Abstract
Introduction
Antiretroviral therapy (ART) is considered the most effective way to prevent perinatal transmission of human immunodeficiency virus (HIV). However, there is little knowledge about the effect of ART on the brain of HIV uninfected children born to HIV infected mothers (HUC). The current study evaluated the brain’s microstructural integrity, and cognitive function in HUC compared to healthy children born to normal mothers (CHNM) and HIV infected children born to HIV infected mothers (HIC) to investigate the effect of in-utero exposure of ART on cerebral gray and white matter.
Materials and methods
Forty nine HIC, 12 HUC and 18 CHNM underwent neuropsychological (NP) assessment and a brain MRI. Diffusion tensor imaging (DTI) data was used to generate fractional anisotropy (FA) and mean diffusivity (MD) maps. Voxel wise comparison for FA and MD was performed between three groups using an analysis of covariance (ANCOVA) including age and sex as covariates, and correction for multiple comparisons (false discovery rate (FDR), p < 0.05 with minimum extended cluster size, 150 voxels). NP test scores were also compared between three groups using ANOVA with Post Hoc Bonferroni multiple comparison corrections (p < 0.05). Significantly changed FA and MD values in different brain regions in HIC and HUC compared to CHNM were used for correlation analysis with NP test scores using Pearson’s correlation.
Results
HIC and HUC groups showed significantly decreased NP test scores in various domain compared to CHNM. Significantly lower NP test scores was observed in HIC than those of HUC. HIC showed decreased FA and increased MD in multiple brain sites as compared to both CHNM and HUC. Decreased FA along with both increased and decreased MD in different brain regions was present in HUC compared to CHNM. Both positive and negative correlation of altered FA and MD values from different brain regions in HIC and HUC with NP test scores was observed.
Conclusion
The presence of brain tissue changes and neurocognitive function deficit in absence of HIV infection in HUC indicates that ART may have a detrimental impact on the developing brain. The findings of the current study underscore the need for screening of ART exposed children for neurodevelopment and cognitive abnormalities at an early stage and call for access to early interventions, and nutritional and care programs.
Keywords: Antiretroviral therapies, Human immunodeficiency virus, Diffusion tensor imaging, Neuropsychological test, Brain
Highlights
-
•
Evaluated brain integrity in HIV uninfected and ART exposed children.
-
•
HIV uninfected and ART exposed children showed altered FA and MD values.
-
•
HIV uninfected and ART exposed showed lower neuropsychological test scores.
-
•
Antiretroviral therapy (ART) affects the neurodevelopment and cognitive function.
1. Introduction
A United Nations report revealed that approximately 37.9 million people are infected with the human immunodeficiency virus (HIV) worldwide. Among them ~18.2 million are women and ~2.8 million are children aged 0–18 years (WHO, 2019). Perinatal HIV transmission is the main route of infection in children. The use of antiretroviral therapy (ART) before and during pregnancy is considered the most effective way to prevent perinatal transmission of HIV (UNICEF, 2016; Rossi and Moschese, 1991). Though ART has improved the health and survival of infected mothers and prevent HIV transmission to children, there are now very large number of children also have been exposed to ART in utero (Afran et al., 2014; Coelho et al., 2017).
Earlier studies based on monotherapy regimen did not indicate an association between ART exposure during pregnancy and growth, cognitive functions, immune function, or neurologic development of HIV uninfected children born to HIV infected mothers (HUC) (Culnane et al., 1999; Funk et al., 2007; Neri et al., 2013; Newell et al., 2003). Currently used polytherapy ART effectively crosses the placental barrier and prevents the perinatal transmission of HIV infection (McCormack and Best, 2014; Ivanovic et al., 2009). However, this approach significantly increases the risk of drug exposure to developing fetus during pregnancy and may lead to impaired growth, neurodevelopmental delays and mitochondrial abnormalities (Calamandrei et al., 2002; Venerosi et al., 2005; Brogly et al., 2007; Barret et al., 2003; Blanche et al., 1999; Williams et al., 2016; Nicholson et al., 2015; Powis et al., 2016).
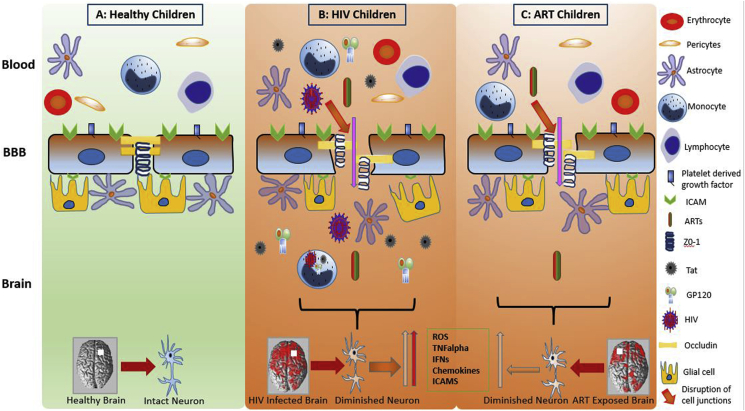
It is well documented that HIV compromises blood-brain barrier (BBB) integrity (Rahimy et al., 2017; McRae, 2016; Zhang et al., 2015; Wallet et al., 2019; Atluri et al., 2015; Louboutin and Strayer, 2012; Bertrand et al., 2019; Patel et al., 2017). The viral proteins affect the endothelial cells by modifying the small unit of GTPases and ERK1/2 and increases the generation of reactive oxygen species (ROS) (Toborek et al., 2003; Zhong et al., 2012; Pu et al., 2005). Increased levels of ROS, proinflammatory cytokines (TNF-α, IL-1β) and C-reactive proteins may further exacerbate inflammation (Brabers and Nottet, 2006; Ross et al., 2009; Younas et al., 2016). These changes result in a leaky BBB by impacting the function of endothelial cells and may subsequently lead to brain tissue damage by affecting the astrocytes and pericytes (Ahmed et al., 2018) (Fig. 1). Similarly, ART can also induce cellular toxicity including mitochondrial damage, loss of BBB integrity, and consequently brain tissue damage (Bertrand et al., 2016; Soontornniyomkij et al., 2014; Chen et al., 2013; Purnell and Fox, 2014; Chang et al., 2008; Cardenas et al., 2009; Ellis et al., 2007) (Fig. 1). For example, Efavirenz impacts the BBB integrity via reduction of tight junction proteins of endothelial cells (Bertrand et al., 2016; Bertrand and Toborek, 2015; Faltz et al., 2017). Another ART “Lopinavir” increases the expression of proinflammatory molecules such as endothelin-1 and sICAM-1 and compromises BBB integrity (Mata-Marin et al., 2013; Auclair et al., 2014). ART induced ROS may also inhibit NADH-linked mitochondrial respiration (Modica-Napolitano, 1993).
Fig. 1.
