Abstract
This work describes the 6-endo-dig cyclization of S-aryl propargyl sulfides to afford 2H-thiochromenes. The substitution at the propargylic position plays a crucial role in allowing intramolecular silver-catalyzed alkyne hydroarylation and N-iodosuccinimide-promoted iodoarylation. Additionally, a PTSA-catalyzed thiolation reaction of propargylic alcohols was developed to synthesize the required tertiary S-aryl propargyl ethers. The applicability of merging these two methods is demonstrated by synthesizing the retinoic acid receptor antagonist AGN194310.
Introduction
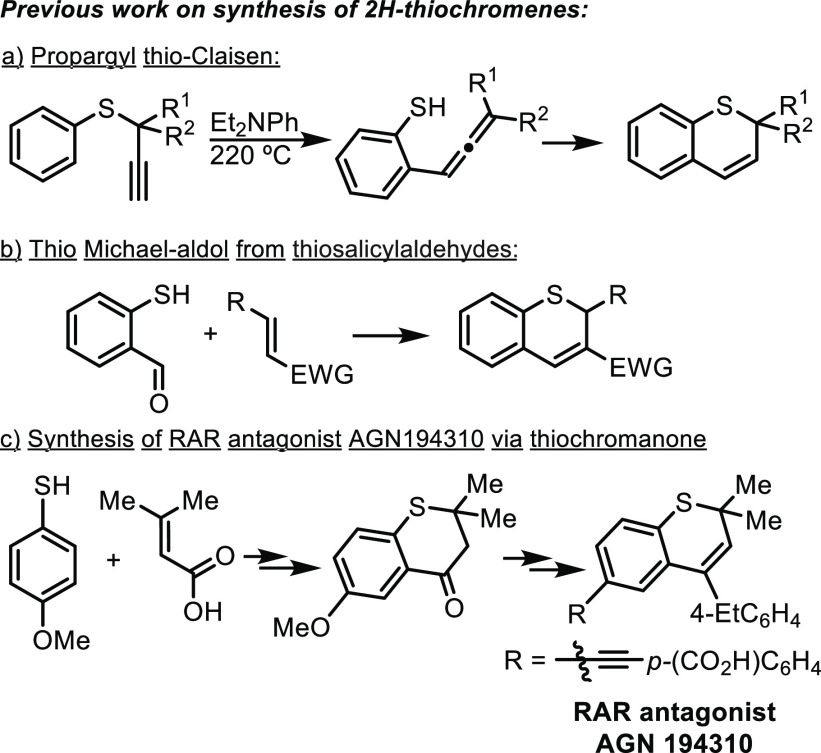
Propargyl N-aryl amines and O-aryl ethers are versatile and precious building blocks.1 The intramolecular alkyne arylation2 of these building blocks provides straightforward access to relevant heterocycles such as hydroquinolines and chromenes,3,4 without requiring a previous arene ortho functionalization. By contrast, this strategy involving C–H bond functionalization remains almost unexplored when applied to the synthesis of 2H-thiocromenes from the related thioethers.5 Intramolecular alkyne hydroarylation of S-aryl propargyl thioethers has been scarcely reported and limited to propargyl Claisen rearrangement using terminal alkynes under harsh reaction conditions (Scheme 1a).6 This rearrangement delivers a reactive allene intermediate7 that evolves into the thiochromene. Therefore, the synthesis of substituted 2H-thiochromenes is commonly accomplished by using thiosalicylaldehydes and alkenes in the presence of an organocatalyst (Scheme 1b).8
Scheme 1. Selected Methodologies for Synthesizing 2H-Thiochromenes.
Alternative classical strategies that do not require ortho prefunctionalization are based on multistep sequences to afford a 4-thiochromanone intermediate.9 Subsequent alcohol formation followed by elimination generates the 2H-thiochromene. This strategy has been employed to synthesize retinoic acid receptor (RAR) antagonist AGN194310 (Scheme 1c).10 Therefore, a more straightforward synthesis of thiochromenes through alkyne arylation of S-aryl propargyl thioethers under mild reaction conditions will be highly appealing.
The lack of electrophilic alkyne arylation methodologies of S-aryl propargyl thioethers is particularly surprising. A possible explanation could be that the sulfur atom favors competitive reaction pathways other than alkyne arylation. For instance, S-phenyl alkyl thioethers react with halonium ions enabling desulfurative halogenation reactions.11 Additionally, in a seminal contribution of the activation of propargyl thioethers by π-acids,12 Wang has reported a thiirenium ion formation after alkyne activation (Scheme 2a). Then, this intermediate evolves through 1,2 migration of the thio group to generate highly reactive metal–carbene species.13 Considering these reports, we hypothesized that tertiary propargyl sulfides, because of the gem-dimethyl effect,14 could react with an adequate alkynophilic reagent affording 2H-thiochromenes by favoring electrophilic alkyne arylation over other competitive pathways. Herein, we report the synthesis of 2H-thiochromenes through iodocyclization or hydroarylation of S-aryl propargyl thioethers (Scheme 2b).
Scheme 2. Alternative Reactivity of Propargyl Sulfides with π-Acid Metal Catalysts.
Results and Discussion
Synthesis of Tertiary S-Aryl Propargyl Thioethers
To tackle our proposed idea, we need to start from tertiary S-aryl propargyl thioethers. However, few examples for synthesizing these types of compounds bearing a quaternary center at the propargylic position have been reported. Despite the advances achieved in the thiolation of propargyl alcohols,5,15 mainly secondary propargylic alcohols have been used. Although an acid catalyst can easily activate tertiary alcohols, the propargylic substitution reaction is more challenging. The generated tertiary propargyl cation intermediate, which could be more adequately represented as an allenium ion, evolves rapidly in different alternative reaction pathways from propargylic substitution, such as competitive elimination or SN′ reactions forming allenes.16 Moreover, thiols could react with propargylic alcohols through other different reactivity patterns that do not implicate a carbocation at the propargylic position, like hydrothiolation of alkynes.17 Whereas thiolation of tertiary propargyl alcohols was efficiently accomplished using alkyl thiols,15a,15c,15f with less nucleophilic thiophenols the reaction takes place with low yields.12 Remarkably, in two recent reports, few examples of S-aryl propargyl thioethers were efficiently synthesized from tertiary propargyl alcohols and an excess of thiophenol (2–3 equiv) by using catalytic amounts of a bimetallic Ir–Sn complex18 or a lithium triflimidate salt.19 To this end, and on the basis of our previous experience in the direct nucleophilic substitutions of propargylic alcohols,15c,16,20 we evaluated the propargylation of thiophenols employing p-toluenesulfonic acid monohydrate (1, PTSA) as a promising, cheap, and easily accessible catalyst. After some optimization,21 this simple Brønsted acid proved to be an efficient catalyst for accomplishing thiolation of a variety of tertiary propargylic alcohols 3 with different thiols 2 (Scheme 3). When dimethyl-substituted propargylic alcohol 3a was tested, under standard conditions using 5 mol % 1 in nitromethane, the desired S-aryl propargyl thioether 4aa was obtained in high yields.
Scheme 3. Synthesis of Propargyl Thioethers 4 from Thiols 2 and Tertiary Propargyl Alcohols 3.
The reaction seemed to be quite general with various thioarenes bearing different electron-donating, neutral, and moderate electron-withdrawing substituents at the para position (4ab–ae, 4jc, 4jk, and 4kd), although a slightly lower yield was observed with a methoxy substituent (4ab). Functional groups at the ortho (4af, 4ag, and 4kl) or meta (4ah) position were well tolerated. Interestingly, thionaphthols (4ai and 4aj) also underwent the PTSA-catalyzed thiolation reaction. Next, we studied different alcohols. Modifications over a methyl group at the propargylic position revealed that phenyl (4ba) and vicinal (4ca) methoxy groups were compatible with the nucleophilic substitution reaction. Diaryl- and dicyclopropyl-substituted tertiary alcohols proved to be more challenging, though the desired propargyl thioethers 4da and 4ea were accessible. Other alcohols bearing one cyclopropyl substituent at the propargylic position also reacted with thiols, providing the desired products (4fa and 4ga). Propargylic alcohols 3 derived from cyclohexanone performed well, affording the corresponding thioethers in high yields (4ha and 4ia). Substrates bearing methoxy groups in the arene moiety R3 were suitable substrates for the thiolation reaction with different thioarenes (4ja, 4jc, 4jk, 4ka, 4kd, and 4kl). Curiously, a methoxy group at the meta position of the arene attached to the alkyne (4ka) provides higher yields than the related substrate bearing a methoxy group at the para position (4aj). Presumably, this moderate difference might be caused by a stronger stabilization of the carbocation intermediate that slows the nucleophilic attack and allows competitive reaction pathways.22 The method was also productive with tertiary alcohols bearing an alkyl group as the alkyne substituent (4la) as well as with alkyl thiols (4bm and 4fn). In addition, to demonstrate the practicability of the method, the reaction was scaled up, affording gram amounts of 4aa (3.78 g, 71% yield) and 4ad (5.21 g, 79% yield).
Synthesis of 2H-Thiochromenes
Once we established an efficient and easily scalable methodology for synthesizing S-aryl propargyl thioethers, we investigated an intramolecular arylation procedure to obtain the desired 2H-thiochromenes. We nonetheless have foreseen that a combination of a suitable electrophilic agent with adequate S-aryl propargyl thioethers will be decisive in achieving the alkyne arylation. As we have already postulated, the Thorpe–Ingold effect could favor the 6-endo-dig cyclization.
To test our hypothesis, we initially focused on electrophilic iodonium reagents based on our previous experience in iodocarbocyclizations.23 Dimethyl-substituted propargylic thioether 4aa, the analogous propargyl unsubstituted compound 5, and secondary propargyl sulfide 6 were evaluated with N-iodosuccinimide (NIS) (Scheme 4). Whereas the reaction of 4aa with NIS gave rise to the desired 3-iodothiochromene 7aa, primary and secondary propargyl thioethers 5 and 6 afforded only disulfide and multiple byproducts, possibly derived from sulfur–halogen substitution;11 the thiochromenes were not observed. This differentiated reactivity of 4aa suggests a crucial gem-dimethyl effect.
Scheme 4. Preliminary Studies of Intramolecular Iodoarylation of S-Aryl Propargyl Thioethers.
Once we demonstrated the feasibility of the process, we continued with the optimization (Table 1). An increase in reaction time and a slight excess of NIS provide 3-iodothiochromene 7aa in higher yields (entries 1 and 2). Lewis or Brønsted acid additives (entries 3–5) do not positively impact the reaction. Other possible iodonium sources, such as molecular iodine in the absence (entry 6) or presence of carbonates (entries 7 and 8), were less efficient. When NBS and NCS replaced NIS, no desired thiochromenes were obtained. Instead, the corresponding disulfide and multiple byproducts possibly derived from sulfur–halogen substitution11 and elimination reactions were obtained.
Table 1. Optimization of the Reaction Conditions for the Iodocarbocyclization Reaction of 4aaa.
| entry | I+ source | equiv | solvent | additive | yield (%)b |
|---|---|---|---|---|---|
| 1 | NIS | 1.1 | CH2Cl2 | – | 51 |
| 2 | NIS | 1.3 | CH2Cl2 | – | 76 (74)c |
| 3d | NIS | 1.1 | CH2Cl2 | BF3·Et2O | 56 |
| 4e | NIS | 1.3 | CH2Cl2 | BF3·Et2O | 60 |
| 5e | NIS | 1.3 | CH2Cl2 | AcOH | 50 |
| 6 | I2 | 1.3 | CH2Cl2 | – | – |
| 7e | I2 | 1.3 | CH2Cl2 | Na2CO3 | 32 |
| 8e | I2 | 1.3 | CH2Cl2 | K2CO3 | 35 |
Reaction conditions: 4aa (0.1 mmol) and NIS (0.13 mmol) in CH2Cl2 (1 mL).
Determined by 1H NMR using CH2Br2 as the internal standard.
Yield after column chromatography in parentheses.
With 0.11 mmol of additive.
With 0.13 mmol of additive.
Next, a selection of S-aryl propargyl thioethers 4 was subjected to the optimized reaction conditions (Table 1, entry 2), affording various 3-iodothiochromenes 7 (Scheme 5). Electron-donating and neutral groups (7aa, 7ab, and 7ae) at the para position of the thioaryl moiety (R4) gave rise to the corresponding 3-iodothiochromenes in high yields. Moderate electron-withdrawing groups such as halogens at the para (7ac–ad and 7kd) and ortho (7kl) position are also well-tolerated. Interestingly, iodoarylation of 4aj takes place selectively in the most activated position of the 2-thionaphthol moiety. Additionally, thioether 4ai also provided access to a tricyclic scaffold (7ai). Modification over the propargylic position was also accomplished, affording spirocyclic compound 7ia. Alkynes bearing methoxy-functionalized arenes in R3 also delivered the desired thiochromenes in variable yields (7ka, 7kd, and 7kl).
Scheme 5. Synthesis of Iodothiochromene Derivatives 7 from Propargyl Thioethers 4.
Once we studied the electrophilic iodoarylation of thioethers 4, we envisioned that metal π-acid catalysts might behave like iodonium reagents allowing the alkyne hydroarylation reaction (Table 2).21 Considering the extraordinary ability of gold(I) complexes to activate alkynes,2b,24 we decided to start our study by employing these types of catalysts. Initial assays with IPrAuNTf2 complexes were unfruitful (entry 1). Gratifyingly, cationic gold complexes generated in situ using silver triflate as a halide scavenger could promote the cyclization generating the desired thiochromene 8aa (entry 2). However, control experiments with the silver salt revealed that AgOTf is indeed the catalyst (entries 3–6).25 Other metal triflates also afforded the desired thiochromene 8aa, although in lower proportions (entries 7 and 8). Next, we studied the nature of the silver catalyst (entries 9–11). Silver salts bearing a less coordinating counteranion gave rise to 8aa in poor yields (entries 9 and 10), possibly due to their lower stability, which results in the formation of a silver mirror. Finally, Brønsted acids were also checked as suitable catalysts. Triflic acid proved to be effective, although lower yields were achieved, presumably due to the degradation of 4aa into a diverse variety of unidentified byproducts (entries 12–14).
Table 2. Optimization of the Reaction Conditions for the Hydroarylation Reaction of 4aaa.
| entry | catalyst | mol % | temp | t (h) | yield (%)b |
|---|---|---|---|---|---|
| 1 | IPrAuNTf2 | 5 | reflux | 24 | – |
| 2 | IPrAuCl/AgOTf | 5 | reflux | 5 | 80 |
| 3 | AgOTf | 5 | reflux | 1 | 79 |
| 4 | AgOTf | 5 | reflux | 5 | 86 (83)c |
| 5 | AgOTf | 5 | rt | 24 | – |
| 6 | AgOTf | 5 | 60 | 24 | 65 |
| 7 | Bi(OTf)3 | 5 | reflux | 5 | 45 |
| 8 | Sc(OTf)3 | 5 | reflux | 5 | 28 |
| 9d | AgSbF6 | 5 | reflux | 5 | 8 |
| 10d | AgBF4 | 5 | reflux | 5 | 5< |
| 11d | AgNTf2 | 5 | reflux | 5 | 21 |
| 12 | TfOH | 5 | reflux | 5 | 40 |
| 13 | TfOH | 1 | reflux | 5 | 36 |
| 14d | TfOH | 0.5 | reflux | 24 | 19 |
Reaction conditions: 4aa (0.1 mmol) in 1,2-dichloroethane (1 mL).
