Abstract
Posttraumatic stress disorder (PTSD) is a highly disabling condition associated with alterations in multiple neurobiological systems, including increases in inflammatory function. Vagus nerve stimulation (VNS) decreases inflammation, however few studies have examined the effects of non-invasive VNS on physiology in human subjects, and no studies in patients with PTSD. The purpose of this study was to assess the effects of transcutaneous cervical VNS (tcVNS) on inflammatory responses to stress. Thirty subjects with a history of exposure to traumatic stress with (N = 10) and without (N = 20) PTSD underwent exposure to stressful tasks immediately followed by active or sham tcVNS and measurement of multiple biomarkers of inflammation (interleukin-(IL)-6, IL-2, IL-1β, Tumor Necrosis Factor alpha (TNFα) and Interferon gamma (IFNγ) over multiple time points. Stressful tasks included exposure to personalized scripts of traumatic events on day 1, and public speech and mental arithmetic (Mental Stress) tasks on days 2 and 3. Traumatic scripts were associated with a pattern of subjective anger measured with Visual Analogue Scales and increased IL-6 and IFNγ in PTSD patients that was blocked by tcVNS (p < .05). Traumatic stress had minimal effects on these biomarkers in non-PTSD subjects and there was no difference between tcVNS or sham. No significant differences were seen between groups in IL-2, IL-1β, or TNFα. These results demonstrate that tcVNS blocks behavioral and inflammatory responses to stress reminders in PTSD.
Keywords: Stress disorders, posttraumatic; Vagus nerve; Inflammation; Interleukin-6; PTSD; Interferon
Highlights
-
•
Non-invasive Vagal Nerve Stimulation (VNS) or sham stimulation was paired with personalized traumatic scripts.
-
•
Blood biomarkers for inflammation were compared between traumatized individuals with and without posttraumatic stress disorder (PTSD).
-
•
VNS blocked stress-induced increases in interleukin-6 (IL-6) and interferon-γ (IFNγ) in patients with PTSD.
1. Introduction
Posttraumatic Stress Disorder (PTSD) is a disabling disorder that affects the quality of life and productivity of millions of Americans (Bremner, 2016). The standard of care for PTSD includes psychotherapy and/or medication (Ballenger et al., 2000; Foa et al., 1999, 2007; Foa and Rothbaum, 1998; Hembree et al., 2003; Lancaster et al., 2016; Schnurr et al., 2007), however current treatments are characterized by high rates of non-completion and/or limitations in efficacy (Ballenger et al., 2004; Davis et al., 2016; Hembree et al., 2003; Schottenbauer et al., 2008). In fact, a report from the Institute of Medicine stated that there is not sufficient evidence to conclude that the first line medication treatment, Selective Serotonin Reuptake Inhibitors (SSRIs), are effective for PTSD (Institute of Medicine of the National Academies, 2014). Based on these facts, new approaches to the treatment of PTSD are needed. Treatments that target the psychobiology of PTSD, involving core changes in brain and autonomic nervous system (Reinertsen et al., 2017; Shah et al., 2013) and immune function (Neigh and Ali, 2016), may have promise for modulating the underlying basis for the disorder (Bremner, 2016; Shah et al., 2013).
Neuromodulation treatments that use electricity are a promising new approach to mental disorders that may act through effects on the underlying neurobiology of these disorders (Adair et al., 2020; Bikson et al., 2016, 2017b; Krames et al., 2018; Schachter and Saper, 1998; Tortella et al., 2015; Woods et al., 2016). Vagal Nerve Stimulation (VNS) is a form of neuromodulation that has been shown to be efficacious in the treatment of epilepsy (Ben-Menachem et al., 1994, 1999; George et al., 1994; Handforth et al., 1998; Salinsky et al., 1999; The Vagus Nerve Stimulation Study Group, 1995) and treatment-refractory major depression (Berry et al., 2013; Brunoni et al., 2013, 2016, 2017; Dell-Osso et al., 2013; George et al., 2000, 2003, 2005; Marangell et al., 2002; Rush et al., 2000, 2005a, 2005b; Sackeim et al., 2001a, 2001b, 2007). FDA-approved VNS for these conditions involves surgical implantation in the brainstem with direct electrical stimulation of the vagus nerve (Aaronson et al., 2017; George et al., 2003; Terry, 2014). VNS has effects that may be beneficial for neurophysiological alterations associated with PTSD, including blocking of sympathetic (Pena et al., 2014; Peña et al., 2013; Schomer et al., 2014) and immune function (Bansal et al., 2012; Borovikova et al., 2000), and enhancement of cognition (Clark et al., 1999; Jacobs et al., 2015; Sackeim et al., 2001a; Sjögren et al., 2002; Smith et al., 2005; Sun et al., 2017; Vonck et al., 2014). The requirement for surgical implantation, however, has limited the widespread implementation of VNS to psychiatry due to cost, inconvenience (Bremner and Rapaport, 2017; Marangell et al., 2002; Sackeim et al., 2001b), and lack of reimbursement by Medicare or other insurance companies (Feldman et al., 2013).
Dysregulated immune function is associated with stress and PTSD (Neigh and Ali, 2016; Passos et al., 2015). Mental stress in the laboratory in human subjects, including patients with coronary artery disease (CAD), is associated with increases in several inflammatory markers, including interleukin-6 (IL-6) (Hammadah et al., 2018; Marsland et al., 2017; Rooks et al., 2016), IL-1β (Lerman et al., 2016; Marsland et al., 2017), IL-10 (Marsland et al., 2017), and tumor necrosis factor (TNF)α (Marsland et al., 2017). Multiple studies show an increase in inflammatory factors at baseline in patients with depression (Akosile et al., 2018; Alcocer-Gómez et al., 2014; Capuron et al., 2008; Felger et al., 2016; Guo et al., 2015; Kiecolt-Glaser et al., 2007; Miller et al., 2009; Miller and Raison, 2016; Su et al., 2009; Vaccarino et al., 2008) and early trauma (Danese et al., 2007, 2008, 2011; Danese and McEwen, 2012; Rooks et al., 2012). Consistent with these studies, PTSD patients show increased inflammation (Gill et al., 2009), including increased baseline concentrations of leukocytes (Boscarino and Chang, 1999; Eswarappa et al., 2019), IL-6 (Gill et al., 2010; Gill et al., 2008; Guo and Tao Liu, 2012; Li et al., 2014; Lindqvist et al., 2017; Miller et al., 2001; Passos et al., 2015; Sutherland et al., 2003; Tucker et al., 2010; Vidovic et al., 2011; von Kanel et al., 2010b), IL1β (Lindqvist et al., 2014; Passos et al., 2015; von Känel et al., 2007), TNF-α (Gill et al., 2010; Lindqvist et al., 2017; Lindqvist et al., 2014; Passos et al., 2015; Sutherland et al., 2003; Vidovic et al., 2011; von Känel et al., 2007), IFNγ (Guo and Tao Liu, 2012; Hoge et al., 2009; Lindqvist et al., 2014; Passos et al., 2015; Woods et al., 2005; Zhou et al., 2014), intercellular adhesion molecule-1 (ICAM-1) (Plantinga et al., 2013; von Kanel et al., 2010a), vascular cell adhesion molecule-1 (VCAM-1) (von Kanel et al., 2010a), hsCRP (Eraly et al., 2014; Eswarappa et al., 2019; Heath et al., 2013; Lindqvist et al., 2017; Miller et al., 2001; Plantinga et al., 2013), and in one study, IL-2, IL-4, IL-8, and IL-10 (Guo and Tao Liu, 2012). Other studies showed no increase in IL-6 (Agorastos et al., 2019; Bruenig et al., 2018; McCanlies et al., 2011; Plantinga et al., 2013; von Känel et al., 2007), CRP (Baumert et al., 2013; Bruenig et al., 2018; Lindqvist et al., 2014; McCanlies et al., 2011; Sutherland et al., 2003; von Kanel et al., 2010b), IL-4 (von Känel et al., 2007), IL-10 (Lindqvist et al., 2017; Lindqvist et al., 2014; von Känel et al., 2007), IL-1β (Lindqvist et al., 2014), or IFN-γ (Bruenig et al., 2018). One study found increased diurnal cerebrospinal fluid (CSF) IL-6 but not plasma IL-6 in PTSD (Baker et al., 2001). Other studies showed altered genotype in genes modulating immune function in PTSD (Guardado et al., 2016). We recently found enhanced IL-6 response to mental stress involving public speaking in CAD patients with PTSD compared to CAD patients without PTSD (Lima et al., 2019). In summary, studies implicate altered immune function in PTSD, with a recent meta-analysis showing the largest effects for IL-6 and IFNγ (Passos et al., 2015).
VNS has effects on inflammation that may be beneficial for PTSD (Borovikova et al., 2000; Brock et al., 2017; Corcoran et al., 2004; Corsi-Zuelli et al., 2017; Das and Basu, 2008; Das, 2007, 2011; Li and Olshansky, 2011). IL-6 and TNF-α are modulable by the vagus nerve (Jan et al., 2010; Marsland et al., 2007). In animal studies VNS blocks lipopolysaccharide (LPS)-induced increases in IL-6, IL-18, IL-1β (Borovikova et al., 2000) and TNF-α (Bansal et al., 2012) but not IL-10 (Borovikova et al., 2000). Studies in patients with epilepsy and implanted VNS devices showed that long-term treatment resulted in decreased LPS-induced IL-6 (De Herdt et al., 2009) and neurotoxic kynurenic metabolites (Majoie et al., 2011) with no effect on IL-6, IL-10, IL-1β, or TNF-α (De Herdt et al., 2009).
A new generation of non-invasive devices have been developed for stimulation of the vagus nerve in the periphery (Bremner and Rapaport, 2017; Polak et al., 2009; Yoo et al., 2013). These non-invasive VNS (nVNS) techniques that stimulate the vagus in the ear (transcutaneous auricular VNS, or taVNS) or neck (transcutaneous cervical VNS (tcVNS)) have the potential for wide-spread implementation in patients with mental disorders (Bremner and Rapaport, 2017), however their effects on neurobiology, including immune function, have not been extensively studied. One study in healthy human subjects showed that tcVNS resulted in decreased TNF-α, IL-1β, IL-8, MIP and MCP-1 (Lerman et al., 2016), while another in PTSD patients showed reductions in TNF-α, but not IL-1β, IL-2 or IL-4 (Brock et al., 2017). tcVNS applied twice daily in an open-label, non-sham controlled study for 26 days in patients with Sjögren’s Syndrome resulted in reductions in baseline levels of Il-6, TNF-α, IL-1β, and MIP (Tarn et al., 2019). No studies have looked at the effects of taVNS or tcVNS on stress-induced changes in immune function. We previously reported that tcVNS in traumatized healthy human subjects with and without PTSD blocked peripheral sympathetic and enhanced parasympathetic responses both at baseline and in response to both personalized traumatic scripts and mental stressors (Gurel et al., 2020a, 2020b, 2020c), and other studies reported that taVNS blocked sympathetic function in patients with co-morbid mild Traumatic Brain Injury (mTBI) and PTSD (Lamb et al., 2017). We hypothesized these effects would be associated with a decrease in inflammation. In the current study, we examined the effects of tcVNS on peripheral cytokine response to personalized traumatic scripts and neutral mental stressors in the form of public speaking and mental arithmetic in traumatized subjects with and without PTSD. We hypothesized that tcVNS would block the effects of stress on IL-6 and IFNγ in PTSD.
