Abstract
Generalized Anxiety Disorder (GAD) presents a high prevalence in the population, leading to distress and disability. Immune system alterations have been associated with anxiety-related behaviors in rodents and GAD patients. CD300f immune receptors are highly expressed in microglia and participate not only in the modulation of immune responses but also in pruning and reshaping synapses. It was recently demonstrated that CD300f might be influential in the pathogenesis of depression in a sex-dependent manner. Here, we evaluated the role of CD300f immune receptor in anxiety, using CD300f knockout mice (CD300f−/−) and patients with GAD. We observed that male CD300f−/− mice had numerous behavioral changes associated with a low-anxiety phenotype, including increased open field central locomotion and rearing behaviors, more exploration in the open arms of the elevated plus-maze test, and decreased latency to eat in the novelty suppressed feeding test. In a cross-sectional population-based study, including 1111 subjects, we evaluated a common single-nucleotide polymorphism rs2034310 (C/T) in the cytoplasmatic tail of CD300f gene in individuals with GAD. Notably, we observed that the T allele of the rs2034310 polymorphism conferred protection against GAD in men, even after adjusting for confounding variables. Overall, our data demonstrate that CD300f immune receptors are involved in the modulation of pathological anxiety behaviors in a sex-dependent manner. The biological basis of these sex differences is still poorly understood, but it may provide significant clues regarding the neuropathophysiological mechanisms of GAD and can pave the way for future specific pharmacological interventions.
Keywords: Generalized anxiety disorder, Immune system, CD300f receptors, Polymorphism
Highlights
-
•
CD300f−/− male mice presented reduced anxiety-like behavior.
-
•
CD300f rs2034310 polymorphism is related to generalized anxiety disorder.
-
•
The T allele protects against generalized anxiety disorder in men.
1. Introduction
Anxiety disorders comprise a heterogeneous spectrum of conditions in which Generalized Anxiety Disorder (GAD) is the most widespread mental health condition (American Psychiatric Association, 2013; DSM-5). The12-month prevalence of GAD is 3.9% (2.1-6.6%), and the lifetime prevalence is estimated to be 12% (8-13.7%) (Murray et al., 2012; Haller et al., 2014). The features of GAD are persistent and excessive worrying, anxiety and tension, allied to somatic symptoms and hypervigilance, for six months or more, that leads to significant distress and disability (Wittchen, 2002).
Anxiety disorders aggregate in families and heritability ranges from 30 to 60% complemented by a combination of environmental factors that shape risk and resilience (Polderman et al., 2015; Craske et al., 2017). Moreover, GAD is twice as prevalent in women, with more disability and worse outcomes (Rubio and López-Ibor, 2007; Vesga-López et al., 2008). On the other hand, men are more likely to have GAD with a comorbid substance use disorder (Rubio and López-Ibor, 2007; Vesga-López et al., 2008). The biological basis of these differences is still poorly understood, but they might be the result of different neuropathophysiological mechanisms and specific etiological factors across sex, including genetic variability, physiological and hormonal differences, and changes in stress responses (Vesga-López et al., 2008).
Immune alterations are frequently observed in GAD patients (Vieira et al., 2010; Copeland et al., 2012; Costello et al., 2019). Notably, genome-wide expression profiles of a community sample identified 631 differentially expressed genes in blood cells of men with GAD, but not women, compared to control individuals. Of these, 123 genes with altered expression were involved in immune responses (Wingo and Gibson, 2015). In mice submitted to psychosocial stress, anxiety occurs in parallel with microglial activation and endothelial dysfunction, leading to monocyte recruitment to the brain (McKim et al., 2018). The immune dysregulation can impact essential brain functions, including neurotransmitter metabolism, cell communication, and synaptic plasticity, as well as the neural circuitry controlling anxiety and emotional regulation (Capuron and Miller, 2011).
The CD300f immunoreceptors (or CLM-1, in rodents) are expressed in peripheral immune cells and in the central nervous system (CNS), especially in microglia and perivascular macrophages (Clark et al., 2009). Activation of CD300f receptors leads to the inhibition of inflammatory process (Keswani et al., 2020; Shiba et al., 2017; Matsukawa et al., 2015) and has neuroprotective properties in diseases related to neurodegeneration and inflammation such as autoimmune encephalomyelitis and excitotoxic brain injury (Martínez-Barriocanal et al., 2017; Zaqueu Lima et al., 2017., Peluffo et al., 2015). Recently, it was reported that CD300f might be influential in the pathogenesis of depression in humans in a sex-dependent manner (Lago-Kaufmann et al., 2020). Here, we explored the involvement of the CD300f immunoreceptor in behavioral paradigms used to evaluate anxiety in rodents. Furthermore, we took advantage of specific single nucleotide polymorphism in the CD300f gene that modifies the receptor phosphorylation and intracellular signaling (Lago-Kaufmann., 2020) to understand the role of these receptors in emotional modulation in humans.
2. Methods
2.1. Behavioral analysis in rodents
Male and female C57/BL6 (originally from The Jackson Laboratory) and global CD300f knockout (CD300f−/− in C57/BL6 background, Xi et al., 2010) mice (4-5-months-old) were obtained from the SPF animal facility of the Institute Pasteur de Montevideo-Uruguay. Animals were housed under a controlled environment (20 ± 1 °C, 12 h-light/dark cycle, free access to food and water). All behavioral analyses were videotaped and scored using the Any Maze software. Mice were handled according to the guidelines of the Institut Pasteur de Montevideo Animal Care Committee (protocols #004-16 and #014-16) and following the guidelines of the European Commission on Animal Care and Uruguayan national law and ethical guidelines on animal care.