Determined by 1H NMR using CH2Br2 as an internal standard.
Yield after column chromatography in parentheses.
No full conversion of 4aa was achieved.
With optimized reaction conditions in hand, we decided to evaluate the reactivity of a selection of tertiary propargyl thioethers 4 (Scheme 6). The hydroarylation was efficiently achieved when the thioaryl moiety bears electron-donating or neutral groups (8aa, 8ab, 8ae, 8ah, 8ah′, 8ha, 8ja, and 8jk), whereas substrates bearing moderate electron-withdrawing groups (4ac and 4ad) were not productive, affording inseparable mixtures of various compounds. The deactivation of the arene makes other alternative reaction pathways competitive. As expected, substitution at the meta position of the thioaryl fragment afforded a mixture of the two different possible regioisomeric thiochromenes 8ah and 8ah′ (∼2:1) derived from the 6-endo cyclization. Analogous behavior was observed utilizing propargyl thioether 4aj obtained from 2-thionaphthol. In this case, linear 8aj and angular 8aj′ thiochromenes were obtained in a 1.2:1 mixture. Propargyl sulfide 4ai derived from 1-thionaphthol gave rise to the complementary angular 2H-thiochromene 8ai. Activated alkynes bearing methoxy-functionalized arene substituents also underwent hydroarylation (8ha, 8ja, and 8jk). Similar to iodocarbocyclization, a spiro[cyclohexane-1,2′-thiochromene] (8ha) could also be accessed.
Scheme 6. Synthesis of Thiochromene Derivatives 8 through Hydroarylation of Propargyl Thioethers 4.
The reaction was performed at 110 °C under microwave irradiation.
Synthesis of AGN194310
To further demonstrate the potential of our methodology, we decided to implement the developed process to synthesize relevant biologically active compounds. Pan-RAR antagonist AGN194310 is a thiochromene derivative that possesses significant activity in RAR signaling10,26 and anticancer activity.27 This compound has been synthesized in 11 steps with an overall yield of 3.5%, involving a critical 4-thiochromenone intermediate.10 In this context, we envisaged that the combination of PTSA-catalyzed thiolation of tertiary propargylic alcohols followed by alkyne hydroarylation could significantly shorten the previously established synthetic route (Scheme 7).
Scheme 7. Synthesis of pan-RAR Antagonist AGN194310 16.

Reaction conditions: (a) 1 mol % PdCl2(PPh3)2, 1 mol % CuI, DIPA, 60 °C; (b) 5 mol % PTSA/MeNO2, rt; (c) ethyl 4-ethynylbenzoate 13, 5 mol % PdCl2(MeCN)2, 10 mol % PtBu3, 5 mol % CuI, Et3N, reflux; (d) 10 mol % AgOTf, 128 °C, MW (150 W), 25 min/1,2-DCE; (e) NaOH, 2:1 THF/Et2O, rt.
The strategy features cheap and readily available starting materials. At the outset, the Sonogashira cross-coupling between commercially available 2-methyl-3-butyn-2-ol 9 and 1-bromo-4-ethylbenzene 10 led quantitatively to 11. PTSA-catalyzed thiolation gave rise to the corresponding tertiary S-aryl propargyl thioether 12 in high yield. Not surprisingly, the alkyne hydroarylation reaction to access the thiochromene core was inefficient with this substrate, likely due to the incompatibility between the bromo-substituted S-aryl propargyl thioethers and the silver catalyst used in the process. Another Sonogashira coupling occurred before the final cyclization to circumvent this issue, furnishing an alternative propargyl sulfide 14 bearing two differentiated alkynes. After careful tuning of the reaction conditions, using microwave irradiation and AgOTf (10 mol %) as a catalyst, selective activation of the propargyl alkyne occurs to generate the thiochromene core. Finally, using a well-established methodology for the ester’s deprotection allowed us to obtain AGN194310. This synthetic sequence affords the pan-RAR antagonist in only five steps in a 22% overall yield.
Conclusions
In summary, herein, we have described the first electrophilic iodo- and hydroarylation of S-aryl propargyl thioethers to synthesize densely substituted 2H-thiochromenes by using NIS and a silver salt as the electrophilic reagent and catalyst, respectively. The applicability of this method was demonstrated by the synthesis of the highly selective retinoic acid receptor antagonist AGN194310. Upon application of this strategy, the synthesis was considerably shortened from 11 to 5 steps. The gem-disubstituent effect plays a crucial role in favoring 6-endo-dig iodo and hydroarylation reactions. The absence of substituents at the propargylic position makes other alternative reaction pathways competitive. Additionally, we have developed a reliable, easily scalable methodology for synthesizing the required S-aryl propargyl thioethers bearing a quaternary center at the propargylic position by employing PTSA as a cheap and readily available catalyst.
Experimental Section
General Methods
All reactions involving air-sensitive compounds were carried out under a N2 atmosphere (99.99%). All glassware was oven-dried (120 °C), evacuated, and purged with nitrogen. Temperatures were reported as bath temperatures. All common reagents and solvents were obtained from commercial suppliers and used without further purification. Non-commercially available propargyl alcohols were prepared following previously described procedures: addition of alkynyl organometallic to a carbonyl16c,28 and/or Sonogashira cross-coupling reaction.29 Solvents were dried following standard methods. Hexane and ethyl acetate were purchased as extra pure grade reagents and used as received. TLC was performed on alumina-backed plates coated with silica gel 60 with the F254 indicator; the chromatograms were visualized by UV light (254 nm) and/or by staining with a Ce/Mo reagent, anisaldehyde, or phosphomolybdic acid solution and subsequent heating. Rf values refer to silica gel. Flash column chromatography was carried out on silica gel 60, 230–400 mesh. 1H and 13C NMR spectra were recorded on a Varian Mercury-Plus (300 MHz for 1H, 75.4 MHz for 13C) or Bruker Avance (300 MHz for 1H, 75.4 MHz for 13C, 282 MHz for 19F) spectrometer at room temperature. NMR splitting pattern abbreviations are as follows: s, singlet; br s, broad singlet; d, doublet; dd, doublet of doublets; dt, doublet of triplets; ddd, doublet of doublet of doublets; t, triplet; td, triplet of doublets; q, quartet; quint, quintet; sext, sextet; m, multiplet. Chemical shifts are reported in parts per million using the residual solvent peak as a reference (CDCl3, 1H δ 7.26 and 13C δ 77.16; DMSO-d6, 1H δ 2.50 and 13C δ 39.50; acetone-d6, 1H δ 2.05 and 13C δ 206.26, 29.84), and the multiplicities of 13C signals were determined by DEPT experiments.
GC-MS spectra were recorded on an Agilent 6890N/5973 Network GC System, equipped with an HP-5MS column or a Thermo 1300GC instrument equipped with an MS 7000ISQ STDNOVPI MS detector, using Chromeleon software. Low-resolution electron impact mass spectra (EI-LRMS) were obtained at 70 eV on a mass spectrometer, and only the molecular ions and/or base peaks, as well as significant peaks in MS, are given. High-resolution mass spectrometry (HRMS) was carried out on a 6545 Q-TOF (Agilent) mass spectrometer (ESI or APCI as ion source) as specified.
Reactions were carried out in common Pyrex round-bottom flasks, and those performed under microwave irradiation in 10 mL microwave vials crimped on top with 20 mm Sil/PTFE septa. When needed, pH values were determined using pH indicator strips (pH 0–14 Universal indicator paper, Merck MColorpHaspt). Microwave irradiation was realized with a CEM Discover S-Class Reactor with a single-mode microwave cavity producing continuous irradiation. Temperature measurements were conducted using an IR sensor located below the microwave cavity floor, and reaction times refer to the total hold time at the indicated temperature. The maximum wattage supplied was 220 W.
General Procedure A for the Synthesis of Propargyl Sulfides 4 from Alcohols 3
Thiol 2 (1.3 equiv, 0.56 mmol) and p-toluenesulfonic acid (4 mg, 0.02 equiv, 5 mol %) were sequentially added to a solution of the corresponding propargyl alcohol 3 (1 equiv, 0.4 mmol) in MeNO2 (0.8 mL, 0.5 M). The mixture was allowed to stir at rt for 30 min, until full depletion of the alcohol was determined by TLC (spots were visualized using Ce/Mo reagent and heat as the staining agent). Then, the reaction was quenched by the addition of aqueous NaOH (0.5 M, 10 mL) and CH2Cl2 (2 mL). The separated aqueous phase was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent, hexane/EtOAc mixture), affording the corresponding propargyl sulfides 4.
(2-Methyl-4-phenylbut-3-yn-2-yl) (p-Tolyl) Sulfide (4aa)
Compound 4aa was prepared according to general procedure A (reaction time, 30 min). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4aa (81% yield, 87 mg). Pale yellow liquid. Rf = 0.22 (hexane). 1H NMR (300 MHz, CDCl3, 25 °C): δ 7.69–7.66 (m, 2H), 7.46–7.42 (m, 2H), 7.38–7.33 (m, 3H), 7.25–7.22 (m, 2H), 2.44 (s, 3H), 1.71 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 139.3 (C), 137.1 (2 × CH), 131.6 (2 × CH), 129.4 (2 × CH), 129.0 (C), 128.2 (2 × CH), 128.0 (CH), 123.4 (C), 94.2 (C), 83.2 (C), 42.5 (C), 30.4 (2 × CH3), 21.4 (CH3). LRMS (EI) m/z (%): 143 (100), 128 (36), 233 (14), 251 (12), 266 (M+, 10). HRMS (ESI+) m/z: [M + H]+ calcd for C18H19S, 267.1202; found, 267.1205.
(4-Methoxyphenyl) (2-Methyl-4-phenylbut-3-yn-2-yl) Sulfide (4ab)
Compound 4ab was prepared according to general procedure A (reaction time, 30 min). The crude product was purified by flash column chromatography on silica gel (50:1 hexane/EtOAc), affording pure 4ab (58% yield, 65 mg). Pale yellow oil. Rf = 0.12 (50:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.68–7.62 (m, 2H), 7.42–7.36 (m, 2H), 7.34–7.30 (m, 3H), 6.95–6.90 (m, 2H), 3.85 (s, 3H), 1.65 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 160.8 (C), 138.8 (2 × CH), 131.6 (2 × CH), 128.3 (2 × CH), 128.0 (CH), 123.4 (C), 114.1 (2 × CH), 94.2 (C), 83.3 (C), 55.4 (CH3), 42.7 (C), 30.3 (2 × CH3), one C peak missed due to overlapping. LRMS (EI) m/z (%): 128 (100), 175 (67), 115 (50), 77 (50), 282 (M+, 27). HRMS (ESI+) m/z: [M + H]+ calcd for C18H19OS, 283.1151; found, 283.1156.
(4-Chlorophenyl) (2-Methyl-4-phenylbut-3-yn-2-yl) Sulfide (4ac)
Compound 4ac was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4ac (81% yield, 90 mg). Pale yellow solid. Mp: 49–51 °C. Rf = 0.24 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.72–7.71 (m, 1H), 7.69–7.68 (m, 1H), 7.44–7.39 (m, 3H), 7.37–7.34 (m, 4H), 1.71 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 138.2 (2 × CH), 135.6 (C), 131.5 (2 × CH), 131.1 (C), 128.7 (2 × CH), 128.3 (2 × CH), 128.1 (CH), 123.1 (C), 93.7 (C), 83.6 (C), 42.9 (C), 30.4 (2 × CH3). LRMS (EI) m/z (%): 143 (100), 128 (32), 286 (M+, 5). HRMS (ESI+) m/z: [M + H]+ calcd for C17H16ClS, 287.0656; found, 287.0651.
(4-Bromophenyl) (2-Methyl-4-phenylbut-3-yn-2-yl) Sulfide (4ad)
Compound 4ad was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4ad (79% yield, 102 mg). White oil. Rf = 0.5 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.63–7.58 (m, 2H), 7.55–7.50 (m, 2H), 7.41–7.33 (m, 5H), 1.68 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 138.4 (2 × CH).131.8 (C), 131.8 (2 × CH), 131.6 (2 × CH), 128.4 (2 × CH), 128.2 (CH), 124.0 (C), 123.1 (C), 93.7 (C), 83.7 (C), 42.9 (C), 30.5 (2 × CH3). LRMS (EI) m/z (%): 143 (100), 128 (38), 108 (15), 330 (M+, 5). HRMS (ESI+) m/z: [M + H]+ calcd for C17H16BrS, 331.0151; found, 331.0149.
(2-Methyl-4-phenylbut-3-yn-2-yl) (Phenyl) Sulfide (4ae)
Compound 4ae was prepared according to general procedure A (reaction time, 30 min). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4ae (70% yield, 71 mg). Pale yellow liquid. Rf = 0.23 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.71–7.75 (m, 2H), 7.35–7.43 (m, 6H), 7.28–7.33 (m, 2H), 1.67 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3, 25 °C): δ 137.1 (2 × CH), 132.6 (C), 131.6 (2 × CH), 129.2 (CH), 128.6 (2 × CH), 128.3 (2 × CH), 128.1 (CH), 123.4 (C), 94.1 (C), 83.4 (C), 42.7 (C), 30.6 (2 × CH3). NMR data are in full agreement with previously described data.19 LRMS (EI) m/z (%): 143 (100), 128 (64), 115 (31), 65 (28), 252 (M+, 3).
(2-Bromophenyl) (2-Methyl-4-phenylbut-3-yn-2-yl) Sulfide (4af)
Compound 4af was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4af (82% yield, 110 mg). Yellow liquid. Rf = 0.2 (hexane). 1H NMR (300 MHz, CDCl3): δ 8.02 (dd, J = 7.72, 1.69 Hz, 1H), 7.72 (dd, J = 7.93, 1.35 Hz, 1H), 7.44–7.40 (m, 2H), 7.37–7.32 (m, 4H), 7.23 (td, J = 7.86, 1.70 Hz, 1H), 1.77 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 137.9 (CH), 134.6 (C), 133.4 (CH), 131.7 (2 × CH), 131.0 (C), 130.1 (CH), 128.4 (2 × CH), δ 128.3 (CH), 127.5 (CH), 123.3 (C), 93.6 (C), 83.9 (C), 44.3 (C), 30.8 (2 × CH3). LRMS (EI) m/z (%): 143 (100), 128 (30), 251 (13), 108 (12), 330 (M+, 10). HRMS (ESI+) m/z: [M + H]+ calcd for C17H16BrS, 331.0151; found, 331.0149.
(2-Chlorophenyl) (2-Methyl-4-phenylbut-3-yn-2-yl) Sulfide (4ag)
Compound 4ag was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4ag (75% yield, 86 mg). Yellow oil. Rf = 0.23 (hexane). 1H NMR (300 MHz, CDCl3): δ 8.03–8.01 (m, 1H), 7.57–7.54 (m, 1H), 7.46–7.43 (m, 2H), 7.37–7.34 (m, 5H), 1.79 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 139.8 (C),138.4 (CH), 132.1 (C), 131.5 (2 × CH), 130.1 (CH), 129.9 (CH), 128.2 (2 × CH), 128.1 (CH), 126.7 (CH), 123.1 (C), 93.5 (C), 83.7 (C), 44.1 (C), 30.7 (2 × CH3). LRMS (EI) m/z (%): 143 (100), 128 (40), 77 (13), 127 (12), 286 (M+, 3). HRMS (ESI+) m/z: [M + H]+ calcd for C17H16ClS, 287.0656; found, 287.0652.
