Abstract
Background:
Multidrug-resistant (MDR) Acinetobacter baumannii is of serious health concern and associated with high mortality. Data regarding the antibiotic resistance pattern of A. baumannii strains in Oman is limited.
Objectives:
To determine the antibiotic resistance pattern of A. baumannii from various clinical samples in a tertiary care hospital in the North-Batinah region of Oman.
Methods:
A. baumannii isolates recovered from various clinical samples in the Microbiology laboratory of Sohar Hospital, Oman, during 2015–2019 were retrospectively analyzed. Organism identification and their antibiotic resistance patterns were performed as recommended by the Clinical and Laboratory Standards Institute.
Results:
A total of 1890 A. baumannii nonduplicate isolates were found from clinical samples of 1326 patients. The isolates were more frequently isolated from elderly patients (40%) and in-patient department patients (67%). Infection/colonization was more common among patients treated in the medicine, intensive-care unit, and surgery departments. A. baumannii strains were highly resistant (50-83%) to most of the tested antibiotics, with the highest against ceftriaxone (83%) and ceftazidime (75%), and lowest against colistin (1%) and tigecycline (8%). Among the isolates, 67% (1265) were MDR strains. Of these, 22%, 32% and 16% were resistant to all six, five and four classes of the tested antibiotics.
Conclusion:
The study found that the frequency of isolation of MDR A. baumannii isolates in the northern region of Oman is high.
Keywords: Acinetobacter baumannii, multidrug resistance, nosocomial infections, Oman
INTRODUCTION
The emergence and continued spread of antimicrobial-resistant bacterial pathogens is a serious concern worldwide.[1,2] Acinetobacter baumannii is a dangerous opportunistic nosocomial pathogen that has emerged in recent years as an important cause of a wide range of infections such as ventilator-associated pneumonia, surgical wound infection, meningitis, and urinary tract infection, especially in immunocompromised patients.[3,4] Its ability to exist and survive in dry and humid surfaces, particularly in the hospital surfaces and equipment, makes controlling the nosocomial transmission extremely difficult.[5,6] In addition, recent emergence of multidrug-resistant (MDR), extensively drug resistant (XDR), and pandrug-resistant clones of A. baumannii in hospital settings and the rapid dissemination of these strains among hospitalized patients has become a serious problem worldwide, as they are difficult to treat and are associated with high mortality.[3,6,7,8,9]
In Oman, a recent surveillance report from the Royal Hospital, Muscat, showed an increasing trend in MDR A. baumannii infection.[10] Another study conducted at Sultan Qaboos University, Muscat, showed a rise in infections associated with nonfermenters such as Pseudomonas and Acinetobacter.[11] A molecular characterization analysis of MDR A. baumannii strains from all six GCC nations revealed that 69% of MDR strains carrying genes encode for OXA-23 and OXA-40 enzymes, which can confer it's resistance against the majority of antibiotics including carbapenems used for treating A. baumannii infections.[11]
The prevalence and antibiotic susceptibility pattern of A. baumannii shows inter- and intra-national variations. Knowledge regarding local prevalence, predisposing factors, and the antibiotic susceptibility pattern of A. baumannii and other MDR pathogens is important for better management of infections.[9] From Oman, there is a limited knowledge regarding the prevalence and antibiotic susceptibility pattern of A. baumannii. Hence, the current study was carried out to determine the extent of antibiotic resistance among A. baumannii isolates recovered from various clinical samples at a tertiary care hospital in North-Batinah region of Oman.
METHODS
This retrospective study included A. baumannii isolates identified from various clinical samples collected at the Microbiology Laboratory at Sohar Hospital, North-Batinah region of Oman, between January 2015 and December 2019. Sohar Hospital is a 400-bed tertiary care public hospital. The data were retrieved from Al-Shifa System of Ministry of Health and the microbiology laboratory records, and included socio-demographic characteristics of the patients, clinical profile and antibiotic resistance patterns of A. baumannii.
The study was approved by the hospital's Research and Ethical Committee and the Ministry of Health, Oman.