2.1.1. Open field test
The apparatus consisted of an acrylic box (40 cm × 60 cm x 50 cm). For the evaluation of exploratory behavior and locomotion, the animals were exposed during 6 min to the arena, and the total distance traveled, time spent in the periphery and in the center of the arena, as well as the number of supported and unsupported rears, and the amount of grooming was recorded (Lago-Kaufmann et al., 2020).
2.1.2. Elevated Plus Maze test
The Elevated Plus Maze (EPM) test was used to assess anxiety status (Lister, 1987). The apparatus consisted of a central square platform (6 × 6 cm) and two open arms (30 × 6 cm) aligned perpendicularly to two closed arms (30 × 6 × 16 cm) and elevated 50 cm above the floor. Mice were individually placed in the central platform and recorded for 5 min. The relative time spent in closed versus opened arms is a measure of anxiety. The number of closed arm entries was used as a measure of spontaneous locomotion.
2.1.3. Novelty suppressed feeding test
The Novelty Suppressed Feeding Test (NSF) measures the latency of mice in approaching and eating food in a new environment following an extended period (18 h) of food deprivation and longer latencies in each parameter considered indicative of anxiety-like behavior. After 18 h of food deprivation, mice were placed in an acrylic box (40 cm × 60 cm x 50 cm) for 10 min with a small piece of mouse chow placed in the center. Immediately after the mouse began to eat the chow, the test ended, and those mice that did not eat within 10 min were considered as zero (Samuels and Hen, 2011).
2.2. Clinical study
2.2.1. Study design and participants
Our sample was derived from a cross-sectional population-based study, including 1111 subjects aged 18–35 years old carried out in the city of Pelotas, Southern Brazil, between June 2011 and May 2013 (Lago-Kaufmann et al., 2020). Sociodemographic and clinical variables were self-reported. Body mass index (BMI) was calculated by the equation: weight (kg)/height (m2). GAD diagnosis was performed using the structured diagnosis interview, the Brazilian version of the Mini International Neuropsychiatric Interview (MINI 5.0), according to DSM-5 criteria (Medical Outcome Systems Inc., Jacksonville, FL, USA). The study was approved by the University’s Ethics Committee (2010/15), and all patients provided written informed consent.
2.2.2. Blood sample collection and genotyping
Ten milliliters of blood were withdrawn (8:00-11:00 a.m.) by venipuncture after the interview. Blood samples were centrifuged (3500 g, at room temperature) for the separation of the leukocytes enriched fraction, and the DNA extraction was performed using a standardized salting-out procedure (Lahiri and Nurnberger, 1991). CD300f rs2034310 (C/T) single nucleotide polymorphism (SNP) has a minor allele frequency of 0.1725 in the European population (http://www.ncbi.nlm.nih.gov/projects/SNP/). Genotyping was performed using forward and reverse primers and probes contained in the 40× Human Custom TaqMan Genotyping Assay (Life Technologies, Foster City, CA, USA). The reactions were carried out using 2 ng of genomic DNA from each subject, TaqMan Genotyping Master Mix 1× (Applied Biosystems), and Custom TaqMan Genotyping Assay 1× in a real-time PCR thermal cycler (7500 Fast Real PCR System; Applied Biosystems), as previously described (Lago-Kaufmann et al., 2020). Fluorescence data files were analyzed using automated allele-calling software (SDS 2.0.1; Applied Biosystems).
2.3. Statistical analysis
Differences between wild-type (WT) and CD300f−/− mice were determined by unpaired Students’ t-test using Graph Pad Prism 6.0 (GraphPad Software Inc., San Diego, USA). The Kolmogorov-Smirnov test assessed data normality, and outlier values were identified using Grubbs’ test. Data are presented as mean ± SEM. Human data were analyzed by SPSS 22.0 (IBM Corporation, Armonk, NY). Variables are presented as number and percentage (%), or mean and standard deviation (SD). The sociodemographic characteristics, according to the genotypes, were compared using Chi-squared or One-Way ANOVA followed by Bonferroni’s post-hoc test. The allele and genotype frequencies were evaluated by direct counting, and the Hardy-Weinberg equilibrium was calculated using the Chi-squared test. The magnitude of the association between different genotypes and GAD was estimated using odds ratio (OR) with 95% confidence intervals (CI), adjusted for age, sex, and ethnicity in the logistic regression analysis. Bonferroni’s correction was used to account for multiple comparisons. p < 0.05 was considered significant.