2. Materials and methods
2.1. Human subjects
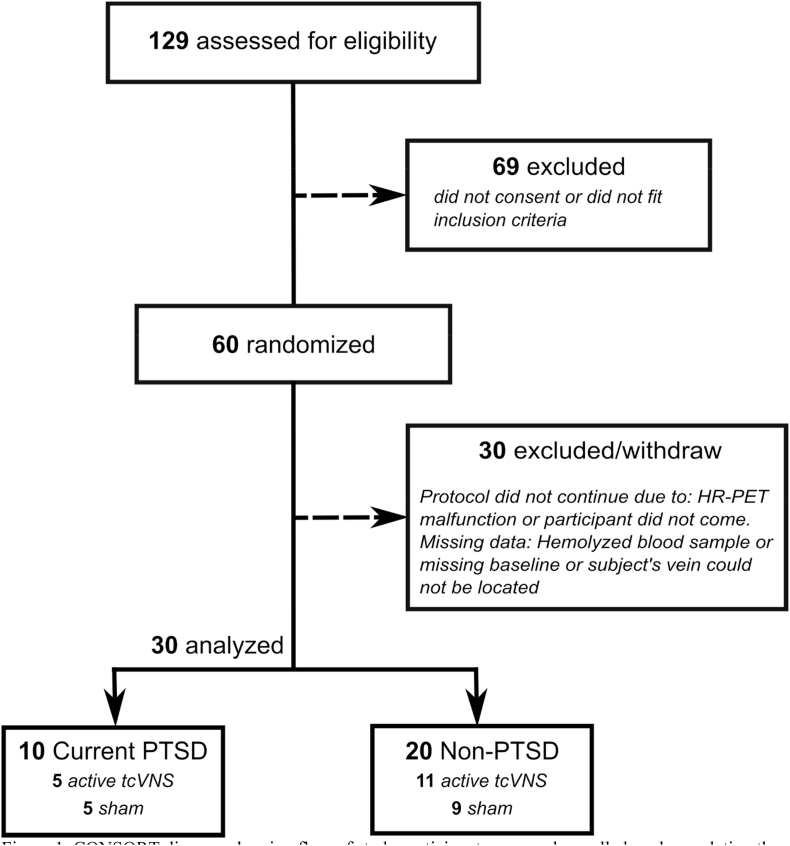
The research reported here (ClinicalTrials.Gov # NCT02992899) was approved by the Institutional Review Boards of Emory University, Georgia Institute of Technology, and the Space and Naval Warfare Systems Command (SPAWAR) Systems Center of the Pacific and the Department of Navy Human Research Protection Program. Subjects provided written, informed consent for participation. Subjects included physically healthy adults age 18–70 with a history of psychological trauma with and without the current diagnosis of posttraumatic stress disorder (PTSD) (Fig. 1). Subjects were excluded with the diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, bulimia or anorexia, as defined by The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013) (American Psychiatric Association, 2013). Subjects were also excluded with current pregnancy, traumatic brain injury (TBI), meningitis, active implanted device, evidence or history of serious medical or neurological illness, such as cardiovascular, gastrointestinal, hepatic, renal, or other systemic illness; carotid atherosclerosis, cervical vagotomy or positive toxicology screen. Psychiatric diagnosis was evaluated with the Structured Clinical Interview for DSM (SCID) (First and Gibbon, 2004). The Clinician Administered PTSD Scale (CAPS) was administered to evaluate for presence and severity of both current and lifetime PTSD (Blake et al., 1995). Among 129 individuals who were screened for eligibility, 60 were enrolled and randomized to active or sham stimulation and 30 did not complete the protocol due to being lost to followup or technical reasons (Fig. 1). Thirty participants with a history of psychological trauma based on DSM criteria including 12 females completed the protocol at Emory University School of Medicine between May 2017 and October 2018. The Structured Clinical Interview for DSM-IV (SCID) was used to evaluate for psychiatric diagnosis (First and Gibbon, 2004). Ten subjects met criteria for current PTSD and 20 had a history of trauma without current PTSD. In the PTSD group, one (10%) met criteria for current co-morbid major depression and five (50%) for a lifetime history of major depression, two (20%) for current generalized anxiety disorder, one (10%) for current panic disorder with agoraphobia, one (10%) for current agoraphobia without panic disorder, one (10%) for current obsessive-compulsive disorder, one (10%) for current social phobia, one (10%) for a lifetime history of sedative/hypnotic abuse, one (10%) for a lifetime history of opioid abuse, and one (10%) for a lifetime history of cocaine abuse. In the non-PTSD group, 2/20 (10%) met criteria for current and lifetime major depression, one (5%) for a lifetime history of alcohol abuse, one (5%) for a lifetime history of marijuana abuse, one (5%) for a lifetime history of stimulant abuse, one (5%) for a lifetime history of opioid abuse, and one (5%) for a lifetime history of hallucinogen/PCP abuse. No subjects met criteria for current alcohol or substance abuse.
Fig. 1.
CONSORT diagram showing flow of study participants screened, enrolled, and completing the protocol.
2.2. Study design
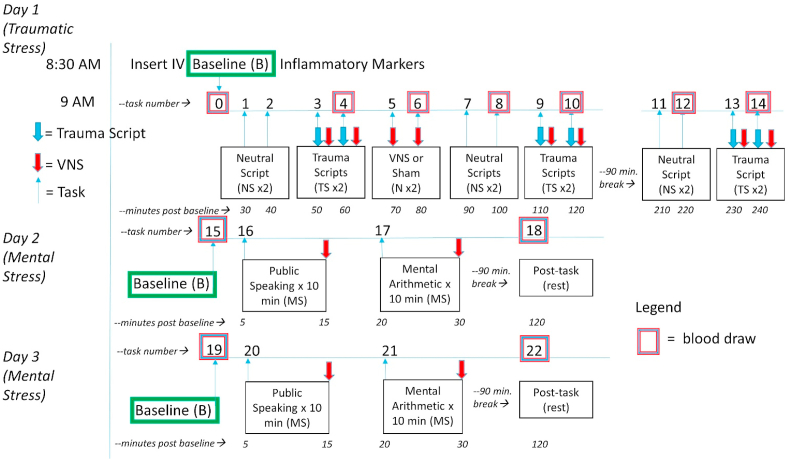
The participants provided their own traumatic experiences, and personalized voice recordings based on these experiences were presented as traumatic stress (Bremner et al., 1999; Orr et al., 1998). Subjects underwent exposure to personalized traumatic scripts in conjunction with tcVNS or sham on day 1, and “neutral” stressful tasks with tcVNS or sham on days 2 and 3 including public speech and mental arithmetic (Fig. 2) (Bremner et al., 2003, 2009; Burg and Soufer, 2014). We have described these paradigms in detail before and they have been shown to reliably produce behavioral and physiological responses consistent with a stress response (Bremner et al., 2003, 2009; Hammadah et al., 2017b). The first day included six traumatic recall scripts (approximately 1-min each) and six neutral scripts presented audibly through headphones. The neutral scripts were designed to induce positive feelings to the subject, such as the description of pleasant scenery. Immediately after the traumatic stress recording ended, stimulation (active or sham) was applied by the researcher from the left side of the neck. Behavioral ratings after each task were performed using Visual Analogue Scales (VAS) rating subjective anger on a 0–100 scale with 100 being most extreme anger and 0 not at all (Southwick et al., 1993). On the same day two stimulation administrations (active or sham) were applied without any stressor. Blood draws were taken on the start of the day (baseline) and after every four scans on this day. The second and third days were identical to each other. Baseline blood draws were taken both mornings. Afterwards, participants underwent a public speech task and mental arithmetic task, as previously described (Gurel et al., 2020b; Hammadah et al., 2017a). Stimulations were applied immediately after the public speech and mental arithmetic tasks. First, the subjects underwent a public speech task for which they were required to provide a 2-min long defense statement in a scenario where they were accused of theft. After hearing the scenario details, they were given 2 min to prepare their defense and 2 min to present their statement. Stimulation was applied immediately after the public speech task. Later, the subjects rested for 8 min in silence. At the end of the 8 min, the subjects were given another task for which they were required to answer series of arithmetic questions for 3 min. A researcher provided negative feedback for incorrect answers and delayed response times. A second stimulation was applied immediately after the arithmetic task. After two mental stressors and two stimulation administrations, the subjects were given a 90-min break. After the break, a second blood draw was taken.
Fig. 2.
Diagram of the study protocol. Traumatized participants with and without PTSD underwent three days of stress, one day (Day 1) with neutral scripts (NS) and personalized traumatic scripts (TS), and two days (Days 2 and 3) with mental stress (MS) involving public speaking and mental arithmetic tasks. Participants underwent randomized, double-blind assignment to tcVNS or sham stimulation which was paired with stress tasks (or no task) on Days 1, 2 and 3. On Day 1 neutral and traumatic scripts lasted about 1 min and occurred in pairs with 10 min in between. Stress tasks were paired with stimulation with tcVNS or sham which began immediately after termination of the task and continued for 2 min followed by a blood draw (purple/blue boxes signify pairing of task/stimulation/blood draw but blood draw actually occurred at the termination of stimulation). On Day 1 participants also underwent stimulation with tcVNS or sham for 2 min in the absence of a task (N) repeated twice with 10 min in between followed by a blood draw. Neutral and traumatic script pairs were repeated followed by a 60 min rest and lunch break, with a repeat of neutral and traumatic script pairs in the afternoon each paired with blood draws. The neutral scripts tasks #11 and #12 were followed by a blood draw (which was about 110 min after the first trauma script pairs at tasks #3 and #4) and the trauma scripts tasks #13 and #14 paired with tcVNS or sham were followed by the final blood draw at 210 min into Day 1 (Traumatic Stress). On Day 2 after a baseline blood draw at rest (task #15) participants underwent mental stress (MS) involving 5 min of public speaking (task #16) with tcVNS or sham at the end, followed by an 8 min rest period, and another 5 min of mental arithmetic (task #17) followed by tcVNS or sham. After a 90 min rest period participants underwent a blood draw at rest (task#18). This was repeated for Day 3 with baseline (task #19, public speaking (task #20), mental arithmetic (task #21) and a blood draw post-task at rest (task #22). The blood draws for all three days were timed to coincide with the roughly 90 min time course of interleukin-6 (IL-6) response to stress based on prior studies. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.3. Blinding
The participants were randomized into active tcVNS or sham groups with pre-numbered devices by the manufacturer who were not involved in the research. Random allocation was carried out by personnel who did not take part in data collection or analyses. The participants and researchers were blinded to the stimulus type. Statistical analyses were carried out by a biostatistician who did not take part in data collection or processing. Stimulus groups was un-blinded for the interpretation of statistical analysis.
2.4. Transcutaneous cervical vagal nerve stimulation
Both active tcVNS and sham stimuli were administered using hand-held GammaCore devices (ElectroCore, Basking Ridge, New Jersey). Stimulation was applied using collar, stainless steel electrodes with a conductive electrode gel placed on the left side of the neck over the carotid sheath as determined by palpation of the carotid artery. Active tcVNS devices produced an alternating current (AC) voltage signal consisting of five 5 kHz sine bursts (1 ms of five sine waves; pulse width = 40 ms) repeating at a rate of 25 Hz. The frequency of 25 Hz was chosen based on prior studies showing optimization of effects on autonomic function and other measures at this frequency (Adair et al., 2020; Badran et al., 2018a, 2018b, 2019; Bikson et al., 2017; Hays et al., 2013, 2014; Hulsey et al., 2017). The sham devices produce an AC biphasic voltage signal consisting of 0.2 Hz square pulses (pulse width = 5 s) eliciting a mild sensation. The peak voltage amplitudes for active and sham device are 30 V and 14 V, for active and sham, respectively. Throughout the protocol, the researcher gradually increased the stimulation intensity with a roll switch to the maximum the participant can tolerate, without pain. Amplitude dosing is dependent on subjective pain perception: researchers slowly increase the amplitude with a roll switch until the subjects instruct to stop. The active group received 17.8 V (± 6.6 SD), and sham group received 13.5 V (± 1.5 SD) averaged across all uses over three days, in this sample. An active stimulation amplitude higher than 15 V using the studied device was previously reported to create vagal somatosensory evoked potentials associated with vagal afferent activation, that are also activated with VNS implants (Nonis et al., 2017). Both active and sham devices delivered 2 min of stimulation. The stimulation intensity was adjustable using a roll switch that ranged from 0 to 5 a.u. (arbitrary units) with a corresponding peak output ranging from 0 to 30 V for active n-VNS, and from 0 to 14 V for the sham device. During each application, the stimulation intensity was increased to the maximum the subject could tolerate, without pain. The stimulation continued at the selected intensity. In this sample for blood draw analysis, the active tcVNS group (n = 16 participants) received 3.00 a.u. (±1.09) mean (±SD) and the sham group (n = 14 participants) received 4.74 a.u. (±0.69) averaged across all fourteen uses over three days, regardless of the disease status. In the active group, patients with PTSD (n = 5) received 2.94 a.u. (±1.09) and participants without PTSD received 3.02 a.u. (±1.09). In the sham group, patients with PTSD (n = 5) received 4.75 a.u. (±0.63) and participants without PTSD (n = 9) received 4.74 a.u. (±0.72). No participants reported lack of sensation.
2.5. Biomarker assay
We performed multiplex assays to measure IL-1β, IL-2, IL-6, TNFα, and IFN-γ purchased from Meso Scale Discovery. All experimental operations were in accordance with standard protocols. R2s of the standard curves for each plate were greater than 0.999.
2.6. Statistical analysis
Analysis of variance (ANOVA) tests were used to compare the demographic characteristics across the tcVNS treatment or sham stimulation group among patients with PTSD and healthy participants. We used ANOVA and linear regression models to measure the association between the cytokine levels and PTSD status, with or without tcVNS treatment effect. The beta coefficients (ß) from the mixed models indicate the adjusted average percent or absolute differences in the changes of parameters from the corresponding rest values, comparing active vs. sham device types. ß were reported along with 95% confidence intervals (CI) and P-values. A two-sided p < 0.05 denoted statistical significance. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and MATLAB (R2017b, Natick, MA).
3. Results
Participant groups were similar in age, body mass index, race, education level and marital status (Table 1). The average age of this population was 30 (SD = 9), and the average BMI was 27 (SD = 5.60). Among all the participants, 18 (50%) were White / Caucasian, 21 (58%) were female, and consistent with prior reports (Kessler et al., 1995), 9/12 (75%) of the PTSD patients were female. All of the PTSD participants randomized to VNS were female (Fisher’s Exact p = 0.045), and the gender proportion in the other groups was similar.
