Abstract
In budding yeast (Saccharomyces cerevisiae), the majority of box H/ACA small nucleolar RNPs (snoRNPs) have been shown to direct site-specific pseudouridylation of rRNA. Among the known protein components of H/ACA snoRNPs, the essential nucleolar protein Cbf5p is the most likely pseudouridine (Ψ) synthase. Cbf5p has considerable sequence similarity to Escherichia coli TruBp, a known Ψ synthase, and shares the “KP” and “XLD” conserved sequence motifs found in the catalytic domains of three distinct families of known and putative Ψ synthases. To gain additional evidence on the role of Cbf5p in rRNA biosynthesis, we have used in vitro mutagenesis techniques to introduce various alanine substitutions into the putative Ψ synthase domain of Cbf5p. Yeast strains expressing these mutated cbf5 genes in a cbf5Δ null background are viable at 25°C but display pronounced cold- and heat-sensitive growth phenotypes. Most of the mutants contain reduced levels of Ψ in rRNA at extreme temperatures. Substitution of alanine for an aspartic acid residue in the conserved XLD motif of Cbf5p (mutant cbf5D95A) abolishes in vivo pseudouridylation of rRNA. Some of the mutants are temperature sensitive both for growth and for formation of Ψ in the rRNA. In most cases, the impaired growth phenotypes are not relieved by transcription of the rRNA from a polymerase II-driven promoter, indicating the absence of polymerase I-related transcriptional defects. There is little or no abnormal accumulation of pre-rRNAs in these mutants, although preferential inhibition of 18S rRNA synthesis is seen in mutant cbf5D95A, which lacks Ψ in rRNA. A subset of mutations in the Ψ synthase domain impairs association of the altered Cbf5p proteins with selected box H/ACA snoRNAs, suggesting that the functional catalytic domain is essential for that interaction. Our results provide additional evidence that Cbf5p is the Ψ synthase component of box H/ACA snoRNPs and suggest that the pseudouridylation of rRNA, although not absolutely required for cell survival, is essential for the formation of fully functional ribosomes.
In eukaryotes the biosynthesis of rRNA occurs in a specialized organelle known as the nucleolus (33, 41, 46, 56). rRNA is transcribed by RNA polymerase I (Pol I) as a single large precursor, which undergoes a series of endo- and exonucleolytic cleavages to produce mature rRNA species. In the yeast Saccharomyces cerevisiae, the 35S pre-rRNA precursor is processed to produce mature 18S, 5.8S, and 25S RNAs (54). The 5S rRNA and ribosomal proteins are imported into the nucleolus for assembly into precursors of the 40S and 60S ribosomal subunits before their export to the cytoplasm (16, 41, 46). An interesting feature of rRNA maturation is the extensive modification the 35S precursor undergoes prior to subsequent cleavage events (29, 40, 39). One such modification, isomerization of uridine to pseudouridine (Ψ), is by far the most abundant posttranscriptional modification of rRNA (29, 40, 39). Formation of Ψ is also known to occur in tRNAs (49), small nuclear RNAs (snRNAs), and small nucleolar RNAs (snoRNAs) (17, 30). Despite the abundance of Ψ in various classes of RNA, very little is known about its role in RNA structure and function.
The conversion of uridine to pseudouridine is thought to involve the breakage of the N1 glycosidic bond, the rotation of the base 180° around the N3-C6 axis, followed by reformation of a covalent bond at position C5 (15, 39). The isomerization reaction is catalyzed by a group of enzymes known as Ψ synthases. A limited number of Ψ synthases have been identified in both prokaryotes and yeast and have been shown to have enzymatic activity in vivo (reviewed in reference 40). Comparative sequence analysis has further identified a number of putative Ψ synthases that share the KP and XLD (where X stands for T, A, or R residues) sequence motifs found in three distinct families of known and putative Ψ synthases (24). Recently, it has been demonstrated that the aspartic acid in the XLD motif is absolutely essential for the catalytic activity of truAp, an Escherichia coli tRNA synthase that catalyzes the conversion of uridine to Ψ at positions 38, 39, and 40 in tRNA (20). Mutation of this residue inhibits Ψ formation in tRNA in vitro.
Prokaryotic Ψ synthases have a high degree of site-specificity in vivo, both for tRNA and rRNA substrates (40, 43). However, site selection for pseudouridylation in eukaryotic rRNAs involves a class of small nucleolar RNAs (snoRNAs) known as box H/ACA snoRNAs (2, 12, 13, 40, 48, 52). The majority of characterized yeast H/ACA snoRNAs have been shown to act as guides to direct site-specific pseudouridylation of rRNA. These snoRNAs form two short regions of complementary to rRNA, resulting in an unpaired pocket that is thought to render a specific uracil residue accessible to the rRNA Ψ synthase. All known guide H/ACA snoRNAs achieve this complementarity through a common hairpin-hinge-hairpin-tail secondary structure motif. The consensus folding includes two evolutionary conserved elements, box H (defined as AGA in yeast and 5′-ANANNA-3′ in other species) located in the hinge region, and box ACA, positioned exactly 3 nucleotides (nt) from the 3′ end of all H/ACA snoRNAs (2). That box H/ACA elements play important roles in site selection was demonstrated by mutational analysis. The site of modification is almost exclusively 14 to 16 nt from the H or ACA box (12, 13, 36). These conserved elements also play a role in snoRNA synthesis and are believed to serve as binding sites for specific proteins (2, 31).
Two yeast H/ACA snoRNAs, snR30 and snR10, are important for normal processing of pre-rRNA (35, 50, 51). Shutdown of snR30 biosynthesis results in defective synthesis of mature 18S rRNA and is lethal (3, 35). However, there is no evidence for involvement of this snoRNA in Ψ formation. Strains lacking snR10 are defective in the processing of the 35S pre-rRNA precursor and exhibit a cold-sensitive phenotype (50). Additionally, snR10 is known to direct synthesis of Ψ in the core of the peptidyltransferase center (36). Interestingly, the remaining 18 H/ACA guide RNAs examined are individually dispensable, although each specifies Ψ formation at one or two specific sites in rRNA (36, 52, 44).
The H/ACA snoRNAs are found as RNP particles (snoRNPs) in the nucleolus. The most recent characterization of the H/ACA snoRNP complexes by two studies has provided some insight into the functions of the protein cofactors (19, 58). These studies have shown independently that all H/ACA snoRNAs form a stable complex with the essential nucleolar proteins Gar1p, Nhp2p, Nop10p, and Cbf5p. Gar1p, the best-characterized member of this complex, is required for stable association of the box H/ACA snoRNAs with the pre-rRNA (2, 12, 13) and is necessary for pre-RNA processing (13, 14). Mutations in Gar1p also inhibit Ψ formation in rRNA (8). Moreover, there is evidence from coimmunoprecipitation experiments (25) for interaction of Gar1p with Cbf5p. Nhp2p was initially characterized as an HMG-like protein (23) and was subsequently shown to be related to the known RNA binding protein, ribosomal protein L32 (55, 19). Nhp2p stably associates with all H/ACA snoRNAs (19, 58) in accordance with its putative RNA binding activities. Nop10p is also required for the stability of the H/ACA snoRNPs, and depletion of Nhp2p or Nop10p results in defective 18S pre-rRNA processing and disruption of Ψ formation in rRNA (19).
The fourth protein constituent of the H/ACA snoRNPs, Cbf5p, has been postulated to have several in vivo functions. Cbf5p was initially isolated as a low-affinity centromere DNA binding protein in vitro (22). CBF5 interacts genetically with the centromere-binding protein gene CBF2/NDC10 and with the meiosis-specific protein kinase gene MCK1 (21). In addition, Cbf5p binds microtubules in vitro, which is consistent with a role in centromere function (22). Interestingly, Cbf5p also functions in ribosome biogenesis. We have shown previously that the temperature-sensitive mutation cbf5-1 prevents rRNA transcription at the nonpermissive temperature and reduces the cytoplasmic pool of 40S and 60S ribosomal subunits (9). In addition, overexpression of SYC1/RRN3, an RNA Pol I-specific transcription factor (59), suppresses the cbf5-1 conditional growth defects (9). Cbf5p is also implicated in rRNA processing because depletion of this protein causes defects in 18S rRNA maturation (25). Moreover, there is evidence that Cbf5p could be an rRNA Ψ synthase. Cbf5p and its protein homologs rat NAP57 (32), Drosophila Nop60B (42), and human dyskerin (18) have sequence homology to E. coli truBp (24), which catalyzes the conversion of uridine to Ψ at position 55 in tRNA (38). Furthermore, Cbf5p shares the KP and XLD motifs found in three distinct families of known and putative Ψ synthases (24). Like Gar1p, Nhp2p, and Nop10p, Cbf5p coimmunoprecipitates with all members of the H/ACA class of snoRNAs, and its depletion inhibits Ψ formation in rRNA (25). However, to date, there is no direct evidence that any of these proteins, including Cbf5p, functions as rRNA Ψ synthases.
In this study, we demonstrate that alteration of selected highly conserved amino acids in the putative Ψ synthase domain of Cbf5p abolishes in vivo pseudouridylation of rRNA. This loss of Ψ correlates with a slow-growth phenotype and abnormally reduced levels of cytoplasmic 40S and 60S subunits. Our results indicate that Cbf5p-dependent pseudouridylation is essential for normal growth of yeast cells; strains lacking Ψ in rRNA are viable but display severe growth defects.
MATERIALS AND METHODS
Plasmids and yeast strains.
Plasmids used are as follows: pBluescript II KS(−) (Stratagene, San Diego, Calif.); pBFG (10) (2μm-derived plasmid with LEU2 marker, and three hemagglutinin [HA] epitope tags expressed under control of the PGK1 promoter); pYHY18-CBF5 (21); pCBF5-BFG (the entire CBF5 open reading frame [ORF] was PCR amplified and cloned into the EcoRI and XhoI sites of pBFG; this plasmid complements the cbf5-null allele); and pNOY103 (2μm-derived plasmid, URA3 marker, with the 35S rRNA gene expressed from the GAL7 promoter [37]), a gift from M. Nomura, University of California, Irvine. Plasmids pcbf5D65A-BFG, pcbf5P67A-BFG, pcbf5L94A-BFG and pcbf5D95A-BFG are described below.