3. Results
3.1. Anxiety-related behaviors in CD300f−/− male mice
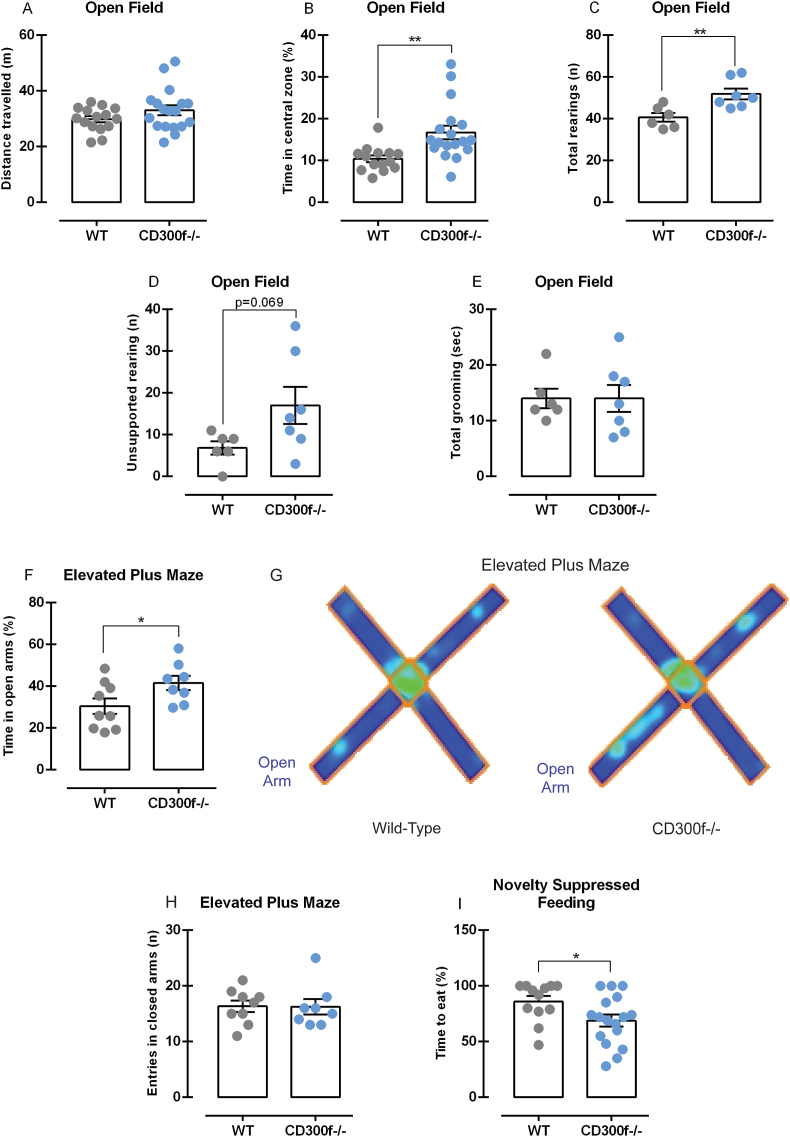
We performed the open field test (OFT) to evaluate locomotion and anxious-like behavior in male WT and CD300−/− mice. No differences in the spontaneous locomotor activity were observed in WT and CD300f−/− mice [t(31) = 1.45, p = 0.157], Fig. 1A. However, as observed in Fig. 1B, CD300f−/− mice spent more time in the center of the OFT [t(30) = 3.22, p = 0.003], which indicates a reduction in anxiety-like behavior. To confirm this hypothesis, we further evaluated other parameters that could indicate less anxiety in a new environment. CD300f−/− male mice presented a higher number of rearing episodes in the OFT compared to WT mice [t(11) = 3.25, p = 0.007, Fig. 1C]. Moreover, rearing behavior can be distinguished between supported rearing, which is related to locomotion and activity and unsupported rearing, related to emotional states (Sturman et al., 2018). In our study, we have noticed that CD300f−/− do not show any changes in supported rearing [t(11) = 0.32, p = 0.758, Supplementary Fig. 1A], but presented a tendency to increased unsupported rearing compared to WT mice [t(11) = 2.01, p = 0.069, Fig. 1D]. Studies have demonstrated that grooming behavior is increased when the animal is habituated to the new environment (Rojas-Carvajal et al., 2018). Here, no differences were observed between CD300f−/− and WT mice in the latency to start grooming [t(10) = 0.44, p = 0.672, Supplementary Fig 1B] or in total time of grooming behavior [t(11) = 0.00, p > 0.999, Fig. 1E]. Defecation in the OFT is another important sign of anxiety. We observed that 66,7% of WT mice defecated in the OFT against 20.0% of CD300f−/− (Supplementary Fig 1C). Additionally, 27.3% of CD300f−/− jumped attempting to escape from the OFT while no WT presented such behavior (Supplementary Fig 1D).
Fig. 1.
Anxiety-related behaviors of CD300f−/− male mice in different behavioral contexts. The total distance traveled in the OF was measured (meters) to evaluate mice spontaneous ambulatory activity (n = 15 WT and 18 CD300f−/−, 1.A), and the % of time spent in the center of the OF was used to evaluate anxious-like behavior (n = 14 WT and 18 CD300f−/−, 1.B). The total rearing (n = 6 WT and 7 CD300f−/−, 1.C) and the number of unsupported (n = 6 WT and 7 CD300f−/−, 1.D) rearing were counted to evaluate explorative behavior and emotionality. Grooming behavior was recorded (seconds) to evaluate mice habituation to a new environment (n = 6 WT and 7 CD300f−/−, 1.E). The % of time spent in the open arms of the EPM (calculated using the total time in the open arms + the total time in the closed arms as 100%) was indicative of anxiety-like behavior (n = 9 WT and 8 CD300f−/−, 1.F). Heatmaps illustrating WT and CD300f−/− mice behavior in the EPM, respectively (1.G). The number of entries in the closed arms of the EPM was indicative of spontaneous locomotion (n = 9 WT and 8 CD300f−/−, 1.H). The latency to start eating in the NSF test was used as an indicative of anxious-like behavior in a new environment (n = 12 WT and 17 CD300f−/−, 1.I). Data are represented as mean ± S.E.M. ∗ represents p < 0.05, and ∗∗ represents p < 0.01 compared to WT. Abbreviations: OF: Open Field; EPM: Elevated Plus Maze; NSF: Novelty Suppressed Feeding; WT: Wild-type mouse; CD300f−/−: CD300f knockout mouse.