Table 1.
| PTSD-VNS (n = 5) | PTSD-Sham (n = 5) | Non-PTSD-VNS (n = 11) | Non-PTSD Sham (n = 9) | Overall (n = 30) | |
| Age | |||||
| Mean (SD) | 29 (8) | 32 (8) | 30 (9) | 34 (12) | 31 (9) |
| Race | |||||
| White | 1 (20%) | 2 (40%) | 6 (55%) | 5 (56%) | 14 (47%) |
| Black | 3 (60%) | 1 (20%) | 3 (27%) | 1 (11%) | 8 (27%) |
| Other | 1 (20%) | 2 (40%) | 2 (18%) | 3 (33%) | 8 (27%) |
| Sex | |||||
| Female | 5 (100%) | 2 (40%) | 5 (45%) | 5 (56%) | 17 (57%) |
| Male | 0 (0%) | 3 (60%) | 6 (55%) | 4 (44%) | 13 (43%) |
| BMI | |||||
| Mean (SD) | 25 (8) | 31 (5) | 27 (6) | 26 (4) | 27 (6) |
| Education Level | |||||
| High school - graduate | 3 (60%) | 2 (40.0%) | 5 (45%) | 2 (22%) | 12 (40%) |
| College graduate | 2 (40%) | 3 (60.0%) | 6 (55%) | 7 (78%) | 18 (60%) |
| Marital Status | |||||
| Never married | 4 (80%) | 2 (40%) | 7 (64%) | 5 (56%) | 18 (60.0%) |
| Married | 0 (0%) | 1 (20%) | 3 (27%) | 2 (22%) | 6 (20.0%) |
| Divorced / Separated | 1 (20%) | 1 (20%) | 1 (9%) | 2 (22%) | 5 (16.7%) |
| Widowed | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | 1 (3.3%) |
| PTSD Score (PCL) | |||||
| Mean (SD) | 44 (11) | 52 (14) | 29 (10) | 30 (11) | 35 (14) |
| PTSDSS Score | |||||
| Mean (SD) | 29 (8) | 24 (17) | 17 (14) | 19 (5) | 18 (14) |
| Anger Index | |||||
| Mean (SD) | 29 (7) | 50 (30) | 26 (13) | 34 (13) | 32 (9) |
| PSS-10 Score | |||||
| Mean (SD) | 24 (4) | 23 (3) | 22 (4) | 21 (8) | 22 (12) |
| ESSI Score | |||||
| Mean (SD) | 24 (5) | 17 (8) | 19 (9) | 21 (5) | 22 (2) |
| CADSS Score | |||||
| Mean (SD) | 3 (4) | 0 (0) | 2 (5) | 1 (3) | 0 (0) |
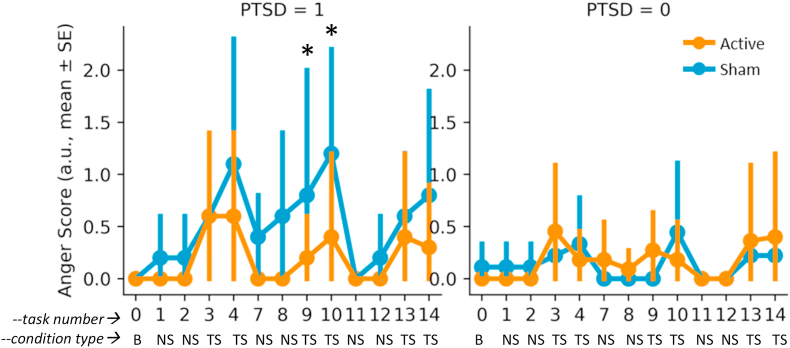
Exposure to personalized traumatic scripts resulted in greater increases in subjective anger on the VAS in PTSD patients compared to traumatized non-PTSD participants, and there was a pattern of greater blunting of response in the tcVNS compared to the sham stimulation group for PTSD patients (Fig. 3). Non-PTSD participants had minimal anger responses for both tcVNS and sham stimulation groups (Fig. 3).
Fig. 3.
Effects of tcVNS (red line) or sham (blue line) on subjective anger as measured with the Visual Analogue Scale (VAS) at baseline (B) and with neutral scripts (NS) and trauma scripts (TS). PTSD patients (left side) had greater anger responses to trauma scripts than non-PTSD traumatized participants, an effect that showed a pattern of being blunted by pairing with active tcVNS. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
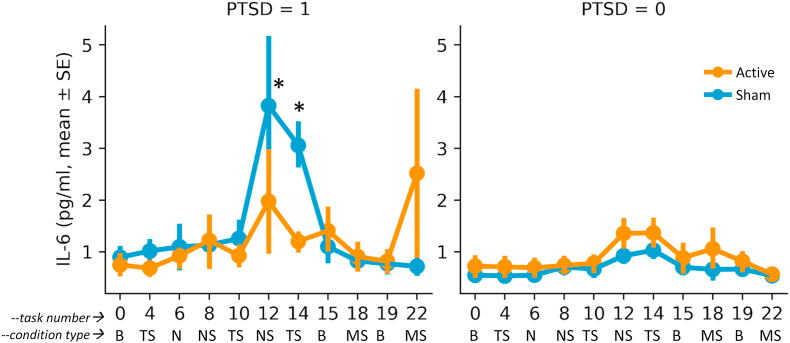
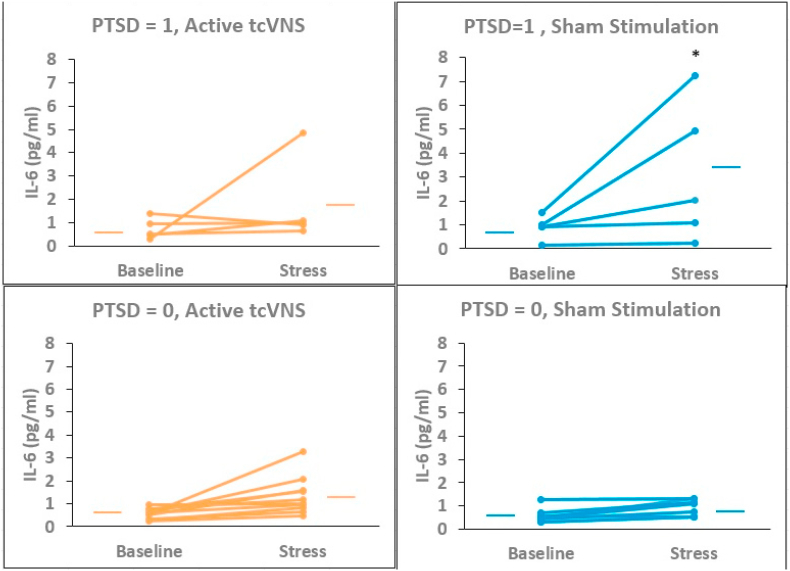
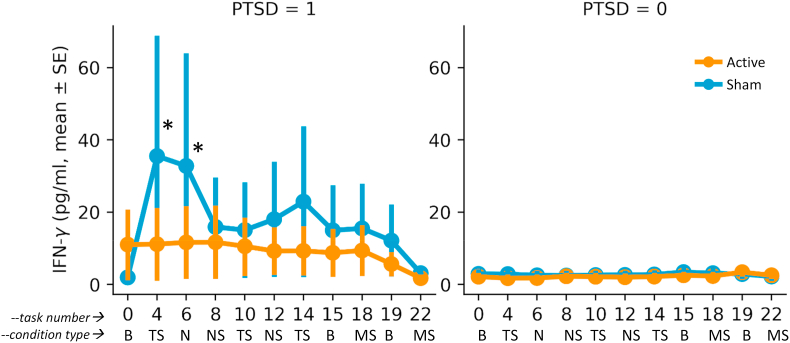
Exposure to personalized traumatic scripts in conjunction with sham stimulation resulted in an increase in IL-6 in PTSD but not non-PTSD participants that was greater following repeated exposure to personalized traumatic scripts (Day 1) than for mental stress (public speaking and mental arithmetic on Days 2 and 3), that peaked about 90 min after exposure to the first traumatic scripts and was blocked by tcVNS (ß = 0.474, 0.009–0.939 95% CI, p = 0.046) (Fig. 4, Fig. 5). There was minimal effect on IL-6 for neutral mental stress (public speaking and mental arithmetic) on days 2 and 3 in either the PTSD or non-PTSD, sham or tcVNS groups. Personalized traumatic scripts resulted in an immediate and marked rise in IFN-γ on Day 1 in the PTSD but not the non-PTSD participants (Fig. 6). The traumatic script-induced increase in IFN-γ was blocked by tcVNS versus sham (ß = -0.246, -0.470 -- -0.022 95% CI, p = 0.032) (Fig. 5). There were no statistically significant differences between tcVNS of sham stimulation groups in IL-2, IL-1β or TNF-α (Table 2).
Fig. 4.
Effects of tcVNS (red line) or sham (blue line) on interleukin-6 (IL-6) response to stress in patients with PTSD (left side) and traumatized participants without PTSD (right side). See Fig. 1 for a complete description of task numbers and condition types. On day 1 (personalized traumatic script day) blood was drawn at baseline (B), after the second presentation (task #4) of two trauma scripts 2 min in length paired with VNS or sham with 10 min between each script/stimulation pairing (TS), after the second (task #6) of two VNS/sham stimulations without task (N), after the second of two neutral scripts (NS) (task #8), after the second presentation (task #10) of two trauma scripts (TS) following the same protocol as before paired with VNS or sham. VNS or sham (TS), after the second of two neutral scripts (task #12), and after the second presentation (task #14) of two trauma scripts (TS) following the same protocol as before paired with tcVNS or sham. Toward the end of Day 1 with repeated TS there was an increase in IL-6 greater in sham versus tcVNS in PTSD patients (∗) that occurred 90 min after the presentation of the first trauma scripts (Time points #12 and #14)(p < .05). On Day 2 (D2) participants underwent a baseline blood draw at rest (task #15) and 90 min after mental stress (MS) in the form of public speaking and mental arithmetic paired with tcVNS or sham (task #18). On Day 3 (D3) participants again underwent a baseline blood draw at rest (task #19) and 90 min after mental stress (MS) using the same protocol as D2 (task #22). There were no significant differences between sham or active on days 2 or three with mental stress (MS, public speaking and mental arithmetic) compared to each days’ baseline in PTSD. Non-PTSD participants showed no difference between active or sham for either trauma scripts (Day 1) or mental stress (Days 2 and 3). Statistical analysis showed a significant day by diagnosis by device effect (p < .05), with secondary analysis showing a significant increase in IL-6 in sham versus tcVNS in the PTSD group with traumatic scripts (Day 1, p < .05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Effects of tcVNS (red lines) or sham (blue lines) on IL-6 in individual traumatized participants with (PTSD = 1, top figures) and without (PTSD = 0, bottom figures) PTSD. Lines connect baseline to post-stress (traumatic scripts) measurements. There was a significant increase in IL-6 in PTSD patients undergoing sham stimulation. Traumatic scripts had little effect on IL-6 in non-PTSD participants. ∗p < .05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Effects of tcVNS (red line) or sham (blue line) on Interferon-γ (IFN-γ) response to stress in patients with PTSD (left side) and traumatized participants without PTSD (right side). Overall there was a marked increase in IFN-γ in the PTSD but not the non-PTSD participants which was most pronounced after the first traumatic script (task #4) and was largely blocked by tcVNS but not sham, resulting in a significant increase in IFN-γ over the three day stress protocol in the sham group versus active tcVNS (∗, p < .05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Mean (SD) Concentrations of Interleukin-2 (IL-2), IL-1β and Tumor Necrosis Factor (TNF)-α Over Time in PTSD and Non-PTSD Participants with Active tcVNS or Sham Stimulation.