(3-Methoxyphenyl) (2-Methyl-4-phenylbut-3-yn-2-yl) Sulfide (4ah)
Compound 4ah was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (20:1 hexane/EtOAc), affording pure 4ah (68% yield, 73 mg). Yellow oil. Rf = 0.35 (20:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.48–7.45 (m, 2H), 7.42–7.41 (m, 1H), 7.39–7.34 (m, 5H), 7.04–7.00 (m, 1H), 3.77 (s, 3H), 1.76 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.3 (C), 133.6 (C), 131.5 (2 × CH), 129.2 (CH), 128.9 (CH), 128.2 (2 × CH), 128.0 (CH), 123.2 (C), 121.5 (CH), 115.3 (CH), 94.1 (C), 83.3 (C), 55.1 (CH3), 42.5 (C), 30.5 (2 × CH3). 139 LRMS (EI) m/z (%): 143 (100), 138 (33), 267 (22), 282 (M+, 15). HRMS (ESI+) m/z: [M + H]+ calcd for C18H19OS, 283.1151; found, 283.1155.
(2-Methyl-4-phenylbut-3-yn-2-yl) (Naphthalen-1-yl) Sulfide (4ai)
Compound 4ai was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4ai (83% yield, 102 mg). Orange oil. Rf = 0.22 (hexane). 1H NMR (300 MHz, CDCl3): δ 8.96–8.93 (m, 1H), 8.18–8.10 (m, 1H), 8.02–7.94 (m, 2H), 7.65–7.54 (m, 3H), 7.34–7.30 (m, 3H), 7.26–7.23 (m, 2H), 1.80 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 137.1 (CH), 136.4 (C), 134.0 (C), 131.3 (CH), 130.2 (CH), 130.1 (C), 128.1 (CH), 127.9 (CH), 127.7 (CH), 127.1 (CH), 126.4 (CH), 125.9 (CH), 125.1 (CH), 123.0 (C), 94.0 (C), 83.5 (C), 43.7 (C), 30.8 (2 × CH3). LRMS (EI) m/z (%): 143 (100), 115 (50), 302 (M+, 35). HRMS (ESI+) m/z: [M + H]+ calcd for C21H19S, 303.1202; found, 303.1201.
(2-Methyl-4-phenylbut-3-yn-2-yl) (Naphthalen-2-yl) Sulfide (4aj)
Compound 4aj was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4aj (71% yield, 88 mg). Colorless solid. Mp: 62–64 °C. Rf = 0.16 (hexane). 1H NMR (300 MHz, CDCl3): δ 8.40 (s, 1H), 7.97–7.92 (m, 4H), 7.63–7.60 (m, 2H), 7.52–7.48 (m, 2H), 7.41–7.38 (m, 3H), 1.85 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 136.8 (CH), 133.6 (CH), 133.4 (C), 133.4 (C), 131.5 (2 × CH), 131.0 (C), 128.2 (2 × CH), 128.0 (CH), 128.0 (CH), 127.9 (CH), 127.7 (CH), 126.8 (CH), 126.3 (CH), 123.2 (C), 94.1 (C), 83.6 (C), 42.9 (C), 30.6 (2 × CH3). LRMS (EI) m/z (%): 143 (100), 115 (43), 128 (40), 302 (M+, 37). HRMS (ESI+) m/z: [M + H]+ calcd for C21H19S, 303.1202; found, 303.1203.
(2,4-Diphenylbut-3-yn-2-yl) (p-Tolyl) Sulfide (4ba)
Compound 4ba was prepared according to general procedure A (reaction time, 30 min). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4ba (90% yield, 120 mg). Colorless oil. Rf = 0.17 (hexane). 1H NMR (300 MHz, CDCl3, 25 °C): δ 7.68–7.64 (m, 2H), 7.44–7.42 (m, 2H), 7.35–7.29 (m, 8H), 7.08–7.05 (m, 2H), 2.34 (s, 3H), 2.02 (s, 3H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 142.6 (C), 139.4 (C), 136.9 (2 × CH), 131.5 (2 × CH), 129. (C), 129.0 (2 × CH), 128.2 (2 × CH), 128.1 (CH), 128.0 (2 × CH), 127.3 (CH), 126.8 (2 × CH), 123.2 (C), 91.9 (C), 86.8 (C), 50.2 (CH), 29.9 (CH3), 21.3 (CH3). LRMS (EI) m/z (%): 205 (100), 127 (18), 328 (M+, 14). HRMS (ESI+) m/z: [M + H]+ calcd for C23H21S, 329.1358; found, 329.1361.
(1-Methoxy-2,4-diphenylbut-3-yn-2-yl) (p-Tolyl) Sulfide (4ca)
Compound 4ac was prepared according to general procedure A (reaction time, 30 min). The crude product was purified by flash column chromatography on silica gel (50:1 hexane/EtOAc), affording pure 4ac (65% yield, 92 mg). Orange oil. Rf = 0.23 (50:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.77–7.74 (m, 2H), 7.47–7.44 (m, 2H), 7.41–7.40 (m, 1H), 7.39–7.31 (m, 7H), 7.09 (d, J = 7.8 Hz, 2H), 4.09 (d, J = 9.7 Hz, 1H), 3.91 (d, J = 9.7 Hz, 1H), 3.45 (s, 3H), 2.36 (s, 3H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 139.6 (C), 139.4 (C), 137.3 (2 × CH), 131.8 (2 × CH), 129.3 (2 × CH), 128.4 (2 × CH), 128.3 (2 × CH), 128.3 (2 × CH), 128.1 (C), 127.8 (2 × CH), 123.2 (C), 89.4 (C), 88.5 (C), 79.0 (CH2), 59.9 (CH3), 55.1 (C), 21.4 (CH3). LRMS (EI) m/z (%): 207 (100), 221 (49), 299 (38), 281 (33), 358 (M+, 5). HRMS (ESI+) m/z: [M + H]+ calcd for C24H23OS, 359.1464; found, 359.1466.
(3-Phenyl-1,1-di-p-tolylprop-2-yn-1-yl) (p-Tolyl) Sulfide (4da)
Compound 4da was prepared according to general procedure A (reaction time, 30 min). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4da (53% yield, 87 mg). Yellow oil. Rf = 0.18 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.62 (d, J = 8.3 Hz, 4H), 7.42–7.38 (m, 2H), 7.35–7.32 (m, 3H), 7.29–7.27 (m, 2H), 7.15 (d, J = 8.1 Hz, 4H), 7.02 (d, J = 7.9 Hz, 2H), 2.37 (s, 6H), 2.33 (s, 3H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 139.7 (2 × C), 139.0 (C), 137.1 (CH), 136.3 (2 × CH), 131.7 (2 × CH), 129.9 (2 × C),129.1 (2 × CH), 128.9 (4 × CH), 128.7 (C), 128.3 (4 × CH), 128.2 (2 × CH), 123.4 (C), 91.6 (C), 88.8 (C), 59.2 (C), 21.4 (CH3), 21.2 (2 × CH3). HRMS (ESI+) m/z: [M + H]+ calcd for C30H27S, 419.1828; found, 419.1833.
(1,1-Dicyclopropyl-3-phenylprop-2-yn-1-yl) (p-Tolyl) Sulfide (4ea)
Compound 4ea was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4ea (64% yield, 80 mg). Yellow oil. Rf = 0.12 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.65 (d, J = 7.02 Hz, 2H), 7.36–7.28 (m, 5H), 7.15 (d, J = 7.9 Hz, 2H), 2.39 (s, 3H), 1.32–1.23 (m, 2H), 0.62–0.40 (m, 8H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 139.2 (C), 137.8 (2 × CH), 131.6 (2 × CH), 129.0 (2 × CH), 128.6 (C), 128.3 (2 × CH), 128.2 (CH), 123.0 (C), 86.8 (C), 85.4 (C), 56.2 (C), 21.5 (CH3), 20.3 (2 × CH), 2.6 (2 × CH2), 2.6 (2 × CH2). LRMS (EI) m/z (%): 195 (100), 136 (54), 318 (M+, 19). HRMS (APCI+) m/z: [M + H]+ calcd for C22H23S, 319.1515; found, 319.1519.
(2-Cyclopropyl-4-phenylbut-3-yn-2-yl) (p-Tolyl) Sulfide (4fa)
Compound 4fa was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4fa (66% yield, 71 mg). Pale yellow oil. Rf = 0.19 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.72 (d, J = 8.10 Hz, 2H), 7.44–7.42 (m, 2H), 7.37–7.35 (m, 3H), 7.24 (d, J = 7.9 Hz, 2H), 2.45 (s, 3H), 1.74 (s, 3H), 1.30–1.23 (m, 1H), 0.71–0.54 (m, 4H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 139.2 (C), 137.4 (2 × CH), 131.5 (2 × CH), 129.1 (2 × CH), 128.7 (C), 128.2 (2 × CH), 128.0 (CH), 123.2 (C), 89.4 (C), 85.2 (C), 49.3 (C), 29.3 (CH3), 21.2 (CH), 21.0 (CH3), 3.6 (CH2), 2.5 (CH2). LRMS (EI) m/z (%): 154 (100), 169 (98), 141 (78), 292 (M+, 8). HRMS (ESI+) m/z: [M + H]+ calcd for C20H21S, 293.1358; found, 293.1361.
[2-Cyclopropyl-4-(4-methoxyphenyl)but-3-yn-2-yl] (p-Tolyl) Sulfide (4ga)
Compound 4ga was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (20:1 hexane/EtOAc), affording pure 4ga (65% yield, 84 mg). Colorless oil. Rf = 0.37 (20:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.65–7.61 (m, 2H), 7.33–7.28 (m, 2H), 7.17–7.16 (m, 2H), 6.88–6.82 (m, 2H), 3.83 (s, 3H), 2.4 (s, 3H), 1.66 (s, 3H), 1.24–1.15 (m, 1H), 0.63–0.46 (m, 4H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.5 (C), 139.2 (C), 137.4 (2 × CH), 133 (2 × CH), 129.2 (2 × CH), 128.6 (C), 115.4 (C), 113.9 (2 × CH), 87.9 (C), 85.1 (C), 55.4 (CH3), 49.6 (C), 29.4 (CH3), 21.4 (CH3), 21.1 (CH), 3.6 (CH2), 2.5 (CH2). LRMS (EI) m/z (%): 91 (100), 231 (76), 199 (64), 115 (61), 322 (M+, 20). HRMS (ESI+) m/z: [M + H]+ calcd for C21H23OS, 323.1464; found, 323.1466.
{1-[(4-Methoxyphenyl)ethynyl]cyclohexyl} (p-Tolyl) Sulfide (4ha)
Compound 4ha was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (10:1 hexane/EtOAc), affording pure 4ha (84% yield, 113 mg). Pale yellow liquid. Rf = 0.39 (10:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.66–7.62 (m, 2H), 7.39–7.34 (m, 2H), 7.21–7.19 (m, 2H), 6.90–6.85 (m, 2H), 3.84 (s, 3H), 2.41 (s, 3H), 2.09–2.04 (m, 2H), 1.78–1.65 (m, 7H), 1.36–1.31 (m, 1H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.4 (C), 139.2 (C), 137.3 (2 × CH), 133.0 (2 × CH), 129.3 (2 × CH), 128.2 (C), 115.8 (C), 113.9 (2 × CH), 91.0 (C), 85.3 (C), 55.4 (CH3), 48.3 (C), 38.8 (2 × CH2), 25.7 (CH2), 23.8 (2 × CH2), 21.4 (CH3). LRMS (EI) m/z (%): 91 (100), 213 (91), 336 (M+, 89). HRMS (ESI+) m/z: [M + H]+ calcd for C22H25OS, 337.1621; found, 337.1626.
[1-(Phenylethynyl)cyclohexyl] (p-Tolyl) Sulfide (4ia)
Compound 4ia was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4ia (87% yield, 106 mg). Colorless oil. Rf = 0.59 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.66–7.63 (m, 2H), 7.44–7.41 (m, 2H), 7.35–7.33 (m, 3H), 7.22–7.19 (m, 2H), 2.42 (s, 3H), 2.13–2.04 (m, 2H), 1.81–1.66 (m, 7H), 1.37–1.33 (m, 1H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 139.2 (C), 137.3 (2 × CH), 131.6 (2 × CH), 129.3 (2 × CH), 128.3 (2 × CH), 128 (C), 127.9 (CH), 123.7 (C), 92.6 (C), 85.5 (C), 48.1 (C), 38.8 (2 × CH2), 25.6 (CH2), 23.7 (2 × CH2), 21.4 (CH3). LRMS (EI) m/z (%): 115 (100), 79 (92), 155 (88), 141 (81), 306 (M+, 71). HRMS (ESI+) m/z: [M + H]+ calcd for C21H23S, 307.1515; found, 307.1519.
[4-(4-Methoxyphenyl)-2-methylbut-3-yn-2-yl] (p-Tolyl) Sulfide (4ja)
Compound 4ja was prepared according to general procedure A (reaction time, 1 h). The crude product was purified by flash column chromatography on silica gel (20:1 hexane/EtOAc), affording pure 4ja (75% yield, 89 mg). Yellow oil. Rf = 0.38 (20:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.61 (d, J = 8.00 Hz, 2H), 7.34–7.31 (m, 2H), 7.21–7.18 (m, 2H), 6.85 (d, J = 8.8 Hz, 2H), 3.83 (s, 3H), 2.41 (s, 3H), 1.65 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.4 (C), 139.3 (C), 137.1 (2 × CH), 133 (2 × CH), 129.4 (2 × CH), 129.2 (C), 115.6 (C), 113.9 (2 × CH), 92.7 (C), 83.1 (C), 55.4 (CH3), 42.7 (C), 30.6 (2 × CH3), 21.4 (CH3). LRMS (EI) m/z (%): 173 (100), 115 (23), 128 (22), 296 (M+, 7). HRMS (ESI+) m/z: [M + H]+ calcd for C19H21OS, 297.1308; found, 297.1311.
(4-Chlorophenyl) [4-(4-Methoxyphenyl)-2-methylbut-3-yn-2-yl] Sulfide (4jc)
Compound 4jc was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (20:1 hexane/EtOAc), affording pure 4jc (82% yield, 103 mg). Orange oil. Rf = 0.38 (20:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.66–7.61 (m, 2H), 7.36–7.28 (m, 4H), 6.88–6.83 (m, 2H), 3.83 (s, 3H), 1.64 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.6 (C), 138.2 (2 × CH), 135.7 (C), 133 (2 × CH), 131.3 (C), 128.8 (2 × CH), 115.3 (C), 114.0 (2 × CH), 92.2 (C), 83.6 (C), 55.4 (CH3), 43.1 (C), 30.6 (2 × CH3). LRMS (EI) m/z (%): 173 (100), 128 (20), 115 (19), 316 (M+, 3). HRMS (ESI+) m/z: [M + H]+ calcd for C18H18ClOS, 317.0761; found, 317.0764.