The following yeast strains were used: YPH274 (MATa/MATα ade2-101/ade2-101 his3-200/his3-200 leu2-1/leu2-1 lys2-801/lys2-801 trp1-1/trp1-1 ura3-52/ura3-52 [Yeast Genetic Stock Center]); YWJ64-ts (same genotype as YPH274 plus cbf5-1/cbf5-1) (9); YCC131 (MATa ade2-1 can1-100 his3-11 leu2-3,112 trp1-1 ura3-1 cbf5::TRP1/pYHY18-CBF5); YCC133, which is isogenic with YCC131 except that it carries pCBF5-BFG; YCC37 (MATα ade2-101ochre his3-Δ200 leu2-Δ1 lys2-801amber trp1-Δ1 ura3-52 cbf5::HIS3/p64-FAT10) (42); YCC35, which is isogenic with YCC37 except that it carries pFLY64-ADNS (2μm plasmid, LEU2 marker, with Nop60B cDNA fused to the ADH1 promoter) (42); YHY64α1/pNOY103 (MATα ade2-101 cbf5-1 his3-Δ200 leu2-1 lys2-801 trp1-1 ura3-52/pNOY103) (strain YHY64α1 [21] was transformed with plasmid pNOY103 [37]); and YZCC12-2 (ade2-101 his3-Δ200 leu2-3,112 syc2::HIS3 trp1-Δ901 ura3-521/pBFG). The mutant strains cbf5D65A, cbf5P67A, cbf5L94A, and cbf5D95A were constructed for this study (see below).
Mutagenesis.
The mutations D65A, P67A, L94A, and D95A were generated by oligonucleotide-directed in vitro mutagenesis by using the Sculptor Mutagenesis Kit (Amersham, Arlington Heights, Ill.). The CBF5 ORF was released from pCBF5-BFG as an EcoRI/XhoI fragment and subcloned into pBluescript II KS(−), previously linearized with the same restriction enzymes and blunt ended with the Klenow DNA Pol I and deoxynucleoside triphosphates (dNTPs). Single-stranded phagemid DNA (1 μg/μl) was annealed to 1.6 pmol of each of the following mutagenic primers per μl: D65A (TGGAAGGTTTAGCTAGATTAATGAC), P67A (GATGGGTTGGAAGCTTTATCTAGA), L94A (TTTTGGATAGCTGTACCAGAGTG-3′), and D95A (GTAACTTTTGGAGCCAATGTACCAG-3′). Primers were synthesized by Genosys Biotechnologies (Woodlands, Tex.). The sequence of each mutagenic primer is complementary to the CBF5 gene, except for the targeted bases (denoted by boldface lettering). Mutations either introduced a restriction site (underline) or destroyed a natural site (italic type). A HindIII restriction site was introduced by a single base change in P67A; a PvuII site was introduced by a triple base change in L94A, while XbaI and BamHI sites were destroyed in D65A and D95A, respectively. Mutations are denoted by the original amino acid and position in the Cbf5p peptide sequence, followed by the substituted amino acid, which in all four cases is alanine. Each mutated sequence was checked by restriction digestion and DNA sequencing before it was subcloned into the pBFG vector. Mutated plasmids were introduced into the yeast strain YCC131 by using the Alkali Yeast Transformation Kit (Bio 101, Inc., La Jolla, Calif.), and transformants were plated onto a medium lacking leucine. Strains that have lost the wild-type YHY18-CBF5 plasmid were selected on 5-fluoroorotic acid plates containing uracil as described previously (47). Plasmids were reisolated from the transformants and digested with appropriate restriction enzymes to check the integrity of each construct.
Northern blot hybridizations.
Total yeast RNA was isolated as described previously (27). Approximately equal aliquots of RNA preparations were separated in 1.2% agarose-formaldehyde gels, transferred to nylon membranes for 24 h by capillary action, and probed with individual 32P-labeled oligonucleotides complementary to various regions of the 35S pre-rRNA transcript as described previously (26).
3H pulse-chase labeling of rRNA.
Pulse-chase labeling of pre-rRNA was done essentially as described previously (9, 53), except that cultures were maintained at 30°C or shifted to 37°C for 2 or 10 h prior to labeling. RNA samples isolated from aliquots containing approximately equal numbers of cells were separated on 1.2% agarose-formaldehyde gels, transferred to nylon membranes, treated with En3Hance as described by the supplier (DuPont/NEN), and exposed to X-ray film. The band intensities were quantitated with the Alpha Innotech Digital Imager, version 3.1 (Alpha Innotech Corp., San Leandro, Calif.).
Immunoprecipitations and Western blots.
Whole-cell protein extracts were prepared by glass bead lysis in a radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride). Immunoprecipitations, SDS-polyacrylamide gel electrophoresis, and immunoblotting were carried out by using an immunoprecipitation kit (Promega, Madison, Wis.) according to the manufacturer’s protocols. Monoclonal antibody HA.11 (Covance-Babco, Richmond, Calif.) at 5 μg/ml was used for immunoprecipitation of HA-tagged wild-type and mutant proteins. However, a 1:80 dilution of the antibody was used for immunodetection of the HA epitope on Western blots. After immunoprecipitation of H+ACA snoRNPs, the sedimented beads were resuspended in 300 μl of immunoprecipitation lysis buffer and deproteinized with 1.5 mg of proteinase K (Boehringer Mannheim, Indianapolis, Ind.) per ml for 30 min at 37°C. Subsequently, total RNA from each sample was prepared as described earlier (2) and analyzed on 8% acrylamide–7 M urea gels (45). RNA was transferred to a nylon membrane by electrophoresis (11). Oligodeoxyribonucleotides (18 to 20 bp) complementary to yeast snR8, snR10, snR31, and snR37 were used as hybridization probes. A 50-pmol mixture of the oligonucleotides was end labeled with polynucleotide kinase (Promega) and 150 μCi of [γ-32P]ATP (Amersham) as described previously (45).
Analysis of Ψ in rRNA.
Control (YCC133) and experimental yeast strains harboring various cbf5 mutant alleles were pregrown at 30°C in yeast extract-peptone containing 2% glucose (YPD) to an optical density at 660 nm (OD660) of 1.0. The cells were pelleted, washed with sterile water, and resuspended in fresh YPD at an OD660 of 0.2. After incubation with shaking at either 30 or 37°C for 60 min (1.5 h shift) or 7 h (10 h shift), cells were pelleted, washed with sterile water, resuspended in 25 ml of low-phosphate medium (57) at an OD660 of 0.5, and incubated for an additional 30 min (1.5 h shift) or 3 h (10 h shift) at either 30 or 37°C with agitation. The culture was centrifuged, and the cells were resuspended and incubated at the permissive or nonpermissive temperature in 3 ml of the same medium containing 1 mCi of [32P]orthophosphate (900 mCi/mmol) for 60 min (1.5-h shift) or 90 min (10-h shift). Total RNA was extracted and fractionated by electrophoresis in 1.0% agarose-formaldehyde gels. The 18S and 25S rRNAs were isolated from the gel by electroelution, ethanol precipitated, and digested with RNase T2 (Sigma R3751) in 5 μl of 50 mM ammonium acetate (pH 4.5)–0.05% SDS–1 mM EDTA for 90 min at 37°C. Aliquots of digested RNA corresponding to about 17,000 or 100,000 cpm, depending upon the experiment, were subjected to two-dimensional thin-layer chromatography (TLC) on plastic-backed cellulose plates (EM Science no. 5577) by using isobutyric acid-NH4OH-H2O (577:38:385, by volume) in the first dimension and 2-propanol–HCl–H2O (70:15:15, by volume) in the second dimension. Patterns were analyzed with a Molecular Dynamics PhosphorImager, and Up:Ψp ratios were determined with ImageQuant v1.1 software. Results are derived from a total of three to four TLC plates per strain, corresponding to at least two independent experiments.
Sucrose gradient sedimentation profiles of cytoplasmic ribosomal subunits.
The protocol was essentially as described previously (1, 9), except that the cultures were examined 2 h after being shifted to the nonpermissive temperature (37°C). The cell extracts were fractionated by sedimentation through 7 to 47% linear sucrose gradients prepared in 50 mM Tris acetate (pH 7.0)–0.5 M KCl–12 mM MgCl2–1 mM dithiothreitol, conditions which cause complete dissociation of ribosomes into the 40S and 60S subunits.
RESULTS
Substitution of conserved amino acid residues in the putative Ψ synthase sequence domains impairs in vivo function of yeast Cbf5p.
Cbf5p has sequence homology to known and putative Ψ synthases (24) and is known to occur in H/ACA snoRNP complexes (25, 58). Thus, Cbf5p is likely to be the enzyme responsible for catalyzing Ψ formation in rRNA. Previous studies demonstrated that depletion of Cbf5p results in loss of pseudouridylation of rRNA in vivo (25). However, depletion of several other protein components of the H/ACA snoRNPs also results in the loss of Ψ in rRNA (8, 19, 58). If Cbf5p is the enzyme responsible for conversion of U to Ψ in rRNA, then single amino acid substitutions in the highly conserved Ψ synthase sequence motifs would be expected to greatly reduce or eliminate Ψ in rRNA.