| IL-2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | 0 | 4 | 6 | 8 | 10 | 12 | 14 | 15 | 18 | 19 | 22 |
| PTSD Active | 0.17 (0.19) | 0.29 (0.19) | 0.19 (0.18) | 0.21 (0.16) | 0.20 (0.18) | 0.31 (0.22) | 0.18 (0.21) | 0.29 (0.19) | 0.34 (0.19) | 0.34 (0.19) | 0.39 (0.16) |
| PTSD Sham | 0.28 (0.21) | 0.31 (0.18) | 0.23 (0.20) | 0.32 (0.18) | 0.30 (0.19) | 0.34 (0.19) | 0.40 (0.16) | 0.22 (0.19) | 0.20 (0.17) | 0.22 (0.17) | 0.21 (0.17) |
| NonPTSD Active | 0.34 (0.22) | 0.23 (0.21) | 0.32 (0.22) | 0.34 (0.22) | 0.29 (0.24) | 0.37 (0.21) | 0.35 (0.20) | 0.23 (0.21) | 0.32 (0.23) | 0.22 (0.22) | 0.39 (0.19) |
| NonPTSD Sham | 0.30 (0.21) | 0.25 (0.22) | 0.24 (0.22) | 0.19 (0.20) | 0.14 (0.15) | 0.24 (0.21) | 0.25 (0.21) | 0.27 (0.20) | 0.27 (0.20) | 0.24 (0.20) | 0.19 (0.18) |
| IL-1β | |||||||||||
| Time | 0 | 4 | 6 | 8 | 10 | 12 | 14 | 15 | 18 | 19 | 22 |
| PTSD Active | 0.06 (0.04) | 0.09 (0.02) | 0.08 (0.04) | 0.08 (0.04) | 0.07 (0.04) | 0.08 (0.04) | 0.09 (0.03) | 0.11 (0.03) | 0.21 (0.27) | 0.12 (0.03) | 0.10 (0.00) |
| PTSD Sham | 0.10 (0.08) | 0.07 (0.04) | 0.07 (0.04) | 0.08 (0.04) | 0.06 (0.03) | 0.03 (0.01) | 0.05 (0.01) | 0.11 (0.08) | 0.09 (0.05) | 0.11 (0.06) | 0.10 (0.05) |
| NonPTSD Active | 0.05 (0.03) | 0.06 (0.04) | 0.08 (0.04) | 0.05 (0.04) | 0.08 (0.07) | 0.07 (0.04) | 0.05 (0.04) | 0.12 (0.08) | 0.14 (0.10) | 0.12 (0.08) | 0.12 (0.09) |
| NonPTSD Sham | 0.08 (0.04) | 0.06 (0.03) | 0.07 (0.04) | 0.09 (0.04) | 0.07 (0.04) | 0.11 (0.08) | 0.09 (0.04) | 0.12 (0.06) | 0.11 (0.06) | 0.09 (0.04) | 0.08 (0.05) |
| TNFα | |||||||||||
| Time | 0 | 4 | 6 | 8 | 10 | 12 | 14 | 15 | 18 | 19 | 22 |
| PTSD Active | 2.60 (0.85) | 2.27 (0.88) | 2.47 (0.76) | 2.41 (0.97) | 2.45 (0.60) | 2.32 (0.62) | 2.27 (0.82) | 2.44 (0.72) | 2.46 (0.64) | 2.55 (0.83) | 1.94 (0.17) |
| PTSD Sham | 2.64 (1.35) | 2.84 (1.83) | 2.50 (1.38) | 2.64 (1.51) | 2.79 (1.58) | 2.55 (1.14) | 2.09 (0.53) | 2.71 (1.42) | 2.73 (1.65) | 2.59 (1.48) | 2.34 (1.46) |
| NonPTSD Active | 2.90 (1.47) | 2.95 (1.53) | 2.89 (1.66) | 2.94 (1.56) | 2.94 (1.38) | 2.74 (1.15) | 2.54 (0.73) | 3.12 (1.52) | 3.17 (1.57) | 3.25 (1.26) | 2.54 (0.29) |
| NonPTSD Sham | 2.22 (0.50) | 2.11 (0.65) | 2.10 (0.68) | 2.23 (0.42) | 2.30 (0.55) | 2.14 (0.52) | 2.23 (0.44) | 3.43 (2.80) | 3.43 (2.69) | 2.67 (1.05) | 2.12 (0.60) |
4. Discussion
Non-invasive transcutaneous cervical vagus nerve stimulation (tcVNS) in this study blocked an increase in the inflammatory marker interleukin-6 (IL-6) and Interferon-γ (IFN-γ) seen with personalized traumatic scripts in PTSD patients administered sham stimulation. Non-PTSD participants with a history of exposure to psychological trauma overall had minimal IL-6 or IFN-γ increases in response to personalized traumatic scripts. Personalized traumatic scripts had much greater effects than mental stress including mental arithmetic and public speaking on IL-6 and INF-γ in PTSD patients and therefore the blocking effects of tcVNS were more prominent. Active tcVNS also blocked subjective anger related to exposure to personalized traumatic scripts in PTSD patients.
The vagus nerve has both afferent fibers that go to the brain and efferent fibers that control peripheral organ, autonomic and immune function. Studies showing that peripheral IL-6 and TNF-α concentrations vary with changes in heart rate variability (HRV, a marker of parasympathetic/sympathetic balance) are consistent with the current findings that the vagus modulates peripheral inflammation (Jan et al., 2010; Marsland et al., 2007) (Jan et al., 2010; Marsland et al., 2007). The current study shows that PTSD patients have an enhanced inflammatory response to stress, with the greatest effects for personalized traumatic scripts. Our finding of blocked IL-6 and IFN-γ responses to stress with tcVNS adds to the growing literature on nVNS affecting central brain and peripheral autonomic function in human (Frangos et al., 2015; Frangos and Komisaruk, 2017; Gurel et al., 2020b, 2020c; Lerman et al., 2016, 2018, 2019; Yakunina et al., 2017) and animal studies (Brock et al., 2017; Chen et al., 2016; Oshinsky et al., 2014).
The effects of tcVNS blocking inflammatory responses and subjective anger to personalized traumatic script suggests clinical relevance for PTSD. Exposure to traumatic events can produce strongly encoded intrusive memories as well as lasting changes in neurobiology, brain circuits involved in the stress response, and symptoms of PTSD (Bremner and Pearce, 2016; Merz et al., 2016). Projections of the vagus through the nucleus tractus solitarius (NTS) extend to the locus coeruleus and hypothalamus, key areas involved in sympathetic hyperarousal in PTSD, as well as brain areas like the amygdala that are involved in the fear response and the medial prefrontal cortex / anterior cingulate, which is involved in both fear extinction and modulation of peripheral neurohormonal responses to stress (Hardy, 1995). tcVNS likely travels through these central pathways to effect changes in peripheral inflammation. Cytokines, inflammasomes, and other inflammatory markers have behavioral effects similar to stress-related psychiatric symptoms (Felger et al., 2013b; Miller and Raison, 2016), so reduction of spikes in IL-6 and IFN-γ that likely occur multiple times a day with traumatic reminders and daily stressors in PTSD patients to will likely benefit symptoms driven by inflammation and lead to improvements in clinical course. Reduction in subjective anger in addition to improving mental health also likely has beneficial health effects. For instance, in our studies of coronary artery disease (CAD) patients, we found not only an increase in mental stress-induced IL-6 in those with co-morbid PTSD (Lima et al., 2019) but also that psychological distress (including an aggregate measure of subjective anger, distress and PTSD) was associated with long-term adverse cardiovascular outcomes.(Pimple et al., 2019) Furthermore, CAD patients with mental stress-induced myocardial ischemia (MSI) had an increase in PTSD (Lima et al., 2020), and subjective anger response to stress was associated with MSI (Pimple et al., 2015).
IL-6 and IFN-γ are pro-inflammatory elements of a complex immune system that is responsible for fighting infections and is also responsive to stress (Miller et al., 2009). Data has accumulated in recent years that elevations in inflammatory markers are associated with stress-related psychiatric disorders, including major depression and PTSD (Miller et al., 2009). Studies in both animals and humans showed that catecholamines released during stress (including mental stress tasks) act through the adrenergic receptor to activate the transcription factor, nuclear factor-κB (NF-κB), which leads to increases in cytokines, including IL-6 (Bierhaus et al., 2003). Raison and Miller have hypothesized that depression and inflammation may have links in evolution (Miller and Raison, 2016; Raison and Miller, 2013). Depression represents an illness-related behavior that serves to conserve energy and may be adaptive in survival, however interpersonal stress may have been a prelude to violent conflicts in primitive societies where an anticipatory outpouring of pro-inflammatory factors may have be critical for survival in the event of life-threatening wounds (Miller and Raison, 2016; Raison and Miller, 2013). Considerable evidence links elevated immune function to major depression and PTSD, and relevant to the current study, one meta-analysis showed that the statistically strongest findings in PTSD were for IL-6 and IFN-γ (Passos et al., 2015). Several studies showed that stress is associated with enhances release of IL-6 (Marsland et al., 2017), including mental stress tasks in patient with PTSD (Lima et al., 2019) and in individuals with early life stress who are vulnerable to the development of depression (Pace et al., 2006). Elevations in IFNγ and IL-6 are associated with decreases in tryptophan, the precursor of serotonin, a key neurotransmitter underlying the neurobiology of depression and PTSD, with associated increased symptoms of depression (Felger et al., 2013a; Raison et al., 2010). Diversion of tryptophan metabolism leads to increased metabolism along the kynurenine pathway, which has been linked to suicide and depression (Myint, 2012). Kynurenine also antagonizes the cholinergic anti-inflammatory effects of VNS (Myint, 2012; Nizri and Brenner, 2013; Olofsson et al., 2015) and is blocked by VNS (Majoie et al., 2011). Kynurenine can be converted to quinolinic acid, which enhances glutamatergic transmission with associated decreases in brain derived neurotrophic factor (BDNF) in the hippocampus, a mechanism implicated in PTSD and depression and the response to antidepressant treatments (Duman, 2004; Duman et al., 2001; Nibuya et al., 1995; Santarelli et al., 2003). These studies indicate that blocking of stress-induced IL-6 elevations with tcVNS may impact the underlying neurobiology of PTSD and have clinical utility for its treatment.
Findings of increased IFN-γ with stress in PTSD that are blocked by tcVNS have relevance for alterations in cell mediated immunity that may underlie symptoms of PTSD. Cell mediated immunity utilizes T cells including CD8+ cytotoxic cells that lyse cells harboring microbes and CD4+ cells that produce cytokines and activate phagocytes that engulf and kill microbes. These latter cells differentiate into Th1 and Th2 subsets, as well as Th17 subsets. Glucocorticoids including cortisol are anti-inflammatory and lower levels of cortisol as seen in patients with PTSD (Bremner et al., 2007; Yehuda et al., 1996) could result in enhancement of Th1 cell function in PTSD patients (Griffin et al., 2014; Zhou et al., 2014). Cytokines produced by Th1 cells include proinflammatory mediators (IFN-γ) and IL-2. IFN-γ is a potent macrophage activator which also has antiviral activity. Th2 cytokines are IL-4, IL-5, IL-10 and IL-13, which are mainly anti-inflammatory. Cytokine production is partly controlled by cholinergic neurotransmission and therefore the vagus nerve (Nizri and Brenner, 2013), and vagal nerve stimulation has been shown to shift the TH1/TH2 balance and dampen pro inflammatory responses (Olofsson et al., 2015). Several lines of evidence link altered cellular immunity to PTSD, including studies in women with PTSD showing enhanced cell mediated immunity (S.N. Wilson et al., 1999) and delayed-type hypersensitivity (DTH) reactions that are consistent with an enhancement of Th1 response and thus increased IFN-γ (Altemus et al., 2003). Other studies have linked DTH responses to elevated IFN-γ (Barth et al., 2003) and have shown increased IFN-γ in PTSD (Lindqvist et al., 2014; Passos et al., 2015; Woods et al., 2005). Vagus nerve stimulation activates T cells that produce acetylcholine, and by binding to the alpa-7 subunit of the cholinergic receptor inhibit NF-κB (Rosas-Ballina et al., 2011). VNS also inhibits High Mobility Group Box 1 (HMGB1), a proinflammatory master mediator, which is increased in PTSD (Huston et al., 2007; Wang et al., 2015). The findings of the current study of an increase in IFN-γ blocked by tcVNS in light of the findings reviewed above add more evidence for a clinically relevant impact on the underlying neurobiology of PTSD.
The current study has several important limitations. We examined multiple biomarkers which introduces the possibility of false positives, even though the primary hypothesis was based on IL-6. The sample size was small, and gender was not evenly distributed between groups. Our original hypothesis was that tcVNS would block IL-6 response to both traumatic script stress and neutral mental stress (public speaking and mental arithmetic). The current study did not find as much of an IL-6 response to neutral mental stress as in our prior study of public speaking stress in patients with Coronary Artery Disease (CAD) and PTSD. That was a different sample, however, including older patients with CAD and more medical comorbidities than the current sample of younger uncomplicated PTSD patients (Lima et al., 2019). The prior study was also the first exposure to stress performed while sitting in a chair as opposed to lying in a scanner, which we have found presents a more direct interpersonal experience in the solicitation of stressful responses within a social context. In prior studies we found a reduction in cardiovascular reactivity on a following day when stress was repeated in a scanner (Bremner et al., 2018). In the current study, neutral mental stress came on subsequent days to personalized traumatic scripts, so a reduction in responsiveness is to be expected. Furthermore, our prior research has shown a more robust biological response in terms of heart rate and blood pressure and cortisol response to traumatic script stress (Elzinga et al., 2003) than neutral mental stress (Bremner et al., 2003) in patients with PTSD. The current findings of great effect on traumatic script stress are in line with these prior results. Also, since this was an exploratory study and nothing is known about the effects of tcVNS on different types of stressors it can be considered as hypothesis generating data for future research. Due to these factors it is possible that findings are related to false positives. Therefore the current findings should be considered exploratory and the results should be replicated in other samples with larger numbers of subjects. Another possible limitation concerns the comparison intervention or “sham stimulation” which involved an active electrical stimulation. Although we have not found that the parameters of the stimulation result in responses consistent with vagus nerve stimulation (Hays et al., 2013; Noble et al., 2017, 2018, 2019; Pena et al., 2014; Souza et al., 2019), it is possible that stimulation occurred in some individuals, or that stimulation of other sensory nerves would have an effect. Use of inert devices as controls could lead to participants perception that they were not getting active interventions, increasing the risk of positive results that are only due to a placebo effect, a constant risk in device research. High frequency voltage signals (such as the active stimulus) pass through the skin with minimal power dissipation due to the low skin-electrode impedance at kHz frequencies; in contrast, lower frequency signals (such as the sham stimulus) are mainly attenuated at the skin-electrode interface due to the high impedance (Rosell et al., 1988). Accordingly, the active device operating at higher frequencies may deliver substantial energy to facilitate stimulation, while the voltage levels appearing at the vagus would be expected to be orders of magnitude lower for the sham device and thus stimulation is unlikely. Nevertheless, since the sham device does deliver relatively high voltage and current levels directly to the skin, it activates skin nociceptors, causing a similar feeling to a pinch. This sensation is necessary for blinding of the participants, and is thought as a critical detail by the authors for the evaluation of the potential treatment in psychiatric populations. Use of sham stimulation is a universal practice, regardless of voltage or current mode. For example, current-mode (i.e., auricular) stimulation studies typically use the earlobe as sham stimulation as the earlobe contains anatomically less nerve innervation (Burger et al., 2019; Kraus et al., 2007; Stavrakis et al., 2020; Verkuil and Burger, 2019; Yakunina et al., 2017, 2018). Compared to ‘no stimulation’ as a sham alternative, investigators think that any sort of sensation is necessary for blinding of the participants, and is thought as a critical detail for the valuation of the potential treatment in psychiatric populations. As this is a psychological study, use of an ‘active sham’ compared to no stimulation might mitigate psychological effects regarding treatment perception. It is important to note that each subject only uses one type of stimulation (either active or sham), devices assigned by staff who do not take part in data collection or analysis. Hence, subjects do not know how the other devices (that they did not use) feel like. If anything, however, factors reviewed above would have diminished our ability to detect differences between active and control devices, rather than increase them.