Effect of human immunodeficiency virus (HIV) and antiretroviral therapy (ART) on blood-brain barrier (BBB), neuron and brain tissue. The brain of healthy children shows intact BBB, and neuron (A). The BBB compromises in HIV infected and ART exposed children, which subsequently leads to brain tissue damage by upregulating the cytokines, pro-inflammatory markers and reactive oxygen species (ROS) (B). BBB is also compromised in children exposed to ART causing neuroinflammation and brain tissue damage (C). More brain tissue damage is obvious in HIV infected and ART exposed children than ART exposed children (B and C).
Magnetic resonance imaging (MRI) has been widely applied modality to evaluate neurodevelopment in children. Diffusion tensor imaging (DTI), a tensor-based MR imaging method, is useful for mapping the microstructural brain tissue integrity (Moseley, 2002; Fellgiebel et al., 2006; Kodl et al., 2008; Pasi et al., 2016; Jahanshad et al., 2015; Ackermann et al., 2016; Li et al., 2015; Uban et al., 2015; Walhovd et al., 2010; Jankiewicz et al., 2017a; Tran et al., 2016; Morie et al., 2017). Neda et al., observed no noticeable changes in DTI indices in the brain of HUC as compared to healthy children born to normal mothers (CHNM) (Jahanshad et al., 2015). A few studies have reported altered diffusion metrics in HUC as compared to CHNM (Jankiewicz et al., 2017a; Tran et al., 2016).
Structural, and functional brain changes associated with impaired cognitive functions are well documented in HIV infected children born to HIV infected mothers (HIC) (Yadav et al., 2017, 2018; Ragin et al., 2005; Wu et al., 2006; Ackermann et al., 2014, 2019; Andronikou et al., 2014, 2015; Patsalides et al., 2002; Cohen et al., 2016). A recent meta-analysis study has showed that HIC and HUC have poor cognitive development and motor outcomes as compared with CHNM (McHenry et al., 2018a). However, limited information is available about brain changes and cognitive function in HUC, moreover, none of the previously published studies have included all three groups (i.e. HIC, HUC, and CHNM) for a straightforward comparison. Hence, there is a clear lack of data regarding neurocognitive performance (NP) and brain changes in the HUC cohort. The current study evaluated brain microstructural tissue integrity, and cognitive function in HUC compared to CHNM and HIC to systematically investigate the effects of in-utero exposure of ART on cerebral gray and white matter, and cognitive function. We hypothesize that ART exposure to HUC may negatively impact the normal brain tissue development and neurocognitive performance.
2. Materials and Methods
The study received Human Ethics Committee and the Institutional Regulatory Board approval. Signed informed consent was obtained from all subjects or their nearest kin before the study. Forty-nine HIC, 12 HUC, and 18 age/sex-matched CHNM of the similar education and socio-economic status were included in the current study (Table 1). Both HIV infected and uninfected subjects were born to HIV infected mother. All subjects were recruited from a national center for HIV prevention and treatment located in north part of India during the period of 2011 to 2013. The parents of these children were mostly referred from primary care centers to the secondary care hospital. The diagnosis and treatment of HIV infected subjects were performed by same healthcare provider. Nutritional intake and psychological support were similar for all HIV infected subjects. The healthy controls were recruited from the same family member or relatives of the infected children. All included children were not having any visible gross brain abnormalities or comorbidity. We excluded those subjects which showed brain abnormalities such as tumor, cyst, or any other mass lesion or brain hyperintensity on T2-weighted and fluid-attenuated inversion recovery and gross brain atrophy on T1-weighted brain images. HIV diagnosis was performed at the government established centers using a standard protocol including an enzyme-linked-immunosorbent-assay (ELISA) test followed by validation from duplicate HIV rapid tests of greater sensitivity and specificity (Yadav et al., 2017).
Table 1.
Demographic, clinical and global diffusion variables of healthy children born to normal mothers (CHNM), HIV uninfected children born to HIV infected mothers (HUC), and HIV infected children (HIC) born to HIV infected mothers.
| Demographic and clinical variables | CHNM (n = 18) | HUC (n = 8) | HIC (n = 22) | P values |
|---|---|---|---|---|
| Age (years) | 12 ± 2.8 | 10 ± 1.8 | 10 ± 2.3 | 0.06 |
| Gender (M/F) | 9/9 | 4/4 | 14/8 | |
| Education | 3.8 ± 2.04 | 4.25 ± 2.12 | 3.6 ± 2.41 | 0.63 |
| Ethnicity | Asian Indian | Asian Indian | Asian Indian | – |
| Peak HIV viral load (log copy/ml) | – | – | 483 ± 240 | – |
| CD4+ T-cell count at the time of MRI ( × 106/L) | – | – | 540 ± 298 | – |
| Global Gray Matter FA | 0.178 ± 0.0066 | 0.176 ± 0.0037 | 0.164 ± 0.0083 | 0.001 |
| Global White Matter FA | 0.325 ± 0.0142 | 0.310 ± 0.0058 | 0.297 ± 0.0161 | 0.001 |
| Global Gray Matter MD ( × 10−3) mm2/s | 0.930 ± 0.031 | 0.922 ± 0.019 | 0.958 ± 0.061 | 0.087 |
| Global White Matter MD( × 10−3) mm2/s | 0.873 ± 0.018 | 0.877 ± 0.022 | 0.909 ± 0.039 | 0.001 |
All HIV-positive children underwent appropriate combination antiretroviral therapy (cART) under the HIV prevention program (Yadav et al., 2018). The initial treatment of cART includes 2 nucleoside/nucleotide (either thymidine analog Zidovudine or Stavudine together with a cytidine analog Lamivudine) and 1 non-nucleoside reverse transcriptase inhibitor (Nevirapine or Efavirenz). All HIV positive mothers received appropriate standard cART regimen. All participating children underwent clinical and neurocognitive assessment, and a brain MRI.
2.1. Neurocognitive performance (NP)
Neurocognitive function of each child was evaluated using a well-established cognitive battery (Revisie Amsterdamse Kinder Intelligentie Test (RAKIT) especially targeted to detect impaired neurocognitive skills (Khire et al., 1992). The neurocognitive battery has 9 test domains (closure, exclusion, memory span, verbal learning, mazes, quantity, learning names, discs, and hidden figures) which can evaluate executive functions, sensory and motor coordination, attention, language, recall power, learning and visual capacity (Khire et al., 1992). The raw scores of neurocognitive test were adjusted to standard scores which were used for the statistical analysis.
