±0.75) | 1.85 (±0.73) | −0.03 | −1.7% | 0.0005 |
| A | 2.09 (±0.73) | 2.01 (±0.69) | −0.08 | −3.7% | <0.0001 | |
| B | 1.73 (±0.68) | 1.72 (±0.69) | −0.01 | −0.7% | 0.4209 | |
| C | 2.01 (±0.79) | 1.97 (±0.76) | −0.04 | −2.2% | 0.1149 | |
| D | 1.51 (±0.76) | 1.58 (±0.8) | 0.07 | 4.7% | 0.0268 | |
| Moderate exacerbations | Total sample | 0.84 (±1.93) | 0.95 (±2.07) | 0.11 | 13.0% | <0.0001 |
| A | 0.08 (±0.27) | 0.21 (±0.74) | 0.13 | 154.7% | <0.0001 | |
| B | 0.08 (±0.27) | 0.29 (±0.9) | 0.22 | 278.2% | <0.0001 | |
| C | 4.23 (±2.43) | 3.83 (±2.86) | −0.40 | −9.4% | 0.0099 | |
| D | 3.72 (±2.69) | 3.76 (±3.07) | 0.03 | 0.9% | 0.7503 | |
| Severe exacerbations | Total sample | 0.07 (±0.39) | 0.1 (±0.46) | 0.03 | 35.7% | 0.0010 |
| A | 0 (±0) | 0.03 (±0.2) | 0.03 | / | <0.0001 | |
| B | 0 (±0) | 0.08 (±0.36) | 0.08 | / | <0.0001 | |
| C | 0.2 (±0.64) | 0.15 (±0.62) | −0.05 | −26.7% | 0.1803 | |
| D | 0.47 (±0.88) | 0.37 (±0.92) | −0.10 | −21.2% | 0.0302 | |
| HRQoL: VAS | Total sample | 58.59 (±19.94) | 57.51 (±20.59) | −1.08 | −1.8% | 0.0006 |
| A | 68.2 (±17.23) | 66.94 (±17.94) | −1.25 | −1.8% | 0.0050 | |
| B | 50.24 (±17.54) | 49.52 (±18.58) | −0.72 | −1.4% | 0.1802 | |
| C | 65.22 (±18.46) | 63.9 (±19.11) | −1.32 | −2.0% | 0.2714 | |
| D | 46.37 (±18.66) | 44.92 (±19.24) | −1.45 | −3.1% | 0.1388 | |
| HRQoL: CAT | Total sample | 19.2 (±7.7) | 19.75 (±7.9) | 0.54 | 2.8% | <0.0001 |
| A | 15.1 (±6.19) | 15.93 (±6.86) | 0.84 | 5.5% | <0.0001 | |
| B | 22.75 (±6.8) | 22.99 (±7.1) | 0.23 | 1.0% | 0.1903 | |
| C | 15.88 (±7.03) | 17.16 (±6.86) | 1.28 | 8.1% | 0.0008 | |
| D | 24.77 (±6.58) | 24.78 (±7.03) | 0.01 | 0.1% | 0.9654 | |
Note: Data are presented as mean (± SD) at baseline and follow-up.
Abbreviations: FEV1, forced expiratory volume in 1 second; HRQoL, health-related quality of life; VAS, visual analog scale; CAT, COPD Assessment Test.
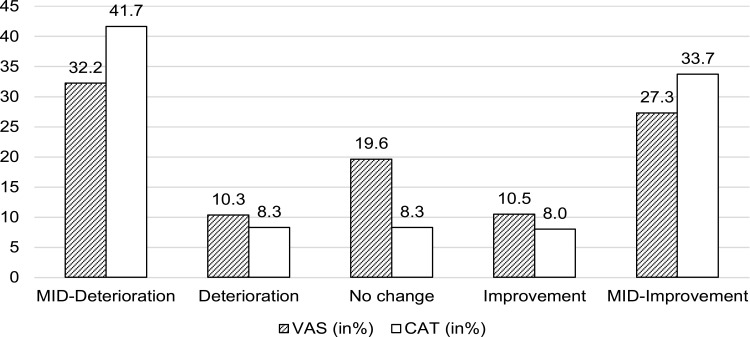
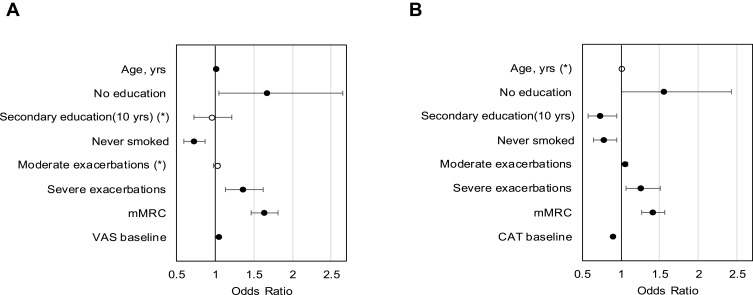
We also observed a significant overall deterioration in the disease-specific (CAT +0.54 units) and generic HRQoL (VAS –1.08 points). The changes were also statistically significant for groups A and B in VAS, and groups A and C in CAT. CAT change was mostly driven by a +1.28 point change in group C. Around one-third of the patients experienced a clinically relevant deterioration in VAS, and almost 42% in CAT (see Figure 2). Determinants of HRQoL deterioration can be seen in Figure 3. A higher baseline HRQoL, age, mMRC, and having severe exacerbations significantly favored a MID deterioration in VAS, while being a never smoker and all education levels compared with the lowest level of education significantly prevented deterioration. For CAT, the results mirrored the findings regarding baseline HRQoL, never smokers, exacerbations, the mMRC, higher and secondary education level.