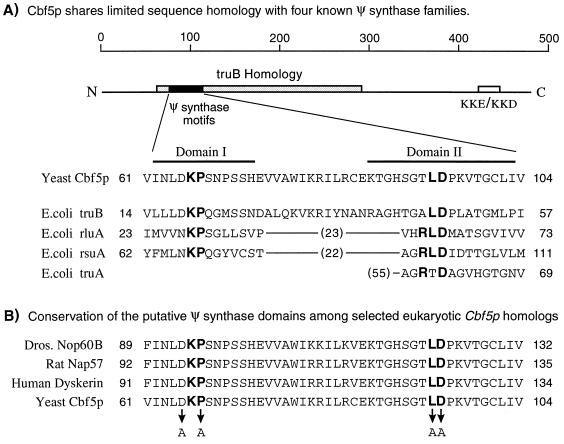
Both the KP and XLD motifs (where X stands for R, A, or T) are conserved among three families of known and putative Ψ synthases (24) and are thought to play important roles in catalysis (Fig. 1A). D65 (immediately adjacent to the KP motif) is more conserved among the truB family of synthases, to which Cbf5p belongs. The aspartic acid in the XLD motif is the only residue conserved in the fourth family, represented by the E. coli truA synthase. Recently, mutation of this particular amino acid residue was shown to abolish the Ψ synthase activity of truAp (20). To address the role of Cbf5p in rRNA pseudouridylation, alanine substitutions were introduced by site-directed mutagenesis at positions D65 (immediately adjacent to the KP motif), P67 (within the KP motif), and L94 and D95 (within the “XLD” motif) (Fig. 1B).
FIG. 1.
Regions of yeast Cbf5p subjected to site-directed mutagenesis. (A) A schematic of the overall sequence of yeast Cbf5p is shown, indicating the location of the truB homology region (shaded box) and the KKE/KKD repeat motif (unshaded box) (22). The highly conserved Ψ synthase domains in Cbf5p and representatives of the four known Ψ synthase families (24) are shown. Domains I and II correspond to motifs I and II described by Koonin (24). The highly conserved KP and XLD sequence motifs in domains I and II are indicated in boldface type. E. coli truA contains no apparent homology to domain I. Numbers in parentheses indicate the number of amino acids between the domains or, in the case of truA, to the N terminus. (B) The putative Ψ synthase catalytic domains in eukaryotic homologs of yeast Cbf5p are shown. The positions of alanine substitutions introduced at conserved residues in yeast Cbf5p are indicated by the arrows.
The mutated cbf5D65A, cbf5P67A, cbf5L94A, and cbf5D95A genes were introduced into a cbf5Δ yeast strain by using a plasmid-shuffle technique as described in Materials and Methods. Briefly, in vitro mutagenesis was carried out on the CBF5 gene cloned in pBluescript KS(−) in E. coli, and the mutated genes were subcloned into the yeast expression vector pBFG, in frame with three HA-epitope tags and under control of the PGK1 promoter. The resulting plasmids were transferred into yeast strain YCC131, which contains a wild-type copy of the CBF5 gene on the pYHY18-CBF5 plasmid covering a chromosomal deletion of the essential CBF5 gene (cbf5::HIS3) (21). Cells harboring both the mutant and the wild-type plasmids were plated for two rounds on medium containing FOA to select for loss of pYHY18-CBF5 (a URA3-bearing plasmid). In all cases, FOA-resistant colonies were obtained (confirmed to be Leu+ and Ura−), indicating that all of the mutant cbf5 strains are viable in the absence of a wild-type copy of CBF5. The presence of the mutated cbf5 gene in these yeast strains was confirmed by rescue of the plasmids in E. coli and restriction enzyme analysis, since the mutations all either created or destroyed restriction sites.
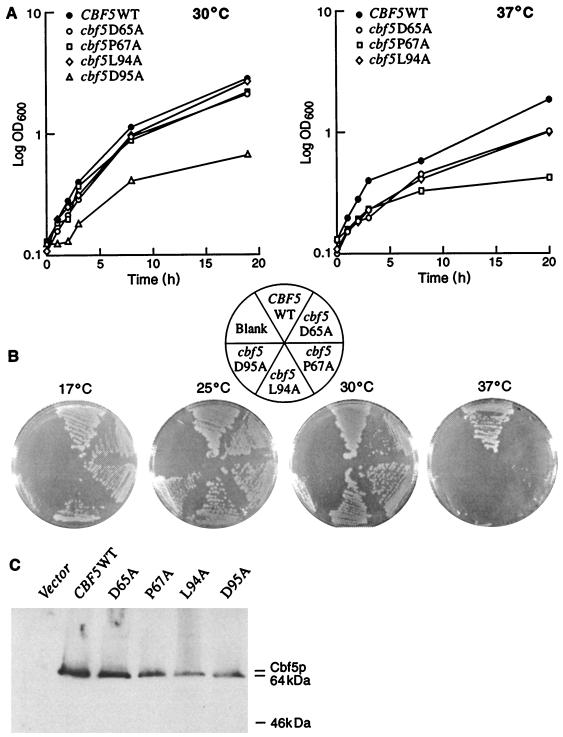
The mutant strains were checked for their growth properties in a rich medium (YPD) at various temperatures. The cbf5D65A, cbf5P67A, and cbf5L94A mutations had minimal effect on growth at 30°C; however, strain cbf5D95A had an unusual slow-growth phenotype at both 30 and 25°C (Fig. 2A and B). Interestingly, all of the mutants are temperature sensitive at 37°C but exhibit different degrees of phenotypic lag. In liquid cultures, strains cbf5D65A and cbf5L94A continue to grow after shiftup to 37°C at a rate somewhat reduced from that of the wild type, with only a brief phenotypic lag (ca. 2 h) (Fig. 2A). In contrast, strain cbf5P67A grows slower than wild type from the onset of the temperature shift and then slows markedly after 3 to 4 h, showing very little further growth after 8 to 9 h. This type of slow or delayed-arrest phenotype is often associated with mutations resulting in defects in rRNA transcription or in ribosome assembly (34). A similar slow-arrest phenotype at 38°C was previously observed with the temperature-sensitive cbf5-1 mutant strain, in which rRNA transcription is severely reduced at the nonpermissive temperature (9). Strain cbf5D95A did not grow at 37°C, either in suspension or on solid medium (Fig. 2). All of the mutants exhibited a cold-sensitive phenotype at 17°C (Fig. 2B), with cbf5D95A again showing the most dramatic phenotype.
FIG. 2.
Yeast cbf5 mutants altered by single amino acid substitutions in the putative Ψ synthase catalytic domain have growth defects. (A) Growth curves of cbf5 mutant and wild-type strains at 30 and 37°C. Cells were grown overnight at 30°C in liquid YPD to an OD600 of ∼0.1. Cultures were either maintained at 30°C or shifted to the restrictive temperature (37°C) to monitor the growth rates over a period of 20 h. (B) The cbf5 mutant and wild-type strains were checked for their growth properties on solid medium at 17, 25, 30, and 37°C after incubation for 4 days. In addition to the heat-sensitive phenotype at 37°C, the mutant strains also exhibited cold-sensitive phenotypes (17°C). (C) Mutationally altered Cbf5p’s are expressed in yeast and accumulate in vivo. Triple HA-tagged wild-type (WT) or mutated Cbf5p’s were expressed from the pBFG vector in a cbf5-null background. Whole-cell extracts were prepared, and aliquots (25 μg total protein) were analyzed on Western blots with monoclonal antibody directed against the triple HA-epitope tag (see Materials and Methods). As negative control, extract from strain YXCC12-2 containing the pBFG vector gave no signal with the anti-HA antibody (leftmost lane). The position of the expected Cbf5p band is indicated.
To determine whether the observed growth defects were due to Cbf5p instability in the mutant strains, we examined the intracellular levels of altered cbf5 proteins by Western blotting. Whole-cell extracts were prepared from the wild-type (YCC133), cbf5D65A, cbf5P67A, and cbf5L94A strains grown for 10 h after a shift to 37°C and from the cbf5D95A strain grown at 30°C. Aliquots containing equal amounts of total protein were analyzed by immunoblotting with monoclonal antibody HA.11 directed against the triple HA-epitope tag. As shown in Fig. 2C, all of the mutationally altered proteins were expressed in vivo, indicating that the growth alterations of the mutant strains were due to inherent functional defect(s).
Pseudouridylation of rRNA is reduced or eliminated in the cbf5 mutants.
If Cbf5p is indeed the Ψ synthase for rRNA, alanine substitutions within the putative Ψ synthase sequence domains should result in reduced Ψ levels in both 18S and 25S rRNA. We determined the Ψ content of rRNA in CBF5 wild type and in the cbf5D65A, cbf5P67A, cbf5L94A, and cbf5D95A mutant strains. De novo-synthesized rRNA was labeled in vivo with [32P]orthophosphate at both 30 and 37°C, the labeled 18S and 25S rRNA species were gel purified and subjected to RNase T2 digestion, and the nucleotide compositions were analyzed by two-dimensional TLC. The Ψp content of each rRNA was compared with the Up content of the same RNA species to obtain a Ψp/Up ratio. The results from analysis of 18S and 25S rRNA samples are presented in Table 1 and Fig. 3. In cells grown at 30°C, Ψ contents of both 18S and 25S rRNA were sharply reduced in mutants cbf5D65A, cbf5L94A, and cbf5D95A. In fact, no Ψ could be detected in rRNAs isolated from strain cbf5D95A under conditions that would permit detection of less than one Ψ per RNA molecule. This result is consistent with the absolute requirement of this aspartate (D95) in the active site of a known prokaryotic Ψ synthase (20). When the labeling was carried out after a shiftup to 37°C, Ψ contents in mutants D65A and L94A were reduced even further. Thus, cbf5L94A 18S and 25S rRNA Ψ levels were reduced to 6 and 5% of wild-type levels, respectively, when cells were labeled 1.5 h after shiftup and were below the limit of detection when labeled 10 h after shiftup (Table 1). However, in mutant cbf5P67A cells grown either at 30 or 37°C, rRNA Ψ levels were found to be consistently above the levels observed in our CBF5 wild-type strain, although it is not certain that the observed differences are significant.
TABLE 1.