To further confirm the reduction in anxiety-related behaviors in male CD300f−/− mice, we used the EPM, which is the gold standard test to assess avoidance and anxiety (Biedermann et al., 2017). The analysis showed that CD300f−/− male mice had increased entries [t(15) = 2.55, p = 0.022, Supplementary Fig 1E] and spent more time in the open arms [t(15) = 2.18, p = 0.045, Fig. 1F, and heatmaps in 1.G] and decreased time in the closed arms [t(15) = 2.18, p = 0.045, Supplementary Fig. 1F] when compared to WT, while no differences were observed in the number of entries in the closed arms [t(15) = 0.04, p = 0.962, Fig. 1H]. Next, we assessed behavior in a new environment in a fasting-state using the NSF test. CD300f−/− mice had a decreased latency to eat compared to WT mice [t(27) = 2.22, p = 0.035, Fig. 1I], but no differences were observed in the time to approach the food pellet [t(26) = 0.37, p = 0.714, Supplementary Fig 1G]. Altogether, these results demonstrate that, when placed in a new environment, CD300f−/− mice present lower anxiety-like behaviors, better habituation, and better emotional state in different behavioral paradigms compared to WT mice.
3.2. Anxiety-related behaviors in CD300f−/− female mice
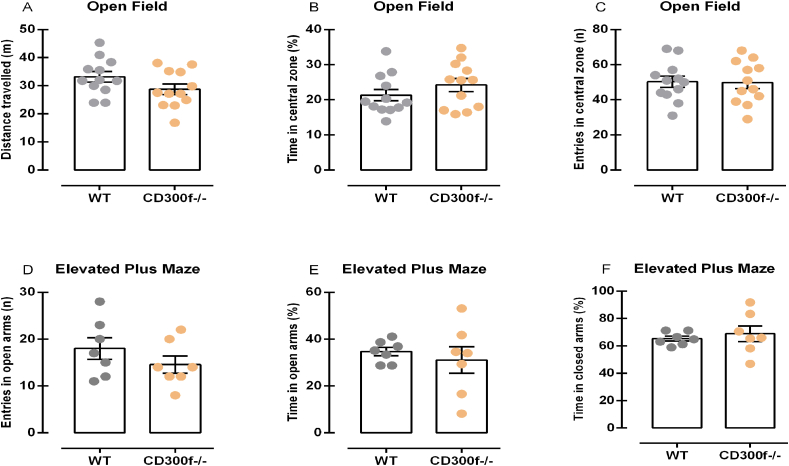
As CD300f has been previously related to major depressive disorder and depression-like behaviors only in females, we explored whether the anxiolytic-like effect observed in CD300f−/− male mice was sex-dependent. Fig. 2 depicts the results of female mice in the OFT and EPM. Statistical analysis demonstrated no differences in the distance traveled [t(22) = 1.660, p = 0.111, Fig. 2A], time spent in the central zone [t(22) = 1.18, p = 0.252, Fig. 2B] and number of entries in the central zone [t(22) = 0.09, p = 0.931, Fig. 2C] of the OFT when comparing WT and CD300−/− female mice. Moreover, we analyzed a separated cohort of female mice in the EPM test. No differences were observed in the number of entries in the open arms [t(12) = 1.16, p = 0.268, Fig. 2D) or in the time spent in the open arms [t(12) = 0.60, p = 0.557, Fig. 2E] or closed arms [t(12) = 0.60, p = 0.557, Fig. 2F] of the EPM when comparing CD300f−/− and WT female mice. These data reveal that female CD300f−/− mice do not display behavior alterations related to anxiety, only depressive-like behavior as previously described (Lago et al., 2020).
Fig. 2.
Anxiety-related behaviors of CD300f−/− female mice in different behavioral contexts. The total distance traveled in the OF was measured (meters) to evaluate female mice spontaneous ambulatory activity (n = 12 WT and 12 CD300f−/−, 2.A) and the % of time spent in the center (n = 12 WT and 12 CD300f−/−, 2.B), as well as the number of entries in the central zone (n = 12 WT and 12 CD300f−/−, 2.C) of the OF, was used to evaluate anxious-like behavior. The number of entries in the open arms of the EPM (n = 7 WT and 7 CD300f−/−, 2.D), the time spent in the open arms (n = 7 WT and 7 CD300f−/−, 2.E) and closed arms (n = 7 WT and 7 CD300f−/−, 2.F) of the EPM (calculated using the total time in the open arms or closed arms + the total time in the closed arms as 100%) was used as indicative of anxiety. Data are represented as mean ± S.E.M. Abbreviations: OF: Open Field; EPM: Elevated Plus Maze; WT: Wild-type mouse; CD300f−/−: CD300f knockout mouse.