2.2. Brain Magnetic resonance imaging
Brain MRI images were collected on a clinical 3-T MRI (GE Healthcare, Wisconsin) using a transmit and receive 8-channel head coil. Three dimensional high resolution T1 weighted brain volumes were collected in the sagittal plane: repetition time (TR) = 8.4 ms; echo time (TE) = 3.32 ms; inversion time (TI) = 400 ms; flip angle (FA) = 13°; matrix size = 512 × 512 and field of view (FOV) = 240 × 240 mm2 with 1 mm thickness. T2 weighted images were acquired in the axial plane with TR = 5660 ms; TE = 98 ms; FA = 90°; matrix size = 256 × 256 and FOV = 240 × 240 mm2 with 3 mm slice thickness. Fluid attenuated inversion recovery (FLAIR) images were also acquired in the axial plane using TR = 9000 ms; TE = 128 ms; TI = 2400 ms; FA = 90°; matrix = 320 × 256; FOV = 240 × 240 mm2 with 3 mm slice thickness. For DTI image acquisition, a dual spin-echo single-shot echo-planar pulse sequence in the axial plane with ramp sampling was performed using the following parameters: TR = 17 s; TE = 88.7 ms; intersection gap = 0; FOV = 240 × 240 mm; image matrix = 256 × 256; NEX = 1; diffusion weighting b-factor = 1000 s/mm2 number of slice = 46; number of direction = 30 with 3 mm slice thickness. 15 HIC who showed hyperintensity on T2 weighted and FLAIR images were excluded from the analysis. Because of incomplete scans or the presence of motion artifacts another 16 children (4 HUC, and 12 HIC) were omitted from the analysis. The data quantification and statistical analysis was performed for a total of 48 children (18 CHNM, 8 HUC, and 22 HIC).
2.3. Diffusion tensor imaging data processing
DTI images were preprocessed using FMRIB Software Library (FSL version 6.0. 0., http://www.fmrib.ox.ac. uk/fsl)) package. The first step was skull stripping of non-diffusion images (B0) from each subject using a brain extraction tool. In the second step, we corrected the data for motion artifact using diffusion sensitizing gradients and geometrical distortions. In the third step, we used motion and distortion corrected images to estimate the diffusion tensor for each voxel and DTI metrics were derived [fractional anisotropy (FA) and mean diffusivity (MD)]. The newest version of FSL (Version 6.0.0) has very robust package (eddy_correct) for distortion correction induced by eddy current and simultaneously it corrects for subject motion. In addition, the FSL software also performs outlier detection to identify slices where signal is lost as a consequence of subject movement during the diffusion encoding and replaces those slices by non-parametric predictions by the Gaussian process.
Normalization and smoothing of FA and MD maps were performed by statistical parametric mapping (SPM) package 12 (Wellcome Department of Cognitive Neurology, UK) and MATLAB (MathWorks, Natick, MA), these normalized and smoothed maps (FA and MD) were used for the voxel-based analysis. In short, non-gradient diffusion-weighted images of each participant were normalized to the standard (Montreal Neurological Institute) space using earlier defined distribution probability of gray, white, and cerebrospinal fluid tissue types maps supplied with SPM12 package (Ashburner and Friston, 2005), and the obtained normalization parameters were applied on FA and MD maps followed by 8 mm Gaussian filter full-width-at-half-maximum smoothing.
3. Statistical analysis
IBM Statistical Package for the Social Sciences (SPSS 22.0) software was used for the statistical analysis. Analysis of variance (ANOVA) and Chi-square tests were performed to examine the demographic and clinical variables among the groups. NP test scores were compared between three groups using ANOVA with Post Hoc Bonferroni multiple comparison corrections (p < 0.05). We compared the normalized and smoothed FA and MD maps voxel-by-voxel between groups using ANCOVA, including age and gender as covariates and correction for multiple comparisons (false discovery rate (FDR) p < 0.05 with minimum extended cluster size, 150 voxels) and F and t value maps were generated.
The FA and MD values from the voxel at peak t value within the clusters were extracted using XYZ coordinates. These FA and MD values were used for the correlation analysis with NP test scores using Pearson’s correlation.
4. Results
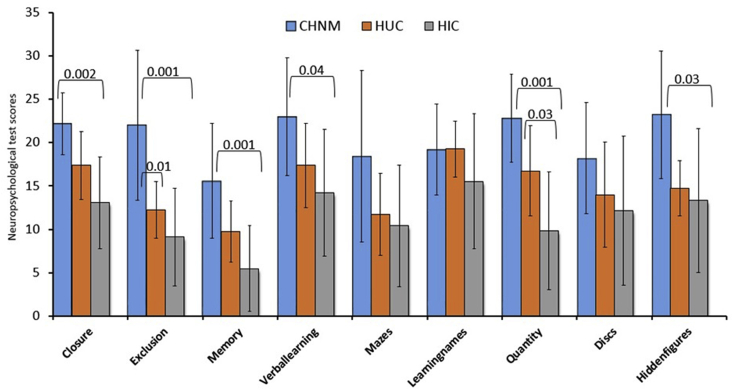
Demographic, clinical, and global brain FA and MD values of CHNM, HUC and HIC are summarized in Table 1. No significant differences in age or gender were observed between groups. HIC showed significantly lower NP test scores in 6 of 9 domains (closure, exclusion, memory, verbal learning, quantities, and hidden-figures), and HUC in 1 of 9 domains (exclusion) as compared to CHNM. HIC also showed significantly lower NP test score in 1 of 9 domains (quantity) compared to HUC (Fig. 2).
Fig. 2.
Bar plots are showing the neuropsychological test (NP) scores (mean ± SD) in healthy children born to normal mothers (CHNM), HIV uninfected children born to HIV infected mothers (HUC) and HIV infected children born to HIV infected mothers (HIC). ANOVA with Post Hoc Bonferroni multiple comparison corrections (p < 0.05) were used for comparison.
4.1. Diffusion changes between HIC and CHNM
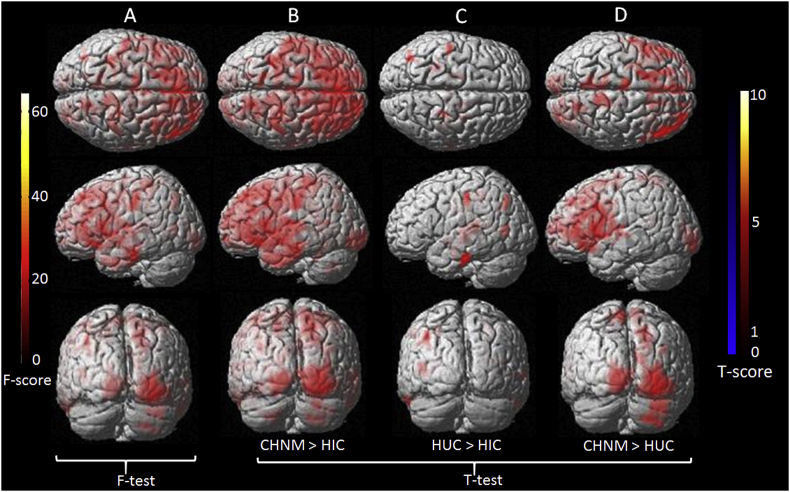
HIC showed significantly decreased FA compared to CHNM in the left cingulum, left cerebellar and left fusiform (Table 3 and Fig. 3 B).