Figure 2.
Proportion of HRQoL change with a Minimal Important Difference (MID).
Notes: MID-Deterioration in VAS ≥–6.9/MID-Improvement in VAS ≥6.9. MID-Deterioration in CAT ≥2/MID-Improvement in CAT ≥–2.
Figure 3.
Determinants of a clinically relevant deterioration in generic HRQoL VAS (A) and disease-specific HRQoL CAT (B).
Note: *Not significant.
Abbreviations: mMRC, Modified Medical Research Council Questionnaire; VAS, visual analog scale; CAT, COPD Assessment Test.
Impact of FEV1 on HRQoL
Around 36% of DMP patients experienced a FEV1 decrease, with group C having the highest (42%) and group D the lowest proportion of deterioration (29%) (Table 3).
Table 3.
Proportion of One-Year Changes in FEV1
| Total | A | B | C | D | |||
|---|---|---|---|---|---|---|---|
| n | 3016 | 1299 | 1118 | 222 | 377 | ||
| FEV1 change | Decrease | 35.9% | 39.2% | 33.1% | 42.3% | 29.2% | |
| Stable | 35.3% | 33.4% | 37.9% | 32.9% | 35.8% | ||
| Increase | 28.7% | 27.4% | 29.0% | 24.8% | 35.0% | ||
Notes: Decrease: ≥–100mL of base value; Increase ≥+100mL of base value.
Abbreviation: FEV1, forced expiratory volume in 1 second.
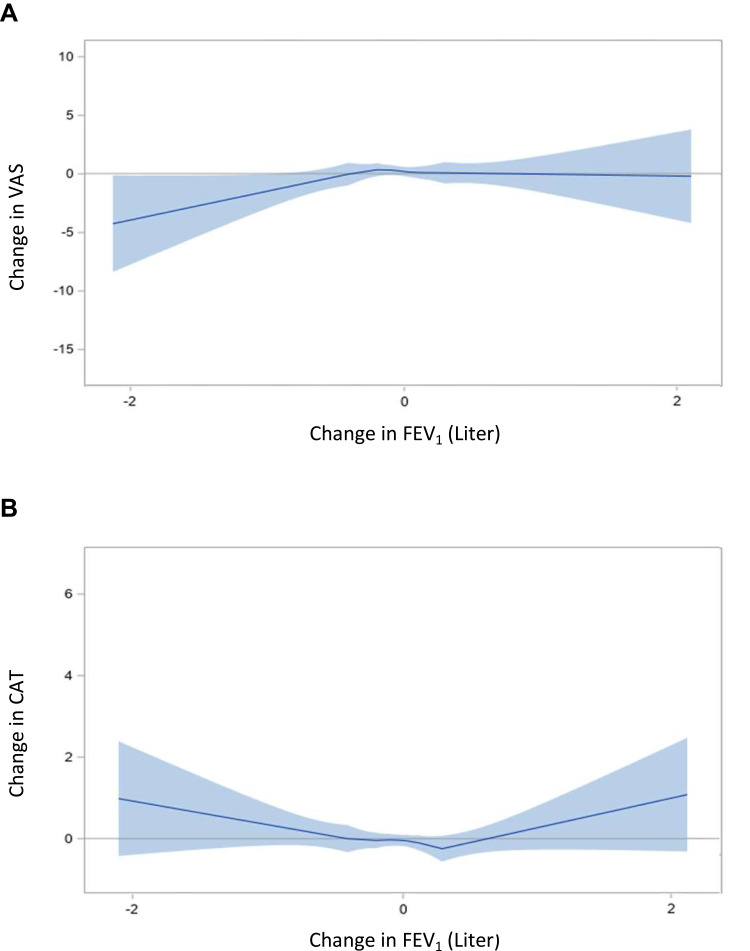
We detected an approximately linear relationship between deterioration in VAS and FEV1 (see Figure 4). The sensitivity analysis showed similar results (Additional File 5).
Figure 4.
Impact of clinical indicators on HRQoL: Linear relationship between change in FEV1 and VAS (A) and CAT (B).
Notes: The estimated smooth functions of the relationship between FEV1 and HRQoL are represented by the solid curves. The shaded areas indicate the 95% confidence intervals. Generalized additive models were adjusted for age, sex, smoking status, education, BMI, number of moderate and severe exacerbation, FEV1 liter change, Charlson Comorbidity index, mMRC, and VAS/CAT baseline.