3.3. Association of CD300f SNP with GAD subjects in a sex-dependent manner
The non-synonymous rs2034310 (C/T) SNP in the cytoplasmic tail of CD300f has been shown to alter receptor signaling (Lago-Kaufmann et al., 2020). We evaluated the association of the SNP with GAD in a population-based study that comprised 1111 individuals, 938 (84.4%) control subjects, and 173 (15.6%) diagnosed with GAD. We found an increased prevalence of GAD in women (73.4%) when compared to men (26.6%), (χ2 = 25.11, df = 1, p < 0.001), in individuals from the intermediate socioeconomic class (χ2 = 18.71, df = 1, p < 0.001), making use of tobacco (χ2 = 43.17, df = 1, p < 0.001) and making use of psychiatric medication (χ2 = 35.00, df = 1, p < 0.001). In addition, an increased prevalence of clinical comorbidities (autoimmune, cardiovascular, metabolic, and infectious diseases) was observed in GAD patients (χ2 = 19.69, df = 1, p < 0.001). No differences in the age [t(1107) = -1.94, p = 0.053], ethnicity (χ2 = 0.61, df = 1, p = 0.433), and BMI [t(1065) = -0.72, p = 0.472] between controls and GAD patients were observed (Table 1).
Table 1.
Sociodemographic and clinical characteristics of the population-based sample according to the GAD diagnosis.
| Characteristics | Generalized Anxiety Disorder (GAD) |
||
|---|---|---|---|
| No | Yes | p-value | |
| Age in years | |||
| (mean ± S.E.M.) | 25.80 ± 0.17 | 26.66 ± 0.39 | 0.053 |
| Sex (% male) | 442 (47.2) | 46 (26. | <0.001 |
| Ethnicity (% Caucasian) | 717 (76.5) | 127 (73.4) | 0.433 |
| Socioeconomic Class | |||
| Low | 169 (18.0) | 52 (30.2) | <0.001 |
| Intermediate | 469 (50.1) | 87 (50.6) | |
| High | 299 (31.9) | 33 (19.2) | |
| Tobacco use (% yes) | 181 (19.4) | 73 (42.4) | <0.001 |
| Body mass index (kg/m2) | 26.01 ± 0.17 | 26.34 ± 0.49 | 0.472 |
| Psychiatric medication | 54 (5.7) | 33 (19.1) | <0.001 |
| Clinical diseases (% yes) | 206 (22.0) | 66 (38.2) | <0.001 |
| Total | 938 (84.4) | 173 (15.6) | |
Data are presented as mean ± standard error of the mean (S.E.M.) or n (%). p-values were computed using χ2 tests or Student’s t-test, as appropriated. p < 0.05 was considered as statistically significant. GAD: Generalized Anxiety Disorder.
When we analyzed the sociodemographic characteristics according to the rs2034310 (C/T) SNP genotypes, no differences were observed for age [F(2,1109) = 1.33, p = 0.265], gender (χ2 = 0.29, df = 2, p = 0.861), socioeconomic class (χ2 = 0.59, df = 4, p = 0.964), tobacco use (χ2 = 1.60, df = 2, p = 0.448), BMI [F(2,1067) = 0.65, p = 0.524], psychiatric medication use (χ2 = 1.21, df = 2, p = 0.547), or presence of clinical conditions (autoimmune, cardiovascular, metabolic and infectious diseases, χ2 = 0.96, df = 2, p = 0.619) (Supplementary Table 1). Ethnicity was significantly associated with genotype (χ2 = 16.86, df = 2, p < 0.001) and a higher frequency of the T allele was observed in non-Caucasian individuals. Additionally, the genotype frequencies agreed with those predicted by Hardy-Weinberg equilibrium for the rs2034310 (C/T) SNP (χ2 = 0.03775, p = 0.846).
We further evaluated the rs2034310 (C/T) SNP genotypes according to the GAD diagnosis. Considering that sex differences can underlie the biological alterations for several diseases, including psychiatric disorders (Hodes and Epperson, 2019), that only male CD300f−/− mice show an anxiolytic-like phenotype, we further stratified our population by sex to analyze the impact of this polymorphism in GAD diagnosis. As depicted in Table 2, in the male population, the CT genotype confers protection against GAD (p = 0.012) even after the adjusted analysis considering sex, age, and ethnicity as confounding variables (p = 0.015). The frequency of the TT genotype in our study, and in the general population, was lower when compared to the other genotypes (Lago-Kaufmann et al., 2020). Then, to evaluate the T allele effect in the GAD diagnosis, we performed a dominant model, where we joined T allele carriers in a unique group (CT/TT). In the dominant model, we observed that men individuals carrying the T allele presented a decreased prevalence of GAD (p = 0.009). Indeed, the T allele was significantly associated with protection against GAD (p = 0.007) in the dominant model, even after adjusted multinomial regression analysis (p = 0.010). Interestingly, when we analyzed the SNP association with GAD diagnosis in women, we did not find any significant association of CT genotype (p = 0.159) or TT genotype (p = 0.997) even after adjusting for the same variables described above (p = 1.107) and (p = 0.820), respectively. We also performed the analysis in the dominant model joining the T allele carriers, however, no association where observed in the unadjusted (p = 0.205) or adjusted (p = 0.129) analysis. Overall, our human data demonstrate that the rs2034310 (C/T) polymorphism is associated with GAD diagnosis and confers protection against GAD in a sex-specific manner.