N-(4-{[4-(4-Methoxyphenyl)-2-methylbut-3-yn-2-yl]thio}phenyl)acetamide (4jk)
Compound 4jk was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (2:1 hexane/EtOAc), affording pure 4jk (70% yield, 95 mg). Pale yellow oil. Rf = 0.12 (2:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 8.04 (s, 1H), 7.61–7.57 (m, 2H), 7.54–7.50 (m, 2H), 7.28–7.25 (m, 2H), 6.80–6.77 (m, 2H), 3.76 (s, 3H), 2.13 (s, 3H), 1.58 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 168.9 (C), 159.4 (C), 139.1 (2 × CH),137.9 (C), 132.9 (2 × CH), 127.5 (C), 119.6 (2 × CH), 115.3 (C), 113.9 (2 × CH), 92.5 (C), 83.2 (C), 55.3 (CH3), 42.9 (C), 30.4 (2 × CH3), 24.6 (CH3). LRMS (EI) m/z (%): 173 (100), 205 (92), 115 (67), 43 (64), 339 (M+, 15). HRMS (ESI+) m/z: [M + H]+ calcd for C20H22NO2S, 340.1366; found, 340.1372.
[4-(3-Methoxyphenyl)-2-methylbut-3-yn-2-yl] (p-Tolyl) Sulfide (4ka)
Compound 4ka was prepared according to general procedure A (reaction time, 1 h). The crude product was purified by flash column chromatography on silica gel (20:1 hexane/EtOAc), affording pure 4ka (93% yield, 110 mg). Yellow oil. Rf = 0.31 (20:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.63 (d, J = 7.9 Hz, 2H), 7.26–7.20 (m, 3H), 7.00 (d, J = 7.6 Hz, 1H), 6.92–6.87 (m, 2H), 3.83 (s, 3H), 2.41 (s, 3H), 1.66 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.3 (C), 139.4 (C), 137.2 (2 × CH), 129.4 (2 × CH), 129.3 (CH), 129.0 (C), 124.4 (C), 124.1 (CH), 116.4 (CH), 114.6 (CH), 94.0 (C), 83.2 (C), 55.3 (CH3), 42.5 (C), 30.43 (2 × CH3), 21.4 (CH3). LRMS (EI) m/z (%): 173 (100), 281 (23), 115 (18), 296 (M+, 16). HRMS (ESI+) m/z: [M + H]+ calcd for C19H21OS, 297.1308; found, 297.1311.
(4-Bromophenyl) [4-(3-Methoxyphenyl)-2-methylbut-3-yn-2-yl] Sulfide (4kd)
Compound 4kd was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (20:1 hexane/EtOAc), affording pure 4kd (70% yield, 102 mg). Pale yellow oil. Rf = 0.36 (20:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.62–7.57 (m, 2H), 7.54–7.50 (m, 2H), 7.30–7.21 (m, 1H), 7.00–6.97 (m, 1H), 6.92–6.88 (m, 2H), 3.83 (s, 3H), 1.67 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.4 (C), 138.4 (2 × CH), 131.8 (C), 131.7 (2 × CH), 129.4 (CH), 124.1 (CH), 124.0 (C), 116.0 (CH), 114.8 (CH), 93.4 (C), 83.7 (C), 55.3 (CH3), 42.9 (C), 30.5 (2 × CH3), one C peak missing due to overlapping. LRMS (EI) m/z (%): 173 (100), 115 (18), 128 (15), 368 (M+, 8). HRMS (ESI+) m/z: [M + H]+ calcd for C18H18BrOS, 361.0256; found, 361.0254.
(2-Fluorophenyl) [4-(3-Methoxyphenyl)-2-methylbut-3-yn-2-yl] Sulfide (4kl)
Compound 4kl was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (20:1 hexane/EtOAc), affording pure 4kl (67% yield, 81 mg). Pale yellow oil. Rf = 0.33 (20:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.80–7.74 (m, 1H), 7.44–7.36 (m, 1H), 7.23–7.13 (m, 3H), 6.97–6.94 (m, 1H), 6.89–6.84 (m, 2H), 3.79 (s, 3H), 1.70 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 164 (C, JC–F = 247.6 Hz), 159.2 (CH), 139.9 (CH), 131.8 (C, JC–F = 8.2 Hz), 129.3 (CH), 124.1 (CH, JC–F = 4.1 Hz), 124.0 (2 × CH), 119.4 (C, JC–F = 18.3 Hz), 116.4 (CH), 115.8 (CH, JC–F = 24.1 Hz), 114.6 (CH), 93.1 (C), 83.4 (C), 55.2 (CH3), 43.7 (C), 30.6 (2 × CH3). 19F NMR (282 MHz, CDCl3): δ −105.0. HRMS (ESI+) m/z: [M + H]+ calcd for C18H18FOS, 301.1057; found, 301.1061.
(2-Methyloct-3-yn-2-yl) (p-Tolyl) Sulfide (4la)
Compound 4la was prepared according to general procedure A (reaction time, 4 h). The crude product was purified by flash column chromatography on silica gel (50:1 hexane/CH2Cl2), affording pure 4la (73% yield, 72 mg). Colorless oil. Rf = 0.33 (50:1 hexane/CH2Cl2). 1H NMR (300 MHz, CDCl3): δ 7.51 (d, J = 8.1 Hz, 2H), 7.15 (dt, J = 7.8, 0.7 Hz, 2H), 2.37 (s, 3H), 2.17 (t, J = 7.0 Hz, 2H), 1.54–1.28 (m, 10H), 0.90 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 139.1 (C), 136.9 (2 × CH), 129.4 (C), 129.3 (2 × CH), 84.8 (C), 83.6 (C), 42.4 (C), 31.0 (CH2), 30.9 (2 × CH3), 22.1 (CH2), 21.4 (CH3), 18.6 (CH2), 13.8 (CH3). LRMS (EI) m/z (%): 123 (100), 246 (M, 55), 231 (30), 216 (33). HRMS (APCI+) m/z: [M + H]+ calcd for C16H23S, 247.1515; found, 247.1519.
(2,4-Diphenylbut-3-yn-2-yl) (Dodecyl) Sulfide (4bm)
Compound 4bm was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4bm (58% yield, 61 mg). Yellow oil. Rf = 0.22 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.83–7.80 (m, 2H), 7.56–7.53 (m, 2H), 7.42–7.36 (m, 5H), 7.32–7.28 (m, 1H), 2.77–2.68 (m, 1H), 2.54–2.45 (m, 1H), 2.00 (s, 3H), 1.56–1.47 (m, 2H), 1.28–1.23 (m, 18H), 0.91 (t, J = 6.73 Hz, 3H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 144.1 (C), 132.3 (2 × CH), 128.9 (2 × CH), 128.8 (2 × CH), 128.5 (C), 127.8 (CH), 127.1 (2 × CH), 123.8 (C), 92.4 (C), 86.1 (C), 47.1 (C), 32.5 (CH2), 32.1 (CH2), 32.0 (CH2), 30.2 (2 × CH2), 30.1 (CH2), 30.0 (CH2), 29.9 (CH2), 29.7 (CH2), 29.6 (CH2), 29.3 (CH2), 14.7 (CH3), 13.3 (CH3). LRMS (EI) m/z (%): 402 (100), 57 (60), 43 (38), 71 (35), 406 (M+, 5). HRMS (ESI+) m/z: [M + H]+ calcd for C28H39S, 407.2767; found, 407.2770.
Benzyl(2-cyclopropyl-4-phenylbut-3-yn-2-yl) Sulfide (4fn)
Compound 4fn was prepared according to general procedure A (reaction time, 2 h). The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 4fn (71% yield, 81 mg). Yellow oil. Rf = 0.17 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.61–7.57 (m, 2H), 7.44–7.23 (m, 6H), 7.16–7.09 (m, 2H), 2.37 (s, 2H), 1.62 (s, 3H), 1.21–1.12 (m, 1H), 0.55–0.45 (m, 4H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 139.3 (C), 137.5 (2 × CH), 131.5 (2 × CH), 129.2 (2 × CH), 128.7 (CH), 128.3 (2 × CH), 128.1 (CH), 123.3 (C), 89.5 (C), 85.2 (C), 49.5 (C), 29.3 (CH2), 21.5 (CH), 21.0 (CH3), 3.7 (CH2), 2.5 (CH2). LRMS (EI) m/z (%): 169 (100), 141 (78), 292 (M+, 12). HRMS (APCI+) m/z: [M + H]+ calcd for C20H21S, 293.1358; found, 293.1356.
Gram Scale Synthesis of Selected Propargyl Sulfides 4
To a solution of propargyl alcohol 2-methyl-4-phenylbut-3-yn-2-ol 3a (3.2 g, 1 equiv, 20 mmol) in MeNO2 (40 mL, 0.5 M) were added p-toluenethiophenol 2a (3.23 g, 1.3 equiv, 26 mmol) or p-bromothiophenol 2d (3.12 mL, 1.3 equiv, 26 mmol) and p-toluenesulfonic acid 1 (76 mg, 0.02 equiv, 5 mol %). The resulting mixture was stirred at rt for 30 min. After the alcohol was consumed, the reaction was quenched with aqueous NaOH (0.5 M, 50 mL). The separated aqueous phase was extracted with CH2Cl2 (3 × 50 mL). The combined organic layers were dried with anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent, hexane/EtOAc mixture) to afford the corresponding propargyl sulfides: (2-methyl-4-phenylbut-3-yn-2-yl) (p-tolyl) sulfide 4aa (3.78 g, 71% yield) or (2-bromophenyl) (2-methyl-4-phenylbut-3-yn-2-yl) sulfide 4ad (5.21 g, 79% yield).
Synthesis of (3-Phenylprop-2-yn-1-yl) (p-Tolyl) Sulfide 5
Primary propargyl sulfide 1-methyl-4-[(3-phenyl-2-propyn-1-yl)thio]-benzene 5 (CAS Registry No. 2306760-67-6) was prepared via a modified version of a previously described procedure.30 Compound 2-propynyl p-tolyl sulfide31 (0.36 g, 1.1 equiv, 2.2 mmol) was dissolved in diisopropylamine (4 mL, 0.5 M). Then iodobenzene (0.22 mL, 1 equiv, 2 mmol), PdCl2Ph3 (28 mg, 2 mol %), and CuI (7.6 mg, 2 mol %) were sequentially added. The reaction mixture was allowed to stir at rt for 3 h. The crude was quenched by addition of brine. The separated aqueous phase was extracted with Et2O (3 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent hexane) to afford the corresponding propargyl sulfide 5, 1-methyl-4-[(3-phenyl-2-propyn-1-yl)thio]benzene (0.31 g, 65% yield, CAS Registry No. 2306760-67-6). NMR data are in full agreement with previously described data.30
1-Methyl-4-[(3-phenyl-2-propyn-1-yl)thio]benzene (5)
Brown oil. Rf = 0.17 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.50–7.49 (m, 2H), 7.46–7.41 (m, 2H), 7.35–7.33 (m, 3H), 7.23–7.20 (m, 2H), 3.86 (s, 2H), 2.41 (s, 3H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 137.4 (C), 137.7 (2 × CH), 137.7 (2 × CH), 137.4 (C), 131.6 (2 × CH), 131.5 (C), 129.8 (2 × CH), 128.3 (2 × CH), 128.2 (CH), 123.1 (C), 85.6 (C), 83.7 (C), 24.6 (CH2), 21.2 (CH3). LRMS (EI) m/z (%): 115 (100), 89 (21), 238 (M+, 20).
Synthesis of (1,3-Diphenylprop-2-yn-1-yl) (p-Tolyl) Sulfide 6
Compound 6 was prepared according to general procedure A. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 6 (88% yield, 111 mg).
(1,3-Diphenylprop-2-yn-1-yl) (p-Tolyl) Sulfide (6)
Pale yellow liquid. Rf = 0.18 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.56–7.35 (m, 12H), 7.19 (d, J = 7.9 Hz, 2H), 5.26 (s, 1H), 2.42 (s, 3H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 138.8 (C) 138.4 (C), 135.1 (2 × CH), 131.7 (2 × CH), 129.7 (C), 129.5 (2 × CH), 128.5 (2 × CH), 128.3 (3 × CH), 128.2 (2 × CH), 127.8 (CH), 123.1 (C),87.7 (C), 86.9 (C), 44.7 (CH), 21.3 (CH3). LRMS (EI) m/z (%): 191 (100), 314 (M+, 35), 207 (18). HRMS (ESI+) m/z: [M + H]+ calcd for C22H19S, 315.1202; found, 315.1203.
General Procedure B for the Synthesis of Iodothiochromenes 7 by Iodoarylation of Propargyl Thioethers 4
N-Iodosuccinimide (58.5 mg, 1.3 equiv, 0.23 mmol) was added to a solution of propargyl thioether 4 (1 equiv, 0.2 mmol) in CH2Cl2 (2 mL, 0.1 M) at 0 °C. The reaction mixture was allowed to warm to rt. Then the reaction mixture was allowed to stir overnight (24 h) until the full depletion of the propargyl sulfide was determined by GC-MS. The reaction was quenched by the addition of saturated aqueous Na2S2O3 (10 mL). The aqueous phase was extracted with CH2Cl2 (3 × 10 mL). The combined organic layer was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. Then, the residual succinimide was precipitated by the addition of Et2O (10 mL) to the crude. The solids were filtered off through a plug of Celite and washed thoroughly with Et2O (3 × 30 mL). The filtrate was concentrated in vacuo, affording crude 3-iodothiochromenes 7, which were purified by column chromatography on silica gel (eluent, hexane/EtOAc mixture) to afford the corresponding pure thiochromenes 7.
3-Iodo-2,2,6-trimethyl-4-phenyl-2H-thiochromene (7aa)
Compound 7aa was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 7aa (75% yield, 58 mg). Yellow oil. Rf = 0.28 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.41–7.38 (m, 2H), 7.37–7.34 (m, 1H), 7.33–7.29 (m, 1H), 7.28–7.25 (m, 2H), 7.11–7.08 (m, 2H), 2.34 (s, 3H), 1.31 (s, 6H). 13C NMR (75.4 MHz, CDCl3): δ 153.7 (C), 152.5 (C), 142.7 (C), 131.0 (C), 129.8 (2 × CH), 129.3 (2 × CH), 128.7 (C), 127.4 (CH), 127.3 (CH), 123.5 (CH), 121.4 (CH), 108.1 (C), 55.1 (C), 24.7 (2 × CH3), 21.2 (CH3). LRMS (EI) m/z (%): 392 (M+, 100), 393 (23), 265 (18). HRMS (ESI+) m/z: [M + O + H]+ calcd for C18H18ISO+, 409.0119; found, 409.0122.32
3-Iodo-6-methoxy-2,2-dimethyl-4-phenyl-2H-thiochromene (7ab)
Compound 7ab was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 7ab (71% yield, 58 mg). Yellow oil. Rf = 0.26 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.39–7.36 (m, 3H), 7.34–7.25 (m, 3H), 6.85–6.82 (m, 2H), 3.81 (s, 3H), 1.27 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.1 (C), 154.5 (C), 152.5 (C), 142.7 (C), 132.4 (2 × CH), 127.3 (CH), 127.2 (CH), 124.8 (C), 123.3 (CH), 121.3 (CH), 114.7 (2 × CH), 105.9 (C), 55.5 (CH3), 55.0 (C), 24.9 (2 × CH3). LRMS (EI) m/z (%): 408 (M+, 100), 281 (76), 142 (50). HRMS (ESI+) m/z: [M + H]+ calcd for C18H18IOS, 409.0118; found, 409.0108.