Bacterial identification method and antibiotic susceptibility testing
A. baumannii isolates were recovered from different clinical samples including blood, urine, respiratory secretions, and others. The isolates were identified by standard microbiological methods and the automated Vitek 2 system (Bio-Mérieux, France). All repeat isolates and the isolates recovered from the regions of most likely colonization such as throat swab, perianal swab, etc., were excluded from the study. Six major classes of antibiotics, namely, beta-lactams, fluoroquinolones, carbapenems, macrolides, aminoglycosides, and sulfamethoxazole-trimethoprim, in addition to colistin and tigecycline, were used to determine the antibiotic susceptibility pattern of isolates.
Antimicrobial susceptibility testing was performed by Kirby-Bauer's disc diffusion method on Muller-Hinton agar with the following antibiotic panel using the Oxoid antibiotic discs: Gentamicin (10 μg), trimethoprim-sulfamethoxazole (1.25/23.75 μg), ciprofloxacin (5 μg), piperacillin-tazobactam (100/10 μg), imipenem (10 μg), meropenem (10 μg), amikacin (10 μg), ceftriaxone (30 μg), ceftazidime (30 μg), colistin (10 μg), and tigecycline (15 μg), as recommended by Clinical and Laboratory Standards Institute (CLSI).[12] For ceftazidime and colistin, a minimum inhibitory concentration (MIC) was determined by the Epsilometer test, as recommended by CLSI.[12]
Strains that showed nonsusceptibility to at least one agent in three or more classes of antibiotics were categorized as MDR organisms. The strains were defined as R0 (absence of resistance to all six classes of antibiotics), R1 (resistant to one class of antibiotics), R2 (resistant to two classes of antibiotics), R3 (resistant to three classes of antibiotics), R4 (resistant to four classes of antibiotics), R5 (resistant to five classes of antibiotics), and R6 (resistant to all six classes of tested antibiotics). In agreement with previous reports, the strains R3-R6 were considered as MDR strains. Furthermore, R6 strains were categorized as XDR A. baumannii.[7,12] Quality control was performed using Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853.
Statistical analysis
Data were cleaned for duplicates and repeat samples. The frequencies of gender and age groups were tallied both in total and year wise. A one sample Chi-square test was done to determine the significance of mode values among the classes. Department and sample wise isolates were classified for descriptive purposes. Year-wise antibiotic susceptibility pattern was tabulated, and the trends plotted for the period 2015–2019.
RESULTS
A total of 1890 nonduplicate A. baumannii isolates were found during the study period from various clinical samples of 1326 patients. Table 1 shows the characteristics of patients and bacterial isolates. There was no gender-wise difference in the frequency of isolation (females: 52%; males: 48%), but it was highest (40%) among those aged >60 years (P < 0.0001).
Table 1.
Characteristics of patients and study isolates
| Parameters | Value, n (%) |
|---|---|
| Number of patients (n=1326) | |
| Number of in-patient department patients | 889 (67) |
| Number of outpatient department patients (including referrals and others) | 437 (33) |
| Gender* | |
| Male | 633 (48) |
| Female | 689 (52) |
| Age* (years) | |
| 0-20 | 210 (16) |
| 21-40 | 408 (31) |
| 41-60 | 200 (15) |
| >60 | 504 (38) |
| Year-wise distribution of isolates | |
| 2015 | 478 (25) |
| 2016 | 316 (17) |
| 2017 | 343 (18) |
| 2018 | 414 (22) |
| 2019 | 339 (18) |
*Gender and age records of 4 patients were not found
Distribution and rate of isolation
Infection/colonization with A. baumannii was more commonly observed among patients admitted in the hospital (67%) than those treated in the outpatient department (24%) and peripheral health centers (9%). Figure 1 shows department-wise distribution of clinical samples. The rate of isolation was highest among patients treated in the Department of Medicine (34%), followed by Intensive Care Units (13%; ICUs), Obstetrics and Gynecology (12%), and Surgery (11%). The rate of isolation from patients treated in other departments was <10%. A. baumannii was predominantly isolated from the skin and soft tissue specimens (551; 29.2%), followed by respiratory secretions (519; 28%), urine (481; 25%), blood (160; 9%), catheter tip (48; 3%), and others (131; 7%) [Figure 2].
Figure 1.
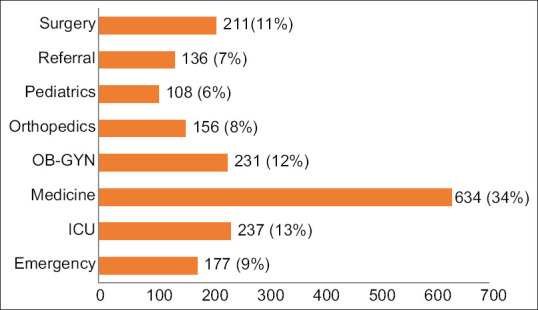
Department-wise distribution of clinical samples
Figure 2.
