Abstract
Cellular senescence, a developmental program central to normal aging and aging pathologies, is robustly regulated at the post-transcriptional level. This regulation involves the interaction of RNA-binding proteins and noncoding RNAs with senescence-associated messenger RNAs (mRNAs). There is increasing evidence that these associations are modulated by chemical modifications of specific mRNA nucleotides which can enhance or reduce the binding of regulatory factors. Recent technological advances in mass spectrometry, next-generation sequencing, and genome mapping have improved markedly the detection of mRNA modifications. Given the rising interest in the epitranscriptomic control of gene expression in aging, we discuss our incipient understanding of the chemical mRNA modifications, specifically m6A and m5C, that influence cellular senescence.
This article is categorized under:
RNA Export and Localization > RNA Localization
RNA Processing > RNA Editing and Modification
Keywords: 5-methylcytosine, aging, mRNA modifications, N6-methyladenosine, senescence
1 |. INTRODUCTION
1.1 |. Cellular senescence
Cellular senescence is a state of permanent growth arrest first identified by Hayflick (1965). Senescence is established in normal tissues in the body following sublethal genomic injury, telomere ablation, oxidative stress, and other types of cell damage, and has been increasingly implicated in aging physiology and pathology. While cellular senescence is beneficial in tissue remodeling, embryonic development, wound healing, and tumor suppression in young persons (Campisi, 2001; Demaria et al., 2014; Prieur & Peeper, 2008; Storer et al., 2013), it has also been linked to the promotion of age-associated functional declines and diseases. Accordingly, in older persons, senescent cells have been implicated in promoting atherosclerosis, liver fibrosis, insulin resistance, Alzheimer’s and Parkinson’s pathologies, chronic obstructive pulmonary disease, age-related chronic inflammation, macular degeneration, and cancer (Aravinthan et al., 2013, 2015; Boccardi, Pelini, Ercolani, Ruggiero, & Mecocci, 2015; Campisi & Robert, 2014; Chinta et al., 2013; Dimri et al., 1995; Ito et al., 2014; Kumar, Seeger, & Voswinckel, 2014).
Cellular senescence is studied in the laboratory using model systems that recapitulate the processes that occur in tissues: damage-induced senescence is triggered by acute stress agents (ionizing radiation, oxidants, chemotherapeutic drugs, hypoxia, etc.), replicative senescence is triggered by proliferative exhaustion in culture, and oncogene-induced senescence is triggered by activated oncogenes. The defining hallmarks of senescence are the presence of β-galactosidase activity at pH 6, the induction of the canonical tumor suppressor pathways TP53(p53)/CDKN1A(p21) and/or CDKN2A(p16)/RB1(RB), and morphological changes such as cell flattening, ambiguous cell borders, and the appearance of vacuoles. Additionally, they exhibit a senescence-associated secretory phenotype (SASP) characterized by the production and secretion of cytokines, chemokines, matrix metalloproteinases, growth factors, and angiogenic factors (Dimri et al., 1995; Kuilman, Michaloglou, Mooi, & Peeper, 2010; Panda, Abdelmohsen, & Gorospe, 2017).
1.2 |. Messenger RNA modifications
The senescence program is tightly controlled to produce proteins that elicit a senescence-associated growth arrest, a stress-response phenotype, morphological changes, and the SASP. These changes in gene expression programs are governed by both transcriptional and post-transcriptional regulators. The transcriptional regulation of senescence is driven by epigenetic modifications in relevant chromatin regions, and by the action of transcriptional regulatory factors including p53, NF-κB, ID1, ID2, E2F, B-MYB, and CEBPβ (Kuilman et al., 2010). Together, they carry out strong and lasting changes in the transcriptional program of senescent cells (Hernandez-Segura et al., 2017).
The post-transcriptional regulation of senescence is carried out by RNA-binding proteins and a diverse group of noncoding regulatory RNAs, including senescence-associated microRNAs and long noncoding RNAs (Abdelmohsen & Gorospe, 2015; Bilsland, Revie, & Keith, 2013; Panda et al., 2017). These factors bind senescence messenger RNAs (mRNAs) forming ribonucleoprotein (mRNP) complexes capable of directing the nuclear processing of precursor (pre-)mRNAs, and the cytoplasmic stability, storage, and translation of mRNAs. Evidence is emerging that the formation of senescence-relevant mRNPs is influenced by specific chemical modifications of the mRNA, in turn affecting mRNA metabolism and protein production, as reviewed here.
1.2.1 |. Post-transcriptional modification of mRNA primary sequences
Pre-mRNA sequences undergo extensive modification before they become mature mRNAs, including 5′-untranslated region (UTR) processing, 3′-UTR processing, alternative splicing, and RNA editing. When nascent pre-mRNA molecules reach up to 30 nucleotides (nt) in length, the 5′ end is often capped with 7′-methylguanosine (m7G) (Venkatesan & Moss, 1982), and most pre-mRNAs are then cleaved at the 3′-UTR and rapidly polyadenylated afterwards, rendering a mature mRNA that contains a 3′ poly(A) tail (E. Wahle & Ruegsegger, 1999). During splicing, pre-mRNA introns are removed and exons are ligated to form a mature mRNA through the action of the spliceosome (Berget, Moore, & Sharp, 1977; Hang, Wan, Yan, & Shi, 2015; M. C. Wahl, Will, & Luhrmann, 2009). RNA can be further edited through the insertion, deletion, or conversion of nucleotides; guanosine-to-adenine (G-to-A) and cytidine-to-uridine (C-to-U) editing have been documented, but the editing most extensively studied on mRNAs is adenosine-to-inosine (A-to-I) nucleotide deamination (Davidson & Shelness, 2000; Nishikura, 2010).
1.2.2 |. Chemical mRNA modifications
Although the first modified RNA nucleotides were identified in the 1950s (Davis & Allen, 1957), only recent technological improvements in mass spectrometry, genome mapping, and next-generation sequencing (NGS) have enabled a precise and systematic characterization of RNA modifications (Helm & Motorin, 2017; Ovcharenko & Rentmeister, 2018; Schaefer, Kapoor, & Jantsch, 2017; Schwartz & Motorin, 2017). As a result, there is resurging interest in identifying RNA modifications that affect mRNP complexes implicated in processes like senescence and aging. Currently, a handful of base-specific modifications have been identified on mRNAs, including N6-methyladenosine (m6A), 5-methylcytidine (m5C), N1-methyladenosine (m1A), and m7G (Table 1) (Limbach, Crain, & McCloskey, 1994; Roundtree, Evans, Pan, & He, 2017; Safra et al., 2017; Schaefer et al., 2017; Venkatesan & Moss, 1982; C. Zhang & Jia, 2018; B. S. Zhao, Roundtree, & He, 2017). Specific proteins known as writers, erasers, and readers work in conjunction to establish, remove, and recognize mRNA modifications, respectively. Chemical modifications of pre-mRNA and mRNA have vast and dynamic regulatory potential because they are inducible and reversible, and because their presence can alter the stability and translation efficiency of mRNAs, in turn affecting the production of key proteins (Ke et al., 2017; Limbach et al., 1994; Roundtree et al., 2017; B. S. Zhao et al., 2017). In this review, we focus on the emerging field of senescence epitranscriptomics by focusing on incipient evidence that nucleotide chemical modification impacts gene expression in cellular senescence.