Findings of the current study that tcVNS blocks inflammatory responses to stress add to our recent studies showing that tcVNS blocks sympathetic arousal associated with exposure to personalized traumatic scripts and/or enhances parasympathetic function (Gurel et al., 2020b, 2020c). These physiological systems are known to be associated with anxiety and other symptoms relevant to PTSD. Other studies in implanted VNS suggest that tcVNS may have useful clinical applications based on its effects on memory and possible enhancement of neuroplasticity and/or facilitation of extinction of conditioned responses to reminders (Bremner, 2016; Bremner and Charney, 2010; Clark et al., 1999; Engineer et al., 2011; Noble et al., 2017; Pena et al., 2014; Peña et al., 2013). Future studies should investigate fundamental questions regarding parameter-specific effects of the stimulation (frequency, amplitude, waveform shape) on neuroinflammatory, cardiovascular, and peripheral function for improved autonomic control and the design of adaptive and personalized therapies (Ardell et al., 2015, 2017; Badran et al., 2018b; Gurel et al., 2020b)
Funding
This work was sponsored by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Targeted Neuroplasticity Training (TNT) program through the Naval Information Warfare Center (NIWC) Cooperative Agreement No. N66001-16-4054.
Declaration of competing interest
J.D.B has research funding support from ElectroCore LLC. Both active and sham stimulation devices used in this study were provided by ElectroCore free of charge.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100138.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aaronson S.T., Sears P., Ruvuna F., Bunker M., Conway C.R., Dougherty D.D., Reimherr F.W., Schwartz T.L., Zajecka J.M. A five-year observational study of patients with treatment-resistant depression treated with VNS therapy or treatment-as-usual: comparison of response, remission, and suicidality. Am. J. Psychiatr. 2017;174:640–648. doi: 10.1176/appi.ajp.2017.16010034. [DOI] [PubMed] [Google Scholar]
- Adair D., Truong D., Esmaeilpour Z., Gebodh N., Borges H., Ho L., Bremner J.D., Badran B.W., Napadow V., Clark V.P., Bikson M. Electrical stimulation of cranial nerves in cognition and disease. Brain Stimul. 2020;13:713–720. doi: 10.1016/j.brs.2020.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A., Hauger R.L., Barkauskas D.A., Lerman I.R., Moeller-Bertram T., Snijders C., Haji U., Patel P.M., Geracioti T.D., Chrousos G.P., Baker D.G. Relations of combat stress and posttraumatic stress disorder to 24-h plasma and cerebrospinal fluid interleukin-6 levels and circadian rhythmicity. Psychoneuroendocrinology. 2019;100:237–245. doi: 10.1016/j.psyneuen.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Akosile W., Voisey J., Lawford B., Colquhounc D., Young R.M., Mehta D. The inflammasome NLRP12 is associated with both depression and coronary artery disease in Vietnam veterans. Psychiatr. Res. 2018;270:775–779. doi: 10.1016/j.psychres.2018.10.051. [DOI] [PubMed] [Google Scholar]
- Alcocer-Gómez E., de Miguel M., Casas-Barquero N., Núñez-Vasco J., Sánchez-Alcazar J.A., Fernández-Rodríguez A., Cordero M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2014;36:111–117. doi: 10.1016/j.bbi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Altemus M., Cloitre M., Dhabhar F.S. Enhanced cellular immune response in women with PTSD related to childhood abuse. Am. J. Psychiatr. 2003;160:1705–1707. doi: 10.1176/appi.ajp.160.9.1705. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Association; Washington, D.C: 2013. The Diagnostic and Statistical Manual of Mental Disorders. (DSM-5) [Google Scholar]
- Ardell J.L., Nier H., Hammer M., Southerland E.M., Ardell C.L., Beaumont E., KenKnight B.H., Armour J.A. Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. J. Physiol. 2017;595:6887–6903. doi: 10.1113/JP274678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardell J.L., Rajendran P.S., Nier H.A., KenKnight B.H., Armour J.A. Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1740–H1752. doi: 10.1152/ajpheart.00557.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran B.W., Alfred B.Y., Adair D.n., Mappin G., DeVries W.H., Jenkins D.D., George M.S., Bikson M. Laboratory administration of transcutaneous auricular Vagus Nerve Stimulation (taVNS): technique, targeting, and considerations. JoVE. 2019 doi: 10.3791/58984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran B.W., Jenkins D.D., DeVries W.H., Dancy M., Summers P.M., Mappin G.M., Bernstein H., Bikson M., Coker-Bolt P., George M.S. Transcutaneous auricular vagus nerve stimulation (taVNS) for improving oromotor function in newborns. Brain Stimul. 2018;11:1198–1200. doi: 10.1016/j.brs.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran B.W., Mithoefer O.J., Summer C.E., LaBate N.T., Glusman C.E., Badran A.W., DeVries W.H., Summers P.M., Austelle C.W., McTeague L.M., Borckardt J.J., George M.S. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul. 2018;11:699–708. doi: 10.1016/j.brs.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D.G., Ekhator N.N., Kasckow J.W., Hill K.K., Zoumakis E., Dashevsky B.A., Chrousos G.P., Geracioti T.D., Jr. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- Ballenger J.C., Davidson J.R., Lecrubier Y., Nutt D.J., Foa E.B., Kessler R.C., McFarlane A.C., Shalev A.Y. Consensus statement on posttraumatic stress disorder from the international consensus group on depression and anxiety. J. Clin. Psychiatr. 2000;61:60–66. [PubMed] [Google Scholar]
- Ballenger J.C., Davidson J.R., Lecrubier Y., Nutt D.J., Marshall R.D., Nemeroff C.B., Shalev A.Y., Yehuda R. Consensus statement update on posttraumatic stress disorder from the international consensus group on depression and anxiety. J. Clin. Psychiatr. 2004;65(Suppl. 1):55–62. [PubMed] [Google Scholar]
- Bansal V., Ryu S.Y., Lopez N., Allexan S., Krzyzaniak M., Eliceiri B., Baird A., Coimbra R. Vagal stimulation modulates inflammation through a ghrelin mediated mechanism in traumatic brain injury. Inflammation. 2012;35:214–220. doi: 10.1007/s10753-011-9307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth H., Berg P.A., Klein R. Method for the in vitro determination of an individual disposition towards Th1- or Th2-reactivity by the application of appropriate stimulatory antigens. Clin. Exp. Immunol. 2003;134:78–85. doi: 10.1046/j.1365-2249.2003.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumert J., Lukaschek K., Kruse J., Thwing Emeny R., Koenig W., von Känel R., Ladwig K.-H., for the KORA investigators No evidence for an association of posttraumatic stress disorder with circulating levels of CRP and IL-18 in a population-based study. Cytokine. 2013;63:201–208. doi: 10.1016/j.cyto.2013.04.033. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E., Hellström K., Waldton C., Augustinsson L.E. Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurology. 1999;52:1265–1267. doi: 10.1212/wnl.52.6.1265. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E., Mañon-Espaillat R., R R., Wilder B.J., Stefan H., Mirza W., Tarver W.B., Wernicke J.F. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia. 1994;35:616–626. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- Berry S.M., Broglio K., Bunker M., Jayewardene A., Olin B., Rush A.J. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med. Devices (Auckl) 2013;6:17–35. doi: 10.2147/MDER.S41017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A., Wolf J., Andrassy M., Rohleder N., Humpert P.M., Petrov D., Ferstl R., von Eynatten M., Wendt T., Rudofsky G., Joswig M., Morcos M., Schwaninger M., McEwen B., Kirschbaum C., Nawroth P.P. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M., Grossman P., Thomas C., Zannou A.L., Jiang J., Adnan T., Mourdoukoutas A.P., Kronberg G., Truong D., Boggio P., Brunoni A.R., Charvet L., Fregni F., Fritsch B., Gillick B., Hamilton R.H., Hampstead B.M., Jankord R., Kirton A., Knotkova H., Liebetanz D., Liu A., Loo C., Nitsche M.A., Reis J., Richardson J.D., Rotenberg A., Turkeltaub P.E., Woods A.J. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016;9:641–661. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M., Paneri B., Mourdoukoutas A., Esmaeilpour Z., Badran B.W., Azzam R., Adair D., Datta A., Fang X.H., Wingeier B., Chao D., Alonso-Alonso M., Lee K., Knotkova H., Woods A.J., Hagedorn D., Jeffery D., Giordano J., Tyler W.J. Limited output transcranial electrical stimulation (LOTES-2017): engineering principles, regulatory statutes, and industry standards for wellness, over-the-counter, or prescription devices with low risk. Brain Stimul. 2017;11(1):34–157. doi: 10.1016/j.brs.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Bikson M., Unal G., Brunoni A., Loo C. What psychiatrists need to know about transcranial direct current stimulation. Psychiatr. Times. 2017:1–3. [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a clinician-administered PTSD scale. J. Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Boscarino J.A., Chang J. Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: research and clinical implications. Psychosom. Med. 1999;61:378–386. doi: 10.1097/00006842-199905000-00019. [DOI] [PubMed] [Google Scholar]
- Bremner D., Vermetten E., Kelley M.E. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J. Nerv. Ment. Dis. 2007;195:919–927. doi: 10.1097/NMD.0b013e3181594ca0. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., editor. Posttraumatic Stress Disorder: from Neurobiology to Treatment. Wiley; Hoboken, New Jersey: 2016. [Google Scholar]
- Bremner J.D., Campanella C., Khan Z., Shah M., Hammadah M., Wilmot K., Al Mheid I., Lima B.B., Garcia E.V., Nye J., Ward L., Kutner M.H., Raggi P., Pearce B.D., Shah A.J., Quyyumi A.A., Vaccarino V. Brain correlates of mental stress-induced myocardial ischemia. Psychosom. Med. 2018;80:515–525. doi: 10.1097/PSY.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Charney D.S. In: Textbook of Anxiety Disorders. Stein D.J., Hollander E., Rothbaum B.O., editors. American Psychiatric Publishing; Arlington, VA: 2010. Neural circuits in fear and anxiety; pp. 55–71. [Google Scholar]
- Bremner J.D., Cheema F.A., Ashraf A., Afzal N., Fani N., Reed J., Musselman D.L., Ritchie J.C., Faber T., Votaw J.R., Nemeroff C.B., Vaccarino V. Effects of a cognitive stress challenge on myocardial perfusion and plasma cortisol in coronary heart disease patients with depression. Stress Health. 2009;25:267–278. doi: 10.1002/smi.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Narayan M., Staib L.H., Southwick S.M., McGlashan T., Charney D.S. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatr. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Pearce B. In: Posttraumatic Stress Disorder: from Neurobiology to Treatment. Bremner J.D., editor. Wiley-Blackwell; Hoboken, New Jersey: 2016. Neurotransmitter, neurohormonal, and neuropeptidal function in stress and PTSD; pp. 181–232. [Google Scholar]
- Bremner J.D., Rapaport M.H. Vagus nerve stimulation: back to the future. Am. J. Psychiatr. 2017;174:609–610. doi: 10.1176/appi.ajp.2017.17040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Vythilingam M., Vermetten E., Adil J., Khan S., Nazeer A., Afzal N., McGlashan T., Anderson G., Heninger G.R., Southwick S.M., Charney D.S. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Brock C., Brock B., Aziz Q., Møller H.J., Pfeiffer Jensen M., Drewes A.M., Farmer A.D. Transcutaneous cervical vagal nerve stimulation modulates cardiac vagal tone and tumor necrosis factor-alpha. Neuro Gastroenterol. Motil. 2017;29:1–4. doi: 10.1111/nmo.12999. [DOI] [PubMed] [Google Scholar]
- Bruenig D., Mehtab D., Morris C.P., Lawford B., Harvey W., McD Young R.S., Voisey J. Correlation between interferon γ and interleukin 6 with PTSD and resilience. Psychiatr. Res. 2018;260:193–198. doi: 10.1016/j.psychres.2017.11.069. [DOI] [PubMed] [Google Scholar]
- Brunoni A.R., Moffa A.H., Fregni F., Palm U., Padberg F., Blumberger D.M., Daskalakis Z.J., Bennabi D., Haffen E., Alonzo A., Loo C.K. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br. J. Psychiatry. 2016;208:522–531. doi: 10.1192/bjp.bp.115.164715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A.R., Moffa A.H., Sampaio-Junior B., Borrione L., Moreno M.L., Fernandes R.A., Veronezi B.P., Nogueira B.S., Aparicio L.V.M., Razza L.B., Chamorro R., Tort L.C., Fraguas R., Lotufo P.A., Gattaz W.F., Fregni F., Benseñor I.M., the ELECT-TDCS Investigators Trial of electrical Direct-Current Therapy versus escitalopram for depression. N. Engl. J. Med. 2017;376:2523–2533. doi: 10.1056/NEJMoa1612999. [DOI] [PubMed] [Google Scholar]