Table 3.
Brain regions with significant between-group differences in FA.
| Condition | Brain area | Voxels | MNI coordinates (mm) |
Peak voxel t score | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| CHNM > HIC | L cingulum | 79393 | 0 | 42 | 8 | 8.94 |
| L cerebellar | 445 | −20 | −60 | −44 | 4.05 | |
| L fusiform | 246 | −40 | −52 | −32 | 3.44 | |
| HUC > HIC | R fusiform | 759 | 28 | −38 | 20 | 6.55 |
| L postcentral gyrus | 193 | −48 | −24 | 40 | 6.54 | |
| Left pulvinar | 1277 | −18 | −34 | 10 | 5.72 | |
| L inferior temporal gyrus | 245 | −60 | −22 | −26 | 5.67 | |
| L angular | 152 | −38 | −70 | 42 | 5.60 | |
| R corpus callosum | 246 | 12 | −28 | 6 | 5.42 | |
| L insula | 999 | −40 | −20 | −4 | 5.35 | |
| L hypothalamus | 661 | −8 | −4 | −6 | 5.20 | |
| R parietal lobe | 212 | 26 | −32 | 48 | 5.11 | |
| R postcentral gyrus | 246 | 50 | −18 | −22 | 4.98 | |
| R parietal lobe | 181 | −24 | −42 | 42 | 4.09 | |
| CHNM > HUC | R inferior occipital gyrus | 4120 | 40 | −74 | −8 | 6.97 |
| R middle frontal gyrus | 13437 | 42 | 50 | 14 | 6.41 | |
| R cuneus | 1552 | 22 | −62 | 36 | 6.14 | |
| R paracentral lobule | 1469 | 10 | −42 | 62 | 6.14 | |
| R insula | 454 | 38 | 16 | −12 | 5.34 | |
| R superior frontal gyrus | 217 | 14 | 32 | 52 | 4.60 | |
| L paracentral lobule | 296 | −6 | −12 | 66 | 4.38 | |
| R supramarginal gyrus | 189 | 36 | −56 | 28 | 4.33 | |
| R superior frontal gyrus | 173 | 16 | 52 | 26 | 3.83 | |
Notes: The statistical thresholds were set at P < 0.05 (corrected for false discovery rate) with a minimum cluster size of 150 voxels. L: Left; R: Right.
Fig. 3.
Voxel-wise one-way ANCOVA findings for fractional anisotropy (FA) between three groups. F-test maps are showing significant difference in FA between three groups (A). T score maps are showing significantly decreased FA value in different brain regions of HIV infected children born to HIV infected mothers (HIC) compared to children born to normal mothers (CHNM) and HIV uninfected children born to HIV infected mothers (HUC) (B, C). T score maps shows decreased FA value in multiple brain sites in HUC children compared to CHNM children (D). ANCOVA (age and gender as covariates with correction for multiple comparisons (false discovery rate, p < 0.05 with minimum extended cluster size, 150 voxels).
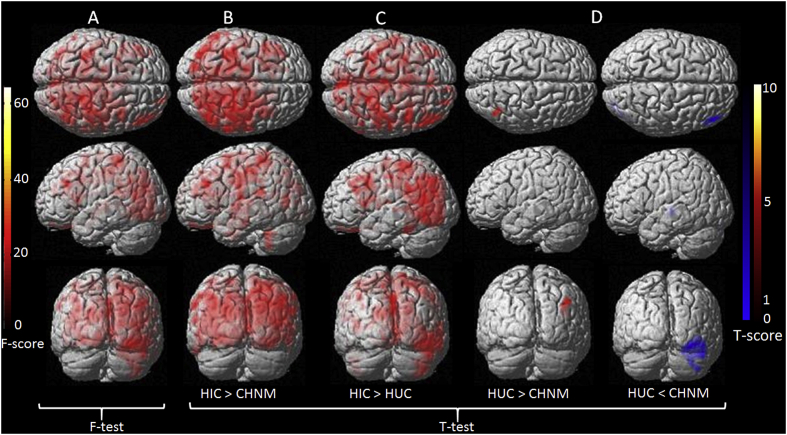
The MD was significantly increased in HIC compared to CHNM in the left precuneus, right medial frontal gyrus and right frontal lobe (Table 4 and Fig. 4 B).
Table 4.
Brain regions with significant between-group differences in MD.
| Condition | Brain area | Voxels | MNI coordinates (mm) |
Peak voxel t score | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| HIC > CHNM | L precuneus | 57704 | −8 | −54 | 14 | 9.14 |
| R medial frontal gyrus | 1404 | 8 | 8 | −22 | 4.09 | |
| R frontal lobe | 224 | −58 | −38 | −28 | 3.48 | |
| HIC > HUC | L extra nuclear | 44787 | −2 | −10 | −10 | 7.13 |
| R superior frontal gyrus | 181 | 24 | 62 | 20 | 4.96 | |
| L middle temporal gyrus | 326 | −44 | −70 | 8 | 4.82 | |
| R inferior temporal gyrus | 508 | 44 | −12 | −36 | 4.24 | |
| L cerebellar tonsil | 348 | −40 | −46 | −52 | 4.12 | |
| R culmen | 178 | 28 | −38 | −30 | 2.99 | |
| HUC > CHNM | R parietal lobe | 218 | 38 | −62 | 32 | 6.43 |
| HUC < CHNM | R cerebelum crus1 | 725 | 46 | −68 | −24 | 5.52 |
| L extra nuclear | 243 | −34 | −22 | −2 | 5.26 | |
| R middle temporal gyrus | 298 | 48 | 30 | −8 | 4.24 | |
Notes: The statistical thresholds were set at P < 0.05 (corrected for false discovery rate) with a minimum cluster size of 150 voxels. L: Left; R: Right.
Fig. 4.
Voxel-wise one-way ANCOVA findings for mean diffusivity (FA) between three groups. F-test maps are showing significant difference in MD between three groups (A). T score maps are showing significantly increased MD value in different brain regions of HIV infected children born to HIV infected mothers (HIC) compared to children born to normal mothers (CHNM) and HIV uninfected children born to HIV infected mothers (HUC) (B and C). T score maps depicts both increased and decreased MD values in HUC compared to CHNM (D). ANCOVA (age and gender as covariates with correction for multiple comparisons (false discovery rate, p < 0.05 with minimum extended cluster size, 150 voxels).
4.2. Diffusion changes between HIC and HUC
Significantly decreased FA in the right fusiform, left postcentral gyrus, left pulvinar, left inferior temporal gyrus, left angular, right corpus callosum, left insula, left hypothalamus, right parietal lobe, right postcentral gyrus, right parietal lobe was observed in HIC compared to HUC (Table 3 and Fig. 3 C).