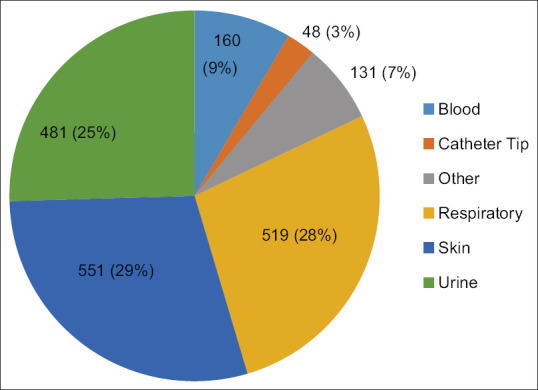
Source of Acinetobacter baumannii isolates
Antibiotic resistance patterns
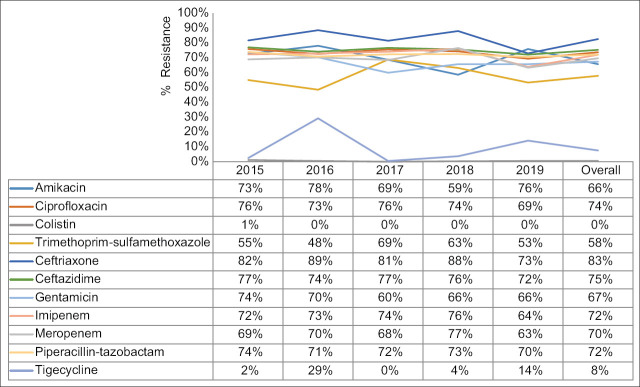
The antibiotic-resistance of A. baumannii to trimethoprim-sulfamethoxazole, amikacin, and gentamicin was 58%, 66%, and 67%, respectively. The resistance to ciprofloxacin, imipenem, meropenem, and piperacillin-tazobactam was in the range of 70%–72%, while the highest resistance was against ceftazidime (75%) and ceftriaxone (83%). The least resistance was observed to colistin (1%) and tigecycline (8%). There was no significant variation in the year-wise frequency and change in the antibiotic-resistant pattern during the study period [Figure 3].
Figure 3.
Year-wise trend and overall antibiotic resistance pattern of Acinetobacter baumannii
Of the total A. baumannii isolates, 67% (n = 1265) were MDR strains, of which 22% (278) were resistant to all the six classes of tested antibiotics (XDR strains). In addition, 32% (596), 16% (304), and 5% (87) were resistant to five, four, and three classes of antibiotics, respectively. There was a gradual increase and decline in the resistance percentage of R3 and R5 strains, respectively, from 2015 to 2019, while the resistance percentage of R4 and R6 strains fluctuated slightly during the study period [Table 2].
Table 2.
Distribution of drug-resistant strains of Acinetobacter baumannii isolated from 2015 to 2019
| Degree of resistance | Percentage | Total (n=1890), n (%) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 2015 (n=478) | 2016 (n=316) | 2017 (n=343) | 2018 (n=414) | 2019 (n=339) | ||
| Non-MDR strains | ||||||
| R0 | 23 | 27 | 25 | 23 | 29 | 471 (25) |
| R1 | 5 | 3 | 3 | 4 | 4 | 73 (4) |
| R2 | 5 | 4 | 3 | 5 | 5 | 81 (4) |
| Total non-MDR strains | 625 (33) | |||||
| MDR strains | ||||||
| R3 | 3 | 4 | 5 | 5 | 6 | 87 (5) |
| R4 | 17 | 19 | 14 | 14 | 16 | 304 (16) |
| R5 | 35 | 32 | 32 | 30 | 29 | 596 (32) |
| R6 | 12 | 12 | 19 | 20 | 11 | 278 (15) |
| Total MDR strains | 1265 (67) | |||||
MDR – Multidrug-resistant
Of the total patients infected/colonized with A. baumannii, MDR strains were recovered from 880 patients (66%), with the majority (759; 86%) being admitted to hospitals. Nearly 35% (312/889) of in-patient department patients infected with MDR strains died [Table 3]; however, the exact cause of death was not analyzed in this study.