6-Chloro-3-iodo-2,2-dimethyl-4-phenyl-2H-thiochromene (7ac)
Compound 7ac was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 7ac (70% yield, 59 mg). Pale yellow oil. Rf = 0.44 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.41–7.29 (m, 5H), 7.26–7.21 (m, 3H), 1.30 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 152.6 (C), 152.4 (C), 142.4 (C), 133.6 (C), 132.2 (C), 129.8 (2 × CH), 129.2 (2 × CH), 127.8 (CH), 127.4 (CH), 123.7 (CH), 121.5 (CH), 110.0 (C), 55.2 (C), 24.5 (2 × CH3). LRMS (EI) m/z (%): 412 (M+, 100), 285 (62), 142 (53). HRMS (APCI+) m/z: [M + H]+ calcd for C17H15ClIS, 412.9622; found, 412.9620.
6-Chloro-3-iodo-2,2-dimethyl-4-phenyl-2H-thiochromene (7ad)
Compound 7ad was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 7ad (67% yield, 63 mg). Yellow oil. Rf = 0.6 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.32–7.42 (m, 6H), 7.16–7.20 (m, 2H), 1.31 (s, 6H). 3C{1H} NMR (75.4 MHz, CDCl3): δ 152.4 (C), 142.4 (C), 134.3 (C), 132.1 (2 × CH), 129.9 (2 × CH), 127.8 (CH), 127.4 (CH), 123.7 (CH), 121.5 (CH), 120.0 (C), 110.0 (C), 55.2 (C), 24.5 (2 × CH3), one C peak is missing due to overlapping. LRMS (EI) m/z (%): 458 (100), 456 (M+, 84), 331 (45). HRMS (APCI+) m/z: [M + H]+ calcd for C17H15BrIS, 456.9117; found, 456.9117.
3-Iodo-2,2-dimethyl-4-phenyl-2H-thiochromene (7ae)
Compound 7ae was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 7ae (74% yield, 56 mg). Pale yellow oil. Rf = 0.33 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.42–7.40 (m, 1H), 7.39–7.37 (m, 1H), 7.36–7.35 (m, 1H), 7.33–7.31 (m, 2H), 7.30–7.27 (m, 2H), 7.26–7.25 (m, 1H), 7.23–7.19 (m, 1H), 1.32 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 153.2 (C), 152.5 (C), 142.6 (C), 134.9 (C), 129.1 (2 × CH), 128.8 (2 × CH), 127.6 (CH), 127.3 (CH), 126.4 (CH), 123.5 (CH), 121.5 (CH), 109.1 (C), 55.2 (C), 24.6 (2 × CH3). LRMS (EI) m/z (%): 378 (M+, 100), 251 (39), 142 (30). HRMS (ESI+) m/z: [M + H]+ calcd for C17H16IS, 379.0012; found, 379.0006.
3-Iodo-2,2-dimethyl-4-phenyl-2H-benzo[h]thiochromene (7ai)
Compound 7ai was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 7ai (60% yield, 52 mg). Pale yellow oil. Rf = 0.26 (hexane). 1H NMR (300 MHz, CDCl3): δ 8.51–8.47 (m, 1H), 7.91–7.88 (m, 1H), 7.79–7.76 (m, 1H), 7.65–7.57 (m, 2H), 7.56–7.53 (m, 2H), 7.42–7.36 (m, 3H), 7.34–7.33 (m, 1H), 1.23 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 153.4 (C), 152.7 (C), 142.6 (C), 134.1 (C), 132.9 (C), 131.2 (C), 128.7 (2 × CH), 128.1 (CH), 127.3 (2 × CH), 126.7 (CH), 126.4 (CH), 125.6 (CH), 125.1 (CH), 123.4 (CH), 121.3 (CH), 107.0 (C), 55.4 (C), 24.7 (2 × CH3). LRMS (EI) m/z (%): 301 (100), 284 (98), 428 (M+, 82). HRMS (ESI+) m/z: [M + H]+ calcd for C21H18IS, 429.0168; found, 429.0165.
2-Iodo-2,2-dimethyl-1-phenyl-3H-benzo[f]thiochromene (7aj)
Compound 7aj was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 7ai (62% yield, 54 mg). Pale yellow oil. Rf = 0.19 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.92–7.65 (m, 4H), 7.58–7.30 (m, 7H), 1.35 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 153.0 (C), 152.6 (C), 142.6 (C), 133.8 (C), 132.3 (C), 132.0 (C), 128.7 (CH), 127.9 (CH), 127.6 (CH), 127.4 (CH), 127.3 (CH), 127.0 (CH), 126.9 (CH), 126.7 (CH), 126.7 (CH), 125.9 (CH), 123.6 (CH), 121.5 (CH), 109.3 (C), 55.2 (C), 24.6 (2 × CH3). LRMS (EI) m/z (%): 301 (100), 284 (90), 428 (M+, 70). HRMS (APCI+) m/z: [M + H]+ calcd for C21H18IS, 429.0168; found, 429.0172.
3′-Iodo-6′-methyl-4′-phenylspiro[cyclohexane-1,2′-thiochromene] (7ia)
Compound 7ia was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 7ia (63% yield, 56 mg). Pale yellow oil. Rf = 0.48 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.80 (d, J = 7.50 Hz, 1H), 7.46–7.39 (m, 2H), 7.34–7.29 (m, 1H), 7.16–7.12 (m, 2H), 7.07 (d, J = 8.2 Hz, 2H), 2.33 (s, 3H), 2.11–2.01 (m, 2H), 1.96–1.79 (m, 5H), 1.50–1.42 (m, 1H), 1.28–1.25 (m, 2H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 153.8 (C), 151.3 (C), 143.3 (C), 135.7 (C), 132 (C), 129.8 (2 × CH), 128.7 (CH), 127.6 (2 × CH), 126.6 (CH), 124.1 (CH), 123.8 (CH), 110.9 (C), 58.5 (CH), 31.9 (2 × CH2), 25.1 (CH2), 22.6 (2 × CH2), 21.1 (CH3). LRMS (EI) m/z (%): 182 (100), 141 (58), 181 (57), 432 (M+, 52). HRMS (ESI+) m/z: [M – I]+ calcd for C21H21S, 305.1364; found, 305.1358.
3-Iodo-4-(3-methoxyphenyl)-2,2,6-trimethyl-2H-thiochromene (7ka)
Compound 7ka was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (100:1 hexane/EtOAc), affording pure 7ka (61% yield, 52 mg). Colorless oil. Rf = 0.21 (100:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.32–7.38 (m, 1H), 7.21–7.24 (m, 2H), 7.03–7.09 (m, 3H), 6.85–6.89 (m, 1H), 3.90 (s, 3H), 2.32 (s, 3H), 1.39 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 155.1 (C), 144.2 (C), 138.2 (C), 136.2 (C), 131.3 (CH), 132.1 (CH), 129.8 (CH), 128.9 (CH), 128.4 (CH), 122.3 (C), 116.2 (CH), 112.2 (C), 110 (CH), 108.9 (C), 55.8 (CH3), 55.6 (C), 25.9 (CH3), 21.8 (2 × CH3). LRMS (EI) m/z (%): 295 (100), 422 (M+, 86), 299 (78), 128 (74). HRMS (APCI+) m/z: [M – I]+ calcd for C19H19OS, 295.1157; found, 295.1152.
6-Bromo-3-iodo-4-(3-methoxyphenyl)-2,2-dimethyl-2H-thiochromene (7kd)
Compound 7kd was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 7kd (72% yield, 68 mg). Colorless oil. Rf = 0.21 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.32–7.37 (m, 3H), 7.11–7.15 (m, 2H), 7.04 (d, J = 7.4 Hz, 1H), 6.86 (d, J = 8.2 Hz, 1H), 3.89 (s, 3H), 1.36 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.6 (C), 144.7 (C), 143.7 (C), 134.2 (C), 132.3 (CH), 132.1 (CH), 130 (CH), 129.7 (CH), 122.2 (CH), 120 (C), 114 (CH), 111.9 (C), 108.9 (CH), 106.5 (C), 55.8 (CH3), 54.6 (C), 24.7 (2 × CH3). LRMS (EI) m/z (%): 299 (100), 488 (44), 486 (M+, 43). HRMS (APCI+) m/z: [M + H]+ calcd for C18H17BrIOS, 486.9223; found, 486.9223.
8-Fluoro-3-iodo-4-(3-methoxyphenyl)-2,2-dimethyl-2H-thiochromene (7kl)
Compound 7kl was prepared according to general procedure B. The crude product was purified by flash column chromatography on silica gel (100:1 hexane/EtOAc), affording pure 7kl (54% yield, 45 mg). Colorless oil. Rf = 0.29 (100:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.26–7.18 (m, 3H), 7.12–7.01 (m, 2H), 6.95 (d, J = 2.4 Hz, 1H), 6.89 (dd J = 8.2, 2.4 Hz, 1H), 3.90 (s, 3H), 1.30 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 162.3 (C), 159.6 (C), 159.0 (C), 152.6 (C), 144.3 (C, JC–F = 73.3 Hz), 131.0 (CH), 128.3 (CH, JC–F = 7.6 Hz), 124.5 (CH, JC–F = 3.7 Hz), 122.1 (CH), 121.8 (C), 115.8 (CH, JC–F = 21.6 Hz), 113.7 (CH), 108.7 (CH), 108.3 (C), 55.8 (CH3), 54.6 (C), 24.7 (2 × CH3). 19F{1H} NMR (282 MHz, CDCl3): δ −110. LRMS (EI) m/z (%): 299 (100), 426 (M+, 42), 300 (14). HRMS (ESI+) m/z: [M + H]+ calcd for C18H17FIOS, 427.0023; found, 427.0023.
General Procedure C for the Synthesis of Thiochromenes 8 by Hydroarylation of Propargyl Thioethers 4
Propargyl sulfide 4 (1 equiv, 0.2 mmol) was dissolved in 1,2-dichloroethane (1 mL, 0.2 M). Then AgOTf (2.6 mg, 0.05 equiv, 0.01 mmol) was added at once. The obtained suspension was allowed to stir at 85 °C in a preheated bath until full depletion of the propargyl thioether was determined by GC-MS. Then, the reaction mixture was allowed to cool to rt, and hexane (2 mL) was added. The mixture was filtered through a plug of silica and washed with hexane. The filtrate was concentrated under reduced pressure. The crude was purified by column chromatography on silica gel (eluent, hexane/EtOAc mixture) to afford the corresponding thiochromenes 8.
General Procedure D for the Synthesis of Thiochromenes 8 by Hydroarylation of Propargyl Thioethers 4 under Microwave Irradiation
Propargyl sulfide 3 (1 equiv, 0.2 mmol) was dissolved in 1,2-dichloroethane (1 mL, 0.2 M) in a microwave tube. Then catalyst AgOTf (2.6 mg, 0.05 equiv, 0.01 mmol) was added at once. The obtained suspension was heated under microwave irradiation at 110 °C for 10 min. Then, the reaction mixture was allowed to cool to rt, and hexane (2 mL) was added. The mixture was filtered through a plug of silica and washed with hexane. The filtrate was concentrated under reduced pressure. The crude was purified by column chromatography on silica gel (eluent, hexane/EtOAc mixture) to afford the corresponding thiochromenes 8.
2,2,6-Trimethyl-4-phenyl-2H-thiochromene (8aa)
Compound 8aa was prepared according to general procedure C. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 8aa (83% yield, 42 mg). Pale yellow oil. Rf = 0.31 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.37–7.33 (m, 3H), 7.31–7.28 (m, 2H), 7.19 (d, J = 1.8 Hz, 1H), 6.94 (d, J = 7.9 Hz, 1H), 6.85 (dd, J = 8.1, 1.8 Hz, 1H), 5.77 (s, 1H), 2.33 (s, 3H), 1.48 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 141.3 (C) 138.8 (C), 137.8 (C), 132.8 (CH), 130.5 (C), 129.4 (2 × CH), 128.8 (C), 128.5 (CH), 128.3 (2 × CH), 127.8 (CH), 127.5 (CH), 126.0 (CH), 40.9 (C), 29.1 (2 × CH3), 21.2 (CH3), LRMS (EI) m/z (%): 251 (100), 250 (20), 266 (M+, 13). HRMS (ESI+) m/z: [M + H]+ calcd for C18H19S, 267.1202; found, 267.1203.
6-Methoxy-2,2-dimethyl-4-phenyl-2H-thiochromene (8ab)
Compound 8ab was prepared according to general procedure D. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 8ab (64% yield, 36 mg). Pale yellow oil. Rf = 0.13 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.38–7.35 (m, 3H), 7.32–7.29 (m, 2H), 6.99 (d, J = 8.6 Hz, 1H), 6.93 (d, J = 2.7 Hz, 1H), 6.59–6.63 (m, 1H), 5.71 (s, 1H), 3.83 (s, 3H), 1.50 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 158.9 (C), 141.3 (C), 138.6 (C), 134.8 (C), 131.4 (CH), 129.3 (2 × CH), 129.1 (CH), 128.3 (2 × CH), 127.5 (CH), 126.4 (C), 112.7 (CH), 111.4 (CH), 55.5 (CH3), 41.3 (C), 29.1 (2 × CH3). LRMS (EI) m/z (%): 267 (100), 268 (18), 282 (M+, 14). HRMS (ESI+) m/z: [M + H]+ calcd for C18H19OS, 283.1151; found, 283.1158.
2,2-Dimethyl-4-phenyl-2H-thiochromene (8ae)
Compound 8ae (CAS Registry No. 132007-64-8) was prepared according to general procedure C. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 8ae (78% yield, 40 mg). NMR spectra are in accordance with previously described data.33 Pale yellow oil. Rf = 0.25 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.40–7.37 (m, 4H), 7.33–7.29 (m, 2H), 7.30–7.14 (m, 1H), 7.07–7.04 (m, 2H), 5.84 (s, 1H), 1.50 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 141 (C), 138.8 (C), 133.7 (C), 132.1 (C), 129.2 (2 × CH), 128.2 (2 × CH), 128.1 (CH), 127.9 (CH), 127.8 (CH), 127.6 (CH), 127.5 (CH), 125.0 (CH), 40.7 (C), 28.9 (2 × CH3). LRMS (EI) m/z (%): 237 (100), 238 (17), 252 (M+, 13).
7-Methoxy-2,2-dimethyl-4-phenyl-2H-thiochromene (8ah)
Compound 8ah was prepared according to general procedure D. The crude product (as a 1.2:1 8ah/8ah′ mixture) was purified by flash column chromatography on silica gel (hexane), affording 8ah (with small traces of 8ah′) (45% yield, 26 mg). Brown oil. Rf = 0.15 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.63–7.60 (m, 1H), 7.42–7.30 (m, 5H), 6.95 (d, J = 2.7 Hz, 1H), 6.85 (dd, J = 8.2, 2.7 Hz, 1H), 6.05 (s, 1H), 3.85 (s, 3H), 1.51 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 157.7 (C), 138.4 (C), 133.6 (C), 132.9 (C), 132.2 (CH), 128.6 (2 × CH), 128.3 (CH), 128.2 (CH), 127.5 (C), 126.8 (2 × CH), 112.8 (CH), 111.0 (CH), 55.5 (CH3), 37.4 (C), 28.8 (2 × CH3). LRMS (EI) m/z (%): 267 (100), 224 (19), 268 (19), 282 (M+, 3). HRMS (ESI+) m/z: [M + H]+ calcd for C18H19OS, 283.1151; found, 283.1156.