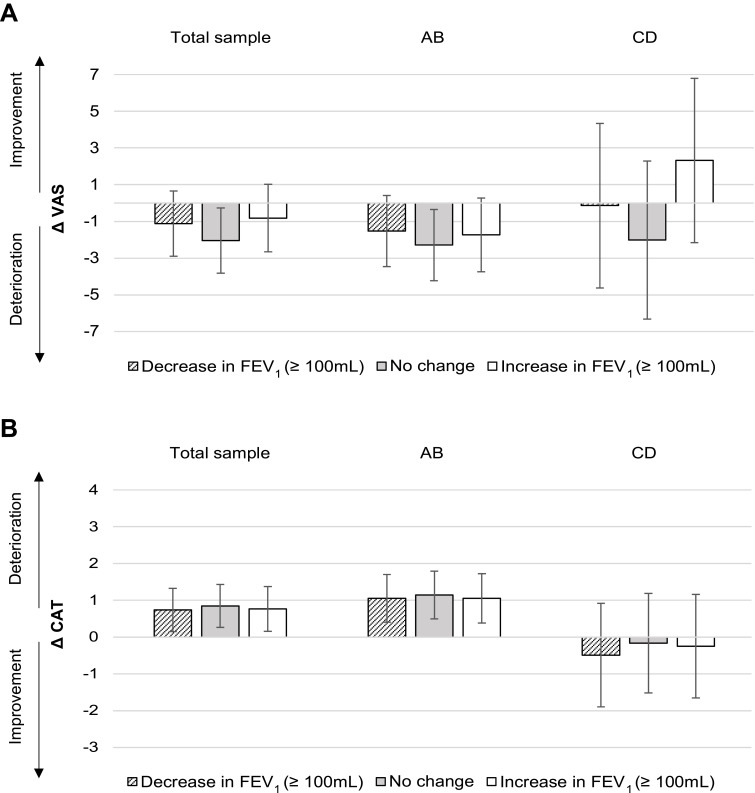
For the total sample, a one-year decrease in FEV1 was associated with a significant deterioration in disease-specific HRQoL (mean change [95% CI]: CAT +0.74 [0.15 to 1.33]) (see Figure 5). At the same time, an increase in FEV1 was also associated with a deterioration in CAT (+0.76 [0.16 to 1.37]), which thus highlights an overall downward trend in HRQoL in our study sample. This trend was also found in groups AB (CAT +1.05) but not in groups CD. Results for changes in VAS were neither significant nor clinically relevant. The sensitivity analysis validated these findings (Additional File 6).
Figure 5.
Change score model: impact of FEV1 on HRQoL.
Notes: Absolute adjusted mean change in VAS (A) and CAT (B) after one year. ABCD groups at baseline. Error bars indicate 95% confidence intervals.
Impact of Exacerbations on HRQoL
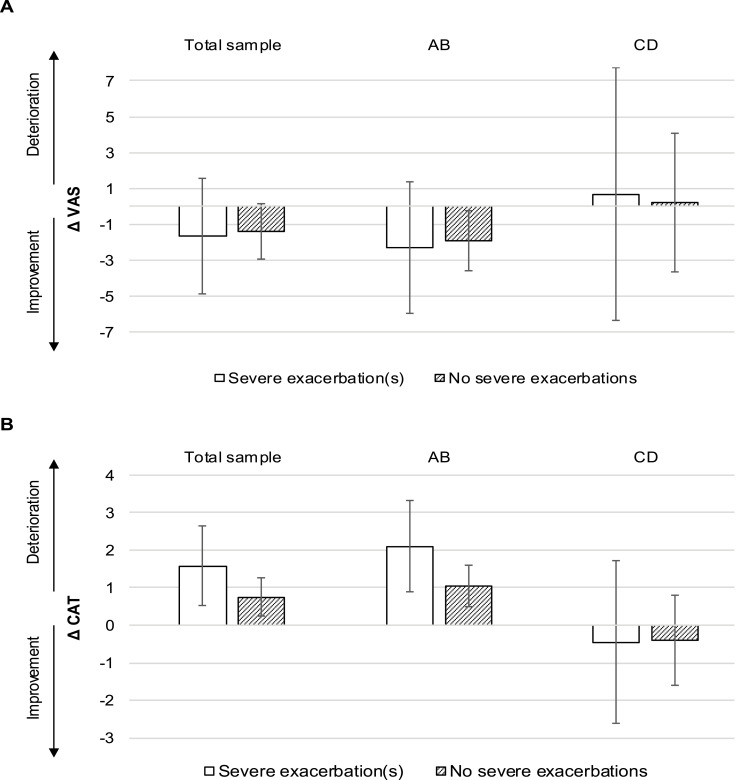
Focusing on patients without severe baseline exacerbations but newly occurring severe exacerbations in the observation period revealed a significant CAT deterioration (mean change [95% CI]: +1.58 [0.52 to 2.64]) that was also clinically relevant in groups AB (+2.1 [0.88 to 3.32]) (see Figure 6). Again, there were no significant results for the VAS model.
Figure 6.
Change score model: impact of exacerbations on HRQoL.
Notes: Absolute adjusted mean change in VAS (A) and CAT (B) after one year. ABCD groups at baseline. Error bars indicate 95% confidence intervals.
In group AB, being male, having a higher mMRC, and a higher Charlson index were linked to a higher probability of experiencing severe exacerbations (Additional File 7). On the other hand, higher FEV1 and BMI values reduced this probability.