Table 2.
Sex differences in the genotype and allele distribution according to GAD diagnosis.
| Genotypes | Generalized Anxiety Disorder (GAD) |
Unadjusted OR (95%)/p∗ | Adjusted OR (95%)/p# | ||
|---|---|---|---|---|---|
| No | Yes | p | |||
| Total population | |||||
| rs2034310 SNP Genotype | |||||
| CC | 520 (55.4) | 114 (65.9) | 0.033 | 1 | 1 |
| CT | 366 (39.0) | 50 (28.9) | 0.623 (0.435-0.892)/0.010 | 0.609 (0.422-0.877)/0.008 | |
| TT | 52 (5.5) | 9 (5.2) | 0.789 (0.378-1.648)/0.529 | 0.738 (0.348-1.565)/0.428 | |
| Dominant model | |||||
| CC | 520 (55.4) | 114 (65.9) | 0.011 | 1 | 1 |
| CT/TT | 418 (44.6) | 59 (34.1) | 0.644 (0.458-0.904)/0.011 | 0.625 (0.442-0.885)/0.008 | |
| Allele- | |||||
| C | 1406 (74.9%) | 278 (80.0) | 0.397 | – | – |
| T | 470 (25.0) | 68 (19.6) | |||
| Women | |||||
| rs2034310 SNP Genotype | |||||
| CC | 277 (56.0) | 79 (62.2) | 0.357 | 1 | 1 |
| CT | 190 (38.4) | 40 (31.5) | 0.738 (0.484-1.127)/0.159 | 0.702 (0.457-1.127)/1.107 | |
| TT | 28 (5.7) | 8 (6.3) | 1.002 (0.439-2.285)/0.997 | 0.907 (0.393-2.096)/0.820 | |
| Dominant model | |||||
| CC | 277 (56.0) | 79 (62.2) | 0.243 | 1 | 1 |
| CT/TT | 218 (44.0) | 48 (37.8) | 0.772 (0.517-1.152)/0.205 | 0.729 (0.484-1.097)/0.129 | |
| Men | |||||
| rs2034310 SNP Genotype | |||||
| CC | 242 (54.8) | 35 (76.1) | 0.020 | 1 | 1 |
| CT | 176 (39.8) | 10 (21.7) | 0.393 (0.189-0.814)/0.012 | 0.404 (0.194-0.841)/0.015 | |
| TT | 24 (5.4) | 1 (2.2) | 0.288 (0.038-2.197)/0.230 | 0.308 (0.040-2.363)/0.257 | |
| Dominant model | |||||
| CC | 242 (54.8) | 35 (76.1) | 0.009 | 1 | 1 |
| CT/TT | 200 (45.2) | 11 (23.9) | 0.380 (0.188-0.768)/0.007 | 0.393 (0.194-0.797)/0.010 | |
Data are presented as number or proportion (%) and computed by χ2 tests comparing patients with GAD and controls. ∗ Unadjusted or # adjusted OR (95% CI/p) values were obtained from logistic regression analysis, and in the total population was adjusted for age, sex, and ethnicity and in women and men subpopulation was adjusted for age and ethnicity. p < 0.05 was considered as statistically significant. GAD: Generalized Anxiety Disorder.
4. Discussion
Dysfunctional microglia are associated with many neurological and psychiatric disorders, yet our knowledge about the pathological mechanisms involved is incomplete (Stein et al., 2017; Ramirez et al., 2017; Li et al., 2014; Wang et al., 2018; McKim et al., 2018). In the CNS, CD300f immunoreceptors are expressed in microglial and perivascular macrophages and regulate not only neuroinflammatory responses, but also important aspects of microglia phenotype, and synaptic connectivity and plasticity through mechanisms not directly related to classical neuroinflammatory cascades (Lago-Kaufmann et al., 2020). It was recently demonstrated that these receptors contribute to mood regulation in female major depressive disorder (Lago-Kaufmann et al., 2020). Here, we set out to address the possibility that CD300f receptors might also be involved in anxiety-related outcomes. Our results demonstrate that the CD300f immunoreceptors modulate behavioral responses associated with anxiety in male mice and humans. While CD300f−/− male mice displayed reduced anxiety-related behaviors in different paradigms, a polymorphism in the CD300f gene was protective against GAD in a population-based study. These responses were observed only in males and expand our previous findings showing sex-specific effects of CD300f in the modulation of psychiatric symptoms.
CD300f−/− mice displayed a decrease in negative valence behaviors associated with anxiety, characterized by an increase in OFT central locomotion, an increase in rearing behaviors, more exploration in the open arms of the EPM test, decreased latency to eat in the NSF test and decrease defecation in the OFT. These tasks explore the neophobic anxiety and the natural aversion of rodents to exposed areas. Thus, the reduction in this natural aversion can indicate a decrease in anxiety-related behaviors (Bailey and Crawley, 2009). Animal models of anxiety do not claim to faithfully reproduce the complexity and heterogeneity of the human conditions. However, they are essential tools to better understand basic differences in anxiety responses and perhaps discover potential new targets for more specific pharmacological intervention (Bourin, 2020).