5-Methoxy-2,2-dimethyl-4-phenyl-2H-thiochromene (8ah′)
Compound 8ah′ was prepared according to general procedure D. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 8ah′ (with small traces of 8ah) (35% yield, 20 mg). Brown oil. Rf = 0.18 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.57–7.54 (m, 2H), 7.38–7.34 (m, 3H), 7.12 (t, J = 8 Hz, 1H), 6.84 (dd, J = 7.9, 1.2 Hz, 1H), 6.75 (dd, J = 8.1, 1.0 Hz, 1H), 5.73 (s, 1H), 3.86 (s, 3H), 1.60 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.1 (C), 138.6 (C), 131.4 (C), 130.4 (CH), 128.6 (2 × CH), 128.2 (CH), 127.2 (CH), 126.9 (C), 126.3 (2 × CH), 125.7 (C), 118.9 (CH), 110.1 (CH), 55.4 (CH3), 37.7 (C), 29.9 (2 × CH3). LRMS (EI) m/z (%): 267 (100), 252 (25), 268 (17), 282 (M+, 6). HRMS (ESI+) m/z: [M + H]+ calcd for C18H19OS, 283.1151; found, 283.1154.
2,2-Dimethyl-4-phenyl-2H-benzo[h]thiochromene (8ai)
Compound 8ai was prepared according to general procedure C. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 8ai (65% yield, 39 mg). Yellow oil. Rf = 0.24 (hexane). 1H NMR (300 MHz, CDCl3): δ 8.37–8.34 (m, 1H), 7.83–7.80 (m, 1H), 7.56–7.51 (m, 3H), 7.42–7.38 (m, 3H), 7.36–7.32 (m, 2H), 7.23 (d, J = 8.7 Hz, 1H), 5.94 (s, 1H), 1.56 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 141.3 (C), 139.9 (C), 133.1 (CH), 130.9 (C), 130.4 (C), 129.7 (C), 129.4 (2 × CH), 128.5 (C), 128.3 (3 × CH), 127.6 (CH), 126.4 (CH), 126.3 (CH), 125.8 (CH), 125.5 (CH), 124.3 (CH), 41.0 (C), 28.7 (2 × CH3). LRMS (EI) m/z (%): 287 (100), 207 (62), 302 (M+, 44). HRMS (ESI+) m/z: [M + H]+ calcd for C21H19S, 303.1202; found, 303.1202.
5-Methoxy-2,2-dimethyl-4-phenyl-2H-thiochromene (8aj)
Compound 8aj was prepared according to general procedure C. The crude (as a 1.25:1 8aj/8aj′ mixture) was purified by flash column chromatography on silica gel (hexane), affording pure 8aj (with small traces of 8aj′) (35% yield, 22 mg). Light brown solid. Mp: 88–90 °C. Rf = 0.20 (hexane). 1H NMR (300 MHz, CDCl3): δ 8.40–8.37 (m, 1H), 7.85–7.82 (m, 1H), 7.62–7.59 (m, 1H), 7.57–7.48 (m, 3H), 7.44–7.42 (m, 2H), 7.37–7.34 (m, 2H), 7.25 (d, J = 8.6 Hz, 1H), 5.81 (s, 1H), 1.58 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 141.3 (C), 139.8 (C), 133.0 (2 × CH), 130.8 (C), 130.3 (C), 129.3 (2 × CH), 128.3 (2 × CH), 127.6 (CH), 126.8 (C), 126.7 (C), 126.3 (CH), 126.2 (CH), 125.7 (CH), 125.4 (CH), 124.2 (CH), 40.9 (C), 28.6 (2 × CH3). LRMS (EI) m/z (%): 287 (100), 288 (20), 302 (M+, 17). HRMS (ESI+) m/z: [M + H]+ calcd for C21H19S, 303.1202; found, 303.1203.
3,3-Dimethyl-1-phenyl-3H-benzo[f]thiochromene (8aj′)
Compound 8aj′ was prepared according to general procedure C. The crude product was purified by flash column chromatography on silica gel (hexane), affording pure 8aj′ (with small traces of 8aj) (33% yield, 19 mg). Cream-colored solid. Mp: 87–89 °C. Rf = 0.17 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.80–7.79 (m, 2H), 7.71 (d, J = 8.5 Hz, 1H), 7.57 (d, J = 8.5 Hz, 1H), 7.29–7.26 (m, 5H), 7.13–7.07 (m, 2H), 6.08 (s, 1H), 1.50 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 143.5 (C), 139.1 (C), 135.5 (CH), 134.8 (C), 132.9 (C), 129.8 (C), 128.5 (2 × CH), 128.4 (CH), 128.1 (CH), 127.9 (C), 127.8 (2 × CH), 127.7 (CH), 127.1 (CH), 126.2 (CH), 125.1 (CH), 124.5 (CH), 41.2 (C), 27.6 (2 × CH3). LRMS (EI) m/z (%): 287 (100), 302 (M+, 31). HRMS (ESI+) m/z: [M + H]+ calcd for C21H19S, 303.1202; found, 303.1203.
4′-(4-Methoxyphenyl)-6′-methylspiro[cyclohexane-1,2′-thiochromene] (8ha)
Compound 8ha was prepared according to general procedure C. The crude product was purified by flash column chromatography on silica gel (100:1 hexane/EtOAc), affording pure 8ha (61% yield, 41 mg). Colorless oil. Rf = 0.23 (100:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.26–7.22 (m, 2H), 7.22–7.21 (m, 1H), 6.97–6.94 (m, 1H), 6.93–6.89 (m, 2H), 6.87–6.84 (m, 1H), 5.82 (s, 1H), 3.87 (s, 3H), 2.33 (s, 3H), 1.91–1.69 (m, 8H), 1.61–1.56 (m, 2H), 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.2 (C), 138.3 (C), 137.6 (C), 133.9 (C), 132.8 (C), 131.4 (C), 131.2 (C), 130.5 (2 × CH), 128.7 (CH), 127.8 (CH), 126.0 (CH), 113.7 (2 × CH), 55.4 (CH3), 45.4 (C), 37.0 (CH2), 29.1 (CH2), 25.9 (CH2), 21.9 (2 × CH2), 21.2 (CH3). LRMS (EI) m/z (%): 293 (100), 336 (M+, 40). HRMS (ESI+) m/z: [M + H]+ calcd for C22H25OS, 337.1621; found, 337.1628.
4-(3-Methoxyphenyl)-2,2,6-trimethyl-2H-thiochromene (8ja)
Compound 8ja was prepared according to general procedure C. The crude product was purified by flash column chromatography on silica gel (40:1 hexane/EtOAc), affording pure 8ja (52% yield, 32 mg). Pale yellow oil. Rf = 0.25 (40:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.25–7.21 (m, 2H), 7.20–7.19 (m, 1H), 6.99–6.96 (m, 1H), 6.94–6.90 (m, 2H), 6.89–6.85 (m, 1H), 5.74 (s, 1H), 3.86 (s, 3H), 2.19 (s, 3H), 1.47 (s, 6H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 159.2 (C), 138.3 (C), 137.7 (C), 133.7 (C), 133.1 (C), 132.1 (CH), 130.7 (C), 130.4 (2 × CH), 129.4 (C), 128.5 (CH), 127.8 (CH), 126.0 (CH), 113.7 (CH), 55.5 (CH3), 41.0 (C), 29.1 (2 × CH3), 21.2 (CH3). LRMS (EI) m/z (%): 281 (100), 282 (20), 296 (M+, 11). HRMS (ESI+) m/z: [M + H]+ calcd for C19H21OS, 297.1309; found, 297.1308.
N-[4-(4-Methoxyphenyl)-2,2-dimethyl-2H-thiochromen-6-yl]acetamide (8jk)
Compound 8jk was prepared according to general procedure C. The crude product was purified by flash column chromatography on silica gel (2:1 hexane/EtOAc), affording pure 8jk (56% yield, 38 mg). Yellow oil. Rf = 0.42 (2:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.54–7.53 (m, 1H), 7.24–7.20 (m, 3H), 7.04 (d, J = 8.5 Hz, 1H), 6.94–6.90 (m, 2H), 5.74 (s, 1H), 3.86 (s, 3H), 2.19 (s, 3H), 1.47 (s, 6H), one H corresponding to the NH group is missing. 13C{1H} NMR (75.4 MHz, CDCl3): δ 168.3 (C), 138 (C), 159.3 (C), 132.1 (C), 130.4 (2 × CH), 129.8 (CH), 128.7 (C), 128.5 (CH), 118.6 (CH), 117.8 (C), 116.4 (C), 114.1 (CH), 113.7 (2 × CH), 55.5 (CH3), 53.9 (CH3), 41.0 (C), 29.1 (2 × CH3). LRMS (EI) m/z (%): 324 (100), 282 (20), 325 (19), 339 (M+, 13). HRMS (ESI+) m/z: [M + H]+ calcd for C20H22NO2S, 340.1366; found, 340.1370.
Synthesis of AGN 194310 and Synthesis of 4-(4-Ethylphenyl)-2-methylbut-3-yn-2-ol (11)
In a Schlenk flask under a N2 atmosphere, 1-bromo-4-ethylbenzene 10 (2.7 mL, 1 equiv, 20 mmol) and 2-methylbut-3-yn-2-ol 9 (2.33 mL, 1.2 equiv, 24 mmol) were dissolved in diisopropylamine (40 mL, 0.5 M). Then PdCl2(PPh3)2 (140 mg, 1 mol %) and CuI (38 mg, 1 mol %) were added to the mixture. The obtained solution was heated at 60 °C overnight in an oil bath. The crude was quenched with brine (50 mL), and the separated aqueous phase was extracted with Et2O (3 × 50 mL). The combined organic layers were dried with anhydrous Na2SO4 and concentrated under reduced pressure, and the filtrate was concentrated under reduced pressure. Then, the crude product was purified by silica gel column chromatography (eluent, 10:1 hexane/EtOAc mixture) to afford the alkynol 4-(4-ethylphenyl)-2-methylbut-3-yn-2-ol3411 (1.74 g, 98% yield, CAS Registry No. 155105-68-3).
4-(4-Ethylphenyl)-2-methylbut-3-yn-2-ol (11)34
Yellow liquid. Rf = 0.3 (10:1 hexane/AcOEt). 1H NMR (300 MHz, CDCl3): δ 7.39–7.35 (m, 2H), 7.17–7.12 (m, 2H), 2.66 (q, J = 7.6 Hz, 2H), 1.66 (s, 6H), 1.25 (t, J = 7.6 Hz, 3H), one H corresponding to the OH group is missing, NMR spectra match those previously reported.3413C{1H} NMR (75.4 MHz, CDCl3): δ 144.6 (C), 127.8 (2 × CH), 131.6 (2 × CH), 120.0 (C), 93.2 (C), 82.3 (C), 65.6 (C), 31.6 (2 × CH3), 28.8 (CH2), 15.4 (CH3). LRMS (EI) m/z (%): 43 (100), 173 (52), 115 (30), 188 (M+, 12).
Synthesis of AGN 194310 and Synthesis of (4-Bromophenyl) [4-(4-Ethylphenyl)-2-methylbut-3-yn-2-yl] Sulfide (12)
First, p-bromothiophenol 2e (1.97 g, 1.3 equiv, 10.4 mmol) and p-toluenesulfonic acid 1 (76 mg, 5 mol %) were added to a previously prepared solution of alkynol 11 (1.46 g, 1 equiv, 8 mmol) in MeNO2 (20 mL, 0.5 M). The mixture was allowed to stir for 30 min until full depletion of the alcohol was determined by TLC; spots were visualized using UV–vis and a Ce/Mo reagent as the staining agent. Then, the reaction was quenched by the addition of aqueous NaOH (0.5 M, 30 mL). The separated aqueous phase was extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent, hexane) to afford pure propargyl sulfide 12 (1.81 g, 70% yield).
(4-Bromophenyl) [4-(4-Ethylphenyl)-2-methylbut-3-yn-2-yl] Sulfide (12)
Yellow liquid. Rf = 0.26 (hexane). 1H NMR (300 MHz, CDCl3): δ 7.78–7.62 (m, 2H), 7.60–7.56 (m, 2H), 7.31–7.27 (m, 2H), 7.17–7.14 (m, 2H), 2.67 (q, J = 7.7 Hz, 2H), 1.64 (s, 6H), 1.26 (t, J = 7.6 Hz, 3H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 144.6 (C), 138.4 (2 × CH), 131.9 (C), 131.8 (2 × CH), 131.6 (2 × CH), 128.0 (2 × CH), 124.0 (C), 120.3 (C), 92.9 (C), 83.8 (C), 43.0 (C), 30.6 (2 × CH3), 28.9 (CH2), 15.5 (CH3). LRMS (EI) m/z (%): 171 (100), 128 (24), 141 (17), 358 (M+, 2). HRMS (APCI+) m/z: [M + H]+ calcd for C19H20BrS+, 359.0464; found, 359.0461.
Synthesis of AGN 194310 and Synthesis of Ethyl 4-[(4-{[4-(4-Ethylphenyl)-2-methylbut-3-yn-2-yl]thio}phenyl)ethynyl]benzoate 14
Anhydrous triethylamine (8 mL, 0.25 M) was added to a mixture of propargyl sulfide 12 (716 mg, 1 equiv, 2 mmol) and ethyl 4-ethynylbenzoate3513 (522 mg, 1.5 equiv, 3.0 mmol) under a N2 atmosphere. Then, PdCl2(MeCN)2 (26 mg, 5 mol %), tri-tert-butylphosphonium tetrafluoroborate (58 mg, 10 mol %), and CuI (19 mg, 5 mol %) were added to the solution. This mixture was allowed to stir at 85 °C overnight (14 h) in an oil bath. The reaction was quenched by the addition of brine (10 mL). The separated aqueous phase was extracted with Et2O (3 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. Then, the crude product was purified by silica gel column chromatography (eluent, 20:1 hexane/EtOAc mixture) to afford ethyl 4-[(4-{[4-(4-ethylphenyl)-2-methylbut-3-yn-2-yl]thio}phenyl)ethynyl]benzoate 14 (750 mg, 83% yield).
4-[(4-{[4-(4-Ethylphenyl)-2-methylbut-3-yn-2-yl]thio}phenyl)ethynyl]benzoate (14)
Pale yellow oil. Rf = 0.23 (20:1 hexane/EtOAc). 1H NMR (300 MHz, CDCl3): δ 1.26 (t, J = 7.6 Hz, 3H), 1.44 (t, J = 7.1 Hz, 3H), 1.68 (s, 6H), 2.67 (q, J = 7.6 Hz, 2H), 4.42 (q, J = 7.1 Hz, 2H), 7.18–7.15 (m, 2H), 7.33–7.31 (m, 2H), 7.64–7.54 (m, 4H), 7.75–7.72 (m, 2H), 8.09–8.06 (m, 2H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 166.1 (C) 144.5 (C), 136.5 (2 × CH), 134.0 (C), 131.7 (2 × CH), 131.6 (2 × CH), 131.5 (2 × CH), 130.1 (C), 129.6 (2 × CH), 127.9 (2 × CH), 127.7 (C), 123.4 (C), 120.3 (C), 93.0 (C), 91.9 (C), 90.2 (C), 83.8 (C), 61.2 (CH2), 43.1 (C), 30.7 (2 × CH3), 28.9 (CH2), 15.5 (CH3), 14.4 (CH3). LRMS (EI) m/z (%): 420 (100), 151 (51), 150 (38), 452 (M+, 3). HRMS (ESI+) m/z: [M + H]+ calcd for C30H29O2S, 453.1902; found, 453.1883.