Pseudo-U content of rRNA in various cbf5 mutantsa
| Strain | 18S rRNA Ψ content (SD) at:
|
25S rRNAΨ content (SD) at:
|
||||
|---|---|---|---|---|---|---|
| 30°C | 37°C (1.5 h) | 37°C (10 h) | 30°C | 37°C (1.5 h) | 37°C (10 h) | |
| YCC133 (CBF5WT) | 100 | 100 | 100 | 100 | 100 | 100 |
| YCC35 (Nop60B) | – | – | – | BD | – | – |
| cbf5-1 | – | – | – | – | – | 110 |
| cbf5D65A | 54 (4) | 40 (3) | 8 | 49 (5) | 28 (2) | 29 (3) |
| cbf5P67A | 127 (3) | – | – | 117 (7) | – | 133 (14) |
| cbf5L94A | 16 (3) | 6 (0.6) | BD | 30 (7) | 5 (0.7) | BD |
| cbf5D95A | BD | – | – | BD | – | – |
Spots corresponding to Up and Ψp from the TLC plates shown in Fig. 3 were quantified by phosphorimager analysis. The Ψ/Up ratios from each mutant strain were then normalized against those for the wild-type strain YCC133 adjusted to 100%. All values were derived from replicate analyses. Numbers in parentheses are standard deviations. –, Not tested; BD, below the limit of detection (<1 Ψp per rRNA molecule).
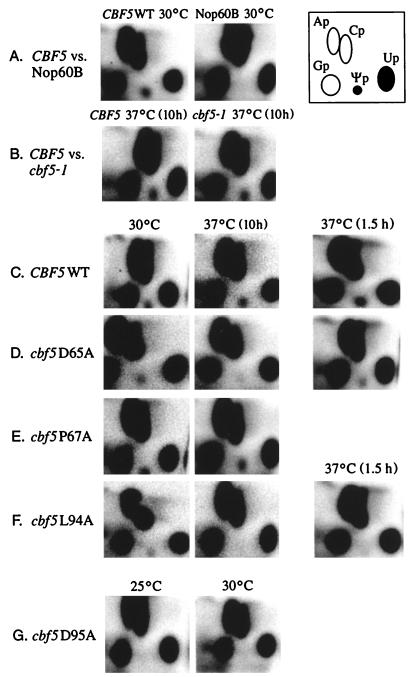
FIG. 3.
Mutations in the conserved Ψ synthase domains of Cbf5p inhibit pseudouridylation of rRNA. In vivo 32P-labeled 25S rRNAs from wild-type or cbf5 mutant cells were extracted from agarose-formaldehyde gel slices, digested with RNase T2, and subjected to two-dimensional TLC (see Materials and Methods). Labeled spots corresponding to Ap, Gp, Cp, Up, and Ψp are indicated in the diagram. The various panels show phosphorimages of TLC-fractionated 25S rRNA hydrolysates prepared from the following strains. (A) YCC37, a wild-type CBF5 expressed from pFAT10 (left panel); YCC35, a Drosophila homolog of CBF5; and Nop60B, expressed from pADNS (right panel). (B) YPH274, a CBF5/CBF5 wild-type isogenic control strain (left) and YWJ64-ts, cbf5-1/cbf5-1 (right) (9). (C) YCC133, a wild-type CBF5 covering the cbf5-null allele, grown at 30°C (left) or 37°C (middle) for 10 h or at 37°C for 1.5 h (right). (D to F) Strains harboring cbf5 mutations D65A, P67A, and L94A, respectively, covering the cbf5-null allele, grown at the permissive temperature 30°C (left) or at the restrictive temperature 37°C for 10 h (middle) or 1.5 h (right). (G) Strain harboring the cbf5D95A point mutation grown at 25°C (left) or 30°C (right). The patterns shown are from analyses with 17,000 cpm of digested RNA per TLC plate. Essentially identical results were obtained with samples containing 100,000 cpm per plate, which increased the sensitivity of Ψ detection to less than one residue per RNA molecule.
With the obvious exception of mutant cbf5P67A, there is a rough correlation between the degree of Ψ depletion and the severity of the growth defects seen in these mutants. For example, strain cbf5D95A shows the most severe growth defect (very slow growth at 30°C and no growth at 37°C) and contains no detectable Ψ in its rRNA. Strains cbf5D65A and cbf5L94A have pronounced temperature-sensitive growth phenotypes and contain markedly reduced levels of Ψ at higher temperatures. Except for the physiological defect associated with the absence of the rluD Ψ synthase in E. coli (43), we know of no other reports that suggest a phenotype associated with the loss of Ψ in rRNA. However, we cannot rule out the possibility that the growth defects associated with these mutations are due to Ψ-independent functions of Cbf5p (see below).
The temperature-sensitive cbf5-1 mutant was recently shown to have a defect in rRNA transcription and a reduced level of 40S and 60S cytoplasmic ribosomal subunits at the nonpermissive temperature 38°C (9). Surprisingly, analysis of Ψ content of 18S rRNA (data not shown) and 25S rRNA from a cbf5-1 strain (YWJ64-ts) labeled after incubation for 10 h at the nonpermissive temperature revealed normal Ψ levels (Table 1 and Fig. 3), findings similar to those observed with an isogenic CBF5 wild type (YPH274) and mutant cbf5P67A. Thus, cbf5-1 and cbf5P67A are examples of Cbf5p structural alterations resulting in a temperature-sensitive growth phenotype in the absence of any effect on Ψ formation in rRNA. The position(s) of amino acid substitution(s) in cbf5-1 is unknown.
When expressed in yeast, Nop60B, the Drosophila homolog of CBF5, was recently shown to partially complement the lethal cbf5::HIS3 null allele, while the rat homolog Nap57 failed to do so (42). Growth of a haploid cbf5::HIS3 strain, harboring the fly Nop60B cDNA expressed from the ADH1 promoter on a 2-μm plasmid, is considerably slower than that of a CBF5 wild-type strain. Nop60B has 63% identity over 380 amino acids to Cbf5p. Although the N-terminal and the C-terminal ends vary in Nop60B, the putative Ψ synthase domain is highly conserved between the two proteins (Fig. 1B). The Ψ content of rRNA in this strain (YCC35) was examined and, surprisingly, YCC35 is also devoid of Ψ in 25S rRNA (Table 1 and Fig. 3A, right panel) or 18S rRNA (data not shown). The severe growth defect seen in YCC35 is quite similar to that of mutant cbf5D95A, which also lacks Ψ in rRNA. However, as with cbf5D95A, it is unclear whether the slow-growth defect of YCC35 is due entirely to loss of Ψ in rRNA or reflects a weak functional complementation of some other Cbf5p function.
The growth defect correlating with Ψ depletion is not relieved by Pol II-driven transcription of rRNA.
We have shown previously that the observed rRNA transcriptional defect in the cbf5-1 conditional mutant can be partially suppressed by expression of rDNA from the Pol II-driven Gal7 promoter on pNOY103 (9). Because rRNA Ψ content is normal in cbf5-1, Cbf5p must play an essential role in Pol I-driven rRNA transcription in addition to its Ψ synthase function. Therefore, it was important to determine whether growth defects of the mutant strains, particularly strains cbf5L94A and cbf5D95A that completely lack Ψ in rRNA, are also suppressed by pNOY103. To this end, we transformed pNOY103 into each mutant strain, and individual transformant colonies were inoculated into glucose (repressed condition) or galactose (induced expression of rDNA) medium to measure relative growth rates. The mutants fell into two classes with respect to suppression by pNOY103 (Table 2). The doubling times of the wild-type, cbf5D65A, and cbf5L94A strains (all containing pNOY103) were identical under repressed or nonrepressed conditions. Interestingly, strain cbf5D95A/pNOY103, which grows slowly, with a doubling time of 4.5 h in glucose, fails to grow upon transfer to galactose medium. However, mutant cbf5P67A/pNOY103 has a doubling time of 3.5 h in glucose and 3.0 h in galactose; thus, the expression of rRNA from a Pol II promoter partially relieves the growth defect of this mutant at 37°C. A similar result was obtained for the cbf5-1/pNOY103 strain (YHY64α1/pNOY103) grown in galactose medium, a result consistent with the suppression results reported previously (9). Class I mutants, which include cbf5D65A, cbf5L94A, and cbf5D95A, are not suppressed by pNOY103, indicating that the growth defect associated with reduction or loss of Ψ in rRNA in these strains is not due to a Pol I-related transcriptional block. The growth defect in class II mutants cbf5P67A and cbf5-1 is partially suppressed by pNOY103, suggesting the presence of a Pol I-specific transcriptional defect in these strains.
TABLE 2.
Growth defects of certain cbf5 mutants are not relieved by Pol II-driven transcription of rRNAa
| Strain (pNOY103) | Temp (°C) | Doubling time (h)
|
|
|---|---|---|---|
| Glucose | Galactose | ||
| YCC133 (CBF5) | 37 | 2.0 | 2.0 |
| Class I | |||
| cbf5D65A | 37 | 3.5 | 3.5 |
| cbf5L94A | 37 | 3.0 | 3.0 |
| cbf5D95A | 30 | 4.5 | Dead |
| Class II | |||
| cbf5P67A | 37 | 3.5 | 3.0 |
| cbf5-1 | 38 | 3.5 | 2.6 |
Doubling times of the indicated yeast strains, all containing plasmid pNOY103 (37), were determined in minimal salts-dextrose growth medium (SD)-glucose or SD-galactose at the indicated temperatures. Class II cbf5 mutants are partially suppressed by plasmid pNOY103; class I mutants are not.
Analysis of pre-rRNA processing in the cbf5 mutant strains.
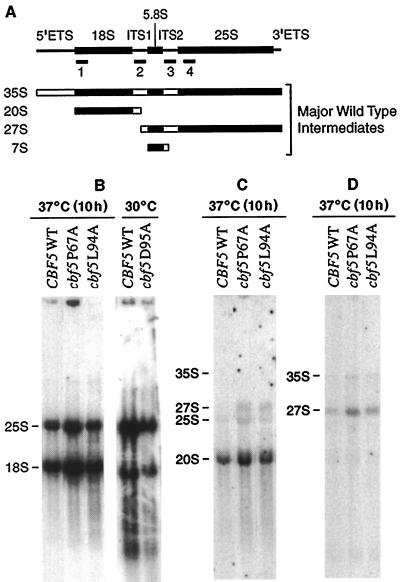
It has been proposed that the majority of pseudouridylation in rRNA occurs on the 35S pre-rRNA prior to the exo- and endonucleolytic cleavage events that yield mature 18S, 5.8S, and 25S rRNA species (6). There is evidence for the presence of at least 30 to 35 Ψ residues in 35S rRNA, suggesting a potential role for pseudouridines in controlling pre-rRNA processing (40). The availability of cbf5 mutants with reduced or completely depleted Ψ in rRNA should allow us to assess the relationship of pseudouridylation and pre-rRNA processing.