The HIC group showed significantly increased MD in the left extra nuclear, right superior frontal gyrus, left middle temporal gyrus, right inferior temporal gyrus, left cerebellar tonsil and right culmen compared to HUC group (Table 4 and Fig. 4 C).
4.3. Diffusion changes between HUC and CHNM
HUC showed significantly decreased FA in the right inferior occipital gyrus, right middle frontal gyrus, right cuneus, right paracentral lobule, right insula, right superior frontal gyrus, left paracentral lobule, right supramarginal gyrus, right superior frontal gyrus compared to CHNM (Table 3 and Fig. 3 D).
Significantly increased MD in the right parietal lobe, while significantly decreased MD in the right cerebellum crus1, left extra nuclear, right middle temporal gyrus was observed in HUC as compared to CHNM (Table 4 and Fig. 4 D).
4.4. Correlation between significant DTI derive metrics from brain regions with cognitive functions
Both positive and negative correlations were observed between altered FA and MD from different brain regions in HIC and HUC with NP test scores (Table 2).
Table 2.
Pearson’s correlation of fractional anisotropy (FA) and mean diffusivity (MD) showing significant difference in different brain regions in HIC and HUC compared to CHNM with neuropsychological test scores.
| Group | Brain sites | Exclusion | Memory | Verbal | Mazes | Quantity | Discs | Hidden | |
|---|---|---|---|---|---|---|---|---|---|
| HIC | FA | L Postcentral | 0.42 | 0.20 | 0.50* | 0.22 | 0.34 | 0.36 | 0.15 |
| R Corpus Callosum | 0.28 | −0.08 | 0.56* | 0.33 | 0.26 | 0.34 | 0.37 | ||
| L Insula | 0.15 | −0.23 | 0.53* | 0.26 | 0.52* | 0.41 | 0.29 | ||
| L Hypothalamus | 0.33 | 0.06 | 0.56* | 0.32 | 0.44 | 0.16 | 0.08 | ||
| MD | R Medial Frontal | 0.40 | 0.48* | 0.05 | 0.02 | −0.01 | 0.31 | 0.35 | |
| R Frontal Lobe | −0.39 | −0.29 | −0.37 | −0.48* | −0.46* | −0.42 | −0.11 | ||
| R Inferior Temporal | −0.23 | −0.14 | −0.17 | −0.52* | −0.21 | −0.17 | 0.05 | ||
| R Culmen | −0.12 | −0.04 | −0.18 | −0.46* | −0.20 | −0.07 | 0.06 | ||
| HUC | FA | R Fusiform | −0.66 | 0.23 | −0.74* | 0.40 | −0.45 | 0.26 | −0.63 |
| L Pulvinar | −0.69 | 0.32 | −0.86* | 0.50 | −0.15 | 0.53 | −0.57 | ||
| L Insula | −0.71* | −0.01 | −0.52 | 0.21 | −0.40 | −0.21 | −0.72* | ||
| L Hypothalamus | 0.04 | 0.97 | 0.17 | 0.61 | 0.32 | 0.61 | 0.05 | ||
| MD | R Cerebelum Crus1 | 0.44 | −0.63 | 0.46 | −0.43 | −0.12 | −0.72* | −0.27 | |
| R Middle Temporal | 0.72* | −0.21 | 0.53 | −0.39 | 0.68 | 0.11 | 0.77* | ||
*Significant (P < 0.05) correlation of neuropsychological test with FA and MD values of various brain regions. L: left; R: right. Only significant correlation of DTI metrics with cognitive domains (two cognitive domains showed no significant correlations).
In HIC, FA from the left postcentral, right corpus callosum, left insula and left hypothalamus significantly correlated with verbal learning (p = 0.03; r = 0.5, p = 0.01; r = 0.56, p = 0.02; r = 0.53, p = 0.01; r = 0.56) and FA from the left insula correlated with quantity (p = 0.02; r = 0.52). The HUC group showed significant negative correlation of FA in the right fusiform and left pulvinar with verbal learning (p = 0.04; r = - 0.74, p = 0.006; r = - 0.86); and left insula with exclusion (p = 0.05; r = - 0.7.) and hidden figures (p = 0.05; r = - 0.76).
In HIC, MD from the right frontal lobe showed negative correlation with mazes (p = 0.04; r = − 0.48) and quantity (p = 0.05; r = − 0.46). MD from the right inferior temporal and culmen showed negative correlation with mazes (p = 0.02; r = − 0.52, p = 0.05; r = − 0.46) while MD from the right medial frontal correlated positively with memory (p = 0.04; r = 0.48).
In HUC, significant positive correlation of MD from the right middle temporal with exclusion (p = 0.04; r = 0.73) and hidden figure (p = 0.03; r = 0.77) was observed, while MD from the right cerebellum crus1 showed negative correlation with discs (p = 0.04; r = - 0.73).
5. Discussion
The changes in FA and MD and cognitive function in HIC as observed in the current study is consistent with the previous findings (Uban et al., 2015; Cohen et al., 2016; Hoare et al., 2012). Significantly decreased FA and altered MD in multiple brain sites with lower NP test scores in HUC as compared to CHNM suggest that exposure of ART may have a detrimental effect on neurodevelopment. However, the changes in DTI metrics as well as NP test scores were comparatively greater in HIC than HUC when compared to those of CHNM. Our findings support the previous observations (Jahanshad et al., 2015; Jankiewicz et al., 2017a; Tran et al., 2016) and suggest that ART exposure may affect the brain and cognitive function of growing children through direct (Children taking the ART) or indirect (Children exposed to ART) pathways.
A study performed by Neda et al., reported no significant difference in neuroanatomical or brain tissue integrity, while, non-significantly lower IQ scores in HUC as compared to CHNM (Jahanshad et al., 2015). Though the above study observed an association between brain microstructural tissue integrity measures and intelligence quotient scores, but they were unable to reach any conclusion due to heterogeneous nature of sample characteristics such as no clear information about maternal history and perinatal antiretroviral drug exposure status. Half population of HUC children were born before 2000 and thus were less likely exposed to ART and among the remaining HUC; 11 children were born between 2000 and 2003 and in that period mothers were only on zidovudine, and 5 children were born after 2004 and mothers were on zidovudine with single-dose nevirapine. Another study performed in normal neonates born to HIV infected mothers showed significantly higher FA in the middle cerebellar peduncles as compared to same age group healthy control (Tran et al., 2016). They found a positive correlation of FA with cognitive functions. Another study reported higher FA and lower MD in the corticospinal tract of HUC children (Jankiewicz et al., 2017b), but it lacks information about the status of HIV infection and treatment of mothers. A straightforward comparison of our study to previous studies is not possible due to the nature of sample characteristics as the current study performed in children with mean age of 10 years.