The results for the change score models and logistic regression were robust in the sensitivity analysis (Additional Files 8 and 9).
Discussion
In this paper, we analyzed the association between a change in lung function or exacerbations and a one-year change in generic and disease-specific HRQoL in COPD patients stratified by GOLD ABCD groups. Overall, we found a small, statistically significant decrease in both HRQoL measures. For the generic VAS, almost one-third of patients deteriorated in a clinically relevant manner; for the disease-specific CAT, this proportion was 42%. In particular, patients in GOLD groups AB (no previous severe exacerbations) who developed at least one severe exacerbation in their follow-up showed a significant and clinically relevant deterioration in disease-specific HRQoL, which highlights a special need for prevention in this subgroup.
An overall deterioration in HRQoL at the population level is well known for other disease-specific and generic measures.16,42,43 In our sample, HRQoL was highest in group A, followed in descending order by groups C, B, and D. This signals a better health status in C over B, even though group C faces a higher exacerbation burden. This is in line with the literature.10,11 We observed the highest decrease rates in group C for disease-specific and in group D for generic HRQoL. The HRQoL deterioration within one year was significantly higher for patients with a more favorable baseline HRQoL, which supports the findings of Habraken et al.44
Further, this overall deterioration was independent of the change direction in FEV1 or severe exacerbations for the total sample, as the HRQoL decreased even with no severe exacerbation or with an increased lung function. This is contrary to the findings of Jones et al,19 who observed a slight increase in disease-specific HRQoL in absence of exacerbations or for a stable FEV1 in a multinational, clinical trial population.
The portion of clinically relevant deteriorations at an individual patient level was higher in our population for the CAT (42%), whereas literature suggest a portion of only one-third for the similar disease-specific SGRQ.45 Predictors for a MID deterioration in HRQoL for both VAS and CAT were found to be a higher HRQoL at baseline, greater breathlessness, and severe exacerbations, whereas being a never smoker decreased the probability of a clinically relevant deterioration. As on the other hand, almost a third of our population presented a HRQoL improvement, more focus should be given to these different phenotypic cohorts in future research.
We are the first to analyze the HRQoL changes in VAS and CAT with regard to changes in FEV1 or exacerbations, stratified by GOLD ABCD groups and based on a large claims dataset. Similar to Lutter et al,16 who evaluated the relationship of FEV1 change and HRQoL change in GOLD groups I–IV within the German COSYCONET cohort, we did not find statistically significant changes in generic HRQoL for lung function declines.16 These authors used the SGRQ to measure disease-specific HRQoL, which limits direct comparability to only generic results. Nonetheless, we also found a statistically significant deterioration in disease-specific CAT for the total sample and groups AB, which did not exceed the MID. On the other hand, our findings diverge from the reference study, as they observed significant improvement in disease-specific and generic HRQoL for an increased FEV1. Regarding disease-specific HRQoL, other studies using SGRQ confirm these results.17,46 In contrast, we detected a significant overall deterioration in HRQoL for our total sample and groups AB in CAT, even for an increased or stable FEV1. This might indicate that FEV1 change categories would be more appropriate with relative changes than absolute changes,47,48 but there is still no consensus yet about a %-MID.47,49 Mannino and Davis,13 for example, defined patients with an annual 3.5% change as rapid decliners. Thus, further research is needed, and our analysis should be reproduced when a consensus is reached on one relative MID, or even one MID per GOLD ABCD group.
Another aspect is that we observed small, non-significant HRQoL improvements over one year for groups CD, independent of lung function development. This can be attributed to relatively small group sizes leading to broad confidence limits, even though we combined both groups to counteract this issue (CD comprised 19.9% of the total sample).
For severe exacerbations, Nishimura et al50 demonstrated a significant decline in disease-specific HRQoL (SGRQ) within 6 months, especially when they occur frequently (>1). Jones et al19 also analyzed the association of change in exacerbation rate on SGRQ over three years. Comparing our results with their placebo group, our results confirm that ≥1 severe exacerbations results in a significant HRQoL decrease, but we did not observe a HRQoL increase for having no severe exacerbations. This again mirrors our observation of an overall downward trend in HRQoL in our study population. For our analysis, we did not measure the change in the absolute number of exacerbations, as no consistent MID exists for exacerbations.51 Therefore, we investigated the change from absence of severe exacerbation at baseline to presence of at least one severe exacerbation during the follow-up. Although we did not find significant changes in VAS, we found a markedly higher and statistically significant HRQoL deterioration in CAT in patients experiencing severe exacerbations. In subgroup GOLD AB, severe exacerbations were associated not only with a significant but also with a clinically relevant deterioration in disease-specific HRQoL. Thus, our results highlight the importance of preventive measures, especially in patients who are in group AB without severe exacerbations in the previous year. For this subgroup, a MID reduction in disease-specific quality of life can be counteracted if one or more severe exacerbations can be prevented. Guo et al21 also recently stressed a need for effective prevention as exacerbations do have a sustainable and lasting impact on HRQoL. In the literature, lower mMRC, fewer exacerbations in history, and FEV1 decrease were shown to affect exacerbation risk. For example, a FEV1 lower than 50% results in a 2.6 higher risk of experiencing a severe exacerbation,52 whereas a 100-mL FEV1 increase reduces exacerbation risk by 10%.53 Countering this, exacerbations were also postulated to have a negative impact on FEV1.21 All in all, the relationship between FEV1 and exacerbations has been shown to be mostly consistent, but exceptions also exist54 and, for example, not all exacerbations inevitably lead to a decline in FEV1.55 As exacerbations and FEV1 appear to be connected, further research with a larger sample size could allow analysis of the combined interactional change in both clinical factors on HRQoL change.