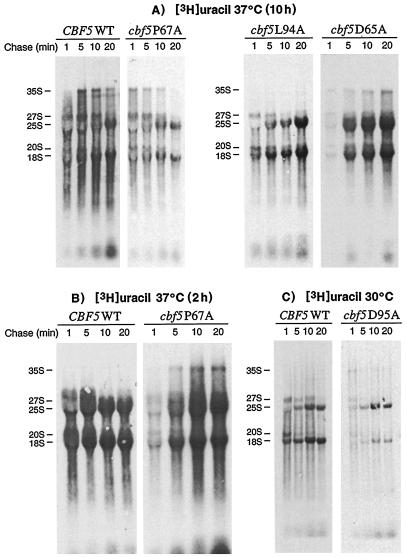
Pulse-chase rRNA labeling experiments were used to analyze the processing of newly synthesized rRNA in the cbf5 mutant strains at permissive and nonpermissive temperatures. Wild-type and mutant strains were grown to mid-log phase at 30°C and transferred to 37°C for 2 or 10 h (with the exception of cbf5D95A, which was maintained at 30°C) prior to pulse-labeling with [3H]uracil. After a chase with an excess of unlabeled uracil, total RNAs isolated from aliquots containing equal numbers of cells were fractionated on 1.2% agarose-formaldehyde gels and visualized by fluorography as described in Materials and Methods. The processing of pre-rRNA in strains cbf5D65A, cbf5P67A, and cbf5L94A shifted to 37°C for 2 or 10 h before labeling was similar to that of the wild-type strain (Fig. 4A and B). The major processing intermediates, 35S, 27S, and 20S pre-rRNAs, were visible in all strains 1 min after initiation of the chase, but were processed at essentially normal rates. There did appear to be some low-level accumulation of these precursors in mutant D65A (Fig. 4A). However, both the 18S and 25S mature species in these three mutants were synthesized at rates almost identical to that of the wild-type strain. The normalized 25S/18S ratios for rRNA samples isolated from cells pulse-labeled 10 h after the shiftup to 37°C (20 min chase) were as follows: P67A, 1.09; D65A, 1.03; L94A, 1.03; and CBF5 wild-type, 1.00. Mutant cbf5P67A incorporated comparatively less [3H]uracil into the newly synthesized rRNA species when labeled after growth for 10 h at 37°C (Fig. 4A), although rRNA synthesis was relatively normal after only 2 h at 37°C (25S/18S ratio of 1.02) (Fig. 4B). This reduced synthesis of rRNA in cbf5P67A coincides with the marked reduction in growth rate of this strain occurring 7 to 8 h after the temperature shift (Fig. 2A). This is expected since rRNA biosynthesis is closely coupled to the growth rate of cells (34). However, there is no evidence for significant accumulation of pre-rRNA precursors in this strain.
FIG. 4.
Pulse-chase labeling of rRNA in the cbf5 mutant strains. Wild-type or cbf5 mutant cells were pulsed with [3H]uracil for 3 min at the permissive temperature or after shift to the nonpermissive temperature and then chased with excess cold uracil for 1, 5, 10, or 20 min as indicated. See Materials and Methods for details. Cultures were preincubated for 10 h at 37°C (A) or for 2 h at 37°C (B) prior to pulse labeling. (C) Wild-type or mutant cbf5D95A cells were grown 48 h at 30°C (to an OD600 of 0.480) before pulse-labeling (3 min) at the same temperature. The expected positions of the various pre-rRNAs and mature 18S and 25S RNAs are indicated.
Pre-rRNA processing was examined in the cbf5D95A mutant cells by pulse-labeling after growth for 48 h at 30°C. Although total RNA was isolated from equal numbers of cells, cbf5D95A contained a reduced amount of 18S and 25S rRNA species compared to wild type on ethidium-stained gels (data not shown). Similarly, the net incorporation of [3H]uracil into newly synthesized rRNA is substantially reduced in this strain (Fig. 4C). The major 35S, 27S and 20S processing intermediates were visible 1 min after initiation of the chase and were processed at normal rates. Interestingly, the ratio of newly synthesized 25S to 18S rRNA is increased to approximately 1.5 (normalized to wild-type CBF5 at 1.0), suggesting a strong defect in 18S accumulation in the D95A mutant (Fig. 4C). However, the relatively small amount of 20S pre-rRNA visible on the blots appears to be normally processed to mature 18S rRNA. Furthermore, the newly synthesized 18S rRNA does not appear to be less stable than wild type, since it accumulated gradually during the 20-min chase. This reduction in the overall synthesis of rRNA in strain cbf5D95A is not improved by supplying 35S precursors transcribed from a Pol II promoter (see above).
It has been reported that genetic depletion of the RNA or protein components of snR30 (35) or snR10 (50) inhibits processing of the 35S large pre-rRNA to yield the 20S pre-rRNA (the immediate precursor to 18S), thereby preventing the synthesis of mature 18S rRNA. This inhibition leads to accumulation of an aberrant 23S precursor. Thus, depletion of Cbf5p or of snR30 RNA results in defective synthesis of 18S rRNA (25, 35). To investigate this possibility in the cbf5 mutants, steady-state levels of precursor and mature rRNA species were examined by Northern blot hybridization with selected hybridization probes to various pre-rRNAs, as well as to mature 18S and 25S rRNAs. The hybridization probes we used are indicated in Fig. 5A. A probe specific to the internal transcribed spacer 1 (ITS1) was used to detect the 20S rRNA, the immediate precursor to 18S rRNA, while a probe complementary to the 5′ region of internal transcribed spacer 2 (ITS2) was used to detect 27S RNA, the precursor to 25S rRNA. Probes specific to 18S and 25S rRNAs were used to detect the mature species. The 35S rRNA is detected by all of the probes. The blots reveal a low-level accumulation of the 20S and 27S precursors in P67A and L94A (Fig. 5C and D), although the increase over wild-type levels is not dramatic. As expected, cbf5D95A cells contained considerably less total rRNA than did the wild type. However, no abnormal accumulation of precursors was apparent; the pre-rRNAs were faintly visible and corresponded to the expected positions of these intermediates in the wild-type strain (data not shown). Both 18S and 25S rRNAs were detected with probes to the mature rRNA species and, as predicted by the pulse-labeling experiments, D95A cells were found to be relatively deficient in 18S rRNA (Fig. 5B). We could not detect the aberrant 23S precursor previously shown to accumulate instead of 20S rRNA upon depletion of Cbf5p or those snoRNAs essential for processing of precursors to 18S rRNA (25). We conclude that none of the cbf5 mutants, even those completely lacking Ψ in rRNA, dramatically accumulate any major processing intermediates under these experimental conditions. Also, there is no direct correlation between loss of Ψ in rRNA and defective synthesis of 18S rRNA, since cbf5L94A, which completely lacks Ψ in rRNA synthesized at 37°C, contains normal levels of 18S rRNA. We cannot eliminate the possibility that cbf5L94A cells contain a low, undetectable level of Ψ at positions critical for 18S rRNA maturation. However, it has been recently demonstrated that, consistent with our own observations, loss of function of Gar1p, another snoRNP-associated protein, results in inhibition of Ψ formation in rRNA independent of effects on 18S rRNA processing (8).
FIG. 5.
Steady-state levels of pre-rRNAs and mature rRNA species in wild-type and selected cbf5 mutant strains. The indicated yeast strains were grown in YPD at 30°C or 37°C to identical cell densities. RNA was extracted from approximately equal numbers of cells, resolved on 1.2% agarose-formaldehyde gels, and transferred to a nylon membrane. (A) Schematic showing the various pre-rRNAs and the hybridization probes used in these experiments. (B) Blots were probed with labeled oligonucleotides 1 (18 nt) and 4 (19 nt), complementary to the 5′ ends of mature 18S and 25S rRNAs. (C) The same membranes were stripped and rehybridized with oligonucleotide 2 (17 nt), which was specific to the 5′ region of ITS1 upstream of cleavage site A2. (D) The same membranes were stripped and reprobed with oligonucleotide 3 (18 nt), specific to the 5′ region of ITS2.
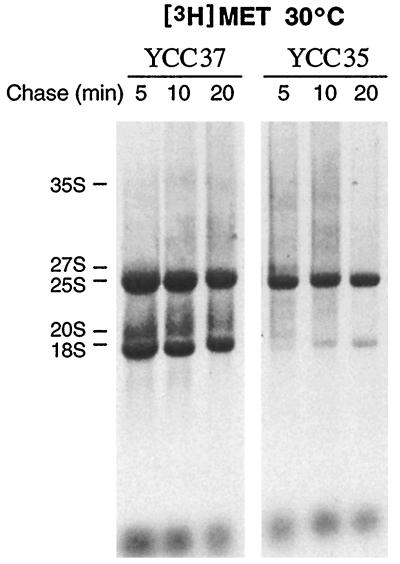
We have also analyzed pre-rRNA processing in strain YCC35 (cbf5::HIS3/Nop60B), which also lacks measurable Ψ in rRNA. Since YCC35 is Ura+, cells were pulse-labeled with [3H-methyl]methionine, which labels the methyl groups on the large precursor. Analysis of the newly synthesized rRNA species in YCC35 gave a result quite similar to that obtained with cbf5D95A. Substantially lower levels of newly synthesized 18S rRNA and its immediate 20S precursor were synthesized in this strain compared to that of the wild-type isogenic strain, YCC37 (Fig. 6). The small amount of labeled 20S seen early in the chase was further processed over time to 18S rRNA. The level of labeled mature 25S rRNA was also slightly less than that seen in the wild-type strain. Both mature rRNA species appeared stable during the 20-min chase period. In terms of the similarity in slow-growth phenotype, lack of Ψ in rRNA, and reduced synthesis of 18S mature rRNA, the defects observed in YCC35 correlate well with those of mutant cbf5D95A.