- Brunoni A.R., Valiengo L., Baccaro A., Zanão T.A., de Oliveira J.F., Goulart A., Boggio P.S., Lotufo P.A., Benseñor I.M., Fregni F. The sertraline versus electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA psychiatr. 2013;70:383–390. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- Burg M.M., Soufer R. Psychological stress and induced ischemic syndromes. Curr. Cardiovasc. Risk Rep. 2014;8:377. doi: 10.1007/s12170-014-0377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A.M., Van der Does W., Thayer J.F., Brosschot J.F., Verkuil B. Transcutaneous vagus nerve stimulation reduces spontaneous but not induced negative thought intrusions in high worriers. Biol. Psychiatr. 2019;142:80–89. doi: 10.1016/j.biopsycho.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Capuron L., Su S., Miller A.H., Bremner J.D., Goldberg J., Vogt G.J., Maisano C., Jones L., Murrah N.V., Vaccarino V. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol. Psychiatr. 2008;64:896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.P., Ay I., de Morais A.L., Qin T., Zheng Y., Sadeghian H., Oka F., Simon B., Eikermann-Haerter K., Ayata C. Vagus nerve stimulation inhibits cortical spreading depression. Pain. 2016;157:797–805. doi: 10.1097/j.pain.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K.B., Naritoku D.K., Smith D.C., Browning R.A., Jensen R.A. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat. Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- Corcoran C., Connor T.J., O’keane V., Garland M.R. The effects of vagus nerve stimulation on pro-and anti-inflammatory cytokines in humans: a preliminary report. Neuroimmunomodulation. 2004;12:307–309. doi: 10.1159/000087109. [DOI] [PubMed] [Google Scholar]
- Corsi-Zuelli F.M.G., Brognara F., Quirino G.F.S., Hiroki C.H., Sobrano Fais R., Del-Ben C.M., Ulloa L., Salgado H.C., Kanashiro A., Loureiro C.M. Neuroimmune interactions in schizophrenia: focus on vagus nerve stimulation and activation of the alpha-7 nicotinic acetylcholine receptor. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Caspi A., Williams B., Ambler A., Sugden K., Mika J., Werts H., Freeman J., Pariante C.M., Moffitt T.E., Arseneault L. Biological embedding of stress through inflammation processes in childhood. Mol. Psychiatr. 2011;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., McEwen B.S. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Danese A., Moffitt T.E., Pariante C.M., Ambler A., Poulton R., Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatr. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Pariante C.M., Caspi A., Taylor A., Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J. Neurosci. Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- Das U.N. Vagus nerve stimulation, depression, and inflammation. Neuropsychopharmacology. 2007;32:2053–2054. doi: 10.1038/sj.npp.1301286. [DOI] [PubMed] [Google Scholar]
- Das U.N. Can vagus nerve stimulation halt or ameliorate rheumatoid arthritis and lupus? Lipids Health Dis. 2011;10:19. doi: 10.1186/1476-511X-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L., Hamner M., Bremner J.D. In: Posttraumatic Stress Disorder: from Neurobiology to Treatment. Bremner J.D., editor. Wiley Blackwell; Hoboken, N,J,: 2016. Pharmacotherapy for PTSD: effects on PTSD symptoms and the brain; pp. 389–412. [Google Scholar]
- De Herdt V., Bogaert S., Bracke K.R., Raedt R., De Vos M., Vonck K., Boon P. Effects of vagus nerve stimulation on pro- and anti-inflammatory cytokine induction in patients with refractory epilepsy. J. Neuroimmunol. 2009;214:104–108. doi: 10.1016/j.jneuroim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Dell-Osso B., Oldani L., Palazzo M.C., Balossi I., Ciabatti M., Altamura A.C. Vagus nerve stimulation in treatment-resistant depression: acute and follow-up results of an Italian case series. J. ECT. 2013;29:41–44. doi: 10.1097/YCT.0b013e3182735ef0. [DOI] [PubMed] [Google Scholar]
- Duman R.S. Depression: a case of neuronal life and death? Biol. Psychiatr. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Duman R.S., Malberg J.E., Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J. Pharmacol. Exp. Therapeut. 2001;299:401–407. [PubMed] [Google Scholar]
- Elzinga B.M., Schmahl C.S., Vermetten E., van Dyck R., Bremner J.D. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Engineer N.D., Riley J.R., Seale J.D., Vrana W.A., Shetake J.A., Sudanagunta S.P., Borland M.S., Kilgard M.P. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly S.A., Nievergelt C.M., Maihofer A.X., Barkauskas D.A., Biswas N., Agorastos A., O’Connor D.T., Baker D.G. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA psychiatr. 2014;71:423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswarapp M., Neylanc Thomas C., Whooley Mary A., Metzlerd Thomas J., Cohen Beth E. Inflammation as a predictor of disease course in posttraumatic stress disorder and depression: a prospective analysis from the Mind Your Heart Study. Brain Behav. Immun. 2019;75:220–227. doi: 10.1016/j.bbi.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Feldman R.L., Dunner D.L., Muller J.S., Stone D.A. Medicare patient experience with vagus nerve stimulation for treatment-resistant depression. J. Med. Econ. 2013;16:63–74. doi: 10.3111/13696998.2012.724745. [DOI] [PubMed] [Google Scholar]
- Felger J.C., Li L., Marvar P.J., Woolwine B.J., Harrison D.G., Raison C.L., Miller A.H. Tyrosine metabolism during interferon-α administration: association with fatigue and CSF dopamine concentrations. Brain Behav. Immun. 2013;31:153–160. doi: 10.1016/j.bbi.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Li Z., Haroon E., Woolwine B.J., Jung M.Y., Hu X., Miller A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatr. 2016;21:1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Mun J., Kimmel H.L., Nye J.A., Drake D.F., Hernandez C.R., Freeman A.A., Rye D.B., Goodman M.M., Howell L.L., Miller A.H. Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology. 2013;38:2179–2187. doi: 10.1038/npp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Gibbon M. In: Comprehensive Handbook of Psychological Assessment. Segal M.J.H.D.L., editor. John Wiley & Sons Inc.; Hoboken, NJ, US: 2004. The structured clinical interview for DSM-IV Axis I disorders (SCID-I) and the structured clinical interview for DSM-IV Axis II disorders (SCID-II) pp. 134–143. [Google Scholar]
- Foa E.B., Davidson J.R.T., Frances A., Culpepper L., Ross R., Ross D. The expert consensus guideline series: treatment of posttraumatic stress disorder. J. Clin. Psychiatr. 1999;60:4–76. [PubMed] [Google Scholar]
- Foa E.B., Hembree E., Rothbaum B.O. Oxford University Press; New York, NY: 2007. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide. [Google Scholar]
- Foa E.B., Rothbaum B.O. The Guilford Press; New York: 1998. Treating the Trauma of Rape: Cognitive-Behavioral Therapy for PTSD. [Google Scholar]
- Frangos E., Ellrich E., Komisaruk B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8:624–636. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangos E., Komisaruk B.R. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul. 2017;10:19–27. doi: 10.1016/j.brs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- George M.S., Rush A.J., Marangell L.B., Sackeim H.A., Brannan S.K., Davis S.M., Howland R., Kling M.A., Moreno F., Rittberg B., Dunner D., Schwartz T., Carpenter L., Burke M., Ninan P., Goodnick P. A one-year comparison of Vagus Nerve Stimulation with treatment as usual for treatment-resistant depression. Biol. Psychiatr. 2005;58:364–373. doi: 10.1016/j.biopsych.2005.07.028. [DOI] [PubMed] [Google Scholar]
- George M.S., Rush A.J., Sackeim H.A., Marangell L. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int. J. Neuropsychopharmacol. 2003;6:73–83. doi: 10.1017/S1461145703003250. [DOI] [PubMed] [Google Scholar]
- George M.S., Sackeim H.A., Rush A.J., Marangell L.B., Nahas Z., Husain M.M., Lissanby S.H., Burt T., Goldman J., Ballenger J.C. Vagus Nerve Stimulation: a new tool for brain research and therapy. Biol. Psychiatr. 2000;47:287–295. doi: 10.1016/s0006-3223(99)00308-x. [DOI] [PubMed] [Google Scholar]
- George R., Salinsky M., Kuzniecky R., Rosenfeld W., Bergen D., Tarver W.B., Wernicke J.F. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term follow-up on the first 67 patients exiting a controlled study. Epilepsia. 1994;35:637–643. doi: 10.1111/j.1528-1157.1994.tb02484.x. [DOI] [PubMed] [Google Scholar]
- Gill J., Luckenbaugh D., Charney D., Vythilingam M. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol. Psychiatr. 2010;68:999–1006. doi: 10.1016/j.biopsych.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Gill J., Vythilingam M., Page G.G. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J. Trauma Stress. 2008;21:530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J.M., Saligan L., Woods S., Page G. PTSD is associated with an excess of inflammatory immune activities. Psychiatr. Care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- Griffin G.D., Charron D., Al-Daccak R. Post-traumatic stress disorder: revisiting adrenergics, glucocorticoids, immune system effects and homeostasis. Clin. Transl. Immunol. 2014;3:e27. doi: 10.1038/cti.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardado P., Olivera A., Rusch H.L., Roy M., Martina C., Lejbman N., Leed H., Gill J.M. Altered gene expression of the innate immune, neuroendocrine, andnuclear factor-kappa B (NF-kB) systems is associated with posttraumatic stress disorder in military personnel. J. Anxiety Disord. 2016;38:9–20. doi: 10.1016/j.janxdis.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Guo H., Callaway J.B., Ting J.P.-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Tao Liu J.-C.G., Jiang Xiang-Ling, Chen Feng, Gao Yun-Suo. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac. J. Trop. Med. 2012:323–325. doi: 10.1016/S1995-7645(12)60048-0. [DOI] [PubMed] [Google Scholar]
- Gurel N.Z., Gazi A.H., Scott K.L., Wittbrodt M.T., Shah A.J., Vaccarino V., Bremner J.D., Inan O.T. Timing considerations for noninvasive Vagal Nerve Stimulation in clinical studies. AMIA Ann. Sympos. Proc. 2020;2019:1061–1070. [PMC free article] [PubMed] [Google Scholar]
- Gurel N.Z., Huang M., Wittbrodt M.T., Jung H., Ladd S.L., Shandhi M.H., Ko Y.-A., Shallenberger L., Nye J.A., Pearce B., Vaccarino V., Shah A.J., Bremner J.D., Inan O.T. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul. 2020;13:47–59. doi: 10.1016/j.brs.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel N.Z., Wittbrodt W.T., Jung H., Ladd S.L., Shah A.J., Vaccarino V., Bremner J.D., Inan O.T. Automatic detection of target engagement in transcutaneous cervical Vagal Nerve Stimulation for traumatic stress triggers. IEEE J. Biomed. Health Inform. 2020;24:1917–1925. doi: 10.1109/JBHI.2020.2981116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M., Al Mheid I., Wilmot K., Ramadan R., Shah A.J., Sun Y., Pearce B., Garcia E.V., Kutner M., Bremner J.D., Esteves F., Raggi P., Sheps D.S., Vaccarino V., Quyyumi A.A. The mental stress ischemia prognosis study (MIPS): objectives, study design, and prevalence of inducible ischemia. Psychosom. Med. 2017;79:311–317. doi: 10.1097/PSY.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M., Alkhoder A., Al Mheid I., Wilmot K., Isakadze N., Abdulhadi N., Chou D., Obideen M., O’Neal W.T., Sullivan S., Samman Tahhan A., Kelli H.M., Ramadan R., Pimple P., Sandesara P., Shah A.J., Ward L., Ko Y.-A., Sun Y., Uphoff I., Pearce B., Garcia E.V., Kutner M., Bremner J.D., Esteves F., Sheps D.S., Raggi P., Vaccarino V., Quyyumi A.A. Hemodynamic, catecholamine, vasomotor and vascular responses: determinants of myocardial ischemia during mental stress. Int. J. Cardiol. 2017;243:47–53. doi: 10.1016/j.ijcard.2017.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M., Sullivan S., Pearce B., Al Mheid I., Wilmot K., Ramadan R., Tahhan A.S., O’Neal W.T., Obideen M., Alkhoder A., Abdelhadi N., Mohamed Kelli H., Ghafeer M.M., Pimple P., Sandesara P., Shah A.J., Hosny K.M., Ward L., Ko Y.A., Sun Y.V., Weng L., Kutner M., Bremner J.D., Sheps D.S., Esteves F., Raggi P., Vaccarino V., Quyyumi A.A. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav. Immun. 2018;68:90–97. doi: 10.1016/j.bbi.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handforth A., DeGiorgio C.M., Schachter S.C., Uthman B.M., Naritoku D.K., Tecoma E.S., Henry T.R., Collins S.D., Vaughn B.V., Gilmartin R.C., Labar D.R., Morris G.L.r., Salinsky M.C., Osorio I., Ristanovic R.K., Labiner D.M., Jones J.C., Murphy J.V., Ney G.C., Wheless J.W. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- Hardy S.G. Medullary projections to the vagus nerve and posterolateral hypothalamus. Anat. Rec. 1995;242:251–258. doi: 10.1002/ar.1092420215. [DOI] [PubMed] [Google Scholar]