For both clinical factors, methodological aspects can explain the different results in VAS and CAT models, as VAS only portrays the current and short-term overall health status, whereas the CAT is COPD specific and refers to the historic and current health status. Therefore, we agree with Lutter et al16 that disease-specific measures are more suitable to assess longitudinal HRQoL in COPD.
With regard to our study design, we need to address limitations, as our study population is exposed to selection bias. First, while our sample included data from an SHI fund with a large market share, we do not have information on COPD patients covered by respective DMPs by other SHI funds, nor on COPD patients not covered by respective DMPs. Second, all participants are voluntarily enrolled in the DMP, which implies that “good risks” are more likely to participate and, non-responders were also shown to be at higher risk. Moreover, the DMP participation is associated with an increased quality of care, which could lead to a HRQoL gap between DMP and non-DMP settings.56 This could result in an underestimated effect on HRQoL and more conservative results than in the whole patient population. Additionally, we only considered patients who did not die. Further, survey results were collected at varying, later times than events documented in claims data, and changes in patients’ health during that time cannot be excluded. Yet plausibility would suggest that patients are more likely to complete the self-administered questionnaire when they are in a more stable state, which tends to reduce this uncertainty.
Regarding our study population and generic HRQoL, we only included patients who had complete data from VAS, but we did not exclude patients with incomplete data in EQ-5D-5L dimensions, as the dimensions were not used in our models. By EQ-5D-5L dimension category, between 31 and 59 missing values occurred per category in the study sample, and an influence on VAS cannot be fully excluded.
Regression to the mean as a result of our repeated measurement of HRQoL, FEV1, and exacerbations is a common issue.57 To account for this, we adjusted all models for baseline values when analyzing changes in clinical factors and changes in HRQoL.
A further problem might be the shortness of our observation period, comprising only about one year, which might be too short to observe the influence of clinical indicators on HRQoL. FEV1 was found to have an important impact on HRQoL, particularly in the long term, and especially for severe exacerbations, an unequal rhythm over the years has been shown,58 suggesting that just one year of observation may not be sufficient to catch the full effect. In addition, our clinical factors retrieved from claims data, such as FEV1, are always from the time before the questionnaires that provided the HRQoL measurements. Nonetheless, this was considered negligible, as it was shown that small health changes often influence HRQoL only temporarily because patients tend to adapt to the impact of changes after a certain time.59,60 Further, we used an annual average of FEV1 values, wherever possible, in an attempt to minimize bias caused by measurement variability.
A possible bias arose through a DMP request switch from liters to percent predicted values for FEV1 within our study period. Several physicians reported values lower than 5 or higher than 120 for FEV1%pred. in wave 2. We reconsidered these values as liter or milliliter values respectively. The remaining values were kept as predicted values, but different calculation methods for the FEV1%pred. exist,61 and we cannot observe the calculation method used by practitioners. For this reason, we worked only with the liter values. We thus accounted for this bias by using only liter values for FEV1 and conducting a sensitivity analysis, which showed robust overall results.
All patients were under their regular treatment, but treatment elements or interventions may have changed, eg, due to severe exacerbations resulting in treatment effects on HRQoL, which were not further considered in this study. Finally, generalizability is limited, because full data availability without any imputation reduced our original sample size considerably, and we analyzed only regional data from Germany.
Nonetheless, our study has some unique advantages, such as a large sample size and the combination of claims data and survey data, which is relatively rare and allowed us to build GOLD ABCD groups. GOLD ABCD groups were found to be more closely associated with disease-specific and generic HRQoL in COPD than GOLD I–IV.10 Second, we were thus able to investigate changes in disease-specific and generic HRQoL in relation to changes in lung function or exacerbations stratified by ABCD groups instead of GOLD I–IV. Also, the combination of EQ-5D and CAT has been shown to be a very useful measure to assess HRQoL in COPD.9,62 Compared with an expected conservative response rate, we perceive our response rate of 29.7% in wave 1 and 62.6% in wave 2 to be good, and it is similar to comparable studies using postal questionnaires within the German DMPs, eg, 32% in DMP Asthma.63
Conclusion
For the longitudinal impact of clinical changes on HRQoL in COPD patients within one year, we conclude that a decline in FEV1 or the occurrence of at least one severe exacerbation is significantly associated with an overall deterioration in disease-specific HRQoL. In particular, patients in COPD groups AB need to be considered further: those experiencing a severe exacerbation within one year reported a clinically relevant deterioration in their disease-specific quality of life. To maintain or rather to improve HRQoL in COPD patients, our results underline the importance of optimal treatment.
Acknowledgments
We would like to thank the AOK Bayern for providing the data and helpful comments on the data. We also thank Dr. med. Frank Powitz, Dr. med. Daniel Pohl, and Boglárka Szentes for their expert opinions and support.