Table 3.
Association of multidrug-resistant infections and death among in-patient department patients
| Year | In-patient deaths/total number of in-patients, n (%) | Resistance pattern (R0-R6) of dead patients (n) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| R0 | R1 | R2 | R3 | R4 | R5 | R6 | ||
| 2015 | 69/207 (32) | 3 | 3 | 2 | 1 | 17 | 31 | 12 |
| 2016 | 65/171 (38) | 8 | 1 | 0 | 5 | 15 | 23 | 13 |
| 2017 | 71/167 (43) | 2 | 1 | 1 | 5 | 9 | 33 | 20 |
| 2018 | 66/171 (39) | 1 | 1 | 0 | 9 | 15 | 22 | 18 |
| 2019 | 67/168 (40) | 2 | 2 | 2 | 3 | 23 | 27 | 8 |
| Overall | 341/889 (38) | 16 | 8 | 5 | 24 | 79 | 138 | 71 |
DISCUSSION
This study found that in the North-Batinah region of Oman, A. baumannii strains have a high level of resistance to most of the tested antibiotics, with the highest against ceftriaxone (83%) and ceftazidime (75%), and the lowest against colistin (1%) and tigecycline (8%). However, there was no significant variation in the year-wise frequency as well as change in the resistance pattern of the isolates during 2015–2019. In contrast, a study from Iran found that between 2012 and 2017 there was a gradual decrease in the frequency of isolation; however, similar to our study, there was a slight fluctuation in the antibiotic resistance pattern during the study period.[13]
There was no gender-based difference in the frequency of isolation of A. baumannii in the current study, which is in contrast with the findings of previous studies showing a higher frequency of isolation among males.[14,15] Medical and surgical interventions such as urinary and central venous catheterization, mechanical ventilation, prior surgery, etc., are potential risk factors associated with A. baumannii infection.[16,17] In the current study, the majority of the patients had been exposed to one or more of these interventions either in the wards or ICUs.
Patients treated in ICUs are generally immunocompromised, and are exposed to various medical and surgical interventions, thereby rendering them more prone to acquiring infection. In the literature, the frequency of A. baumannii isolation has been reported to be highest among ICU patients compared with other settings.[16,17] In contrast to these studies, the A. baumannii strains in this study were commonly isolated from clinical samples of patients treated in the medical department compared with the intensive care unit. This could be due to significant difference in number of patients who had undergone medical intervention at these two departments. Regarding the site, the frequency of A. baumannii isolation was highest from pus and wound swab (skin and soft tissue specimens) which is consistent with the reports of Oberoi et al. and Sharma et al.[18,19]
The unrestricted use of broad-spectrum cephalosporins in clinical practice due to their low toxicity compared to other drugs and easy availability in community pharmacies has played a key role in the emergence of resistance to these drugs, as also observed in the present study.[20] Studies from Middle Eastern countries have reported that the emergence of cephalosporin-resistant A. baumannii strains in the region is predominantly due to extended beta-lactamase production through acquisition of blaCTX-M, blaGES, blaOXA, and blaNDMβ-lactamase genes.[21,22] In addition, previous studies from Oman have indicated high level increase in resistance to piperacillin-tazobactam in the past 20 years, while relatively lower resistance was reported against aminoglycosides (gentamicin and amikacin) and quinolones.[23] However, resistance rate of A. baumannii to aminoglycosides, quinolones, and trimethoprim-sulfamethoxazole was also significantly high in our study. This is congruent with previous studies conducted worldwide.[24,25] The emergence of high-level resistance could be due to antibiotic pressure resulting from increased prescription of these drugs in recent years in Oman.