FIG. 6.
Strain YCC35, with Nop60B (the Drosophila homolog of yeast CBF5) covering the cbf5Δ null, is defective in synthesis of 18S rRNA. Strain YCC35 and the isogenic CBF5 wild-type strain YCC37 were grown for 48 h at 30°C prior to the pulse-labeling with l-[3H-methyl]methionine for 3 min at 30°C. The cultures were chased with excess unlabeled methionine for 5, 10, or 20 min. RNA was isolated from equivalent numbers of cells, separated on 1.2% agarose-formaldehyde gels, and visualized by fluorography. The expected positions of the various pre- and mature rRNAs are indicated.
Mutational alteration of the Ψ synthase domain in Cbf5p can affect formation of snoRNP complexes.
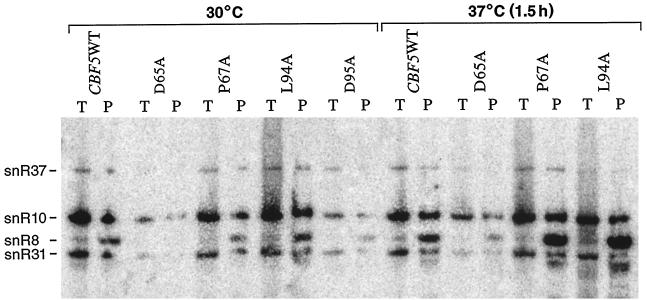
Results from various laboratories indicate that Cbf5p, Gar1p, Nhp2p, and Nop10p combine with various box H/ACA snoRNAs to form the RNP complexes (H/ACA snoRNPs) required for pseudouridylation of rRNA (8, 19, 25, 58). In addition, Cbf5p has been shown to associate with all members of the box H/ACA snoRNAs (25). The growth defects seen in our cbf5 mutants possibly could result from an inability of the altered cbf5 proteins to complex properly with the snoRNAs. We therefore asked whether any of the amino acid substitutions in Cbf5p altered the physical association of the mutated proteins with the H/ACA snoRNAs. We utilized a coimmunoprecipitation strategy as described in Materials and Methods. Briefly, mutant and wild-type strains were grown overnight at 30°C, and aliquots were transferred to 37°C and grown for an additional 1.5 h (except cbf5D95A was maintained at 30°C). Cell lysates were prepared and immunoprecipitated with monoclonal antibody HA.11 (see Materials and Methods) directed against the triple HA-tagged Cbf5p. Total RNA was extracted from the immunoprecipitates, fractionated on denaturing gels, and analyzed on Northern blots with a mixture of labeled hybridization probes specific for four H/ACA snoRNAs: snR8, snR10, snR31, and snR37. In mutant L94A and P67A cells kept either at 30 or 37°C for 1.5 h, snoRNP particle content is at wild-type levels, indicating that the physical association of these mutated Cbf5ps with the snoRNAs is relatively normal (Fig. 7). However, mutant D65A cell extracts, prepared from cells grown at either temperature, contain decreased quantities of the four analyzed snoRNAs (Fig. 7, lanes marked D65A [T]); although most of these snoRNAs appear to be associated with the immunoprecipitated Cbf5p-containing snoRNP particles (Fig. 7, lanes marked D65A [P]). Similarly, extracts prepared from D95A cells grown at 30°C are quite deficient in both total snoRNAs and snoRNP particles. Thus, the synthesis and/or the stability of snoRNAs is impaired in both cbf5D65A and cbf5D95A. The latter results confirm and extend previously reported observations indicating that Cbf5p is required for stable association of snoRNAs in the H/ACA snoRNP complexes (25, 58). It is clear, however, that the observed severe impairment of Ψ formation in mutant L94A rRNA is not due to lack of intact snoRNP particles, since snoRNP content is relatively normal in this strain (at least for the four snoRNAs analyzed) at 30°C and after 1.5 h at 37°C. Prolonged (10 h) incubation of the cbf5 mutant strains at high temperatures does cause an overall reduction in snoRNP particle content (data not shown), possibly due to secondary effects or inherent instability of the altered snoRNP particles.
FIG. 7.
Association of HA-epitope-tagged mutant cbf5 proteins with selected box H/ACA snoRNAs. Yeast extracts were prepared from cells expressing wild-type or mutationally altered cbf5 proteins after growth at the permissive (30°C) or the nonpermissive (37°C) temperature for 1.5 h. Immunoprecipitations were carried out with monoclonal antibody directed against the triple HA-epitope tags. RNA was purified from equal volumes of total extracts (T) or immunoprecipitated beads (P) and analyzed on 8% acrylamide–7 M urea gels. RNA was transferred to a nylon membrane by electrophoresis, fixed by UV irradiation, and probed with a mixture of 32P-end-labeled snR8, snR10, snR31, and snR37 oligonucleotides. For details, see Materials and Methods. The expected positions of these snoRNAs are indicated.
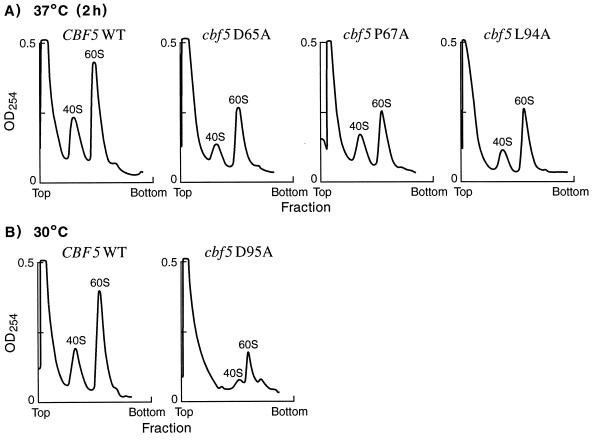
Cytoplasmic ribosomal subunit profiles in the cbf5 mutant strains.
Defects in ribosome assembly and/or transport can be detected by measuring the levels of mature ribosomal subunits in the cytoplasm. Cell extracts were prepared from cbf5 mutant strains and wild-type cells after incubation for 2 h at the nonpermissive temperature (for strain cbf5D95A, after overnight growth at 30°C). Ribosome profiles were obtained by sedimentation of cell extracts through sucrose gradients under conditions leading to dissociation of intact ribosomes to the 40S and 60S subunits (see Materials and Methods). Mutant cbf5D65A, cbf5P67A and cbf5L94A cells contain approximately wild-type levels of the ribosomal subunits after 2 h at 37°C, as judged by the relative ratios of the 40S and 60S subunit peaks (Fig. 8A). However, the ribosomal subunit profiles were severely altered in extracts prepared from cbf5D95A cells. The level of the 60S ribosomal subunit peak is severely reduced, and the 40S peak is almost nonexistent in this mutant (Fig. 8B). Also, the relative positions of the two subunit peaks are altered in D95A extracts, suggesting the presence of abnormal particles, such as subunit precursors or degradation products. As expected from the rather severe effects on rRNA synthesis seen in the pulse-labeling experiments (Fig. 4C), the D95A mutation also has a dramatic effect on the production of mature cytoplasmic ribosomes.
FIG. 8.
Sedimentation profiles of 40S and 60S cytoplasmic ribosomal subunits from cells expressing wild-type or mutated cbf5 genes. Exponentially growing cells (30°C) were either shifted to 37°C for 2 h (A) or maintained at 30°C (B) prior to harvesting. Approximately 20 OD260 U of each cell extract was sedimented through a 7 to 47% linear sucrose gradient under conditions that dissociate ribosomes into the 40S and 60S subunits, which were analyzed as described in Materials and Methods.
DISCUSSION
In yeast cells the products of two genes, PUS4 and CBF5, have considerable sequence homology to truBp, the pseudouridine synthase that catalyzes the formation of Ψ55 in E. coli tRNA (38). Recently, Pus4p was shown to be the yeast counterpart of E. coli truBp, catalyzing the formation of Ψ55 in both mitochondrial and cytoplasmic tRNAs (4). Therefore, it is reasonable to expect that Cbf5p should also have pseudouridylation activity. Moreover, results from various laboratories have provided evidence that Cbf5p, together with Gar1p, Nhp2p, and Nop10p, are the core protein components of the H/ACA snoRNPs (19, 25, 28, 58). The majority of H/ACA snoRNPs, by virtue of their association with guide RNAs, are implicated in directing site-specific pseudouridylation of rRNA (12, 36, 40, 44). Depletion in yeast of individual H/ACA snoRNP proteins reduces Ψ levels in rRNA, suggesting that a Ψ synthase activity is an integral component of these snoRNPs. Among these essential snoRNP proteins, only Cbf5p shares sequence similarity with known or putative Ψ synthases. In the present study, we provide further evidence indicating that Cbf5p is the Ψ synthase component of the H/ACA snoRNPs. Substitution of certain highly conserved amino acids in the putative Ψ synthase domains of Cbf5p greatly reduces Ψ levels in rRNA. In fact, alteration in Cbf5p of D95 in the XLD motif, previously shown to be essential for the catalytic activity of truAp, an E. coli tRNA Ψ synthase (20), appears to result in complete loss of Ψ in yeast rRNA (Table 1). However, two of these mutations (D65A and D95A) result in reduction of both rRNA Ψ content and snoRNP levels. In these strains, it is possible that the impairment of Ψ synthesis could be due to the lack of intact snoRNP particles. However, mutant cbf5L94A shows nearly complete loss of Ψ in both 18S and 25S rRNAs, with no observable reduction of cellular snoRNP levels (Table 1 and Fig. 7). The most reasonable explanation of these results is that Cbf5p indeed is the Ψ synthase component of the snoRNP particles.