Funding Statement
The LQ-DMP project is funded by the Innovation Fund of the Federal Joint Committee (Gemeinsamer Bundesausschuss) of Germany (support code 01VSF16025).
Abbreviations
BMI, Body mass index; COPD, Chronic obstructive pulmonary disease; CAT, COPD assessment test; CCQ, Clinical COPD Questionnaire; COSYCONET, COPD and systemic consequences – comorbidities network; DMP, Disease management program; EQ-VAS, Euro-Qol visual analog scale; EQ-5D, Euro-Qol 5 dimensions questionnaire; FEV1, Forced expiratory volume in 1 second; FEV1%pred., FEV1% predicted; GAM, Generalized additive model; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; HRQoL, Health-related quality of life; MID, Minimal important difference; MMRC, Modified British Medical Research Council questionnaire; PROM, Patient-reported outcome measure; SD, Standard deviation; SGRQ, Saint George’s respiratory questionnaire; SHI, Statutory health insurance.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available according to the data protection concept approved by the responsible data security officials and the ethics committee.
Ethics Approval and Consent to Participate
The study complies with the Declaration of Helsinki and has been approved by the ethics committee of the Ludwig-Maximilians-University, Munich, Germany (vote no. 17-358). All participants provided written informed consent at the time of inclusion in the disease management program.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr Claus F Vogelmeier reports grants, personal fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, grants from Chiesi, GlaxoSmithKline, Grifols, and Menarini, grants from Novartis, Nuvaira, and MedUpdate, outside the submitted work. Prof. Dr. Reiner Leidl reports grants from Innovation Fund, Federal Joint Committee, Germany, during the conduct of the study. The authors declare that they have no other competing interests.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. The top 10 causes of death; 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed September3, 2021.
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leidy NK, Murray LT, Jones P, Sethi S. Performance of the EXAcerbations of chronic pulmonary disease tool patient-reported outcome measure in three clinical trials of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(3):316–325. doi: 10.1513/AnnalsATS.201309-305OC [DOI] [PubMed] [Google Scholar]
- 5.Mackay AJ, Kostikas K, Murray L, et al. Patient-reported outcomes for the detection, quantification, and evaluation of chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2018;198(6):730–738. doi: 10.1164/rccm.201712-2482CI [DOI] [PubMed] [Google Scholar]
- 6.Gemeinsamer Bundesausschuss. Richtlinie Des Gemeinsamen Bundesausschusses Zur Zusammenführung Der Anforderungen an Strukturierte Behandlungsprogramme Nach § 137f Absatz 2 SGB V (DMP-Anforderungen-Richtlinie/DMPA-RL) [Directive of the Federal Joint Committee on the Combination of Requirements for Structured Treatment Programs Pursuant to Section 137f (2) of the German Social Code, Book V (DMP Requirements Directive/DMPA Directive)]; 2021. Available from: https://www.g-ba.de/downloads/62-492-2416/DMP-A-RL_2020-11-20_iK-2021-02-25.pdf. Accessed September 6, 2021.
- 7.Wilke S, Jones PW, Müllerova H, et al. One-year change in health status and subsequent outcomes in COPD. Thorax. 2015;70(5):420–425. doi: 10.1136/thoraxjnl-2014-205697 [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Oh YM, Jo M. Health-related quality of life in chronic obstructive pulmonary disease patients in Korea. Health Qual Life Outcomes. 2014;12(57):1–7. doi: 10.1186/1477-7525-12-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wacker ME, Jörres RA, Karch A, et al. Assessing health-related quality of life in COPD: comparing generic and disease-specific instruments with focus on comorbidities. BMC Pulm Med. 2016;16(1):1–11. doi: 10.1186/s12890-016-0238-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boland MRS, Tsiachristas A, Kruis AL, Chavannes NH, Rutten-van Mölken MPMH. Are GOLD ABCD groups better associated with health status and costs than GOLD 1234 grades? A Cross-Sectional Study. Prim Care Respir J. 2014;23(1):30–37. doi: 10.4104/pcrj.2014.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agusti A, Edwards LD, Celli B, et al. Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J. 2013;42(3):636–646. doi: 10.1183/09031936.00195212 [DOI] [PubMed] [Google Scholar]
- 12.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648. doi: 10.1136/bmj.1.6077.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannino DM, Davis KJ. Lung function decline and outcomes in an elderly population. Thorax. 2006;61(6):472–477. doi: 10.1136/thx.2005.052449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tantucci C, Modina D. Lung function decline in COPD. Int J COPD. 2012;7:95. doi: 10.2147/COPD.S27480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas ET, Guppy M, Straus SE, Bell KJL, Glasziou P. Rate of normal lung function decline in ageing adults: a systematic review of prospective cohort studies. BMJ Open. 2019;9(6):e028150. doi: 10.1136/bmjopen-2018-028150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutter JI, Jörres RA, Kahnert K, et al. Health-related quality of life associates with change in FEV1 in COPD: results from the COSYCONET cohort. BMC Pulm Med. 2020;20(148):1–12. doi: 10.1186/s12890-020-1147-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Loge C, Taugaut B, Fofana F, et al. Relationship between FEV1 and patient-reported outcomes changes: results of a meta-analysis of randomized trials in stable COPD. Chronic Obstr Pulm Dis J COPD Found. 