A study by Kerr SJ et al., 2014 observed significant lower verbal Intelligence Quotient, full-scale IQ, and Bead Memory in HUC than those of CHNM (Kerr et al., 2014). Neda et al., have also showed significant lower NP test scores in HUC as compared to CHNM similar to Kerr and Puthanakit T studies (Jahanshad et al., 2015; Kerr et al., 2014; Puthanakit et al., 2013). Few studies evaluated the developmental differences in HUC have found marginal effects of ART on cognitive performance (McHenry et al., 2018b).
The current study observed a significant correlation of FA and MD with NP test scores. The presence of significant correlations of NP test scores with DTI metrics suggest that the neuronal changes have an impact on cognitive functions in both HIC and HUC children. Further, the higher brain tissue damage in HIC than HUC could be the synergistic effect of HIV and ART.
Due to the cross-sectional design of our study we were not able to assess the impact of ART or HIV on brain development over time (changes with age). Moreover, the small sample size could be considered as a limitation of the current study. We envisage that a larger sample size may improve the correlation between DTI metrics and NP test scores and provide a clear view of the effects of ART on brain development. Nevertheless, the strength of the current study is the comprehensive neurocognitive test battery and inclusion of three groups (HIC, HUC, and CHNM) for a straightforward comparison.
6. Conclusions
Lower FA indicates loss of brain tissue integrity (neuronal damage or demyelination), and altered MD denotes the presence of cerebral edema and/or neuro-inflammation, which may subsequently responsible for the lower cognitive functions in HIC and HUC. The presence of brain tissue changes and neurocognitive deficits in the absence of HIV infection in HUC emphasize that ART may have a detrimental impact on developing brain. The findings of the current study underscore the need for screening of ART exposed children for the neurodevelopment and cognitive abnormalities at an early stage and call for access to early interventions, and nutritional and care programs.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Declaration of competing interest
All the listed coauthors have no conflict of interest.
Acknowledgements
This study was funded by Department of Science and Technology (SR/CSI/02/2 0 10, G), New Delhi, India. Sidra Medicine provided data processing platform. This work was supported by funding from Sidra Medicine to Mohammad Haris (50610110002), Santosh Kumar Yadav (5011043002) and Ajaz A. Bhat (5011041002).
References
- Ackermann C., Andronikou S., Laughton B., Kidd M., Dobbels E., Innes S. White matter signal abnormalities in children with suspected HIV-related neurologic disease on early combination antiretroviral therapy. Pediatr. Infect. Dis. J. 2014;33:e207–212. doi: 10.1097/INF.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann C., Andronikou S., Saleh M.G., Laughton B., Alhamud A.A., van der Kouwe A. Early antiretroviral therapy in HIV-infected children is associated with diffuse white matter structural abnormality and corpus callosum sparing. AJNR Am. J. Neuroradiol. 2016;37:2363–2369. doi: 10.3174/ajnr.A4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann C., van Toorn R., Andronikou S. Human immunodeficiency virus-related cerebral white matter disease in children. Pediatr. Radiol. 2019;49:652–662. doi: 10.1007/s00247-018-4310-x. [DOI] [PubMed] [Google Scholar]
- Afran L., Garcia Knight M., Nduati E., Urban B.C., Heyderman R.S., Rowland-Jones S.L. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin. Exp. Immunol. 2014;176:11–22. doi: 10.1111/cei.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed D., Roy D., Cassol E. Examining relationships between metabolism and persistent inflammation in HIV patients on antiretroviral therapy. Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/6238978. 6238978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronikou S., Ackermann C., Laughton B., Cotton M., Tomazos N., Spottiswoode B. Correlating brain volume and callosal thickness with clinical and laboratory indicators of disease severity in children with HIV-related brain disease. Child’s Nerv. Syst. : ChNS : Off. J. Int. Soc. Pediatr. Neurosurg. 2014;30:1549–1557. doi: 10.1007/s00381-014-2434-3. [DOI] [PubMed] [Google Scholar]
- Andronikou S., Ackermann C., Laughton B., Cotton M., Tomazos N., Spottiswoode B. Corpus callosum thickness on mid-sagittal MRI as a marker of brain volume: a pilot study in children with HIV-related brain disease and controls. Pediatr. Radiol. 2015;45:1016–1025. doi: 10.1007/s00247-014-3255-y. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Atluri V.S., Hidalgo M., Samikkannu T., Kurapati K.R., Jayant R.D., Sagar V. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front. Cell. Neurosci. 2015;9:212. doi: 10.3389/fncel.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair M., Afonso P., Capel E., Caron-Debarle M., Capeau J. Impact of darunavir, atazanavir and lopinavir boosted with ritonavir on cultured human endothelial cells: beneficial effect of pravastatin. Antivir. Ther. 2014;19:773–782. doi: 10.3851/IMP2752. [DOI] [PubMed] [Google Scholar]
- Barret B., Tardieu M., Rustin P., Lacroix C., Chabrol B., Desguerre I. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. Aids. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- Bertrand L., Toborek M. Dysregulation of endoplasmic reticulum stress and autophagic responses by the antiretroviral drug Efavirenz. Mol. Pharmacol. 2015;88:304–315. doi: 10.1124/mol.115.098590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand L., Dygert L., Toborek M. Antiretroviral treatment with Efavirenz disrupts the blood-brain barrier integrity and increases stroke severity. Sci. Rep. 2016;6:39738. doi: 10.1038/srep39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand L., Cho H.J., Toborek M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain : J. Neurol. 2019;142:502–511. doi: 10.1093/brain/awy339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche S., Tardieu M., Rustin P., Slama A., Barret B., Firtion G. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- Brabers N.A., Nottet H.S. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur. J. Clin. Invest. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Brogly S.B., Ylitalo N., Mofenson L.M., Oleske J., Van Dyke R., Crain M.J. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. Aids. 2007;21:929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- Calamandrei G., Valanzano A., Puopolo M., Aloe L. Developmental exposure to the antiretroviral drug zidovudine increases brain levels of brain-derived neurotrophic factor in mice. Neurosci. Lett. 2002;333:111–114. doi: 10.1016/s0304-3940(02)01023-6. [DOI] [PubMed] [Google Scholar]