- Hays S.A., Khodaparast N., Ruiz A., Sloan A.M., Hulsey D.R., Rennaker R.L., Kilgard M.P. The timing and amount of vagus nerve stimulation during rehabilitative training affect post-stroke recovery of forelimb strength. Neuroreport. 2014;25:682–688. doi: 10.1097/WNR.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays S.A., Rennaker R.L., Kilgard M.P. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog. Brain Res. 2013;207:275–299. doi: 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath N.M., Chesney S.A., Gerhart J.I., Goldsmith R.E., Luborsky J.L., Stevens N.R., Hobfoll S.E. Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine. 2013;63:172–178. doi: 10.1016/j.cyto.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree E.A., Foa E.B., Dorfan N.M., Street G.P., Kowalski J., Tu X. Do patients drop out prematurely from exposure therapy for PTSD? J. Trauma Stress. 2003;16:555–562. doi: 10.1023/B:JOTS.0000004078.93012.7d. [DOI] [PubMed] [Google Scholar]
- Hoge E.A., Brandstetter K., Moshier S., Pollack M.H., Wong K.K., Simon N.M. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress. Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- Hulsey D.R., Riley J.R., Loerwald K.W., Rennaker R.L., Kilgard M.P., Hays S.A. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Neurology. 2017;289:21–30. doi: 10.1016/j.expneurol.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston J.M., Gallowitsch-Puerta M., Ochani M., Ochani K., Yuan R., Rosas-Ballina M., Ashok M., Goldstein R.S., Chavan S., Pavlov V.A. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 2007;35:2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies . National Academies of Science, Engineering and Medicine: Health and Medicine Division; Washington, D.C: 2014. Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment. [PubMed] [Google Scholar]
- Jacobs H.I.L., Riphagen J.M., Razat C.M., Wiese S., Sack A.T. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol. Aging. 2015;36:1860–1867. doi: 10.1016/j.neurobiolaging.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Jan B.U., Coyle S.M., Macor M.A., Reddell M., Calvano S.E., Lowry S.F. Relationship of basal heart rate variability to in vivo cytokine responses following endotoxin. Shock. 2010;33:363–368. doi: 10.1097/SHK.0b013e3181b66bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatr. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Belury M.A., Porter K., Beversdorf D.Q., Lemeshow S., Glaser R. Depressive symptoms, omega-6 fatty acids, and inflammation in older adults. Psychosom. Med. 2007;69:217–224. doi: 10.1097/PSY.0b013e3180313a45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krames E., Peckham P.H., Rezai A., editors. Neuromodulation: Comprehensive Textbook of Principles, Technologies, and Therapies. Academic Press (an imprint of Elsevier); London, United Kingdom: 2018. [Google Scholar]
- Kraus T., Hösl K., Kiess O., Schanze A., Kornhuber J., Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J. Neural. Transm. 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- Lamb D.G., Porges E.C., Lewis G.F., Williamson J.B. Non-invasive Vagal Nerve Stimulation effects on hyperarousal and autonomic state in patients with posttraumatic stress disorder and history of mild traumatic brain injury: preliminary evidence. Front. Med. 2017;4:124. doi: 10.3389/fmed.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster C.L., Teeters J.B., Gros D.F., Back S.E. Posttraumatic stress disorder: overview of evidence-based assessment and treatment. J. Clin. Med. 2016;5:105. doi: 10.3390/jcm5110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman I., Davis B., Huang M., Huang C., Sorkin L., Proudfoot J., Zhong E., Kimball D., Rao R., Simon B., Spadoni A. bioRxiv; 2018. Noninvasive Vagus Nerve Stimulation Alters Neural Response and Physiological Autonomic Tone to Noxious Thermal Challenge; p. 367979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman I., Davis B., Huang M., Huang C., Sorkin L., Proudfoot J., Zhong E., Kimball D., Rao R., Simon B., Spadoni A., Strigo I., Baker D.G., Simmons A.N. Noninvasive vagus nerve stimulation alters neural response and physiological autonomic tone to noxious thermal challenge. PLoS One. 2019;14 doi: 10.1371/journal.pone.0201212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman I., Hauger R., Sorkin L., Proudfoot J., Davis B., Huang A., Lam K., Simon B., Baker D.G. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation. 2016;19:283–290. doi: 10.1111/ner.12398. [DOI] [PubMed] [Google Scholar]
- Li W., Olshansky B. Inflammatory cytokines and nitric oxide in heart failure and potential modulation by vagus nerve stimulation. Heart Fail. Rev. 2011;16:137–145. doi: 10.1007/s10741-010-9184-4. [DOI] [PubMed] [Google Scholar]
- Li X., Wilder-Smith C.H., Kan M.E., Lu J., Cao Y., Wong R.K. Combat-training stress in soldiers increases S100B, a marker of increased blood-brain-barrier permeability, and induces immune activation. Neuroendocrinol. Lett. 2014;35 [PubMed] [Google Scholar]
- Lima B.B., Hammadah M., Pearce B.D., Shah A., Moazzami K., Kim J.H., Sullivan S., Levantsevych O., Lewis T.T., Weng L., Elon L., Li L., Raggi P., Bremner J.D., Quyyumi A., Vaccarino V. Association of posttraumatic stress disorder with mental stress-induced myocardial ischemia in adults after myocardial infarction. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B.B., Hammadah M., Wilmot K., Pearce B.D., Shah A., Levantsevych O., Kaseer B., Obideen M., Gafeer M.M., Kim J.H., Sullivan S., Lewis T.T., Weng L., Elon L., Li L., Bremner J.D., Raggi P., Quyyumi A., Vaccarino V. Posttraumatic Stress Disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav. Immun. 2019;75:26–33. doi: 10.1016/j.bbi.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D., Dhabhar F.S., Mellon S.H., Yehuda R., Grenon M., Flory J.D., Bierer L.M., Abu-Amara D., Coy M., Makotkine I., Reus V.I., Bersani F.S., Marmar C.R., Wolkowitz O.M. Increased pro-inflammatory milieu in combat related PTSD – a new cohort replication study. Brain Behav. Immun. 2017;59:260–264. doi: 10.1016/j.bbi.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Lindqvist D., Wolkowitz O.M., Mellon S., Yehuda R., Flory J.D., Henn-Haase C., Bierer L.M., Abu-Amara D., Coy M., Neylan T.C., Makotkine I., Reus V.I., Yan X., Taylor N.M., Marmar C.R., Dhabhar F.S. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav. Immun. 2014;42:81–88. doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Majoie H.J.M., Rijkers K., Berfelo M.W., Hulsman J.A.R.J., Myint A., Schwarz M., Vles J.S.H. Vagus nerve stimulation in refractory epilepsy: effects on pro-and anti-inflammatory cytokines in peripheral blood. Neuroimmunomodulation. 2011;18:52–56. doi: 10.1159/000315530. [DOI] [PubMed] [Google Scholar]
- Marangell L.B., Rush A.J., George M.S., Sackeim H.A., Johnson C.R., Husain M.M., Nahas Z., Lisanby S.H. Vagus Nerve Stimulation (VNS) for major depressive episodes: longer-term outcome. Biol. Psychiatr. 2002;51:280–287. doi: 10.1016/s0006-3223(01)01343-9. [DOI] [PubMed] [Google Scholar]
- Marsland A.L., Gianaros P.J., Prather A.A., Jennings J.R., Neumann S.A., Manuck S.B. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom. Med. 2007;69:709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- Marsland A.L., Walsh C., Lockwood K., John-Henderson N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav. Immun. 2017;64:208–219. doi: 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCanlies E.C., Araia S.K., Joseph P.N., Mnatsakanova A., Andrew M.E., Burchfiel C.M., JM V. C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine. 2011;55:74–78. doi: 10.1016/j.cyto.2011.03.025. C-reactive protein,interleukin-6,and posttraumatic, stress disorder symptomology inurban police officers.Cytokine 55, 2011. [DOI] [PubMed] [Google Scholar]
- Merz C.J., Elzinga B.M., Schwabe L. In: Posttraumatic Stress Disorder: from Neurobiology to Treatment. Bremner J.D., editor. John Wiley & Sons; Hoboken, New Jersey: 2016. Stress, fear, and memory in healthy individuals; pp. 159–178. [Google Scholar]
- Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathphysiology of depression. Biol. Psychiatr. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.J., Sutherland A.G., Hutchison J.D., Alexander D.A. C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: a pilot study. Cytokine. 2001;13:253–255. doi: 10.1006/cyto.2000.0825. [DOI] [PubMed] [Google Scholar]
- Myint A.M. Kynurenines: from the perspective of major psychiatric disorders. FEBS J. 2012;279:1375–1385. doi: 10.1111/j.1742-4658.2012.08551.x. [DOI] [PubMed] [Google Scholar]
- Neigh G.H., Ali F.F. Co-morbidity of PTSD and immune system dysfunction: opportunities for treatment. Curr. Opin. Pharmacol. 2016;29:104–110. doi: 10.1016/j.coph.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M., Morinobu S., Duman R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizri E., Brenner T. Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids. 2013;45:73–85. doi: 10.1007/s00726-011-1192-8. [DOI] [PubMed] [Google Scholar]
- Noble I.J., Gonzalez I.J., Meruva V.B., Callahan K.A., Belfort B.D., Ramanathan K.R., Meyers E., Kilgard M.P., Rennaker R.L., McIntyre C.K. Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Transl. Psychiatry. 2017;7:1–8. doi: 10.1038/tp.2017.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble L.J., Meruva V.B., Hays S.A., Rennaker R.L., Kilgard M.P., McIntyre C.K. Vagus nerve stimulation promotes generalization of conditioned fear extinction and reduces anxiety in rats. Brain Stimul. 2018;12:9–18. doi: 10.1016/j.brs.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble L.J., Souza R.R., McIntyre C.K. Vagus nerve stimulation as a tool for enhancing extinction in exposure-based therapies. Psychopharmacology (Berl.) 2019;236:355–367. doi: 10.1007/s00213-018-4994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonis R., D’Ostilio K., Schoenen J., Magis D. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: an electrophysiological study in healthy volunteers. Cephalagia. 2017;37:1285–1293. doi: 10.1177/0333102417717470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson P.S., Levine Y.A., Caravaca A., Chavan S.S., Pavlov V.A., Faltys M., Tracey K.J. Single-pulse and unidirectional electrical activation of the cervical vagus nerve reduces tumor necrosis factor in endotoxemia. Bioelectron. Med. 2015;2:37–42. [Google Scholar]
- Orr S.P., Lasko N.B., Metzger L.J., Ahern C.E., Berry N.J., Pitman R.K. Psychophysiologic assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. J. Consult. Clin. Psychol. 1998;66:906–913. doi: 10.1037//0022-006x.66.6.906. [DOI] [PubMed] [Google Scholar]
- Oshinsky M.L., Murphy A.L., Hekierski H., Cooper M., Simon B.J. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155 doi: 10.1016/j.pain.2014.02.009. 2037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace T.W.W., Mletzko T.C., Alagbe O., Musselman D.L., Nemeroff C.B., Miller A.H., Heim C.M. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatr. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Passos C.I., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., Salum G., Magalhães P.V., Kapczinski F., Kauer-Sant’Anna M. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatr. 2015;2:1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- Pena D.F., Childs J.E., Willett S., Vital A., McIntyre C.K., Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front. Behav. Neurosci. 2014;8:1–8. doi: 10.3389/fnbeh.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña D.F., Engineer N.D., McIntyre C.K. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol. Psychiatr. 2013;73:1071–1077. doi: 10.1016/j.biopsych.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimple P., Lima B.B., Hammadah M., Wilmot K., Ramadan R., Levantsevych O., Sullivan S., Kim J.H., Kaseer B., Shah A.J., Ward L., Raggi P., Bremner J.D., Hanfelt J., Lewis T., Quyyumi A.A., Vaccarino V. Psychological distress and subsequent cardiovascular events in individuals with coronary artery disease. JAHA. 2019;8 doi: 10.1161/JAHA.118.011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimple P., Shah A., Rooks C., Bremner J.D., Nye J., Ibeanu I., Murrah N., Shallenberger L., Kelley M., Raggi P., Vaccarino V. Association between anger and mental stress-induced myocardial ischemia. Am. Heart J. 2015;169:115–121. doi: 10.1016/j.ahj.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga L., Bremner J.D., Miller A.A., Jones D.P., Veledar E., Goldberg J., Vaccarino V. Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav. Immun. 2013;30:125–132. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak T., Markulin F., Ehlis A.-C., Langer J.B.M., Ringel T.M., Fallgatter A.J. Far field potentials from brain stem after transcutaneous vagus nerve stimulation: optimization of stimulation and recording parameters. J. Neural. Transm. 2009;116:1237–1242. doi: 10.1007/s00702-009-0282-1. [DOI] [PubMed] [Google Scholar]