2016;3(2):519. doi: 10.15326/jcopdf.3.2.2015.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai K, Makita H, Suzuki M, et al. Differential changes in quality of life components over 5 years in chronic obstructive pulmonary disease patients. Int J COPD. 2015;10:745. doi: 10.2147/COPD.S77586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones PW, Anderson JA, Calverley PMA, et al. Health status in the TORCH study of COPD: treatment efficacy and other determinants of change. Respir Res. 2011;12(71):1–8. doi: 10.1186/1465-9921-12-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menn P, Weber N, Holle R. Health-related quality of life in patients with severe COPD hospitalized for exacerbations - comparing EQ-5D, SF-12 and SGRQ. Health Qual Life Outcomes. 2010;8(39):39. doi: 10.1186/1477-7525-8-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J, Chen Y, Zhang W, Tong S, Dong J. Moderate and severe exacerbations have a significant impact on health-related quality of life, utility, and lung function in patients with chronic obstructive pulmonary disease: a meta-analysis. Int J Surg. 2020;78:28–35. doi: 10.1016/j.ijsu.2020.04.010 [DOI] [PubMed] [Google Scholar]
- 22.Esteban C, Quintana JM, Moraza J, et al. Impact of hospitalisations for exacerbations of COPD on health-related quality of life. Respir Med. 2009;103(8):1201–1208. doi: 10.1016/j.rmed.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 23.Siebeling L, Musoro JZ, Geskus RB, et al. Prediction of COPD-specific health-related quality of life in primary care COPD patients: a Prospective Cohort Study. NPJ Prim Care Respir Med. 2014;24(14060):1–7. doi: 10.1038/npjpcrm.2014.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellou V, Belbasis L, Konstantinidis AK, Tzoulaki I, Evangelou E. Prognostic models for outcome prediction in patients with chronic obstructive pulmonary disease: systematic review and critical appraisal. Br Med J. 2019;367:l5358. doi: 10.1136/bmj.l5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esteban C, Arostegui I, Aramburu A, et al. Predictive factors over time of health-related quality of life in COPD patients. Respir Res. 2020;21(138):1. doi: 10.1186/s12931-020-01395-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bundesministerium für Gesundheit. Mitgliederstatistik KM6; 2017. Available from: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Statistiken/GKV/Mitglieder_Versicherte/KM6_2017.xlsx. Accessed September3, 2021.
- 27.Fletcher CM. Standardized questionnaire on respiratory symptoms: a statement prepared for, and approved by, the medical research council’s committee on the aetiology of chronic bronchitis. Br Med J. 1960;2(5213):1665.13688719 [Google Scholar]
- 28.Kirsch F, Schramm A, Schwarzkopf L, et al. Direct and indirect costs of COPD progression and its comorbidities in a structured disease management program: results from the LQ-DMP study. Respir Res. 2019;20(1):1–5. doi: 10.1186/s12931-019-1179-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 30.Mittal R, Chhabra SK. GOLD classification of COPD: discordance in criteria for symptoms and exacerbation risk assessment. COPD J Chronic Obstr Pulm Dis. 2017;14(1):1–6. doi: 10.1080/15412555.2016.1230844 [DOI] [PubMed] [Google Scholar]
- 31.Smid DE, Franssen FME, Gonik M, et al. Redefining cut-points for high symptom burden of the global initiative for chronic obstructive lung disease classification in 18,577 patients with chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2017;18(12):1097.e11–1097.e24. doi: 10.1016/j.jamda.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 32.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nolan CM, Longworth L, Lord J, et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax. 2016;71(6):493–500. doi: 10.1136/thoraxjnl-2015-207782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kon SSC, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi: 10.1016/S2213-2600(14)70001-3 [DOI] [PubMed] [Google Scholar]
- 35.Smid DE, Franssen FME, Houben-Wilke S, et al. Responsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: a prospective analysis. J Am Med Dir Assoc. 2017;18(1):53–58. doi: 10.1016/j.jamda.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 36.Szentes BL, Schwarzkopf L, Kirsch F, Schramm A, Leidl R. Measuring quality of life in COPD patients: comparing disease-specific supplements to the EQ-5D-5L. Expert Rev Pharmacoeconomics Outcomes Res. 2020;20(5):523–529. doi: 10.1080/14737167.2019.1662302 [DOI] [PubMed] [Google Scholar]
- 37.Zanini A, Aiello M, Adamo D, et al. Estimation of minimal clinically important difference in EQ-5D visual analog scale score after pulmonary rehabilitation in subjects with COPD. Respir Care. 2015;60(1):88–95. doi: 10.4187/respcare.03272 [DOI] [PubMed] [Google Scholar]