Synthesis of AGN 194310 and Synthesis of Ethyl 4-{[4-(4-Ethylphenyl)-2,2-dimethyl-2H-thiochromen-6-yl]ethynyl}benzoate 15
To a solution of propargyl sulfide 14 (181 mg, 1 equiv, 0.4 mmol) in 1,2-dichloroethane (2 mL, 0.2 M) was added AgOTf (5.2 mg, 10 mol %). The reaction mixture was heated under microwave irradiation (128 °C, 150 W, 20 min). After that, the crude was allowed to cool to room temperature, hexane (4 mL) was added, and the mixture was filtered through a plug of silica and washed with hexane. The filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent, 20:1 hexane/EtOAc mixture) to afford ethyl 4-{[4-(4-ethylphenyl)-2,2-dimethyl-2H-thiochromen-6-yl]ethynyl}benzoate 15 (98 mg, 55% yield, CAS Registry No. 229961-27-7). NMR data were in full agreement with previously reported spectra.10
4-{[4-(4-Ethylphenyl)-2,2-dimethyl-2H-thiochromen-6-yl]ethynyl}benzoate (15)
Pale yellow oil. Rf = 0.28 (20:1 hexane/AcOEt). 1H NMR (300 MHz, CDCl3): δ 8.07–8.03 (m, 2H), 7.61–7.51 (m, 4H), 7.23–7.20 (m, 4H), 7.09 (d, J = 8.1 Hz, 1H), 5.88 (s, 1H), 4.41 (q, J = 7.1 Hz, 2H), 2.72 (q, J = 7.6 Hz, 2H), 1.50 (s, 6H), 1.43 (t, J = 7.1 Hz, 3H), 1.30 (t, J = 7.6 Hz, 3H). 13C{1H} NMR (75.4 MHz, CDCl3): δ 166.2 (C),138.5 (C), 137.9 (C), 134.5 (C), 133.8 (C), 133.5 (C), 132.3 (C), 131.6 (2 × CH), 131.5 (C), 131.0 (CH), 130.0 (C), 127.9 (2 × CH), 127.8 (CH), 121.9 (CH), 129.6 (2 × CH), 129.3 (2 × CH), 128.3 (CH), 92.1 (C), 89.6 (C), 61.3 (CH2), 41.0 (C), 29.0 (2 × CH3), 28.7 (CH2), 15.7 (CH3), 14.5 (CH3).
Synthesis of AGN 194310 and Synthesis of 4-{[4-(4-Ethylphenyl)-2,2-dimethyl-2H-thiochromen-6-yl]ethynyl}benzoic Acid 16
A solution of thiochromene 15 (90 mg, 0.2 mmol) in THF (2 mL) was treated with aqueous NaOH (2 mL, 4 M). The solution was allowed to stir at rt overnight. Then, the mixture was acidified with HCl [10% (w/w) aqueous solution]. The separated aqueous phase was extracted with EtOAc (3 × 10 mL). The combined organic layers were washed with water and brine, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent, 100:1:5 CH2Cl2/MeOH/HCOOH), affording retinoid acid antagonist AGN194310 16 (62 mg, 73% yield, CAS Registry No. 229961-45-9). NMR data were in full agreement with previously reported data.10
4-{[4-(4-Ethylphenyl)-2,2-dimethyl-2H-thiochromen-6-yl]ethynyl}benzoic Acid (16, AGN194310)
Pale yellow oil that solidifies upon refrigeration. Rf = 0.28 (100:1:5 CH2Cl2/MeOH/HCOOH). 1H NMR [300 MHz, (CD3)2CO]: δ 8.09 (d, J = 8.4 Hz, 2H), 7.72–7.69 (m, 2H), 7.59–7.58 (m, 1H), 7.59–7.58 (m, 1H), 7.33–7.28 (m, 3H), 7.24–7.21 (m, 2H), 7.10 (d, J = 8.1 Hz, 2H), 5.97 (s, 1H), 2.71 (d, J = 7.6 Hz, 2H), 1.49 (s, 6H), 1.27 (t, J = 7.6 Hz, 3H), one H corresponding to the COOH group is missing. 13C{1H} NMR [75.4 MHz, (CD3)2CO]: δ 167.1 (C), 144.8 (C), 139.0 (C),138.6 (C), 135.6 (C), 134.9 (C), 134.3 (C), 133.1 (C), 132.5 (2 × CH), 131.4 (CH), 130.7 (2 × CH), 129.9 (2 × CH), 129.1 (CH), 128.8 (2 × CH), 128.6 (CH), 128.3 (C), 122.7 (CH), 92.3 (C), 90.2 (C), 41.6 (C), 29.2 (CH2), 29.0 (2 × CH3), 16.1 (CH3).
Acknowledgments
The authors gratefully acknowledge Junta de Castilla y León and FEDER (BU291P18 and BU049P20) and Ministerio de Ciencia e Innovación and FEDER (CTQ2016-75023-C2-1-P) for financial support. The project leading to these results has received funding from “la Caixa” Foundation, under Agreement LCF/PR/PR18/51130007> (CAIXA-UBU001). N.V., F.M.-L., and S.S.-P. thank Junta de Castilla y León and FSE and FEDER for predoctoral (N.V. and F.M.-L.) and postdoctoral (S.S.-P.) contracts, respectively.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.1c00333.
Copies of 1H and 13C{1H} NMR spectra for all new compounds (PDF)
FAIR data, including the primary NMR FID files, for compounds 4aa–4aj, 4ba, 4bm, 4aa–4ka, 4jc, 4jk, 4kd, 4kl, 4fn, 5, 6, 7aa–7ae, 7ai, 7ia, 7kd, 7kl, 8aa, 8ab, 8ae, 8ah, 8ah′, 8ai, 8aj, 8aj′, 8ha, 8ja, 8jk, 11, 12, 13, 14, 15, and 16 (ZIP)
FAIR data, including the primary NMR FID files, for compounds 4al, 7aj, and 7ka (ZIP)
The authors declare no competing financial interest.
Supplementary Material
References
- a Hashmi A. S. K. Gold-catalyzed synthesis of N,O-heterocycles. Pure Appl. Chem. 2010, 82, 657–668. 10.1351/PAC-CON-09-10-17. [DOI] [Google Scholar]; b Lauder K.; Toscani A.; Scalacci N.; Castagnolo D. Synthesis and Reactivity of Propargylamines in Organic Chemistry. Chem. Rev. 2017, 117, 14091–14200. 10.1021/acs.chemrev.7b00343. [DOI] [PubMed] [Google Scholar]; c Khan T.; Yaragorla S. Iodocyclization of Propargyl Alcohols: Highly Facile Approach to Hetero/Carbocyclic Iodides. Eur. J. Org. Chem. 2019, 2019, 3989–4012. 10.1002/ejoc.201900474. [DOI] [Google Scholar]
- a Yamamoto Y. Synthesis of heterocycles via transition-metal-catalyzed hydroarylation of alkynes. Chem. Soc. Rev. 2014, 43, 1575–1600. 10.1039/C3CS60369E. [DOI] [PubMed] [Google Scholar]; b Dorel R.; Echavarren A. M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. 10.1021/cr500691k. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Boyarskiy V. P.; Ryabukhin D. S.; Bokach N. A.; Vasilyev A. V. Alkenylation of Arenes and Heteroarenes with Alkynes. Chem. Rev. 2016, 116, 5894–5986. 10.1021/acs.chemrev.5b00514. [DOI] [PubMed] [Google Scholar]
- For selected hydroarylation examples, see:; a Pastine S. J.; Youn S. W.; Sames D. PtIV-Catalyzed Cyclization of Arene-Alkyne Substrates via Intramolecular Electrophilic Hydroarylation. Org. Lett. 2003, 5, 1055–1058. 10.1021/ol034177k. [DOI] [PubMed] [Google Scholar]; b Nishizawa M.; Takao H.; Yadav V. K.; Imagawa H.; Sugihara T. Mercuric Triflate-(TMU)3-Catalyzed Cyclization of ω-Arylalkyne Leading to Dihydronaphthalenes. Org. Lett. 2003, 5, 4563–4565. 10.1021/ol035622e. [DOI] [PubMed] [Google Scholar]; c Martin-Matute B.; Nevado C.; Cárdenas D. J.; Echavarren A. M. Intramolecular Reactions of Alkynes with Furans and Electron Rich Arenes Catalyzed by PtCl2: The Role of Platinum Carbenes as Intermediates. J. Am. Chem. Soc. 2003, 125, 5757–5766. 10.1021/ja029125p. [DOI] [PubMed] [Google Scholar]; d Nevado C.; Echavarren A. M. Intramolecular hydroarylation of alkynes catalyzed by platinum or gold: Mechanism and endo selectivity. Chem. - Eur. J. 2005, 11, 3155–3164. 10.1002/chem.200401069. [DOI] [PubMed] [Google Scholar]; e Menon R. S.; Findlay A. D.; Bissember A. C.; Banwell M. G. The Au(I)-Catalyzed Intramolecular Hydroarylation of Terminal Alkynes Under Mild Conditions: Application to the Synthesis of 2H-Chromenes, Coumarins, Benzofurans, and Dihydroquinolines. J. Org. Chem. 2009, 74, 8901–8903. 10.1021/jo902032p. [DOI] [PubMed] [Google Scholar]; f Suárez-Pantiga S.; Palomas D.; Rubio E.; González J. M. Consecutive C-H Functionalization Reactions of Arenes: Synthesis of Carbo- and Heteropolycyclic Skeletons. Angew. Chem., Int. Ed. 2009, 48, 7857–7861. 10.1002/anie.200902989. [DOI] [PubMed] [Google Scholar]; g Komeyama K.; Igawa R.; Takaki K. Cationic iron-catalyzed intramolecular alkyne-hydroarylation with electron-deficient arenes. Chem. Commun. 2010, 46, 1748–1750. 10.1039/b920695g. [DOI] [PubMed] [Google Scholar]; h Arcadi A.; Blesi F.; Cacchi S.; Fabrizi G.; Goggiamani A.; Marinelli F. Gold versus silver catalyzed intramolecular hydroarylation reactions of [(3-arylprop-2-ynyl)oxy]benzene derivatives. Org. Biomol. Chem. 2012, 10, 9700–9708. 10.1039/c2ob26763b. [DOI] [PubMed] [Google Scholar]; i Ding D.; Mou T.; Feng M.; Jiang X. Utility of Ligand Effect in Homogenous Gold Catalysis: Enabling Regiodivergent π-Bond-Activated Cyclization. J. Am. Chem. Soc. 2016, 138, 5218–5221. 10.1021/jacs.6b01707. [DOI] [PubMed] [Google Scholar]; j Alonso-Marañón L.; Sarandeses L. A.; Martínez M. M.; Pérez Sestelo J. Sequential In-catalyzed intramolecular hydroarylation and Pd-catalyzed cross-coupling reactions using bromopropargyl aryl ethers and amines. Org. Chem. Front. 2017, 4, 500–505. 10.1039/C6QO00721J. [DOI] [Google Scholar]; k Arcadi A.; Ciogli A.; Fabrizi G.; Fochetti A.; Franzini R.; Ghirga F.; Goggiamani A.; Iazzetti A. Synthesis of pyrano[2,3-f]chromen-2-ones vs. pyrano[3,2-g]chromen-2-ones through site controlled gold-catalyzed annulations. Org. Biomol. Chem. 2019, 17, 10065–10072. 10.1039/C9OB01695C. [DOI] [PubMed] [Google Scholar]; l Cervi A.; Vo Y.; Chai C. L. L.; Banwell M. G.; Lan P.; Willis A. C. Gold(I)-Catalyzed Intramolecular Hydroarylation of Phenol-Derived Propiolates and Certain Related Ethers as a Route to Selectively Functionalized Coumarins and 2H-Chromenes. J. Org. Chem. 2021, 86, 178–198. 10.1021/acs.joc.0c02011. [DOI] [PubMed] [Google Scholar]
- For a selection of iodoarylation reactions, see:; a Barluenga J.; Trincado M.; Marco-Arias M.; Ballesteros A.; Rubio E.; González J. M. Intramolecular iodoarylation reaction of alkynes: easy access to derivatives of benzofused heterocycles. Chem. Commun. 2005, 2008–2010. 10.1039/B500303B. [DOI] [PubMed] [Google Scholar]; b Zhang X.; Campo M. A.; Yao T.; Larock R. C. Synthesis of substituted quinolines by electrophilic cyclization of N-(2-alkynyl)anilines. Org. Lett. 2005, 7, 763–766. 10.1021/ol0476218. [DOI] [PubMed] [Google Scholar]; c Worlikar S. A.; Kesharwani T.; Yao T.; Larock R. C. Synthesis of 3,4-disubstituted 2H-benzopyrans through C-C bond formation via electrophilic cyclization. J. Org. Chem. 2007, 72, 1347–1353. 10.1021/jo062234s. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Moran-Poladura P.; Suárez-Pantiga S.; Piedrafita M.; Rubio E.; González J. M. Regiocontrolled gold(I)-catalyzed cyclization reactions of N-(3-iodoprop-2-ynyl)-N-tosylanilines. J. Organomet. Chem. 2011, 696, 12–15. 10.1016/j.jorganchem.2010.09.014. [DOI] [Google Scholar]; For the synthesis of related iodoheterocycles by hydroarylation, see:; e Moran-Poladura P.; Rubio E.; González J. M. Gold(I)-catalyzed hydroarylation reaction of aryl(3-iodoprop-2-yn-1-yl) ethers: synthesis of 3-iodo-2H-chromene derivative. Beilstein J. Org. Chem. 2013, 9, 2120–2128. 10.3762/bjoc.9.249. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Nakamura Y.; Sakata Y.; Hosoya T.; Yoshida S. Synthesis of Functionalized Benzopyran/Coumarin-Derived Aryne Precursors and Their Applications. Org. Lett. 2020, 22, 8505–8510. 10.1021/acs.orglett.0c03106. [DOI] [PubMed] [Google Scholar]; g Johannsen T.; Golz C.; Alcarazo M. α-Cationic Phospholes: Synthesis and Applications as Ancillary Ligands. Angew. Chem., Int. Ed. 2020, 59, 22779–22784. 10.1002/anie.202009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizer S. A.; Sycheva E. S.; Al Quntar A. A. A.; Kurmankulov N. B.; Yerzhanov K. B.; Dembitsky V. M. Propargylic Sulfides: Synthesis, Properties, and Application. Chem. Rev. 2015, 115, 1475–1502. 10.1021/cr4001435. [DOI] [PubMed] [Google Scholar]
- a Rodighiero P.; Pastorini G.; Chilin A.; Marotto A. Synthesis of methyl derivatives of linear and angular thienocoumarins and thiopyranocoumarins. J. Heterocycl. Chem. 1998, 35, 847–852. 10.1002/jhet.5570350411. [DOI] [Google Scholar]; b Chen Y.; Zhang Q.; Zhang B.; Xia P.; Xia Y.; Yang Z.-Y.; Kilgore N.; Wild C.; Morris-Natschke S. L.; Lee K.-H. Anti-AIDS agents. Part 56: Synthesis and anti-HIV activity of 7-thia-di-O-(−)-camphanoyl-(+)-cis-khellactone (7-thia-DCK) analogs. Bioorg. Med. Chem. 2004, 12, 6383–6387. 10.1016/j.bmc.2004.09.038. [DOI] [PubMed] [Google Scholar]; c Kenny R. S.; Mashelkar U. C.; Rane D. M.; Bezawada D. K. Intramolecular electrophilic hydroarylation via Claisen rearrangement: synthesis of chromenes, heterothiochromenes and hetero-dihydrothiochromenes. Tetrahedron 2006, 62, 9280–9288. 10.1016/j.tet.2006.06.079. [DOI] [Google Scholar]