TABLE 1.
Common modifications of mRNA nucleotides
| Symbol | Description | Initial report in mammals |
|---|---|---|
| m6A | N6-methyladenosine | ✓ |
| Am | 2′-O-methyladenosine | ✓ |
| m6Am | N6,2′-O-dimethyladenosine | ✓ |
| m62Am | N6,N6,2′-O-trimethyladenosine | |
| A-I | Adenosine to inosine | ✓ |
| m5C | 5-Methylcytidine | |
| Cm | 2′-O-methylcytidine | ✓ |
| m7G | 7-Methylguanosine | ✓ |
| Gm | 2′-O-methylguanosine | ✓ |
| m2,7G | N2,7-dimethylguanosine | |
| m2,2,7G | N2,N2,7-trimethylguanosine | |
| Um | 2′-O-methyluridine | ✓ |
| ac4c | N4-acetylcytidine | |
| m1A | N1-methyladenosine | |
| m3Um | 3,2′-O-dimethyluridine | |
| Ψ | Pseudouridine |
Among the most prominent RNA modifications, those that have been linked to senescence thus far are highlighted in bold.
Abbreviation: mRNA, messenger RNA.
2 |. RNA METHYLATION AT m6A IN SENESCENCE
2.1 |. N6-methyladenosine (m6A) writers, erasers, readers
N6-methyladenosine (m6A) is considered one of the most abundant base modifications in eukaryotic mRNA (Bokar, Rath-Shambaugh, Ludwiczak, Narayan, & Rottman, 1994; Desrosiers, Friderici, & Rottman, 1974). Initial studies suggested that methyltransferase-like 3 (METTL3), and 14 (METTL14), Wilms Tumor 1-Associated Protein (WTAP), and KIAA1429 form a multimeric methyltransferase “writer” complex that is responsible for the addition of the methyl group in m6A (Bokar et al., 1994; Bokar, Shambaugh, Polayes, Matera, & Rottman, 1997; Geula et al., 2015; J. Liu et al., 2014; Ping et al., 2014). More recent studies revealed that RBM15, Hakai, and ZC3H13 also interact with WTAP and contribute functionally to establishing m6A modifications (Knuckles et al., 2018; Ruzicka et al., 2017; Y. Yang, Hsu, Chen, & Yang, 2018). Although METTL3 has direct methyltransferase capacity (K. I. Zhou & Pan, 2016), knockdown studies indicate that recruitment of METTL14, WTAP, and KIAA1429 is also necessary for establishing the full in vivo m6A methylation program in mammals (Schwartz et al., 2014; Sledz & Jinek, 2016; P. Wang, Doxtader, & Nam, 2016; X. Wang, Feng, et al., 2016).
Most m6A residues are found within a “GGACU” consensus sequence (J. Liu et al., 2014), which is highly enriched in the 3′-UTRs, near stop codons, and in the last exon (Meyer et al., 2012), suggesting a potential role for m6A in modulating translation efficiency, nuclear export, alternative splicing, mRNA turnover, and microRNA biogenesis and metabolism (Alarcon, Goodarzi, et al., 2015; Alarcon, Lee, Goodarzi, Halberg, & Tavazoie, 2015; Geula et al., 2015; N. Liu et al., 2015; Meyer et al., 2015; X. Wang et al., 2015; X. Zhao et al., 2014; Zheng et al., 2013; J. Zhou et al., 2015). m6A modifications are dynamic and reversible, indicating that they may have important adaptive roles. The AlkB homologue 5 (ALKBH5) is a nuclear demethylase that exhibits m6A demethylation capacity as an “eraser” (Zheng et al., 2013). Several studies have suggested an eraser role of m6A for FTO (Gerken et al., 2007; Vujovic et al., 2013; Wei et al., 2018; X. Zhao et al., 2014), but it has also been suggested that FTO may predominantly demethylate m6Am instead (Mauer et al., 2017).
The m6A modification is recognized specifically by a class of RNA-binding proteins known as “readers”. It is through readers that m6A can affect different cellular processes by interacting with protein complexes to induce signaling events and catalyze enzymatic reactions (Cao, Li, Yin, & Flavell, 2016). The proteins YTHDC1, YTHDF1, YTHDF2, and YTHDF3 have been identified as important m6A readers (Cao et al., 2016; Y. Wang et al., 2014; K. I. Zhou & Pan, 2018). In one example, YTHDF2-induced destabilization of methylated mRNAs was shown to influence stem cell differentiation by regulating the expression of pluripotency factors (Geula et al., 2015; Y. Wang et al., 2014). Indeed, germline Ythdf2−/− knockout mice showed an impaired maternal transcriptome and lower oocyte competence (Ivanova et al., 2017). In another example, J. Zhou et al. (2015) proposed that YTHDF2 may modulate the heat shock response by promoting HSP70 translation through its interaction with the m6A modification in the 5′-UTR of Hsp70 mRNA; however, subsequent studies revealed that m6A modification at the HSP70 5′-UTR enabled the eukaryotic initiation factor 3 (eIF3)-mediated recruitment of the 43S complex and the start of translation independently of eIF4E (Meyer et al., 2015).
In addition, two members of the HNRNP family, HNRNPC and HNRNPG, displayed higher binding affinity to RNA following m6A-induced alterations in RNA structure. In the nucleus, m6A methylation was reported to alter the local RNA structure, increasing the binding of HNRNPC (N. Liu et al., 2015) and HNRNPG (N. Liu et al., 2017), and possibly affecting their actions in alternative splicing. HNRNPA2B1 was suggested to be a nuclear reader of m6A methylation and influenced both microRNA processing and alternative splicing (Alarcon, Goodarzi, et al., 2015). However, it was recently found that HNRNPA2B1 bound to motifs located near m6A modifications due to the higher access enabled by m6A-mediated RNA unfolding (Wu et al., 2018). Thus, HNRNPA2B1 is not itself an m6A reader, but rather responds to the presence of m6A by recognizing local structural changes induced by the chemical modification. Interestingly, m6A has been reported to also prevent the binding of other proteins, as shown for G3BP1, in turn affecting mRNA stability (Edupuganti et al., 2017).