Carbapenems such as imipenem and meropenem have been the mainstay in the treatment of A. baumannii infections since the past two decades. However, an overwhelming increasing trend of carbapenem resistance ranging from 50 to 100% reported worldwide is of serious concern because it drastically limits the therapeutic options.[26,27] Outbreaks caused by carbapenem-resistant A. baumannii (CRAB) have been reported worldwide including in the Middle East.[28] The number of studies reporting the emergence of CRAB have increased in the past two decades, indicating widespread dissemination of resistant clones worldwide.[28] The two most noted clones were designated as global clone 1 (GC1) and GC2.[28] In the present study, nearly three-fourth of the A. baumannii strains were resistant to imipenem (72%) and meropenem (70%), denoting an epidemiological concern. Alteration in efflux pump and acquisition of genes encoding for production carbapenem-hydrolyzing oxacillinase are the major resistance mechanisms observed in CRAB.[28,29] Plasmids, transposons, and integrons are the major exchangeable genetic elements that carry resistance genes. Molecular characterization in Middle Eastern region and worldwide revealed blaCTX-M, blaGES, blaOXA, and blaNDM as the most commonly observed genes encoding for carbapenem resistance.[28,29,30] These results raise questions regarding the validity of carbapenems as first-line empirical drugs for treating Acinetobacter infections. Currently, treatment options for CRAB infections are very limited, with colistin and tigecycline as the last remaining options available.[31] The present study results reinforce this, as the A. baumannii strains were highly susceptible to colistin and tigecycline. This also highlights the need to judiciously use these drugs to avoid resistance.
In the current study, we also assessed the extent of drug resistance in A. baumannii isolates. The resistance pattern revealed that 67% of A. baumannii isolates were MDR strains, of which 22% were XDR strains. Most of the MDR strains were isolated from patients hospitalized in ICUs, surgery and medicine department, as they are often exposed to different medical interventions. The findings of the present study were consistent with the global studies, which have reported high prevalence of MDR and XDR strains in hospitalized, especially critically ill, patients.[32,33] In addition, the frequency of death among admitted patients infected with MDR strains was significantly high (91%) compared to those infected with non-MDR strains; however, the exact cause of death was not recorded in the current study, and further studies are required that can determine the mortality rates directly associated with such strains. Another study from Oman has reported significantly high mortality among patients infected with MDR strains compared to non-MDR strains. Additionally, longer length of hospital stays, critical care admission, and the presence of comorbidities have contributed to increased mortality.[34,35,36]
A major limitation of this study is lack of molecular studies (genotyping) and determination of MIC values. Therefore, it is recommended for a prospective study to determine the MIC values and genotyping of A. baumannii strains to identify prevalent drug-resistant clones in Oman.
CONCLUSION
The results of the study suggest high frequency of isolation of MDR A. baumannii isolates in the northern region of Oman. This highlights the need for further strengthening the surveillance to identify potential colonizers and reservoirs of the microorganisms combined with antibiotic stewardship program and for developing highly effective infection control procedures in controlling emergence and spread of drug-resistant strains in hospitals.
Ethical considerations
The study was approved by the institutional Research and Ethical Committee and the Ministry of Health, Oman (Approval no.: MH/DHSG/NBG/1923195718/2019; Date: 30/09/2019). The study adhered to the Declaration of Helsinki, 2013.
Peer review
This article was peer-reviewed by three independent and anonymous reviewers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors express their sincere gratitude to microbiology laboratory and the IT staff of Sohar Hospital for their support for data collection.
REFERENCES
- 1.Antimicrobial Resistance. [Last accessed on 2020 Oct 13]. Available from: https://www.who.int/publications-detail-redirect/global-action-plan-on-antimicrobialresistance .
- 2.Sannathimmappa MB, Nambiar V, Aravindakshan R. A cross-sectional study to evaluate the knowledge and attitude of medical students concerning antibiotic usage and antimicrobial resistance. Int J Acad Med. 2021;7:113–9. [Google Scholar]
- 3.Ayoub Moubareck C, Hammoudi Halat D. Insights into Acinetobacter baumannii: A review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics (Basel) 2020;9:E119. doi: 10.3390/antibiotics9030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halaji M, Rezaei A, Zalipoor M, Faghri J. Investigation of Class I, II, and III integrons among Acinetobacter baumannii isolates from hospitalized patients in Isfahan, Iran. Oman Med J. 2018;33:37–42. doi: 10.5001/omj.2018.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohan BS, Lava R, Nambiar V, Krishnakumar M, Srikrishna R. Multidrug-resistant (MDR) Acinetobacter: A major nosocomial pathogen challenging physicians. Int J Biol Med Res. 2013;4:2726–8. [Google Scholar]
- 6.Kaur A, Gill AK, Singh S. Prevalence and antibiogram of nonfermenting Gram negative bacilli isolates obtained from various clinical samples in a tertiary care hospital, Bathinda, Punjab, India. Int J Res Med Sci. 2018;6:1228–34. [Google Scholar]
- 7.Girija AS, Priyadharsini JV. CLSI based antibiogram profile and the detection of MDR and XDR strains of Acinetobacter baumannii isolated from urine samples. Med J Islam Repub Iran. 2019;33:3. doi: 10.34171/mjiri.33.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assimakopoulos SF, Karamouzos V, Lefkaditi A, Sklavou C, Kolonitsiou F, Christofidou M, et al. Triple combination therapy with high-dose ampicillin/sulbactam, high-dose tigecycline and colistin in the treatment of ventilator-associated pneumonia caused by pan-drug resistant Acinetobacter baumannii: A case series study. Infez Med. 2019;27:11–6. [PubMed] [Google Scholar]
- 9.Clark NM, Zhanel GG, Lynch JP., 3rd Emergence of antimicrobial resistance among Acinetobacter species: A global threat. Curr Opin Crit Care. 2016;22:491–9. doi: 10.1097/MCC.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 10. [Last accessed on 2020 Dec 29]. Oman: Antimicrobial Resistance (AMR) National Action Plan. Available from: https://www.who.int/publications/m/item/omanantimicrobial-resistance-(amr)-national-action-plan .