- Raison C.L., Kelley K.W., Lawson M.A., Woolwine B.J., Vogt G., Spivey J.R., Saito K., Miller A.H. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: relationship to CNS immune responses and depression. Mol. Psychiatr. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Miller A.H. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Mol. Psychiatr. 2013;18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinertsen E., Nemati S., Vest A.N., Vaccarino V., Lampert R., Shah A.J., Clifford G.D. Heart rate-based window segmentation improves accuracy of classifying posttraumatic stress disorder using heart rate variability measures. Physiol. Meas. 2017;38:1061–1076. doi: 10.1088/1361-6579/aa6e9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks C., Veledar E., Goldberg J., Bremner J.D., Vaccarino V. Early trauma and inflammation: role of familial factors in a study of twins. Psychosom. Med. 2012;74:146–152. doi: 10.1097/PSY.0b013e318240a7d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks C.R., Ibeanu I., Shah A., Pimple P., Murrah N., Shallenberger L., Pace T., Bremner J.D., Raggi P., Vaccarino V. Young women post-MI have higher plasma concentrations of interleukin-6 before and after stress testing. Brain Behav. Immun. 2016;51:92–98. doi: 10.1016/j.bbi.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M., Olofsson P.S., Ochani M., Valdés-Ferrer S.I., Levine Y.A., Reardon C., Tusche M.W., Pavlov V.A., Andersson U., Chavan S., Mak T.W., Tracey K.J. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell J., Colominas J., Riu P., Pallas-Areny R., Webster J.G. Skin impedance from 1 Hz to 1 MHz. IEEE Trans. Biomed. Eng. 1988;35:649–651. doi: 10.1109/10.4599. [DOI] [PubMed] [Google Scholar]
- Rush A.J., George M.S., Sackeim H.A., Marangell L.B., Husain M., Giller C., Nahas Z., Haines S., Simson R.K., Goodman R., Burt T. Vagus Nerve Stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol. Psychiatr. 2000;47:276–286. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Marangell L.B., Sackeim H.A., George M.S., Brannan S.K., Davis S.M., Howland R., Kling M.A., Rittberg B.R., Burke W.J., Rapaport M.H., Zajecka J., Nierenberg A.A., Husain M.M., Ginsberg D., Cooke R.G. Vagus Nerve Stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol. Psychiatr. 2005;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Sackheim H.A., Marangell L.B., George M.S., Brannan S.K., Davis S.M., Lavori P., Howland R., Kling M.A., Rittberg B., Carpenter L., Ninan P., Moreno F., Schwartz T., Conway C., Burke M., Barry J.J. Effects of 12 Months of Vagus Nerve Stimulation in treatment-resistant depression: a naturalistic study. Biol. Psychiatr. 2005;58:355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Sackeim H.A., Brannan S.K., Rush A.J., George M.S., Marangell L.B., Allen J. Durability of antidepressant response to vagus nerve stimulation (VNS) Int. J. Neuropsychopharmacol. 2007;10:817–826. doi: 10.1017/S1461145706007425. [DOI] [PubMed] [Google Scholar]
- Sackeim H.A., Keilp J.G., Rush A.J., George M.S., Marangell L.B., Dormer J.S., Burt T., Lisanby S.H., Husain M., Collum M., Oliver N.C., Zboyan H.A. The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry Neuropsychol. Behav. Neurol. 2001;14:53–62. [PubMed] [Google Scholar]
- Sackeim H.A., Rush A.J., George M.S., Marangell L.B., Husain M.M., Nahas Z., Johnson C.R., Seidman S., Giller C., Haines S., Simpson R.K., Goodman R.R. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–728. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Salinsky M.C., Uthman B.M., Ristanovic R.K., Wernicke J.F., Tarver W.B. Vagus nerve stimulation for the treatment of medically intractable seizures. Results of a 1-year open-extension trial. The Vagus Nerve Stimulation Study Group. Arch. Neurol. 1999;53:1176–1180. doi: 10.1001/archneur.1996.00550110128021. [DOI] [PubMed] [Google Scholar]
- Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schachter S.C., Saper C.B. Vagus nerve stimulation. Epilepsia. 1998;39:677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Schnurr P.P., Friedman M.J., Engel C.C., Foa E.B., Shea T., Chow B.K., Resick P.A., Thurston V., Orsillo S.M., Haug R., Turner C., Bernardy N. Cognitive behavioral therapy for posttraumatic stress disorder in women. J. Am. Med. Assoc. 2007;297:820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- Schomer A.C., Nearing B.D., Schachter S.C., Verrier R.L. Vagus nerve stimulation reduces cardiac electrical instability assessed by quantitative T-wave alternans analysis in patients with drug-resistant focal epilepsy. Epilepsia. 2014;55:1996–2002. doi: 10.1111/epi.12855. [DOI] [PubMed] [Google Scholar]
- Schottenbauer M.A., Glass C.R., Arnkoff D.B., Tendick V., Gray S.H. Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry. 2008;71:134–168. doi: 10.1521/psyc.2008.71.2.134. [DOI] [PubMed] [Google Scholar]
- Shah A.J., Lampert R., Goldberg J., Veledar E., Bremner J.D., Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol. Psychiatr. 2013;73:1103–1110. doi: 10.1016/j.biopsych.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren M.J., Hellstrom P.T., Jonsson M.A., Runnerstam M., Silander H.C., Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: a pilot study. J. Clin. Psychiatr. 2002;63:972–980. doi: 10.4088/jcp.v63n1103. [DOI] [PubMed] [Google Scholar]
- Smith D.C., Modglin A.A., Roosevelt R.W., Neese S.L., Jensen R.A., Browning R.A., Clough R.W. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J. Neurotrauma. 2005;22:1485–1502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick S.M., Krystal J.H., Morgan C.A., Johnson D., Nagy L.M., Nicolaou A., Heninger G.R., Charney D.S. Abnormal noradrenergic function in posttraumatic stress disorder. Arch. Gen. Psychiatr. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- Souza R.R., Robertson N.M., Pruitt D.T., Gonzales P.A., Hays S.A., Rennaker R.L., Kilgard M.P., McIntyre C.K. Vagus nerve stimulation reverses the extinction impairments in a model of PTSD with prolonged and repeated trauma. Stress. 2019;22(4):509–520. doi: 10.1080/10253890.2019.1602604. [DOI] [PubMed] [Google Scholar]
- Stavrakis S., Stoner J.A., Humphrey M.B., Morris L., Filiberti A., Reynolds J.C., Elkholey K., Javed I., Twidale N., Riha P., Varahan S., Scherlag B.J., Jackman W.M., Dasari T.W., Po S.S. TREAT af (transcutaneous electrical vagus nerve stimulation to suppress atrial fibrillation): a randomized clinical trial. JACC. Clin. Electrophysiol. 2020;6:282–291. doi: 10.1016/j.jacep.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Miller A.H., Snieder H., Bremner J.D., Ritchie J., Maisano C., Jones L., Murrah N.V., Goldberg J., Vaccarino V. Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: the Twins Heart Study. Psychosom. Med. 2009;71:152–158. doi: 10.1097/PSY.0b013e31819082ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Perakyla J., Holm K., Haapasalo J., Lehtimaki K., Ogawa K.H., Peltola J., Hartikainen K.M. Vagus nerve stimulation improves working memory performance. J. Clin. Exp. Neuropsychol. Vagus Nerve stimul. improv. Work. Memory performance. 2017:954–964. doi: 10.1080/13803395.2017.1285869. [DOI] [PubMed] [Google Scholar]
- Sutherland A.G., Alexander D.A., Hutchison J.D. Disturbance of pro-inflammatory cytokines in post-traumatic psychopathology. Cytokine. 2003;24:219–225. doi: 10.1016/j.cyto.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tarn J., Legg S., Mitchell S., Simon B., Ng W.-F. The effects of noninvasive vagus nerve stimulation on fatigue and immune responses in patients with primary Sjögren’s Syndrome. Neuromodulation. 2019;22:580–585. doi: 10.1111/ner.12879. [DOI] [PubMed] [Google Scholar]
- Terry R.S. 2014. Vagus Nerve Stimulation for Epilepsy. [Google Scholar]
- The Vagus Nerve Stimulation Study Group A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology. 1995;45:224–230. doi: 10.1212/wnl.45.2.224. [DOI] [PubMed] [Google Scholar]
- Tortella G., Casati R., Aparicio L.V.M., Mantovani A., Senço N., D’Urso G., Brunelin J., Guarienti F., Lorencini Selingardi P.M., Muszkat D., de Sampaio Pereira B., Valiengo L., Moffa A.H., Simis M., Borrione L., Brunoni A.R. Transcranial direct current stimulation in psychiatric disorders. World J. Psychiatr. 2015;5:88–102. doi: 10.5498/wjp.v5.i1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P., Jeon-Slaughter H., Pfefferbaum B., Khan Q., Davis N.J. Emotional and biological stress measures in Katrina survivors relocated to Oklahoma. Am. J. Disaster Med. 2010;5:113–125. doi: 10.5055/ajdm.2010.0013. [DOI] [PubMed] [Google Scholar]
- Vaccarino V., Brennan M.L., Miller A.H., Bremner J.D., Ritchie J.C., Lindau F., Veledar E., Su S., Murrah N.V., Jones L., Jawed F., Dai J., Goldberg J., Hazen S.L. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol. Psychiatr. 2008;64:476–483. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuil B., Burger A.M. Transcutaneous vagus nerve stimulation does not affect attention to fearful faces in high worriers. Behav. Res. Ther. 2019;113:25–31. doi: 10.1016/j.brat.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Vidovic A., Gotovac K., Vilibic M., Sabioncello A., Jovanovic T., Rabatic S., Folnegovic-Smalc V., Dekaris D. Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation. 2011;18:199–211. doi: 10.1159/000322869. [DOI] [PubMed] [Google Scholar]
- von Kanel R., Abbas C.C., Begre S., Saner H., Gander M.L., Schmid J.P. Posttraumatic stress disorder and soluble cellular adhesion molecules at rest and in response to a trauma-specific interview in patients after myocardial infarction. Psychiatr. Res. 2010;179:312–317. doi: 10.1016/j.psychres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- von Kanel R., Begre S., Abbas C.C., Saner H., Gander M.L., Schmid J.P. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation. 2010;17:39–46. doi: 10.1159/000243084. [DOI] [PubMed] [Google Scholar]
- von Känel R., Hepp U., Kraemer B., Traber R., Keel M., Mica L., Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Vonck K., Raedt R., Naulaerts J., De Vogelaere F., Thiery E., Van Roost D., Aldenkamp B., Miatton M., Boon P. Vagus nerve stimulation. 25 years later! what do we know about the effects on cognition? Neurosci. Biobehav. Rev. 2014;45:63–71. doi: 10.1016/j.neubiorev.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Wang X.-W., Karki A., Du D.-Y., Zhao X.-J., Xiang X.-Y., Lu Z.-Q. Plasma levels of high mobility group box 1 increase in patients with posttraumatic stress disorder after severe blunt chest trauma: a prospective cohort study. J. Surg. Res. 2015;193:308–315. doi: 10.1016/j.jss.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Wilson S.N., van der Kolok B., Burbridge J., Fisler R., Kradin R. Phenotype of blood lymphocytes in PTSD suggests chronic immune activation. Psychosomatics. 1999;40(3):222–225. doi: 10.1016/S0033-3182(99)71238-7. [DOI] [PubMed] [Google Scholar]
- Woods A.B., Page G.G., O’Campo P., Pugh L.C., Ford D., Campbell J.C. The mediation effect of posttraumatic stress disorder symptoms on the relationship of intimate partner violence and IFN-gamma levels. Am. J. Community Psychol. 2005;36:159–175. doi: 10.1007/s10464-005-6240-7. [DOI] [PubMed] [Google Scholar]
- Woods A.J., Antal A., Bikson M., Boggio P.S., Brunoni A.R., Celnik P., Cohen L.G., Fregni F., Herrmann C.S., Kappenman E.S., Knotkova H., Liebetanz D., Miniussi C., Mirandam P.C., Paulus W., Priori A., Reato D., Stagg C., Wenderoth N., Nitsche M.A. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016;127:1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunina N., Kim S.S., Nam E.-C. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation. 2017;20:290–300. doi: 10.1111/ner.12541. [DOI] [PubMed] [Google Scholar]
- Yakunina N., Kim S.S., Nam E.C. BOLD fMRI effects of transcutaneous vagus nerve stimulation in patients with chronic tinnitus. PLoS One. 2018;28 doi: 10.1371/journal.pone.0207281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Teicher M.H., Trestman R.L., Levengood R.A., Siever L.J. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol. Psychiatr. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Yoo P.B., Lubock N.B., Hincapie J.G., Ruble S.B., Hamann J.J., Grill W.M. High-resolution measurement of electrically-evoked vagus nerve activity in the anesthetized dog. J. Neural. Eng. 2013;10 doi: 10.1088/1741-2560/10/2/026003. [DOI] [PubMed] [Google Scholar]
- Zhou J., Nagarkatti P., Zhong Y., Ginsberg J.P., Singh N.P., Zhang J., Nagarkatti M. Dysregulation in microRNA expression Is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.