All of the cbf5 mutants examined in this study showed some degree of growth impairment, especially at high temperatures (Fig. 2). In some of these mutants, a rough correlation is seen between the severity of these growth defects and the amount of Ψ depletion in rRNA. Strains cbf5D65A and cbf5L94A are temperature sensitive both for growth and for insertion of Ψ in rRNA. Mutant cbf5D95A, which lacks Ψ in rRNA even at permissive temperatures, shows the most severe growth defect. These results suggest that pseudouridylation of rRNA may be required for normal growth of yeast cells. However, strain cbf5P67A and the cbf5-1 conditional mutant exhibit temperature-sensitive growth phenotypes in the absence of any effect on Ψ formation. Clearly, Cbf5p and the snoRNPs are involved in functions other than Ψ formation, and thus we cannot exclude the possibility that the slow-growth defect observed in these cbf5 mutants could be due to inactivation of non-Ψ-related function(s). Recently, it has been shown that RluDp, an E. coli Ψ synthase required for formation of the universally conserved Ψ1915 and Ψ1917 modifications in rRNA, is essential for normal growth; the absence of RluDp results in severe growth defects in E. coli (43). However, no growth defects have been previously associated with loss of function of other known rRNA Ψ synthases.
Expression of rRNA from a Pol II-dependent promoter in pNOY103 was previously shown to suppress the temperature-sensitive growth phenotype associated with defects in rRNA synthesis in the cbf5-1 conditional mutant (9). However, mutations resulting in Ψ depletion in mutants cbf5D65A, cbf5L94A, and cbf5D95A were not suppressed by the presence of pNOY103, indicating that the growth defects associated with Ψ depletion do not result from transcriptional problems. In fact, cbf5D95A/pNOY103, which contains no Ψ in rRNA, does not grow upon transfer to galactose medium, possibly because expression of rRNA from a Pol II promoter increases the intracellular pool of undermodified rRNA which might be toxic to cells. In contrast, the cbf5P67A mutation is partially suppressed by pNOY103, indicating that this mutant may have a transcriptional defect analogous to that observed in cbf5-1. Alteration of a highly conserved proline in the Ψ synthase domain could result in significant conformational changes in Cbf5p, since proline is known to occur at bends in the peptide backbone (7). The exact mechanism by which Cbf5p exerts its effects upon Pol I-dependent rRNA transcription is still unknown.
The majority of Ψ has been shown to be inserted in the large pre-rRNA precursor in both prokaryotic and eukaryotic rRNA prior to nucleolytic cleavages, suggesting a possible role of this modification in processing (6). Recently, it has been demonstrated that a conditional mutation (gar1.1) in Gar1p results in inhibition of 18S rRNA production and depletion of ribosomal pseudouridines at the nonpermissive temperature (8). No direct correlation was observed between rRNA processing and rRNA pseudouridylation, however, since depletion of snR30 or U3 similarly prevented 18S rRNA processing even though Ψ content in the 35S pre-rRNA was normal (8). Our results indicate that lack of Ψ in rRNA does not result in dramatic accumulation of precursors, since the Ψ-depleted strains cbf5L94A and cbf5D65A, rRNA processing is relatively normal compared to that of the wild-type strain. However, as with gar1.1, a severe reduction in net synthesis of mature 18S rRNA is seen in cbf5D95A and in the cbf5Δ strain expressing the Drosophila Cbf5p homology (YCC35), both of which lack Ψ in rRNA. No processing intermediates were seen to accumulate in these strains; the residual 20S pre-rRNA, the immediate precursor to 18S, was processed to mature 18S during a 20-min chase period. In a Cbf5p-depleted strain, the defect in synthesis of 18S rRNA was previously shown to be accompanied by accumulation of an aberrant 23S RNA (25). However, we have not detected this intermediate in strain cbf5D95A. The basis for the drastically reduced levels of both 18S and 25S rRNAs in this mutant is still unclear, although it seems likely that rRNAs lacking proper Ψ modifications might be intrinsically unstable in vivo. For example, Ψ could stabilize the secondary structure of rRNA and facilitate interaction with ribosomal proteins during the assembly process. In addition, a severe reduction in mature cytoplasmic ribosomal subunits is seen in cbf5D95A cells (Fig. 8). This is undoubtedly in large part a direct result of the defect in 18S rRNA biosynthesis. Some or all of these effects could stem from loss of non-Ψ-related snoRNP functions, such as chaperoning the folding of pre-rRNA, which in turn could affect rRNA transcription, processing, modification, assembly of rRNP complexes, and transport to the cytoplasm.
The conserved H/ACA box elements are required for synthesis and accumulation of snoRNAs and may serve as binding sites for individual snoRNP proteins (2, 31, 5). In two of the cbf5 mutants (D65A and D95A), coimmunoprecipitation experiments indicate that both snoRNA and snoRNP levels are severely reduced, a finding consistent with the previous observation that Cbf5p is required for the stability of H/ACA snoRNAs (25). These results suggest that Cbf5p may also play a role in synthesis and/or accumulation of this class of RNAs. One possible explanation would be that Cbf5p directly interacts with the conserved H/ACA boxes to provide metabolic stability to the snoRNA and to participate in assembly of the snoRNP particle. Binding to the H/ACA elements could place the catalytic domain of Cbf5p in direct contact with the substrate uridine, located invariantly 14 to 16 residues from either the H or the ACA box. Another interesting possibility is that Cbf5p could be involved in pseudouridylation of snoRNAs, since it is known certain snoRNAs contain Ψ (17, 30). However, in other Ψ synthase active site mutants (L94A and P67A), snoRNA and snoRNP contents are normal. Thus, the relationship between Ψ synthase activity of Cbf5p and the ability of the protein to participate in the formation of intact snoRNP particles is still unclear.
Finally, to prove conclusively that Cbf5p functions as a Ψ synthase, the catalytic activity of this protein must be demonstrated in vitro. This might be difficult, because the catalytic activity could require the presence of an intact snoRNP complex. However, the recent identification of the core protein components of the H/ACA snoRNPs could eventually lead to the development of an in vitro assay for Ψ formation and further information on the role of Ψ in rRNA structure and function.
ACKNOWLEDGMENTS
We thank Mary Baum for technical advice and assistance in preparing the manuscript, Dottie McLaren for preparing the figures, and M. Nomura (University of California, Irvine) for providing plasmid pNOY103.
This research was supported by National Institutes of Health research grants CA11034 from the National Cancer Institute (J.C.), GM33783 (L.C.) and GM19351 (M.J.F.). J.C. is an American Cancer Society Research Professor.
REFERENCES
- 1.Baim S B, Pietras D F, Eustice D C, Sherman F. A mutation allowing mRNA secondary structure diminishes translation of Saccharomyces cerevisiae iso-1-cytochrome c. Mol Cell Biol. 1985;5:1839–1846. doi: 10.1128/mcb.5.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balakin A G, Smith L, Fournier M J. The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell. 1996;86:823–834. doi: 10.1016/s0092-8674(00)80156-7. [DOI] [PubMed] [Google Scholar]
- 3.Bally M, Hughes J, Cesareni C. snR30: a new essential small nuclear RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1988;16:5291–5303. doi: 10.1093/nar/16.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker H F, Motorin Y, Planta R J, Grosjean H. The yeast gene YNL292w encodes a pseudouridine synthase (Pus4) catalyzing the formation of Ψ55 in both mitochondria and cytoplasmic tRNAs. Nucleic Acids Res. 1997;25:4493–4499. doi: 10.1093/nar/25.22.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bortolin M-L, Ganot P, Kiss T. Elements essential for accumulation and function of small nucleolar RNAs directing site-specific pseudouridylation of ribosomal RNAs. EMBO J. 1999;18:457–469. doi: 10.1093/emboj/18.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand R C, Klootwijk J, Sibum C P, Planta R J. Pseudouridylation of yeast ribosomal precursor RNA. Nucleic Acids Res. 1979;7:121–134. doi: 10.1093/nar/7.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branden C, Tooze J. Introduction to protein structure. New York, N.Y: Garland Publishing, Inc.; 1991. Motifs of protein structure; pp. 13–14. [Google Scholar]
- 8.Bousquet-Antonelli C, Henry Y, Gélugne J-P, Caizergues-Ferrer M, Kiss T. A small nucleolar RNP protein is required for pseudouridylation of eukaryotic ribosomal RNAs. EMBO J. 1997;16:4770–4776. doi: 10.1093/emboj/16.15.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadwell C, Yoon H-J, Zebarjadian Y, Carbon J. The yeast nucleolar protein Cbf5p is involved in rRNA biosynthesis and interacts genetically with the RNA polymerase I transcription factor RRN3. Mol Cell Biol. 1997;17:6175–6183. doi: 10.1128/mcb.17.10.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chardin P, Camonis J H, Gale N W, Van Aelst L, Schlessinger J, Wigler M H, Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds GRB2. Science. 1993;260:1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- 11.Fabrizio P, Esser S, Kastner B, Lührmann R. Isolation of S. cerevisiae snRNPs: comparison of U1 and U4/U6.U5 to their human counterparts. Science. 1994;264:261–265. doi: 10.1126/science.8146658. [DOI] [PubMed] [Google Scholar]
- 12.Ganot P, Bortolin M-L, Kiss T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell. 1997;89:799–809. doi: 10.1016/s0092-8674(00)80263-9. [DOI] [PubMed] [Google Scholar]