2.2 |. m6A mRNA methylation in senescence
Li et al. (2017) recently reported a link between m6A methylation and cell senescence. Using human cervical carcinoma HeLa cells, the authors showed that METTL3/METTL14-dependent m6A methylation of p21 mRNA increased the levels of p21 protein without affecting total p21 mRNA levels, implicating m6A methylation in controlling the efficiency of p21 translation. Interestingly, the authors found that METTL3/METLL14 elicited m6A modification of p21 mRNA in HCT116 p53−/− cells, indicating that this regulation and the ensuing rise in p21 levels were independent of p53 status. Residues A2044 and A2061 in the p21 3′-UTR were identified as methylation sites. Exposure of HCT116 p53−/− cells to hydrogen peroxide significantly increased the levels of p21 and METTL3/METLL14 and the activity of senescence-associated β-galactosidase [(SA)-β-gal], further supporting a p53-independent role for METTL3/METLL14 in triggering senescence in response to oxidative damage (Figure 1).
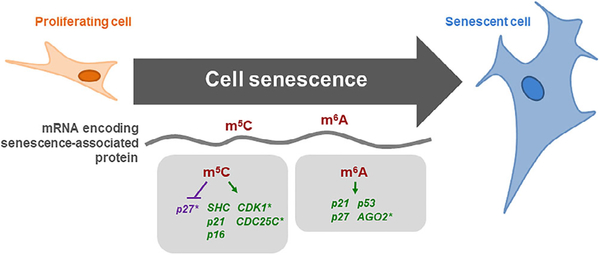
FIGURE 1.
Senescence-associated changes in m6A and m5C modifications. The main modifications reported in mRNAs encoding senescence-associated proteins, m6A and m5C, are indicated (gray rectangles). Each modification can promote (green) or repress (purple) protein expression from the respective mRNAs. *mRNA methylation at m6A and m5C decreases as cells progress towards senescence. mRNA, messenger RNA
Lewinska, Adamczyk-Grochala, Deregowska, and Wnuk (2017) studied the exposure of three different breast cancer cell lines, MCF-7, MDA-MB-231, and SK-BR-3, to sublethal concentrations of sulforaphane (SFN), which triggered cell cycle arrest. Exposure to SFN increased p53, p21, and p27 protein levels, but not the levels of the respective mRNAs, and elevated (SA)-β-gal activity. Interestingly, exposure to SFN caused DNA hypomethylation, decreased global RNA m6A methylation levels, and altered the abundance of many microRNAs. Although the underlying mechanisms are not well understood at present, the authors hypothesized that SFN elicited senescence by lowering global methylation, which increased genetic instability and possibly contributed to cell cycle arrest, in conjunction with reduced m6A RNA levels and modified microRNA pools (Lewinska, Adamczyk-Grochala, Deregowska, et al., 2017).
In another study, Min et al. (2018) showed that global m6A methylation levels decreased in peripheral blood mononuclear cells (PBMCs) of older individuals when compared to younger Caucasian males. The authors found that DROSHA, a specific endoribonuclease that cleaves the 3′ end of pre-microRNA hairpins during the genesis of microRNAs (Slezak-Prochazka, Durmus, Kroesen, & van den Berg, 2010), and AGO2, a major component of the RNA-induced silencing complex (RISC), were both encoded by highly methylated mRNAs in young PBMCs. However, in aged PBMCs AGO2 mRNA methylation selectively decreased, while DROSHA mRNA methylation remained constant. These findings led the authors to suggest that there is selective, age-specific methylation of mRNAs. The decreased AGO2 mRNA methylation in older PBMCs was tentatively linked to lower levels of WTAP, a writer of m6A methylation, as other methyltransferases, demethylases, and m6A-binding proteins were only moderately affected (Min et al., 2018). The fact that AGO2 mRNA methylation is selectively reduced in aged PBMCs further supports the existence of mRNA-specific m6A modifications in aging (Figure 1).
Subsequently, the same authors showed that the hypomethylation of AGO2 mRNA correlated with decreased AGO2 protein levels in proliferating human diploid fibroblasts (HDFs), a model of cell senescence (Min et al., 2018). m6A methylation was lower in both older PBMCs and late-passage HDFs. However, differently from the findings in older PBMCs, late-passage HDFs not only exhibited lower levels of AGO2 mRNAs, but also displayed reduced abundance of DROSHA and DICER1 mRNAs. Reducing AGO2 mRNA methylation at m6A by METTL3 silencing destabilized AGO2 mRNA and decreased the levels of a subset of microRNAs, suggesting that lowering AGO2 impaired the production of specific microRNAs in both old PBMCs and late-passage HDFs. Interestingly, members of let-7 family of microRNAs, previously found to trigger senescence (Peng et al., 2012), were found to be downregulated in both old PBMCs and in HDFs in which MTLL3 or METTL14 were silenced. In summary, m6A methylation of AGO2 mRNA diminished AGO2 protein levels with aging and cell senescence. While the exact m6A writers that mediate AGO2 mRNA methylation in aging are not yet known, the accumulating evidence points to an important role of AGO2 mRNA methylation in aging- and senescence-associated microRNA function (Table 2). At the moment, specific senescence-associated mRNAs influenced by m6A erasers and readers remain to be identified.
TABLE 2.
mRNA modifications influencing senescence and/or age-related changes
| Modification | mRNAs modified (region) | Writers | Processes affected | Impact of modification on senescence and/or aging | Refs. |
|---|---|---|---|---|---|
| m6A | p21 (3′-UTR) | METTL3/METTL14 | Translation ↑ | Senescence ↑ | Li et al. (2017) |
|
p53 (unknown) p21 (unknown) p27 (unknown) |
Unknown | Translation ↑ | Senescence ↑ | Lewinska, Adamczyk-Grochala, Deregowska, et al. (2017) | |
| AGO2 (unknown) | METTL3/WTAP | Translation ↑ (PBMCs) | Aging ↓ | Min et al. (2018) | |
| METTL3 | Stability ↑ (HDFs) | Senescence? | |||
| m5C | p21 (3′-UTR) | NSUN2 | Translation ↑ | Senescence ↑ | Li et al. (2017) |
| p16 (3′-UTR) | NSUN2 | Stability ↑ | Senescence ↑ | Zhang et al. (2012) | |
| CDK1 (3′-UTR) | NSUN2 | Translation ↑ | Senescence ↓ | Xing et al. (2015) | |
| CDC25C | NSUN2 | Translation ↑ | Senescence ↓ | Xing et al. (2015) | |
| p27 (5′-UTR) | NSUN2 | Translation ↓ | Senescence ↓ | Tang et al. (2015) | |
| SHC (5′-UTR,CR,3′-UTR) | NSUN2 | Translation ↑ | Senescence ↑ | Cai et al. (2016) | |
| TERC | Unknown | Telomerase activity↑ | Proliferation↑ | Tang et al. (2018) |
The ribonucleotide modifications (column 1), modified mRNAs and region of the mRNAs that are modified (column 2), enzymes involved (column 3), and consequences on gene regulatory processes (column 4) are indicted. The impact of the modifications on senescence or aging is indicated (column 5). ↑, increased; ↓, decreased.
Abbreviations: mRNA, messenger RNA; UTR, untranslated region.