- 38.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 39.Hastie TJ, Tibshirani RJ. Generalized additive models. Stat Sci. 1986;1(3):297–318. doi: 10.1201/9780203753781 [DOI] [PubMed] [Google Scholar]
- 40.Sheskin DJ. Handbook of Parametric and Nonparametric Statistical Procedures. 4th ed. Chapman & Hall/CRC; 2007. doi: 10.1201/9780429186196 [DOI] [Google Scholar]
- 41.Donohue JF. Minimal clinically important differences in COPD lung function. COPD J Chronic Obstr Pulm Dis. 2005;2(1):111–124. doi: 10.1081/COPD-200053377 [DOI] [PubMed] [Google Scholar]
- 42.Spencer S, Calverley PMA, Burge PS, Jones PW. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(1):122–128. doi: 10.1164/ajrccm.163.1.2005009 [DOI] [PubMed] [Google Scholar]
- 43.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Mishima M. Longitudinal deteriorations in patient reported outcomes in patients with COPD. Respir Med. 2007;101(1):146–153. doi: 10.1016/j.rmed.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 44.Habraken JM, Van Der Wal G, Riet T, Weersink EJM, Tobene F, Bindels PJE. Health-related quality of life and functional status in end-stage COPD: a Longitudinal Study. Eur Respir J. 2011;37(2):280–288. doi: 10.1183/09031936.00149309 [DOI] [PubMed] [Google Scholar]
- 45.Monteagudo M, Rodríguez-Blanco T, Llagostera M, et al. Factors associated with changes in quality of life of COPD patients: a Prospective Study in primary care. Respir Med. 2013;107(10):1589–1597. doi: 10.1016/j.rmed.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 46.Westwood M, Bourbeau J, Jones PW, Cerulli A, Capkun-Niggli G, Worthy G. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res. 2011;12(40):1–9. doi: 10.1186/1465-9921-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiley JP, Ram JS, Croxton TL, Weinmann GG. Challenges associated with estimating minimal clinically important differences in COPD - the NHLBI perspective. COPD J Chronic Obstr Pulm Dis. 2005;2(1):43–46. doi: 10.1081/COPD-200050649 [DOI] [PubMed] [Google Scholar]
- 48.Raimondi GA. FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(12):1676–1677. doi: 10.1164/rccm.201701-0001LE [DOI] [PubMed] [Google Scholar]
- 49.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–469. doi: 10.1183/09031936.00099306 [DOI] [PubMed] [Google Scholar]
- 50.Nishimura K, Sato S, Tsukino M, et al. Effect of exacerbations on health status in subjects with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2009;7(1):69. doi: 10.1186/1477-7525-7-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189(3):250–255. doi: 10.1164/rccm.201310-1863PP [DOI] [PubMed] [Google Scholar]
- 52.Cao Z, Ong KC, Eng P, Tan WC, Ng TP. Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188–195. doi: 10.1111/j.1440-1843.2006.00819.x [DOI] [PubMed] [Google Scholar]
- 53.Zhudenkov K, Palmér R, Jauhiainen A, et al. Longitudinal FEV1 and exacerbation risk in COPD: quantifying the association using joint modelling. Int J COPD. 2021;16:101–111. doi: 10.2147/COPD.S284720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soler-Cataluña JJ, Martínez-García MÁ, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178(4):332–338. doi: 10.1164/rccm.200712-1869OC [DOI] [PubMed] [Google Scholar]
- 56.Mehring M, Donnachie E, Fexer J, Hofmann F, Schneider A. Disease Management Programs for patients with COPD in Germany: a longitudinal evaluation of routinely collected patient records. Respir Care. 2014;59(7):1123–1132. doi: 10.4187/respcare.02748 [DOI] [PubMed] [Google Scholar]
- 57.Suissa S. Lung function decline in COPD trials: bias from regression to the mean. Eur Respir J. 2008;32(4):829–831. doi: 10.1183/09031936.00103008 [DOI] [PubMed] [Google Scholar]
- 58.Han MLK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi: 10.1016/S2213-2600(17)30207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allison PJ, Locker D, Feine JS. Quality of life: a dynamic construct. Soc Sci Med. 1997;45(2):221–230. doi: 10.1016/S0277-9536(96)00339-5 [DOI] [PubMed] [Google Scholar]
- 60.Huber MB, Reitmeir P, Vogelmann M, Leidl R. EQ-5D-5L in the general German population: comparison and evaluation of three yearly cross-section surveys. Int J Environ Res Public Health. 2016;13(3):343. doi: 10.3390/ijerph13030343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sood A, Dawson BK, Henkle JQ, Hopkins-Price P, Qualls C. Effect of change of reference standard to NHANES III on interpretation of spirometric “abnormality”. Int J COPD. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2695189/2007;2(3):361. [PMC free article] [PubMed] [Google Scholar]
- 62.Sundh J, Johansson G, Larsson K, et al. Comorbidity and health-related quality of life in patients with severe chronic obstructive pulmonary disease attending Swedish secondary care units. Int J COPD. 2015;10:173. doi: 10.2147/COPD.S74645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bücker B, Löscher S, Schürer C, Schaper K, Abholz -H-H, Wilm S. Asthma in Deutschland: Versorgungslage aus Patientensicht. Eine Fragebogenstudie zum disease-management-programm asthma [Ambulatory care of patients with asthma in Germany and disease management program for asthma from the view of statutory health insured]. DMW Dtsch Medizinische Wochenschrift. 2015;140(6):e60–e66. doi: 10.1055/s-0041-101012 [DOI] [PubMed] [Google Scholar]