From the epidemiological point of view, our study shows that the T allele of CD300f rs2034310 SNP independently confers protection against GAD in humans. This effect is probably mediated by a dominant effect, as only one T allele is needed in heterozygosis. The C/T exchange produces a non-synonymous substitution of Arg to Gln in the cytoplasmic tail of the receptor that abrogates the protein kinase C delta (PKC-δ) phosphorylation of a threonine in its cytoplasmic tail. The CD300f immune receptor contains in its complex cytoplasmic tail several signaling motifs, including immunoreceptor tyrosine-based inhibition motifs (ITIM), and another tyrosine motif that can be involved in the internalization of this receptor from the cell surface. In fact, internalization indicates that this receptor is involved in processes such as phagocytosis, exposure of antigens, regulation of effector mechanisms, and even viral infection (Choi et al., 2011). In addition, activating antibodies against the CD300f receptor led to the inhibition of the transcription factor NFκB-mediated TLR-2, 3, 4, and 9 signaling and expression of interleukin-8 and metalloproteinase-9 (Lee et al., 2011). Indeed, these pathways were already observed to be altered in anxiety models (Décarie-Spain et al., 2018; Zimmerman et al., 2012), and its inhibition could be associated with low anxiety in the CD300f−/− male mice. However, additional studies are needed to understand the functional implications of this substitution in those pathways.
According to the data we obtained in our population-based study, the T allele of CD300f rs2034310 SNP was protective against GAD in men but not women. Previously published epidemiological studies show that women are more susceptible to GAD than men. However, these conditions have singular clinical manifestations and treatment responses in man and woman, suggesting distinctive biological mechanisms (Craske et al., 2017; Kaczkurkin et al., 2016). We still have a limited understanding of the role of sex differences in most of the anxiety disorders, including GAD and a range of candidate mechanisms have been proposed, including sex chromosome-dependent gene expression and brain development (Donner and Lowry, 2013).
The role of CD300f in behavioral modulation is still largely unexplored. Recently we demonstrated the involvement of CD300f immunoreceptors in major depressive disorder and the T allele presence as a protective mechanism only in women (Lago-Kaufmann et al., 2020). However, CD300f immunoreceptors have also been putatively linked to a familial case of Autism Spectrum Disorder (Inoue et al., 2015). Interestingly, this condition shows a sex ratio heavily skewed towards males and is associated with anxiety-related symptoms and difficulties with behavioral avoidance. Thus, the potential role of CD300f in this and other psychiatric conditions still needs to be explored.
The mechanisms underlying the sex-specific effects of CD300f are largely unknown. Our previous work only showed mild changes in proinflammatory molecules of naïve CD300−/− female and male mice brain, including increased mRNA for il6, il1rn, CD163, and siglec1, and no changes in microglial homeostatic or disease-associated microglial (DAM) genes besides spp1 (Lago-Kaufmann). Moreover, no important changes in the inflammatory response were observed in the spinal cord after a sciatic nerve crush injury in CD300f−/− mice (Lago-Kaufmann). On the other hand, the absence of CD300f affected synaptic plasticity, noradrenaline levels in the hippocampus, and microglial immunometabolic phenotype (Lago-Kaufmann et al., 2020). Thus, these mechanisms should be compared between male and female mice in future experiments to unravel the intriguing question of how is it possible that a unique modification in a single immunoreceptor contributes to the generation of two different psychiatric manifestations in males and females.
The impact of immunoreceptors in brain-related disorders is a huge field of interest, and this is the first study to examine the role of CD300f receptors in anxiety. Collectively, our results from CD300f−/− mice and individuals with GAD suggest a sex-specific role for CD300f receptors in anxiety-related behaviors in men. Understanding the link between CD300f signaling and behavioral impact in a sex-dependent manner is of great importance to establish the complete contribution of this new target to psychiatric disorders.
Declaration of competing interest
None.