3 |. RNA METHYLATION AT m5C IN SENESCENCE
3.1 |. 5-Methylcytidine (m5C) writers, erasers, readers
Despite the fact that the 5-methylcytinidine (m5C) modification was first identified in DNA in the 1950s (Davis & Allen, 1957) and in mRNA in the 1970s (Sommer et al., 1976), broad interest in the presence of m5C modifications in mRNA has only emerged relatively recently (Amort et al., 2017; Squires et al., 2012; X. Yang et al., 2017). In general, m5C methylation was found predominantly in noncoding RNAs, in both the 5′-UTR and 3′-UTR of mRNAs, and in the vicinity of AGO-binding regions. Thus far, two prominent m5C mRNA methylation writers have been identified, DNMT2 (DNA methyltransferase 2) and NSUN2 (NOP2/Sun RNA Methyltransferase Family Member 2), although a general RNA motif has not yet been identified (Squires et al., 2012). Although DNMT2 and NSUN2 have been mostly studied in m5C tRNA methylation (Blanco et al., 2011; Brzezicha et al., 2006; Goll et al., 2006; Schaefer et al., 2010; Squires et al., 2012), NSUN2 has also been implicated in directly methylating coding transcripts, as shown for CINP and NAPRT1 mRNAs (Squires et al., 2012). Despite a considerable amount of work done on DNMT2, its exact role in m5C mRNA methylation is not fully understood at present, particularly in light of recent evidence that Dnmt2 silencing in mouse fibroblasts promotes global DNA and RNA hypermethylation (Lewinska, Adamczyk-Grochala, Kwasniewicz, & Wnuk, 2017). While ALYREF was recently identified as an m5C reader (X. Yang et al., 2017), the lack of a more comprehensive knowledge of m5C writers, erases, and readers adds to the challenges of elucidating the mechanisms that govern m5C modification in mRNA.
3.2 |. m5C mRNA methylation in senescence
Although relatively less is known about the enzymology of m5C mRNA methylation as compared to m6A mRNA methylation, m5C mRNA modification has also been shown to modulate cellular senescence (Figure 1). Similar to the induction of senescence by m6A-regulated rise in p21 levels independently of p53, NSUN2-mediated m5C methylation of p21 mRNA at C2079 (a residue in the p21 3′-UTR) also contributed to enhancing p21 protein abundance (Li et al., 2017); in fact, m6A and m5C modifications of p21 mRNA synergistically elevated the levels of p21 protein, which in turn promoted senescence (Li et al., 2017). In addition, NSUN2 also increased p16 expression levels by methylating the p16 3′-UTR, thereby stabilizing p16 mRNA (X. Zhang et al., 2012), even though the specific m5C site was not identified. These results further implicate NSUN2 in the induction of senescence via both the p53/p21 and the p16/RB pathways.
The role of NSUN2 in senescence is nonetheless complex, as there is evidence that NSUN2 can both promote and suppress senescence depending on the substrate mRNA. Xing et al. (2015) reported that NSUN2 affected the production levels of CDK1, p27, and CDC25C in different ways. NSUN2 was first reported to promote cell growth and delay replicative senescence by elevating CDK1 protein levels. In HeLa cells, Xing et al. found that NSUN2 raised m5C methylation of the CDK1 3′-UTR, increasing its presence in polysomes and its translation efficiency, eventually elevating CDK1 protein levels. Moreover, NSUN2-dependent methylation of CDK1 mRNA increased its translation in a cell cycle-dependent manner, peaking during the S and G2/M cell cycle phases (Xing et al., 2015). Interestingly, the same authors subsequently discovered that NSUN2 further delayed replicative senescence (Tang et al., 2015) by methylating the C64 residue at the 5′-UTR of p27 mRNA (without affecting total p27 mRNA levels) and reducing p27 translation. In senescent IDH4 fibroblasts (Shay, West, & Wright, 1992), the authors observed decreased levels of NSUN2, CKD1, and CDC25C proteins, while the levels of p27 and p21 proteins increased. Similarly, in human diploid 2BS fibroblasts expressing reduced levels of NSUN2 through shRNA-mediated silencing, p27 protein levels increased, while CKD1 and CDC25C protein levels decreased. As expected, NSUN2 knockout promoted growth arrest and increased in (SA)-β-gal production(Tang et al., 2015). In line with previous observations in HeLa cells, methylation of CKD1 and p27 mRNAs was diminished in replicative senescence, further linking NSUN2-induced m5C methylation to senescence and aging.
Paradoxically, later reports showed that in human umbilical vein endothelial cells (HUVECs), NSUN2 instead promoted senescence by increasing the translation and production of SHC (Cai et al., 2016), a family of proteins that function as adaptors in the Ras intracellular signaling cascade. Among the three isoforms of SHC, p66SHC in particular enhanced senescence by promoting the formation of reactive oxygen species (ROS), while at the same time its expression correlated negatively with mammalian longevity (Luzi, Confalonieri, Di Fiore, & Pelicci, 2000; Xu et al., 2014; W. Zhang et al., 2010). NSUN2 methylated SHC mRNA in multiple sites—the 5′-UTR, the coding region (CR), and the 3′-UTR—both in vivo and in vitro. Methylation of SHC mRNA by NSUN2 enhanced SHC translation leading to the activation of p38MAPK, G1 cell cycle arrest, and increased ROS levels, implicating NSUN2 in the induction of oxidative stress-induced senescence. Furthermore, by increasing SHC mRNA methylation, NSUN2 increased SHC production in a p53-independent manner, playing a role in HUVEC oxidative stress and premature senescence. Similar observations were reported in high glucose-induced premature senescence in HUVEC cells. However, since replicatively senescent cells had low levels of NSUN2 and high levels of SHC, the authors proposed that NSUN2 may not regulate SHC levels in replicative senescence, but likely played a different modulatory role in response to oxidant damage (Cai et al., 2016).
Replicative senescence is characterized by telomere shortening (Nelson, McBryan, Jeyapalan, Sedivy, & Adams, 2014). Recently, m5C methylation of TERC, a noncoding RNA that forms part of the telomerase holoenzyme, was shown to promote the complexing of TERC with the protein telomerase (TERT). Given that the RNA-binding protein HuR increases the interaction between TERT-TERC and thereby helps to maintain telomere length (Tang et al., 2018), the reduction of HuR levels in senescent cells may help explain the loss of telomerase activity with senescence. Understanding the contribution of mRNA methylation on the integrity of telomeres will shed further light into the role of chemical mRNA modifications in replicative senescence.
The role of DNMT2 in senescence is even less well understood. Lewinska, Adamczyk-Grochala, Kwasniewicz, et al. (2017) reported that Dnmt2 gene ablation may help mediate cellular senescence in mouse fibroblasts. Compared with WT mice, Dnmt2-null mice displayed several senescence-associated traits, such as inhibition of cell proliferation via elevated p53 and p21 protein levels, moderately shorter telomeres, ROS accumulation, genomic instability, and increased (SA)-β-gal production. Interestingly, reducing DNMT2 levels promoted global DNA and RNA hypermethylation and increased the levels of other methyltransferases including DNMT1, DNMT3a, and DNMT3b. Thus, the previously suggested role of DNMT2 as writer of m5C for tRNA and mRNA (Dev et al., 2017; Khoddami & Cairns, 2013) seems to be more complex in aging, as suggested by the finding of RNA and DNA hypermethylation in Dnmt2-silenced cells. In a related study, silencing DNMT2 in human WI-38 and BJ fibroblasts rendered them more sensitive to oxidative stress, inhibited cell proliferation, and led to the differential upregulation of many microRNAs related to proliferation and tumorigenesis (Lewinska et al., 2018). However, at this time the specific mRNA substrates methylated at m5C by DNMT2 that influence the senescent phenotype are not known.