- Cardenas V.A., Meyerhoff D.J., Studholme C., Kornak J., Rothlind J., Lampiris H. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J. Neurovirol. 2009;15:324–333. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Yakupov R., Nakama H., Stokes B., Ernst T. Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J. Neuroimmune Pharmacol. : Off. J. Soc. NeuroImmune Pharmacol. 2008;3:95–104. doi: 10.1007/s11481-007-9092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Clifford D.B., Deng L., Wu K., Lee A.J., Bosch R.J. Peripheral neuropathy in ART-experienced patients: prevalence and risk factors. J. Neurovirol. 2013;19:557–564. doi: 10.1007/s13365-013-0216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho A.V., Tricarico P.M., Celsi F., Crovella S. Antiretroviral treatment in HIV-1-Positive mothers: neurological implications in virus-free children. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Caan M.W., Mutsaerts H.J., Scherpbier H.J., Kuijpers T.W., Reiss P. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology. 2016;86:19–27. doi: 10.1212/WNL.0000000000002209. [DOI] [PubMed] [Google Scholar]
- Culnane M., Fowler M., Lee S.S., McSherry G., Brady M., O’Donnell K. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. Pediatric AIDS Clinical Trials Group Protocol 219/076 Teams. Jama. 1999;281:151–157. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- Ellis R., Langford D., Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Faltz M., Bergin H., Pilavachi E., Grimwade G., Mabley J.G. Effect of the anti-retroviral drugs Efavirenz, Tenofovir and emtricitabine on endothelial cell function: role of PARP. Cardiovasc. Toxicol. 2017;17:393–404. doi: 10.1007/s12012-016-9397-4. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A., Mazanek M., Whybra C., Beck M., Hartung R., Muller K.M. Pattern of microstructural brain tissue alterations in Fabry disease: a diffusion-tensor imaging study. J. Neurol. 2006;253:780–787. doi: 10.1007/s00415-006-0118-y. [DOI] [PubMed] [Google Scholar]
- Funk M.J., Belinson S.E., Pimenta J.M., Morsheimer M., Gibbons D.C. Mitochondrial disorders among infants exposed to HIV and antiretroviral therapy. Drug Saf. 2007;30:845–859. doi: 10.2165/00002018-200730100-00004. [DOI] [PubMed] [Google Scholar]
- Hoare J., Fouche J.-P., Spottiswoode B., Donald K., Philipps N., Bezuidenhout H. A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naïve “slow progressors.”. J. Neurovirol. 2012;18:205–212. doi: 10.1007/s13365-012-0099-9. [DOI] [PubMed] [Google Scholar]
- Ivanovic J., Nicastri E., Anceschi M.M., Ascenzi P., Signore F., Pisani G. Transplacental transfer of antiretroviral drugs and newborn birth weight in HIV-infected pregnant women. Curr. HIV Res. 2009;7:620–625. doi: 10.2174/157016209789973628. [DOI] [PubMed] [Google Scholar]
- Jahanshad N., Couture M.C., Prasitsuebsai W., Nir T.M., Aurpibul L., Thompson P.M. Brain imaging and neurodevelopment in HIV-uninfected Thai children born to HIV-infected mothers. Pediatr. Infect. Dis. J. 2015;34:e211–216. doi: 10.1097/INF.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankiewicz M., Holmes M.J., Taylor P.A., Cotton M.F., Laughton B., van der Kouwe A.J.W. White matter abnormalities in children with HIV infection and exposure. Front. Neuroanat. 2017;11:88. doi: 10.3389/fnana.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankiewicz M., Holmes M.J., Taylor P.A., Cotton M.F., Laughton B., van der Kouwe A.J.W. White matter abnormalities in children with HIV infection and exposure. Front. Neuroanat. 2017;11 doi: 10.3389/fnana.2017.00088. 88-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr S.J., Puthanakit T., Vibol U., Aurpibul L., Vonthanak S., Kosalaraksa P. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care. 2014;26:1327–1335. doi: 10.1080/09540121.2014.920949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khire U.B.H., Rene A.C., Bharat S., Athavale U., Kher R. 1992. Indian Child Intelligence Test (ICIT), Technical Manual, Adaptation of Revised Amsterdamse Kinder Intelligentie Test. [Google Scholar]
- Kodl C.T., Franc D.T., Rao J.P., Anderson F.S., Thomas W., Mueller B.A. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57:3083–3089. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wu G., Wen Z., Zhang J., Lei H., Gui X. White matter development is potentially influenced in adolescents with vertically transmitted HIV infections: a tract-based spatial statistics study. AJNR Am. J. Neuroradiol. 2015;36:2163–2169. doi: 10.3174/ajnr.A4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louboutin J.P., Strayer D.S. Blood-brain barrier abnormalities caused by HIV-1 gp120: mechanistic and therapeutic implications. TheScientificWorldJOURNAL. 2012;2012:482575. doi: 10.1100/2012/482575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Marin J.A., Mendez-Cruz R., Arroyo-Anduiza C.I., Mata-Marin L.A., Gaytan-Martinez J., Asbun-Bojalil J. Effect of antiretroviral therapy on inflammatory markers of endothelial dysfunction in HIV treatment-naive infected patients. J. Med. Virol. 2013;85:1321–1326. doi: 10.1002/jmv.23624. [DOI] [PubMed] [Google Scholar]
- McCormack S.A., Best B.M. Protecting the fetus against HIV infection: a systematic review of placental transfer of antiretrovirals. Clin. Pharmacokinet. 2014;53:989–1004. doi: 10.1007/s40262-014-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry M.S., McAteer C.I., Oyungu E., McDonald B.C., Bosma C.B., Mpofu P.B. Neurodevelopment in young children born to HIV-infected mothers: a meta-analysis. Pediatrics. 2018;141 doi: 10.1542/peds.2017-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry M.S., McAteer C.I., Oyungu E., McDonald B.C., Bosma C.B., Mpofu P.B. Neurodevelopment in young children born to HIV-infected mothers: a meta-analysis. Pediatrics. 2018;141 doi: 10.1542/peds.2017-2888. e20172888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae M. HIV and viral protein effects on the blood brain barrier. Tissue Barriers. 2016;4 doi: 10.1080/21688370.2016.1143543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica-Napolitano J.S. AZT causes tissue-specific inhibition of mitochondrial bioenergetic function. Biochem. Biophys. Res. Commun. 1993;194:170–177. doi: 10.1006/bbrc.1993.1800. [DOI] [PubMed] [Google Scholar]
- Morie K.P., Yip S.W., Zhai Z.W., Xu J., Hamilton K.R., Sinha R. White-matter crossing-fiber microstructure in adolescents prenatally exposed to cocaine. Drug Alcohol Depend. 2017;174:23–29. doi: 10.1016/j.drugalcdep.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging - a review. NMR Biomed. 2002;15:553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- Neri D., Somarriba G.A., Schaefer N.N., Chaparro A.I., Scott G.B., Lopez Mitnik G. Growth and body composition of uninfected children exposed to human immunodeficiency virus: comparison with a contemporary cohort and United States National Standards. J. Pediatr. 2013;163:249–254. doi: 10.1016/j.jpeds.2012.12.034. e241-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell M.L., Borja M.C., Peckham C., European Collaborative S. Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111:e52–60. doi: 10.1542/peds.111.1.e52. [DOI] [PubMed] [Google Scholar]