- 11.Balkhair A, Al-Farsi YM, Al-Muharrmi Z, Al-Rashdi R, Al-Jabri M, Neilson F, et al. Epidemiology of multi-drug resistant organisms in a teaching hospital in Oman: A one-year hospital-based study. ScientificWorldJournal. 2014;2014:157102. doi: 10.1155/2014/157102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. CLSI Supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 13.Rezaee P, Hamzeh A, Mohammadi M. Acinetobacter baumannii antibiotics resistance in Iran. J Bacteriol Mycol Open Access. 2019;7:159–62. [Google Scholar]
- 14.Sharma RK, Mamoria VP. A prospective study on prevalence and antibiotic susceptibility pattern of Acinetobacter baumannii in clinical samples obtained from patients admitted in various wards and intensive care units. J Mahatma Gandhi Univ Med Sci Technol. 2017;2:122–7. [Google Scholar]
- 15.Al-rahmany D, Golchinheydari S, Ghazi IM. Risk factors associated with the mortality of Acinetobacter baumannii. Access Microbiol. 2020;2:7. [Google Scholar]
- 16.Ashuthosh KC, Hegde A, Rao P, Manipura R. Multidrug-resistant Acinetobacter baumannii – The modern menace: A retrospective study in a tertiary hospital in Mangalore. Infect Drug Resist. 2020;13:2181–7. doi: 10.2147/IDR.S249123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abd El-Baky RM, Farhan SM, Ibrahim RA, Mahran KM, Hetta HF. Antimicrobial resistance pattern and molecular epidemiology of ESBL and MBL producing Acinetobacter baumannii isolated from hospitals in Minia, Egypt. Alexandria J Med. 2020;56:4–13. [Google Scholar]
- 18.Oberoi A, Aggarwal A, Lal M. A decade of an underestimated nosocomial pathogen – Acinetobacter in a tertiary care hospital in Punjab. JK Sci. 2009;11:24–6. [Google Scholar]
- 19.Sharma AK, Kumari K, Kumar M, Prasad A. Speciation and antimicrobial resistance pattern of Acinetobacter species from clinical isolates in tertiary care hospital. Int J Med Res Prof. 2018;4:139–43. [Google Scholar]
- 20.Bush K, Bradford PA. β-Lactams and β-Lactamase inhibitors: An overview. Cold Spring Harb Perspect Med. 2016;6:a025247. doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dandachi I, Chaddad A, Hanna J, Matta J, Daoud Z. Understanding the epidemiology of multi-drug resistant gram-negative bacilli in the middle east using a one health approach. Front Microbiol. 2019;10:1941. doi: 10.3389/fmicb.2019.01941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dortet L, Poirel L, Al Yaqoubi F, Nordmann P. NDM-1, OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. Clin Microbiol Infect. 2012;18:E144–8. doi: 10.1111/j.1469-0691.2012.03796.x. [DOI] [PubMed] [Google Scholar]
- 23.Nasser M, Palwe S, Bhargava RN, Feuilloley MG, Kharat AS. Retrospective analysis on antimicrobial resistance trends and prevalence of β-lactamases in Escherichia coli and ESKAPE pathogens isolated from Arabian patients during 2000–2020. Microorganisms. 2020;8:1626. doi: 10.3390/microorganisms8101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boral B, Unaldi Ö, Ergin A, Durmaz R, Eser ÖK Acinetobacter Study Group. A prospective multicenter study on the evaluation of antimicrobial resistance and molecular epidemiology of multidrug-resistant Acinetobacter baumannii infections in intensive care units with clinical and environmental features. Ann Clin Microbiol Antimicrob. 