4 |. CONCLUSIONS AND PERSPECTIVES
It is well established that epitranscriptomic changes, including chemical modifications of RNA, are key regulators of gene expression programs RNA and cell physiology. Early reports identified many chemical base modifications in tRNAs, while m6A and m5C were found to be among the most prominent chemical modifications in mRNAs (Davis & Allen, 1957; Roundtree et al., 2017; Schaefer et al., 2017; B. S. Zhao et al., 2017). However, systematic studies of mRNA base modifications have only been possible thanks to the recent technological improvements in mass spectrometry, NGS, and genome mapping (Schaefer et al., 2017). Ongoing research on mRNA modifications is predominantly focused on the contribution of mRNA methylation and editing in cancer, obesity, and other age-associated pathologies (Batista, 2017; Cao et al., 2016; Deng et al., 2018). Indeed, a growing body of evidence indicates that mRNA modifications may contribute to physiological aging and diseases of aging.
NGS-based techniques, currently the best approaches to detect mRNA methylation, require PCR amplification and digestion of the amplified fragments for library preparation and analysis (Schwartz & Motorin, 2017). The recent development of third-generation (or long-read) sequencing technologies, including those by Pacific Biosciences, Oxford Nanopore, Quantapore, and Stratos, is expected to increase the ease of identifying mRNA modifications. Nanopore sequencing, which was first developed to measure RNA modifications on the 16S rRNA of Escherichia coli (Smith, Jain, Mulroney, Garalde, & Akeson, 2017), holds great promise as a technology to identify mRNA methylation on individual transcripts (Garalde et al., 2018) as this field progresses.
As reviewed here, two common chemical mRNA modifications, m6A and m5C, can modulate senescence in different cell culture models through various mechanisms. Writers of methylation at m6A (MELLT3/MELLT14 and WTAP), and m5C (NSUN2), have been shown to regulate the levels of senescence-associated proteins p21 and p27 by methylating p21 and p27 mRNAs (Lewinska, Adamczyk-Grochala, Deregowska, et al., 2017; Li et al., 2017; Tang et al., 2015). Interestingly, p21 protein levels were synergistically increased by methylation of p21 mRNA at m6A and m5C (Li et al., 2017). Furthermore, NSUN2 was reported to increase SHC levels, thereby contributing to premature senescence following exposure to oxidative stress or high glucose (Cai et al., 2016). mRNA methylation was also reported to regulate the expression of a subset of cyclins and cyclin-dependent kinases, in turn triggering cell cycle arrest and promoting senescence (Lewinska, Adamczyk-Grochala, Deregowska, et al., 2017; Xing et al., 2015). In addition, the methylation-induced degradation of AGO2 mRNA was found to affect the differential expression of many microRNAs and further played a role in promoting senescence (Min et al., 2018). Moreover, Dnmt2-null mouse fibroblasts were found to have enhanced global m5C mRNA methylation, accompanied by the presence of several senescence-associated hallmarks, such as high levels of p53 and p21, shorter telomeres, and elevated (SA)-β-gal activity (Lewinska, Adamczyk-Grochala, Kwasniewicz, et al., 2017). Given the accumulating evidence of m6A and m5C methylation of mRNAs encoding key senescence-regulatory factors, a comprehensive analysis of mRNA methylation in different senescent models is needed. Genetic animal models will also be essential for investigating the full impact of senescence-associated mRNA methylation in vivo and on aging physiology.
Finally, while other epitranscriptomic changes, such as nucleotide conversions (C-to-U, G-to-A, and A-to-I) have not been studied in the context of senescence, dysregulated A-to-I editing has been linked to pathologies that increase in aging, including amyotrophic lateral sclerosis, glioblastoma multiforme, hepatocellular carcinoma, type 2 diabetes mellitus, and transient forebrain ischemia (Slotkin & Nishikura, 2013). In A-to-I editing, adenosine undergoes a conversion to inosine through the deamination of the C6 carbon of adenine catalyzed by the ADAR (adenosine deaminase acting on RNA) family of proteins (Nishikura, 2010), changing the sequence of the translated protein, since inosine behaves chemically similar to guanosine. A-to-I editing is also involved in many other processes such as post-transcriptional regulation in organ development, innate immunity, and autoimmunity (Mannion et al., 2014; Pestal et al., 2015; Stellos et al., 2016). Nicholas et al. examined two transcripts that undergo A-to-I editing, CYFIP2 and GABRA3 mRNAs, in 25 frontal cortex tissue samples from donors ranging between 22 and 103 years of age. They found that only CYFIP2 mRNA A-to-I editing decreased with age, indicating that mRNA A-to-I editing decreased selectively for certain transcripts and proposed that this selectivity might arise from the differential expression of ADAR family members with age (Nicholas et al., 2010). Whether nucleotide conversions occur during senescence remains to be investigated.
In closing, thanks to the development of improved animal models and detection technologies, our knowledge of the influence of RNA metabolism on physiology and disease is increasing rapidly. The same tools will be instrumental in current efforts to advance our understanding of the RNA modifications that drive cellular senescence and aging.
ACKNOWLEDGMENT
This work was supported in its entirety by the National Institute on Aging Intramural Research Program, NIH (AG000393–11).
Funding information
National Institute on Aging, NIH, Grant/Award Number: AG000393-11
Footnotes
RELATED WIREs ARTICLES
The dynamic RNA modification 5-methylcytosine and its emerging role as an epitranscriptomic mark. mRNA methylation by NSUN2 in cell proliferation.
Dynamic and reversible RNA N6 -methyladenosine methylation.