- 13.Ganot P, Caizergues-Ferrer M, Kiss T. The family of box ACA small nucleolar RNAs is defined by an evolutionarily conserved secondary structure and ubiquitous sequence elements essential for RNA accumulation. Genes Dev. 1997;11:941–956. doi: 10.1101/gad.11.7.941. [DOI] [PubMed] [Google Scholar]
- 14.Girard J-P, Lehtonen H, Caizergues-Ferrer M, Amalric F, Tollervey D, Lapeyre B. GAR1 is an essential small nucleolar RNP protein required for pre-rRNA processing in yeast. EMBO J. 1992;11:673–682. doi: 10.1002/j.1460-2075.1992.tb05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldwasser E, Heinrikson R L. The biochemistry of pseudouridine. In: Davidson N J, Cohen W E, editors. Progress in nucleic acid research and molecular biology. Vol. 5. New York, N.Y: Academic Press, Inc.; 1966. pp. 399–416. [DOI] [PubMed] [Google Scholar]
- 16.Green R, Noller H F. Ribosomes and translation. Annu Rev Biochem. 1997;66:679–716. doi: 10.1146/annurev.biochem.66.1.679. [DOI] [PubMed] [Google Scholar]
- 17.Gu J, Chen Y, Reddy R. Small RNA database. Nucleic Acids Res. 1998;26:160–162. doi: 10.1093/nar/26.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heiss N S, Knight S W, Vulliamy T J, Klauck S M, Wiemann S, Mason P J, Pouska A, Dokal L. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 19.Henras A, Henry Y, Bousquet-Antonelli C, Noaillac-Depeyre J, Gélugne J-P, Caizergues-Ferrer M. Nhp2p and Nop10p are essential for the function of H/ACA snoRNPs. EMBO J. 1998;17:7078–7090. doi: 10.1093/emboj/17.23.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Pookanjanatavip M, Gu X, Santi D V. A conserved aspartate of tRNA pseudouridine synthase is essential for activity and a probable nucleophilic catalyst. Biochemistry. 1998;10:3651–3657. doi: 10.1021/bi971874+. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W, Lim M-Y, Yoon H-J, Thorner J, Martin G S, Carbon J. Overexpression of the yeast MCK1 protein kinase suppresses conditional mutations in centromere-binding protein genes CBF2 and CBF5. Mol Gen Genet. 1995;246:360–366. doi: 10.1007/BF00288609. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W, Middleton K, Yoon H-J, Fouquet C, Carbon J. An essential yeast protein, Cbf5p, binds in vitro to centromeres and microtubules. Mol Cell Biol. 1993;13:4887–4893. doi: 10.1128/mcb.13.8.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolodrubetz D, Burgum A. Sequence and genetic analysis of NHP2: a moderately abundant high mobility group-like nuclear protein with an essential function in Saccharomyces cerevisiae. Yeast. 1991;7:79–90. doi: 10.1002/yea.320070202. [DOI] [PubMed] [Google Scholar]
- 24.Koonin E V. Pseudouridine synthases: four families of enzymes containing a putative uridine-binding motif also conserved in dUTPases and dCTP deaminases. Nucleic Acids Res. 1996;24:688–693. doi: 10.1093/nar/24.12.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lafontaine D L, Bousquet-Antonelli J C, Henry Y, Caizergues-Ferrer M, Tollervey D. The box H+ACA snoRNAs carry Cbf5p, the putative pseudouridine synthase. Genes Dev. 1998;12:527–537. doi: 10.1101/gad.12.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafontaine D, Vandenhaute J, Tollervey D. The 18S rRNA dimethylase Dim1p is required for pre-ribosomal RNA processing. Genes Dev. 1995;9:2470–2481. doi: 10.1101/gad.9.20.2470. [DOI] [PubMed] [Google Scholar]
- 27.Lindahl L, Archer R H, Zengel J M. A new rRNA processing mutant of Saccharomyces cerevisiae. Nucleic Acids Res. 1991;20:295–301. doi: 10.1093/nar/20.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lübben B, Fabrizio P, Kastner B, Lührmann R. Isolation and characterization of the small nucleolar ribonucleoprotein particle snR30 from Saccharomyces cerevisiae. J Biol Chem. 1995;270:11549–11554. doi: 10.1074/jbc.270.19.11549. [DOI] [PubMed] [Google Scholar]
- 29.Maden B E H. The numerous modified nucleotides in eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1990;39:241–300. doi: 10.1016/s0079-6603(08)60629-7. [DOI] [PubMed] [Google Scholar]
- 30.Massenet S, Mougin A, Branlant S. Posttranscriptional modifications in the U small nuclear RNAs. In: Grosjean H, Benne R, editors. Modification and editing of RNA. Washington, D.C: ASM Press; 1998. pp. 201–227. [Google Scholar]
- 31.Maxwell E S, Fournier M J. The small nucleolar RNAs. Annu Rev Biochem. 1995;35:897–934. doi: 10.1146/annurev.bi.64.070195.004341. [DOI] [PubMed] [Google Scholar]
- 32.Meier U T, Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994;127:1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mélèse T, Xhu Z. The nucleolus: an organelle formed by the act of building a ribosome. Curr Opin Cell Biol. 1995;7:319–324. doi: 10.1016/0955-0674(95)80085-9. [DOI] [PubMed] [Google Scholar]
- 34.Moritz M, Pulaski B A, Woolford J L., Jr Assembly of 60S ribosomal subunits is perturbed in temperature-sensitive yeast mutant defective in ribosomal protein L16. Mol Cell Biol. 1991;11:5681–5692. doi: 10.1128/mcb.11.11.5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrissey J P, Tollervey D. Yeast snR30 is a small nucleolar RNA required for 18S rRNA synthesis. Mol Cell Biol. 1993;13:2469–2477. doi: 10.1128/mcb.13.4.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni J, Tien A L, Fournier M J. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell. 1997;89:565–573. doi: 10.1016/s0092-8674(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 37.Nogi Y, Vu L, Nomura M. An approach for isolation of mutants defective in 35S ribosomal RNA synthesis in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1991;88:7026–7030. doi: 10.1073/pnas.88.16.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nurse K, Wrezesinski J, Bakin A, Lane B G, Ofengand J. Purification, cloning, and properties of the tRNA Ψ55 synthase from Escherichia coli. RNA. 1995;1:102–112. [PMC free article] [PubMed] [Google Scholar]
- 39.Ofengand J, Bakin A, Wrzesinski J, Nurse K, Lane B G. The pseudouridine residues of ribosomal RNA. Biochem Cell Biol. 1995;73:915–924. doi: 10.1139/o95-099. [DOI] [PubMed] [Google Scholar]
- 40.Ofengand J, Fournier M J. The pseudouridine residues of rRNA: number, location, biosynthesis, and function. In: Grosjean H, Benne R, editors. Modification and editing of RNA. Washington, D.C: ASM Press; 1998. pp. 229–253. [Google Scholar]
- 41.Pederson T. Survey and summary: the plurifunctional nucleolus. Nucleic Acids Res. 1998;26:3871–3876. doi: 10.1093/nar/26.17.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips B, Billin A N, Cadwell C, Buchholz R, Erickson C, Merriam J R, Carbon J, Poole S J. The Nop60B Gene of Drosophila encodes an essential nucleolar protein that functions in yeast. Mol Gen Genet. 1998;260:20–29. doi: 10.1007/s004380050866. [DOI] [PubMed] [Google Scholar]
- 43.Raychaudhuri S, Conrad J, Hall B G, Ofengand J. A pseudouridine synthase required for the formation of two universally conserved pseudouridines in ribosomal RNA is essential for normal growth of Escherichia coli. RNA. 1998;4:1407–1417. doi: 10.1017/s1355838298981146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samarsky D A, Fournier M J. A comprehensive database for the small nucleolar RNAs from Saccharomyces cerevisiae. Nucleic Acids Res. 1999;27:161–164. doi: 10.1093/nar/27.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 46.Sheer U, Weisenberger D. The nucleolus. Curr Opin Cell Biol. 1994;6:354–359. doi: 10.1016/0955-0674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 47.Sikorski R, Boeke J D. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- 48.Smith C M, Steitz J A. Sno storm in the nucleolus: new roles for myriad small RNPs. Cell. 1997;89:669–672. doi: 10.1016/s0092-8674(00)80247-0. [DOI] [PubMed] [Google Scholar]
- 49.Sprinzl M, Steegborn C, Hübel F, Steinberg S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1996;24:68–72. doi: 10.1093/nar/24.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tollervey D. A yeast small nuclear RNA is required for normal processing of pre-ribosomal RNA. EMBO J. 1987;6:4169–4175. doi: 10.1002/j.1460-2075.1987.tb02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tollervey D, Guthrie C. Deletion of a yeast small nucleolar RNA gene impairs growth. EMBO J. 1985;4:3873–3878. doi: 10.1002/j.1460-2075.1985.tb04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tollervey D, Kiss T. Function and synthesis of small nucleolar RNA. Curr Opin Cell Biol. 1997;9:337–342. doi: 10.1016/s0955-0674(97)80005-1. [DOI] [PubMed] [Google Scholar]
- 53.Tollervey D, Lehtonen H, Carmo-Fonesca M, Hurt E C. The small nucleolar RNP protein NOP1 (fibrillarin) is required for pre-rRNA processing in yeast. EMBO J. 1991;10:573–583. doi: 10.1002/j.1460-2075.1991.tb07984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venema J, Tollervey D. Processing of pre-ribosomal RNA in Saccharomyces cerevisiae. Yeast. 1995;11:1629–1650. doi: 10.1002/yea.320111607. [DOI] [PubMed] [Google Scholar]
- 55.Vilardell J, Warner J R. Ribosomal protein L32 of Saccharomyces cerevisiae influences both the splicing of its own transcript and the processing of rRNA. Mol Cell Biol. 1997;17:1959–1965. doi: 10.1128/mcb.17.4.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warner J R. The nucleolus and ribosome formation. Curr Opin Cell Biol. 1990;2:521–527. doi: 10.1016/0955-0674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 57.Warner J R. Labeling of RNA and phosphoproteins in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:423–428. doi: 10.1016/0076-6879(91)94033-9. [DOI] [PubMed] [Google Scholar]
- 58.Watkins N J, Gottschalk A, Neubauer G, Kastner B, Fabrizio P, Mann M, Lührmann R. Cbf5p, a potential peudouridine synthase, and Nhp2p, a putative RNA binding protein, are present together with Gar1p in all H box/ACA motif snoRNPs and constitute a common bi-partite structure. RNA. 1998;4:1549–1568. doi: 10.1017/s1355838298980761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamato R T, Nogi Y, Dodd J A, Nomura M. RRN3 gene of Saccharomyces cerevisiae encodes an essential RNA polymerase I transcription factor which interacts with the polymerase independently of DNA template. EMBO J. 1996;15:3964–3973. [PMC free article] [PubMed] [Google Scholar]