2019;18:19. doi: 10.1186/s12941-019-0319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azimi T, Maham S, Fallah F, Azimi L, Gholinejad Z. Evaluating the antimicrobial resistance patterns among major bacterial pathogens isolated from clinical specimens taken from patients in Mofid Children's Hospital, Tehran, Iran: 2013–2018. Infect Drug Resist. 2019;12:2089–102. doi: 10.2147/IDR.S215329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zilberberg MD, Kollef MH, Shorr AF. Secular trends in Acinetobacter baumannii resistance in respiratory and blood stream specimens in the United States, 2003 to 2012: A survey study. J Hosp Med. 2016;11:21–6. doi: 10.1002/jhm.2477. [DOI] [PubMed] [Google Scholar]
- 27.Tal-Jasper R, Katz DE, Amrami N, Ravid D, Avivi D, Zaidenstein R, et al. Clinical and epidemiological significance of carbapenem resistance in Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2016;60:3127–31. doi: 10.1128/AAC.02656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamidian M, Nigro SJ. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb Genom. 2019;5:e000306. doi: 10.1099/mgen.0.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolain JM, Loucif L, Al-Maslamani M, Elmagboul E, Al-Ansari N, Taj-Aldeen S, et al. Emergence of multidrug-resistant Acinetobacter baumannii producing OXA-23 Carbapenemase in Qatar. New Microbes New Infect. 2016;11:47–51. doi: 10.1016/j.nmni.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcántar-Curiel MD, Rosales-Reyes R, Jarillo-Quijada MD, Gayosso-Vázquez C, Fernández-Vázquez JL, Toledano-Tableros JE, et al. Carbapenem-resistant Acinetobacter baumannii in three tertiary care hospitals in Mexico: Virulence profiles, innate immune response and clonal dissemination. Front Microbiol. 2019;10:1–19. doi: 10.3389/fmicb.2019.02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirmohammadlou N, Zeighami H, Haghi F, Kashefieh M. Resistance pattern and distribution of carbapenemase and antiseptic resistance genes among multidrug-resistant Acinetobacter baumannii isolated from intensive care unit patients. J Med Microbiol. 2018;67:1467–73. doi: 10.1099/jmm.0.000826. [DOI] [PubMed] [Google Scholar]
- 32.Reza H. The frequency of multidrug-resistance and extensively drug-resistant Acinetobacter baumannii in west of Iran. J Clin Microbiol Infect Dis. 2018;1:4–8. [Google Scholar]
- 33.Azizi O, Shahcheraghi F, Salimizand H, Modarresi F, Shakibaie MR, Mansouri SH, et al. Molecular analysis and expression of bap gene in biofilm-forming multi-drug-resistant Acinetobacter baumannii. Rep Biochem Mol Biol. 2016;5:62–72. [PMC free article] [PubMed] [Google Scholar]
- 34.Al Rahmany D, Albeloushi A, Alreesi I, Alzaabi A, Alreesi M, Pontiggia L, et al. Exploring bacterial resistance in Northern Oman, a foundation for implementing evidence-based antimicrobial stewardship program. Int J Infect Dis. 2019;83:77–82. doi: 10.1016/j.ijid.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Alrahmany D, Omar AF, Harb G, El Nekidy WS, Ghazi IM. Acinetobacter baumannii infections in hospitalized patients, treatment outcomes. Antibiotics (Basel) 2021;10:630. doi: 10.3390/antibiotics10060630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sannathimmappa MB, Nambiar V, Aravindakshan R, Al-Kasaby NM. Profile and antibiotic-resistance pattern of bacteria isolated from endotracheal secretions of mechanically ventilated patients at a tertiary care hospital. J Educ Health Promot. 2021;10:195. doi: 10.4103/jehp.jehp_1517_20. [DOI] [PMC free article] [PubMed] [Google Scholar]