Further Reading
Tuorto, F., Liebers, R., Musch, T., Schaefer, M., Hofmann, S., Kellner, S., … Lyko, F. (2012). RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nature Structural & Molecular Biology, 19(9), 900–905. https://doi.org/10.1038/nsmb.2357
REFERENCES
- Abdelmohsen K, & Gorospe M (2015). Noncoding RNA control of cellular senescence. WIREs RNA, 6(6), 615–629. 10.1002/wrna.1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, & Tavazoie SF (2015). HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell, 162(6), 1299–1308. 10.1016/j.cell.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon CR, Lee H, Goodarzi H, Halberg N, & Tavazoie SF (2015). N6-methyladenosine marks primary microRNAs for processing. Nature, 519(7544), 482–485. 10.1038/nature14281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amort T, Rieder D, Wille A, Khokhlova-Cubberley D, Riml C, Trixl L, … Lusser A (2017). Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biology, 18(1), 1. 10.1186/s13059-016-1139-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravinthan A, Challis B, Shannon N, Hoare M, Heaney J, & Alexander GJ (2015). Selective insulin resistance in hepatocyte senescence. Experimental Cell Research, 331(1), 38–45. 10.1016/j.yexcr.2014.09.025 [DOI] [PubMed] [Google Scholar]
- Aravinthan A, Pietrosi G, Hoare M, Jupp J, Marshall A, Verrill C, … Alexander GJ (2013). Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. PLoS One, 8(9), e72904. 10.1371/journal.pone.0072904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ (2017). The RNA modification N(6)-methyladenosine and its implications in human disease. Genomics, Proteomics, & Bioinformatics, 15(3), 154–163. 10.1016/j.gpb.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget SM, Moore C, & Sharp PA (1977). Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proceedings of the National Academy of Sciences of the United States of America, 74(8), 3171–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland AE, Revie J, & Keith W (2013). MicroRNA and senescence: The senectome, integration and distributed control. Critical Reviews in Oncogenesis, 18(4), 373–390. [DOI] [PubMed] [Google Scholar]
- Blanco S, Kurowski A, Nichols J, Watt FM, Benitah SA, & Frye M (2011). The RNA-methyltransferase Misu (NSun2) poises epidermal stem cells to differentiate. PLoS Genetics, 7(12), e1002403. 10.1371/journal.pgen.1002403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi V, Pelini L, Ercolani S, Ruggiero C, & Mecocci P (2015). From cellular senescence to Alzheimer’s disease: The role of telomere shortening. Ageing Research Reviews, 22, 1–8. 10.1016/j.arr.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Bokar JA, Rath-Shambaugh ME, Ludwiczak R, Narayan P, & Rottman F (1994). Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. The Journal of Biological Chemistry, 269(26), 17697–17704. [PubMed] [Google Scholar]
- Bokar JA, Shambaugh ME, Polayes D, Matera AG, & Rottman FM (1997). Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA, 3(11), 1233–1247. [PMC free article] [PubMed] [Google Scholar]
- Brzezicha B, Schmidt M, Makalowska I, Jarmolowski A, Pienkowska J, & Szweykowska-Kulinska Z (2006). Identification of human tRNA: m5C methyltransferase catalysing intron-dependent m5C formation in the first position of the anticodon of the pre-tRNA Leu (CAA). Nucleic Acids Research, 34(20), 6034–6043. 10.1093/nar/gkl765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Hu Y, Tang H, Hu H, Pang L, Xing J, … Wang W (2016). RNA methyltransferase NSUN2 promotes stress-induced HUVEC senescence. Oncotarget, 7(15), 19099–19110. 10.18632/oncotarget.8087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J (2001). Cellular senescence as a tumor-suppressor mechanism. Trends in Cell Biology, 11(11), S27–S31. [DOI] [PubMed] [Google Scholar]
- Campisi J, & Robert L (2014). Cell senescence: Role in aging and age-related diseases. Interdisciplinary Topics in Gerontology, 39, 45–61. 10.1159/000358899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Li HB, Yin Z, & Flavell RA (2016). Recent advances in dynamic m6A RNA modification. Open Biology, 6(4), 160003. 10.1098/rsob.160003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Lieu CA, Demaria M, Laberge RM, Campisi J, & Andersen JK (2013). Environmental stress, ageing and glial cell senescence: A novel mechanistic link to Parkinson’s disease? Journal of Internal Medicine, 273(5), 429–436. 10.1111/joim.12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson NO, & Shelness GS (2000). APOLIPOPROTEIN B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annual Review of Nutrition, 20, 169–193. 10.1146/annurev.nutr.20.1.169 [DOI] [PubMed] [Google Scholar]
- Davis FF, & Allen FW (1957). Ribonucleic acids from yeast which contain a fifth nucleotide. The Journal of Biological Chemistry, 227(2), 907–915. [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, … Campisi J (2014). An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental Cell, 31(6), 722–733. 10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Su R, Weng H, Huang H, Li Z, & Chen J (2018). RNA N(6)-methyladenosine modification in cancers: Current status and perspectives. Cell Research, 28(5), 507–517. 10.1038/s41422-018-0034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R, Friderici K, & Rottman F (1974). Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proceedings of the National Academy of Sciences of the United States of America, 71(10), 3971–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev RR, Ganji R, Singh SP, Mahalingam S, Banerjee S, & Khosla S (2017). Cytosine methylation by DNMT2 facilitates stability and survival of HIV-1 RNA in the host cell during infection. The Biochemical Journal, 474(12), 2009–2026. 10.1042/BCJ20170258 [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, … Pereira-Smith O (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America, 92(20), 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, Lu Z, … Vermeulen M (2017). N(6)-methyladenosine (m(6)A) recruits and repels proteins to regulate mRNA homeostasis. Nature Structural & Molecular Biology, 24(10), 870–878. 10.1038/nsmb.3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garalde DR, Snell EA, Jachimowicz D, Sipos B, Lloyd JH, Bruce M, … Turner DJ (2018). Highly parallel direct RNA sequencing on an array of nanopores. Nature Methods, 15(3), 201–206. 10.1038/nmeth.4577 [DOI] [PubMed] [Google Scholar]
- Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, … Schofield CJ (2007). The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science, 318(5855), 1469–1472. 10.1126/science.1151710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, … Hanna JH (2015). Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science, 347(6225), 1002–1006. 10.1126/science.1261417 [DOI] [PubMed] [Google Scholar]
- Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, … Bestor TH (2006). Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science, 311(5759), 395–398. 10.1126/science.1120976 [DOI] [PubMed] [Google Scholar]
- Hang J, Wan R, Yan C, & Shi Y (2015). Structural basis of pre-mRNA splicing. Science, 349(6253), 1191–1198. 10.1126/science.aac8159 [DOI] [PubMed] [Google Scholar]
- Hayflick L (1965). The limited in vitro lifetime of human diploid cell strains. Experimental Cell Research, 37, 614–636. [DOI] [PubMed] [Google Scholar]
- Helm M, & Motorin Y (2017). Detecting RNA modifications in the epitranscriptome: Predict and validate. Nature Reviews Genetics, 18(5), 275–291. 10.1038/nrg.2016.169 [DOI] [PubMed] [Google Scholar]
- Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, & Demaria M (2017). Unmasking transcriptional heterogeneity in senescent cells. Current Biology, 27(17), 2652–2660. 10.1016/j.cub.2017.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito TK, Yokoyama M, Yoshida Y, Nojima A, Kassai H, Oishi K, … Minamino T (2014). A crucial role for CDC42 in senescence-associated inflammation and atherosclerosis. PLoS One, 9(7), e102186. 10.1371/journal.pone.0102186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova I, Much C, Di Giacomo M, Azzi C, Morgan M, Moreira PN, … O’Carroll D (2017). The RNA m(6)A reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Molecular Cell, 67(6), 1059–1067. 10.1016/j.molcel.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Pandya-Jones A, Saito Y, Fak JJ, Vagbo CB, Geula S, … Darnell RB (2017). m(6)A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes & Development, 31(10), 990–1006. 10.1101/gad.301036.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoddami V, & Cairns BR (2013). Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nature Biotechnology, 31(5), 458–464. 10.1038/nbt.2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, … Roignant JY (2018). Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes & Development, 32 (5–6), 415–429. 10.1101/gad.309146.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, & Peeper DS (2010). The essence of senescence. Genes & Development, 24(22), 2463–2479. 10.1101/gad.1971610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Seeger W, & Voswinckel R (2014). Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. American Journal of Respiratory Cell and Molecular Biology, 51(3), 323–333. 10.1165/rcmb.2013-0382PS [DOI] [PubMed] [Google Scholar]
- Lewinska A, Adamczyk-Grochala J, Deregowska A, & Wnuk M (2017). Sulforaphane-induced cell cycle arrest and senescence are accompanied by DNA hypomethylation and changes in microRNA profile in breast cancer cells. Theranostics, 7(14), 3461–3477. 10.7150/thno.20657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinska A, Adamczyk-Grochala J, Kwasniewicz E, Deregowska A, Semik E, Zabek T, & Wnuk M (2018). Reduced levels of methyltransferase DNMT2 sensitize human fibroblasts to oxidative stress and DNA damage that is accompanied by changes in proliferation-related miRNA expression. Redox Biology, 14, 20–34. 10.1016/j.redox.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinska A, Adamczyk-Grochala J, Kwasniewicz E, & Wnuk M (2017). Downregulation of methyltransferase Dnmt2 results in condition-dependent telomere shortening and senescence or apoptosis in mouse fibroblasts. Journal of Cellular Physiology, 232(12), 3714–3726. 10.1002/jcp.25848 [DOI] [PubMed] [Google Scholar]
- Li Q, Li X, Tang H, Jiang B, Dou Y, Gorospe M, & Wang W (2017). NSUN2-mediated m5C methylation and METTL3/METTL14-mediated m6A methylation cooperatively enhance p21 translation. Journal of Cellular Biochemistry, 118(9), 2587–2598. 10.1002/jcb.25957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbach PA, Crain PF, & McCloskey JA (1994). Summary: The modified nucleosides of RNA. Nucleic Acids Research, 22(12), 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, … He C (2014). A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nature Chemical Biology, 10(2), 93–95. 10.1038/nchembio.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Dai Q, Zheng G, He C, Parisien M, & Pan T (2015). N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature, 518(7540), 560–564. 10.1038/nature14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, & Pan T (2017). N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Research, 45(10), 6051–6063. 10.1093/nar/gkx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi L, Confalonieri S, Di Fiore PP, & Pelicci PG (2000). Evolution of Shc functions from nematode to human. Current Opinion in Genetics & Development, 10(6), 668–674. [DOI] [PubMed] [Google Scholar]
- Mannion NM, Greenwood SM, Young R, Cox S, Brindle J, Read D, … O’Connell MA (2014). The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Reports, 9(4), 1482–1494. 10.1016/j.celrep.2014.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, … Jaffrey SR (2017). Reversible methylation of m(6)Am in the 5′ cap controls mRNA stability. Nature, 541(7637), 371–375. 10.1038/nature21022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, … Jaffrey SR (2015). 5′ UTR m(6)A promotes cap-independent translation. Cell, 163(4), 999–1010. 10.1016/j.cell.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, & Jaffrey SR (2012). Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell, 149(7), 1635–1646. 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KW, Zealy RW, Davila S, Fomin M, Cummings JC, Makowsky D, … Yoon JH (2018). Profiling of m6A RNA modifications identified an age-associated regulation of AGO2 mRNA stability. Aging Cell, 17(3), e12753. 10.1111/acel.12753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DM, McBryan T, Jeyapalan JC, Sedivy JM, & Adams PD (2014). A comparison of oncogene-induced senescence and replicative senescence: Implications for tumor suppression and aging. Age, 36(3), 9637. 10.1007/s11357-014-9637-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas A, de Magalhaes JP, Kraytsberg Y, Richfield EK, Levanon EY, & Khrapko K (2010). Age-related gene-specific changes of A-to-I mRNA editing in the human brain. Mechanisms of Ageing and Development, 131(6), 445–447. 10.1016/j.mad.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K (2010). Functions and regulation of RNA editing by ADAR deaminases. Annual Review of Biochemistry, 79, 321–349. 10.1146/annurev-biochem-060208-105251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovcharenko A, & Rentmeister A (2018). Emerging approaches for detection of methylation sites in RNA. Open Biology, 8(9). 10.1098/rsob.180121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda AC, Abdelmohsen K, & Gorospe M (2017). SASP regulation by noncoding RNA. Mechanisms of Ageing and Development, 168, 37–43. 10.1016/j.mad.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CH, Liu JH, Woung LC, Lin TJ, Chiou SH, Tseng PC, … Chen SJ (2012). MicroRNAs and cataracts: Correlation among let-7 expression, age and the severity of lens opacity. The British Journal of Ophthalmology, 96(5), 747–751. 10.1136/bjophthalmol-2011-300585 [DOI] [PubMed] [Google Scholar]
- Pestal K, Funk CC, Snyder JM, Price ND, Treuting PM, & Stetson DB (2015). Isoforms of RNA-editing enzyme ADAR1 independently control nucleic acid sensor MDA5-driven autoimmunity and multi-organ development. Immunity, 43(5), 933–944. 10.1016/j.immuni.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, … Yang YG (2014). Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Research, 24(2), 177–189. 10.1038/cr.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur A, & Peeper DS (2008). Cellular senescence in vivo: A barrier to tumorigenesis. Current Opinion in Cell Biology, 20(2), 150–155. 10.1016/j.ceb.2008.01.007 [DOI] [PubMed] [Google Scholar]
- Roundtree IA, Evans ME, Pan T, & He C (2017). Dynamic RNA modifications in gene expression regulation. Cell, 169(7), 1187–1200. 10.1016/j.cell.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka K, Zhang M, Campilho A, Bodi Z, Kashif M, Saleh M, … Fray RG (2017). Identification of factors required for m(6) A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. The New Phytologist, 215(1), 157–172. 10.1111/nph.14586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safra M, Sas-Chen A, Nir R, Winkler R, Nachshon A, Bar-Yaacov D, … Schwartz S (2017). The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature, 551(7679), 251–255. 10.1038/nature24456 [DOI] [PubMed] [Google Scholar]