- Nicholson L., Chisenga M., Siame J., Kasonka L., Filteau S. Growth and health outcomes at school age in HIV-exposed, uninfected Zambian children: follow-up of two cohorts studied in infancy. BMC Pediatr. 2015;15:66. doi: 10.1186/s12887-015-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasi M., van Uden I.W., Tuladhar A.M., de Leeuw F.E., Pantoni L. White matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease: clinical consequences. Stroke. 2016;47:1679–1684. doi: 10.1161/STROKEAHA.115.012065. [DOI] [PubMed] [Google Scholar]
- Patel S., Leibrand C.R., Palasuberniam P., Couraud P.O., Weksler B., Jahr F.M. Effects of HIV-1 tat and methamphetamine on blood-brain barrier integrity and function in vitro. Antimicrob. Agents Chemother. 2017:61. doi: 10.1128/AAC.01307-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsalides A.D., Wood L.V., Atac G.K., Sandifer E., Butman J.A., Patronas N.J. Cerebrovascular disease in HIV-infected pediatric patients: neuroimaging findings. AJR Am. J. Roentgenol. 2002;179:999–1003. doi: 10.2214/ajr.179.4.1790999. [DOI] [PubMed] [Google Scholar]
- Powis K.M., Smeaton L., Hughes M.D., Tumbare E.A., Souda S., Jao J. In-utero triple antiretroviral exposure associated with decreased growth among HIV-exposed uninfected infants in Botswana. Aids. 2016;30:211–220. doi: 10.1097/QAD.0000000000000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu H., Tian J., Andras I.E., Hayashi K., Flora G., Hennig B. HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J. Cerebr. Blood Flow Metabol. : Off. J. Int. Soc. Cerebr. Blood Flow Metabol. 2005;25:1325–1335. doi: 10.1038/sj.jcbfm.9600125. [DOI] [PubMed] [Google Scholar]
- Purnell P.R., Fox H.S. Efavirenz induces neuronal autophagy and mitochondrial alterations. J. Pharmacol. Exp. Therapeut. 2014;351:250–258. doi: 10.1124/jpet.114.217869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthanakit T., Ananworanich J., Vonthanak S., Kosalaraksa P., Hansudewechakul R., van der Lugt J. Cognitive function and neurodevelopmental outcomes in HIV-infected Children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatr. Infect. Dis. J. 2013;32:501–508. doi: 10.1097/INF.0b013e31827fb19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin A.B., Wu Y., Storey P., Cohen B.A., Edelman R.R., Epstein L.G. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J. Neurovirol. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimy E., Li F.Y., Hagberg L., Fuchs D., Robertson K., Meyerhoff D.J. Blood-brain barrier disruption is initiated during primary HIV infection and not rapidly altered by antiretroviral therapy. J. Infect. Dis. 2017;215:1132–1140. doi: 10.1093/infdis/jix013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A.C., Rizk N., O’Riordan M.A., Dogra V., El-Bejjani D., Storer N. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin. Infect. Dis. : Off. Publ. Infect. Dis. Soc. Am. 2009;49:1119–1127. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P., Moschese V. Mother-to-child transmission of human immunodeficiency virus. Faseb. J. : Off. Publ. Fed. Am. Soc. Exp. Biol. 1991;5:2419–2426. doi: 10.1096/fasebj.5.10.2065890. [DOI] [PubMed] [Google Scholar]
- Soontornniyomkij V., Umlauf A., Chung S.A., Cochran M.L., Soontornniyomkij B., Gouaux B. HIV protease inhibitor exposure predicts cerebral small vessel disease. Aids. 2014;28:1297–1306. doi: 10.1097/QAD.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toborek M., Lee Y.W., Pu H., Malecki A., Flora G., Garrido R. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J. Neurochem. 2003;84:169–179. doi: 10.1046/j.1471-4159.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- Tran L.T., Roos A., Fouche J.P., Koen N., Woods R.P., Zar H.J. White matter microstructural integrity and neurobehavioral outcome of HIV-exposed uninfected neonates. Medicine. 2016;95:e2577. doi: 10.1097/MD.0000000000002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban K.A., Herting M.M., Williams P.L., Ajmera T., Gautam P., Huo Y. White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. Aids. 2015;29:1035–1044. doi: 10.1097/QAD.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF Eliminating mother-to-child- transmission: UNICEF data: UNICEF. https://datauniceforg/topic/hivaids/emtct/#.2016
- Venerosi A., Valanzano A., Puopolo M., Calamandrei G. Neurobehavioral effects of prenatal exposure to AZT: a preliminary investigation with the D1 receptor agonist SKF 38393 in mice. Neurotoxicol. Teratol. 2005;27:169–173. doi: 10.1016/j.ntt.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Westlye L.T., Moe V., Slinning K., Due-Tonnessen P., Bjornerud A. White matter characteristics and cognition in prenatally opiate- and polysubstance-exposed children: a diffusion tensor imaging study. AJNR Am. J. Neuroradiol. 2010;31:894–900. doi: 10.3174/ajnr.A1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallet C., De Rovere M., Van Assche J., Daouad F., De Wit S., Gautier V. Microglial cells: the main HIV-1 reservoir in the brain. Front. Cell. Infect. Microbiol. 2019;9:362. doi: 10.3389/fcimb.2019.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO HIV/AIDS. https://wwwwhoint/news-room/fact-sheets/detail/hiv-aids.2019
- Williams P.L., Hazra R., Van Dyke R.B., Yildirim C., Crain M.J., Seage G.R., 3rd Antiretroviral exposure during pregnancy and adverse outcomes in HIV-exposed uninfected infants and children using a trigger-based design. Aids. 2016;30:133–144. doi: 10.1097/QAD.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Storey P., Cohen B.A., Epstein L.G., Edelman R.R., Ragin A.B. Diffusion alterations in corpus callosum of patients with HIV. AJNR Am. J. Neuroradiol. 2006;27:656–660. [PMC free article] [PubMed] [Google Scholar]
- Yadav S.K., Gupta R.K., Garg R.K., Venkatesh V., Gupta P.K., Singh A.K. Altered structural brain changes and neurocognitive performance in pediatric HIV. Neuroimage Clin. 2017;14:316–322. doi: 10.1016/j.nicl.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S.K., Gupta R.K., Hashem S., Bhat A.A., Garg R.K., Venkatesh V. Changes in resting-state functional brain activity are associated with waning cognitive functions in HIV-infected children. Neuroimage Clin. 2018;20:1204–1210. doi: 10.1016/j.nicl.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younas M., Psomas C., Reynes J., Corbeau P. Immune activation in the course of HIV-1 infection: causes, phenotypes and persistence under therapy. HIV Med. 2016;17:89–105. doi: 10.1111/hiv.12310. [DOI] [PubMed] [Google Scholar]
- Zhang Y.L., Ouyang Y.B., Liu L.G., Chen D.X. Blood-brain barrier and neuro-AIDS. Eur. Rev. Med. Pharmacol. Sci. 2015;19:4927–4939. [PubMed] [Google Scholar]
- Zhong Y., Zhang B., Eum S.Y., Toborek M. HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation. J. Neurosci. : Off. J. Soc. Neurosci. 2012;32:143–150. doi: 10.1523/JNEUROSCI.4266-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]