- For related thiochromene synthesis from allene intermediates, see:; Zhang H.; Wang B.; Yi H.; Zhang Y.; Wang J. Rh(II)-Catalyzed [2,3]-Sigmatropic Rearrangement of Sulfur Ylides Derived from Cyclopropenes and Sulfides. Org. Lett. 2015, 17, 3322–3325. 10.1021/acs.orglett.5b01542. [DOI] [PubMed] [Google Scholar]
- a Wang W.; Li H.; Wang J.; Zu L. Enantioselective Organocatalytic Tandem Michael-Aldol Reactions: One-Pot Synthesis of Chiral Thiochromenes. J. Am. Chem. Soc. 2006, 128, 10354–10355. 10.1021/ja063328m. [DOI] [PubMed] [Google Scholar]; b Choudhury A. R.; Mukherjee S. A Catalytic Michael/Horner-Wadsworth-Emmons Cascade Reaction for Enantioselective Synthesis of Thiochromenes. Adv. Synth. Catal. 2013, 355, 1989–1995. 10.1002/adsc.201300281. [DOI] [Google Scholar]; c Ahlemeyer N. A.; Birman V. B. Asymmetric Catalytic Synthesis of Thiochromenes via an Acyl Transfer-Initiated Cascade. Org. Lett. 2016, 18, 3454–3457. 10.1021/acs.orglett.6b01639. [DOI] [PubMed] [Google Scholar]
- a Tércio J.; Ferreira B.; Catani V.; Comasseto J. V. Synthesis of 2,2-Dimethyl-2H-thiochromenes, the Sulfur Analogs of Precocenes. Synthesis 1987, 1987, 149–153. 10.1055/s-1987-27866. [DOI] [Google Scholar]; b Gabbutt C. D.; Hartley D. J.; Hepworth J. D.; Heron B. M.; Kanjia M.; Rahman M. M. Synthesis and reactivity of some 4-bromo- 2H-chromenes and 2H-thiochromenes. Tetrahedron 1994, 50, 2507–2522. 10.1016/S0040-4020(01)86967-2. [DOI] [Google Scholar]
- Johnson A. T.; Wang L.; Standeven A. M.; Escobar M.; Chandraratna R. A. S. Synthesis and biological activity of high-affinity retinoic acid receptor antagonists. Bioorg. Med. Chem. 1999, 7, 1321–1338. 10.1016/S0968-0896(99)00055-3. [DOI] [PubMed] [Google Scholar]
- a Canestrari D.; Cioffi C.; Biancofiore I.; Lancianesi S.; Ghisu L.; Ruether M.; O’Brien J.; Adamo M. F. A.; Ibrahim H. Sulphide as a leaving group: highly stereoselective bromination of alkyl phenyl sulphides. Chem. Sci. 2019, 10, 9042–9050. 10.1039/C9SC03560E. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Canestrari D.; Lancianesi S.; Badiola E.; Strinna C.; Ibrahim H.; Adamo M. F. A. Desulfurative Chlorination of Alkyl Phenyl Sulfides. Org. Lett. 2017, 19, 918–921. 10.1021/acs.orglett.7b00077. [DOI] [PubMed] [Google Scholar]
- Peng L.; Zhang X.; Zhang S.; Wang J. Au-Catalyzed Reaction of Propargylic Sulfides and Dithioacetals. J. Org. Chem. 2007, 72, 1192–1197. 10.1021/jo0618674. [DOI] [PubMed] [Google Scholar]
- Harris R. J.; Widenhoefer R. A. Gold carbenes, gold-stabilized carbocations, and cationic intermediates relevant to gold-catalysed enyne cycloaddition. Chem. Soc. Rev. 2016, 45, 4533–4551. 10.1039/C6CS00171H. [DOI] [PubMed] [Google Scholar]
- Jung M. E.; Piizzi G. gem-Disubstituent Effect: Theoretical Basis and Synthetic Applications. Chem. Rev. 2005, 105, 1735–1766. 10.1021/cr940337h. [DOI] [PubMed] [Google Scholar]
- a Inada Y.; Nishibayashi Y.; Hidai M.; Uemura S. Ruthenium-Catalyzed Propargylic Substitution Reaction of Propargylic Alcohols with Thiols: A General Synthetic Route to Propargylic Sulfides. J. Am. Chem. Soc. 2002, 124, 15172–15173. 10.1021/ja027754t. [DOI] [PubMed] [Google Scholar]; b Georgy M.; Boucard V.; Campagne J.-M. Gold(III)-Catalyzed Nucleophilic Substitution of Propargylic Alcohols. J. Am. Chem. Soc. 2005, 127, 14180–14181. 10.1021/ja0534147. [DOI] [PubMed] [Google Scholar]; c Sanz R.; Martínez A.; Álvarez-Gutiérrez J. M.; Rodríguez F. Metal-free catalytic nucleophilic substitution of propargylic alcohols. Eur. J. Org. Chem. 2006, 2006, 1383–1386. 10.1002/ejoc.200500960. [DOI] [Google Scholar]; d Zhan Z.-P.; Yang W.-Z.; Yang R.-F.; Yu J.-L.; Li J.-P.; Liu H.-J. BiCl3-Catalyzed propargylic substitution reaction of propargylic alcohols with C-, O-, S- and N-centered nucleophiles. Chem. Commun. 2006, 3352–3354. 10.1039/b606470a. [DOI] [PubMed] [Google Scholar]; e Zhan Z.-P.; Yu J.-L.; Liu H.-J.; Cui Y.-Y.; Yang R.-F.; Yang W.-Z.; Li J.-P. A General and Efficient FeCl3-Catalyzed Nucleophilic Substitution of Propargylic Alcohols. J. Org. Chem. 2006, 71, 8298–8301. 10.1021/jo061234p. [DOI] [PubMed] [Google Scholar]; f Hui H.-H.; Zhao Q.; Yang M.-Y.; She D.-B.; Chen M.; Huang G.-S. Copper(II) bromide catalyzed novel preparation of propargylic ethers and sulfides by SN1-type substitution between propargylic alcohols and alcohols or thiols. Synthesis 2008, 2008, 191–196. 10.1055/s-2007-990951. [DOI] [Google Scholar]; g Biswas S.; Samec J. S. M. The Efficiency of the Metal Catalysts in the Nucleophilic Substitution of Alcohols is Dependent on the Nucleophile and Not on the Electrophile. Chem. - Asian J. 2013, 8, 974–981. 10.1002/asia.201201178. [DOI] [PubMed] [Google Scholar]
- a Sanz R.; Miguel D.; Martínez A.; Álvarez-Gutiérrez J. M.; Rodríguez F. Brønsted Acid Catalyzed Propargylation of 1,3-Dicarbonyl Derivatives. Synthesis of Tetrasubstituted Furans. Org. Lett. 2007, 9, 727–730. 10.1021/ol0631298. [DOI] [PubMed] [Google Scholar]; b Sanz R.; Gohain M.; Miguel D.; Martínez A.; Rodríguez F. Synthesis of 3-Allenylindoles and 3-Dienylindoles by Brønsted Acid Catalyzed Allenylation of 2-Arylindoles with Tertiary Propargylic Alcohols. Synlett 2009, 2009, 1985–1989. 10.1055/s-0029-1217543. [DOI] [Google Scholar]; c Sanz R.; Miguel D.; Martínez A.; Gohain M.; García-García P.; Fernández-Rodríguez M. A.; Álvarez E.; Rodríguez F. Brønsted Acid Catalyzed Alkylation of Indoles with Tertiary Propargylic Alcohols: Scope and Limitations. Eur. J. Org. Chem. 2010, 2010, 7027–7039. 10.1002/ejoc.201001055. [DOI] [Google Scholar]; d Cabrera-Lobera N.; Velasco N.; Sanz R.; Fernández-Rodríguez M. A. Brønsted acid-catalyzed synthesis of tetrasubstituted allenes and polysubstituted 2H-chromenes from tertiary propargylic alcohols. Tetrahedron 2019, 75, 4071–4080. 10.1016/j.tet.2019.05.023. [DOI] [Google Scholar]
- a Biswas S.; Samec J. S. M. A gold(I)-catalyzed route to α-sulfenylated carbonyl compounds from propargylic alcohols and aryl thiols. Chem. Commun. 2012, 48, 6586–6588. 10.1039/c2cc32042h. [DOI] [PubMed] [Google Scholar]; b Biswas S.; Dahlstrand C.; Watile R. A.; Kalek M.; Himo F.; Samec J. S. M. Atom-Efficient Gold(I)-Chloride-Catalyzed Synthesis of α-Sulfenylated Carbonyl Compounds from Propargylic Alcohols and Aryl Thiols: Substrate Scope and Experimental and Theoretical Mechanistic Investigation. Chem. - Eur. J. 2013, 19, 17939–17950. 10.1002/chem.201302485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity A. K.; Chatterjee P. N.; Roy S. Multimetallic Ir-Sn3-catalyzed substitution reaction of π-activated alcohols with carbon and heteroatom nucleophiles. Tetrahedron 2013, 69, 942–956. 10.1016/j.tet.2012.10.086. [DOI] [Google Scholar]
- Croft R. A.; Mousseau J. J.; Choi C.; Bull J. A. Lithium-Catalyzed Thiol Alkylation with Tertiary and Secondary Alcohols: Synthesis of 3-Sulfanyl-Oxetanes as Bioisostere. Chem. - Eur. J. 2018, 24, 818–821. 10.1002/chem.201705576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sanz R.; Martínez A.; Miguel D.; Álvarez-Gutiérrez J. M.; Rodríguez F. Brønsted acid-catalyzed nucleophilic substitution of alcohols. Adv. Synth. Catal. 2006, 348, 1841–1845. 10.1002/adsc.200606183. [DOI] [Google Scholar]; b Sanz R.; Miguel D.; Álvarez-Gutiérrez J. M.; Rodríguez F. Brønsted acid catalyzed C3-selective propargylation and benzylation of indoles with tertiary alcohols. Synlett 2008, 2008, 975–978. 10.1055/s-2008-1072584. [DOI] [Google Scholar]
- For additional experiments, see the Supporting Information.
- As the reaction occurs under an air atmosphere, if the nucleophilic attack is slowed, noticeable competitive oxidation could happen.
- a Sanz R.; Martínez A.; García-García P.; Fernández-Rodríguez M. A.; Rashid M. A.; Rodríguez F. Halocyclization of o-(alkynyl)styrenes. Synthesis of 3-halo-1H-indenes. Chem. Commun. 2010, 46, 7427–7429. 10.1039/c0cc02590a. [DOI] [PubMed] [Google Scholar]; b García-García P.; Sanjuán A. M.; Rashid M. A.A.; Martínez-Cuezva A.; Fernández-Rodríguez M. A.; Rodríguez F.; Sanz R. Synthesis of Functionalized 1H-Indenes and Benzofulvenes through Iodocyclization of o-(Alkynyl)styrenes. J. Org. Chem. 2017, 82, 1155–1165. 10.1021/acs.joc.6b02788. [DOI] [PubMed] [Google Scholar]
- a Hashmi A. S. K. Gold-catalyzed organic reactions. Chem. Rev. 2007, 107, 3180–3211. 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]; b Campeau D.; León Rayo D. F.; Mansour A.; Muratov K.; Gagosz F. Gold-Catalyzed Reactions of Specially Activated Alkynes, Allenes, and Alkenes. Chem. Rev. 2020, 10.1021/acs.chemrev.0c00788. [DOI] [PubMed] [Google Scholar]
- a Fang G.; Bi X. Silver-catalysed reactions of alkynes: recent advances. Chem. Soc. Rev. 2015, 44, 8124–8173. 10.1039/C5CS00027K. [DOI] [PubMed] [Google Scholar]; b Li M.; Wu W.; Jiang H. Recent Advances in Silver-Catalyzed Transformations of Electronically Unbiased Alkenes and Alkynes. ChemCatChem 2020, 12, 5034–5050. 10.1002/cctc.202000743. [DOI] [Google Scholar]
- Walkley C. R.; Yuan Y. D.; Chandraratna R. A. S; McArthur G. A. Retinoic acid receptor antagonism in vivo expands the numbers of precursor cells during granulopoiesis. Leukemia 2002, 16, 1763–1772. 10.1038/sj.leu.2402625. [DOI] [PubMed] [Google Scholar]
- Keedwell R. G.; Zhao Y.; Hammond L. A.; Wen K.; Qin S.; Atangan L. I.; Shurland D. L.; Wallace D. M. A.; Bird R.; Reitmair A.; Chandraratna R. A. S.; Brown G. An antagonist of retinoic acid receptors more effectively inhibits growth of human prostate cancer cells than normal prostate epithelium. Br. J. Cancer 2004, 91, 580–588. 10.1038/sj.bjc.6602024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Lara F.; Suárez A.; Suárez-Pantiga S.; Tapia M. J.; Sanz R. Straight access to highly fluorescent angular indolocarbazoles via merging Au- and Mo-catalysis. Org. Chem. Front. 2020, 7, 1869–1877. 10.1039/D0QO00405G. [DOI] [Google Scholar]
- Sonogashira K.; Tohda T.; Hagihara H. A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16 (50), 4467–4470. 10.1016/S0040-4039(00)91094-3. [DOI] [Google Scholar]
- Zhang X.; Liu Z.; Yang X.; Dong Y.; Virelli M.; Zanoni G.; Anderson E. A.; Bi X. Use of trifluoroacetaldehyde N-tfsylhydrazone as a trifluorodiazoethane surrogate and its synthetic applications. Nat. Commun. 2019, 10, 284. 10.1038/s41467-018-08253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martzel T.; Lohier J. F.; Gaumont A. C.; Brière J. F.; Perrio S. Sulfinate-organocatalyzed (3 + 2) annulation reaction of propargyl or allenyl sulfones with activated amines. Eur. J. Org. Chem. 2018, 2018, 5069–5073. 10.1002/ejoc.201800749. [DOI] [Google Scholar]
- It seems that rapid oxidation of the substrate occurs during HRMS (ESI+) analysis.
- Festal D.; Nioche J. Y.; Descours D.; Bellemin R.; Decerprit J. Eur. Pat. Appl. EP 380392 A2 19900808, 1990.
- Li T.; Wang Z.; Zhang M.; Zhang H.-J.; Wen T.-B. Rh/Cu-catalyzed multiple C-H, C-C and C-N bond cleavage: facile synthesis of pyrido[2,1-a]indoles from 1-(pyridine-2-yl)-1H-indoles and γ-substituted propargyl alcohols. Chem. Commun. 2015, 51, 6777–6780. 10.1039/C5CC01412C. [DOI] [PubMed] [Google Scholar]
- Compound 13 was synthesized according to a previously described procedure:; Yashima E.; Matsushima T.; Okamoto Y. Chirality Assignment of Amines and Amino Alcohols Based on Circular Dichroism Induced by Helix Formation of a Stereoregular Poly((4-carboxyphenyl)acetylene) through Acid-Base Complexation. J. Am. Chem. Soc. 1997, 119 (27), 6345–6359. 10.1021/ja964470y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.