- Schaefer M, Kapoor U, & Jantsch MF (2017). Understanding RNA modifications: The promises and technological bottlenecks of the ‘epitranscriptome’. Open Biology, 7(5). 10.1098/rsob.170077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Pollex T, Hanna K, Tuorto F, Meusburger M, Helm M, & Lyko F (2010). RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes & Development, 24(15), 1590–1595. 10.1101/gad.586710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, & Motorin Y (2017). Next-generation sequencing technologies for detection of modified nucleotides in RNAs. RNA Biology, 14(9), 1124–1137. 10.1080/15476286.2016.1251543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, … Regev A (2014). Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Reports, 8(1), 284–296. 10.1016/j.celrep.2014.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, West MD, & Wright WE (1992). Re-expression of senescent markers in deinduced reversibly immortalized cells. Experimental Gerontology, 27(5–6), 477–492. [DOI] [PubMed] [Google Scholar]
- Sledz P, & Jinek M (2016). Structural insights into the molecular mechanism of the m(6)A writer complex. eLife, 5. 10.7554/eLife.18434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak-Prochazka I, Durmus S, Kroesen BJ, & van den Berg A (2010). MicroRNAs, macrocontrol: Regulation of miRNA processing. RNA, 16(6), 1087–1095. 10.1261/rna.1804410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin W, & Nishikura K (2013). Adenosine-to-inosine RNA editing and human disease. Genome Medicine, 5(11), 105. 10.1186/gm508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Jain M, Mulroney L, Garalde DR, & Akeson M (2017). Reading canonical and modified nucleotides in 16S ribosomal RNA using nanopore direct RNA sequencing. bioRxiv. 10.1101/132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S, Salditt-Georgieff M, Bachenheimer S, Darnell JE, Furuichi Y, Morgan M, & Shatkin AJ (1976). The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Research, 3(3), 749–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, … Preiss T (2012). Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Research, 40(11), 5023–5033. 10.1093/nar/gks144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellos K, Gatsiou A, Stamatelopoulos K, Perisic Matic L, John D, Lunella FF, … Dimmeler S (2016). Adenosine-to-inosine RNA editing controls cathepsin S expression in atherosclerosis by enabling HuR-mediated post-transcriptional regulation. Nature Medicine, 22(10), 1140–1150. 10.1038/nm.4172 [DOI] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, … Keyes WM (2013). Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell, 155(5), 1119–1130. 10.1016/j.cell.2013.10.041 [DOI] [PubMed] [Google Scholar]
- Tang H, Fan X, Xing J, Liu Z, Jiang B, Dou Y, … Wang W (2015). NSun2 delays replicative senescence by repressing p27 (KIP1) translation and elevating CDK1 translation. Aging, 7(12), 1143–1158. 10.18632/aging.100860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Wang H, Cheng X, Fan X, Yang F, Zhang M, … Wang W (2018). HuR regulates telomerase activity through TERC methylation. Nature Communications, 9(1), 2213. 10.1038/s41467-018-04617-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S, & Moss B (1982). Eukaryotic mRNA capping enzyme-guanylate covalent intermediate. Proceedings of the National Academy of Sciences of the United States of America, 79(2), 340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujovic P, Stamenkovic S, Jasnic N, Lakic I, Djurasevic SF, Cvijic G, & Djordjevic J (2013). Fasting induced cytoplasmic FTO expression in some neurons of rat hypothalamus. PLoS One, 8(5), e63694. 10.1371/journal.pone.0063694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl MC, Will CL, & Luhrmann R (2009). The spliceosome: Design principles of a dynamic RNP machine. Cell, 136(4), 701–718. 10.1016/j.cell.2009.02.009 [DOI] [PubMed] [Google Scholar]
- Wahle E, & Ruegsegger U (1999). 3′-End processing of pre-mRNA in eukaryotes. FEMS Microbiology Reviews, 23(3), 277–295. 10.1111/j.1574-6976.1999.tb00400.x [DOI] [PubMed] [Google Scholar]
- Wang P, Doxtader KA, & Nam Y (2016). Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Molecular Cell, 63(2), 306–317. 10.1016/j.molcel.2016.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, … Yin P (2016). Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature, 534(7608), 575–578. 10.1038/nature18298 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, … He C (2015). N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell, 161(6), 1388–1399. 10.1016/j.cell.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, & Zhao JC (2014). N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nature Cell Biology, 16(2), 191–198. 10.1038/ncb2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Liu F, Lu Z, Fei Q, Ai Y, He PC, … He C (2018). Differential m(6)A, m(6)Am, and m(1)A demethylation mediated by FTO in the cell nucleus and cytoplasm. Molecular Cell, 71(6), 973–985. 10.1016/j.molcel.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Su S, Patil DP, Liu H, Gan J, Jaffrey SR, & Ma J (2018). Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nature Communications, 9(1), 420. 10.1038/s41467-017-02770-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Yi J, Cai X, Tang H, Liu Z, Zhang X, … Wang W (2015). NSun2 promotes cell growth via elevating cyclin-dependent kinase 1 translation. Molecular and Cellular Biology, 35(23), 4043–4052. 10.1128/MCB.00742-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Pang L, Cai X, Liu X, Yuan S, Fan X, … Wang W (2014). Let-7-repressesed Shc translation delays replicative senescence. Aging Cell, 13(1), 185–192. 10.1111/acel.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yang Y, Sun BF, Chen YS, Xu JW, Lai WY, … Yang YG (2017). 5-Methylcytosine promotes mRNA export – NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Research, 27(5), 606–625. 10.1038/cr.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hsu PJ, Chen YS, & Yang YG (2018). Dynamic transcriptomic m(6)A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Research, 28(6), 616–624. 10.1038/s41422-018-0040-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, & Jia G (2018). Reversible RNA modification N(1)-methyladenosine (m(1)A) in mRNA and tRNA. Genomics, Proteomics, & Bioinformatics, 16(3), 155–161. 10.1016/j.gpb.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ji W, Yang L, Xu Y, Yang J, & Zhuang Z (2010). Epigenetic enhancement of p66Shc during cellular replicative or premature senescence. Toxicology, 278(2), 189–194. 10.1016/j.tox.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu Z, Yi J, Tang H, Xing J, Yu M, … Wang W (2012). The tRNA methyltransferase NSun2 stabilizes p16INK(4) mRNA by methylating the 3′-untranslated region of p16. Nature Communications, 3, 712. 10.1038/ncomms1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BS, Roundtree IA, & He C (2017). Post-transcriptional gene regulation by mRNA modifications. Nature Reviews Molecular Cell Biology, 18(1), 31–42. 10.1038/nrm.2016.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, … Yang YG (2014). FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Research, 24(12), 1403–1419. 10.1038/cr.2014.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, … He C (2013). ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Molecular Cell, 49(1), 18–29. 10.1016/j.molcel.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, & Qian SB (2015). Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature, 526(7574), 591–594. 10.1038/nature15377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KI, & Pan T (2016). Structures of the m(6)A methyltransferase complex: Two subunits with distinct but coordinated roles. Molecular Cell, 63(2), 183–185. 10.1016/j.molcel.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KI, & Pan T (2018). An additional class of m(6)A readers. Nature Cell Biology, 20(3), 230–232. 10.1038/s41556-018-0046-y [DOI] [PMC free article] [PubMed] [Google Scholar]