Acknowledgements
This work has been supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES) and PRONEX-FAPERGS (08/2009 - Pronex 10/0055-0), International Brain Research Organization (IBRO)- LARC PROLAB grant. Comisión Sectorial de Investigación Científica (CSIC), Uruguay; Programa de Desarrollo de las Ciencias Básicas (PEDECIBA), Uruguay; Fondo de Convergencia Estructural del MERCOSUR (COF 03/1111); Banco de Seguros del Estado, Uruguay; Agencia Nacional de Investigación e Innovación (ANII); International Center for Genetic Engineering and Biotechnology (ICGEB-CRP/URY19-01). FNK received International Society for Neurochemistry (ISN) and IBRO awards, and a CAPES fellowship to perform this project. KJ, LMS, RAS, GG, MPK are CNPq Research Fellows. We thank all members of the transgenic animal unit (UATE) of the Institut Pasteur de Montevideo for assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100191.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- American Psychiatric Association . fifth ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders: Diagnostic and Statistical Manual of Mental Disorders. 2013. [DOI] [Google Scholar]
- Bailey K.R., Crawley J.N. In: Methods of Behavior Analysis in Neuroscience. second ed. Buccafusco J.J., editor. CRC Press/Taylor & Francis.; Boca Raton (FL: 2009. Anxiety-related behaviors in mice. [Google Scholar]
- Biedermann S.V., Biedermann D.G., Wenzlaff F., Kurjak T., Nouri S., Auer M.K., Wiedemann K., Briken P., Haaker J., Lonsdorf T.B., Fuss J. An elevated plus-maze in mixed reality for studying human anxiety-related behavior. BMC Biol. 2017;15:125. doi: 10.1186/s12915-017-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M. Experimental anxiety model for anxiety disorders: relevance to drug discovery. Adv. Exp. Med. Biol. 2020;1191:169–184. doi: 10.1007/978-981-32-9705-0_11. [DOI] [PubMed] [Google Scholar]
- Capuron L., Miller A.H. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.C., Simhadri V.R., Tian L., Gil-Krzewska A., Krzewski K., Borrego F., Coligan J.E. Cutting edge: mouse CD300f (CMRF-35–Like Molecule-1) recognizes outer membrane-exposed phosphatidylserine and can promote phagocytosis. J. Immunol. 2011;187:3483–3487. doi: 10.4049/jimmunol.1101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G.J., Ju X., Tate C., Hart D.N. The CD300 family of molecules are evolutionarily significant regulators of leukocyte functions. Trends Immunol. 2009;30:209–217. doi: 10.1016/j.it.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Copeland W.E., Shanahan L., Worthman C., Angold A., Costello E.J. Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychol. Med. 2012;42:2641–2650. doi: 10.1017/S0033291712000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello H., Gould R.L., Abrol E., Howard R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-027925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske M.G., Stein M.B., Eley T.C., Milad M.R., Holmes A., Rapee R.M., Wittchen H.U. Anxiety disorders. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décarie-Spain L., Sharma S., Hryhorczuk C., Issa-Garcia V., Barker P.A., Arbour N., Alquier T., Fulton S. Nucleus accumbens inflammation mediates anxiodepressive behavior and compulsive sucrose seeking elicited by saturated dietary fat. Mol. Metab. 2018;10:1–13. doi: 10.1016/j.molmet.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner N.C., Lowry C.A. Sex differences in anxiety and emotional behavior. Pflügers Archiv. 2013;465:601–626. doi: 10.1007/s00424-013-1271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller H., Cramer H., Lauche R., Gass F., Dobos G.J. The prevalence and burden of subthreshold generalized anxiety disorder: a systematic review. BMC Psychiatr. 2014;14:128. doi: 10.1186/1471-244X-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes G.E., Epperson C.N. Sex differences in vulnerability and resilience to stress across the life span. Biol. Psychiatr. 2019;86:421–432. doi: 10.1016/j.biopsych.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue E., Watanabe Y., Egawa J., Sugimoto A., Nunokawa A., Shibuya M., Igeta H., Someya T. Rare heterozygous truncating variations and risk of autism spectrum disorder: whole-exome sequencing of a multiplex family and follow-up study in a Japanese population. Psychiatr. Clin. Neurosci. 2015;69:472–476. doi: 10.1111/pcn.12274. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin A.N., Moore T.M., Ruparel K., Ciric R., Calkins M.E., Shinohara R.T., Elliott M.A., Hopson R., Roalf D.R., Vandekar S.N., Gennatas E.D., Wolf D.H., Scott J.C., Pine D.S., Leibenluft E., Detre J.A., Foa E.B., Gur R.E., Gur R.C., Satterthwaite T.D. Elevated amygdala perfusion mediates developmental sex differences in trait anxiety. Biol. Psychiatr. 2016;80:775–785. doi: 10.1016/j.biopsych.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani T., Roland J., Herbert F., Delcroix-Genete D., Bauderlique-Le, Roy H., Gaayeb L., Cazenave P.A., Pied S. Expression of CD300lf by microglia contributes to resistance to cerebral malaria by impeding the neuroinflammation. Gene Immun. 2020;21:45–62. doi: 10.1038/s41435-019-0085-9. [DOI] [PubMed] [Google Scholar]
- Lago N., Kaufmann F.N., Negro-Demontel M.L., Alí-Ruiz D., Ghisleni G., Rego N., Arcas-García A., Vitureira N., Jansen K., Souza L.M., Silva R.A., Lara D.R., Pannunzio B., Abin-Carriquiry J.A., Amo-Aparicio J., Martin-Otal C., Naya H., McGavern D.B., Sayós J., López-Vales R., Kaster M.P., Peluffo H. CD300f immunoreceptor is associated with major depressive disorder and decreased microglial metabolic fitness. Proc. Natl. Acad. Sci. U.S.A. 2020;117:6651–6662. doi: 10.1073/pnas.1911816117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri D.K., Nurnberger J., Jr. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.M., Kim E.J., Suk K., Lee W.H. CD300F blocks both MyD88 and TRIF-mediated TLR signaling through activation of Src homology region 2 domain-containing phosphatase 1. J. Immunol. 2011;186:6296–6303. doi: 10.4049/jimmunol.1002184. [DOI] [PubMed] [Google Scholar]
- Li Z., Ma L., Kulesskaya N., Vôikar V., Tian L. Microglia are polarized to M1 type in high-anxiety inbred mice in response to lipopolysaccharide challenge. Brain Behav. Immun. 2014;38:237–248. doi: 10.1016/j.bbi.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Martínez-Barriocanal Á., Arcas-García A., Magallon-Lorenz M., Ejarque-Ortíz A., Negro-Demontel M.L., ComasCasellas E., Schwartz S., Jr., Malhotra S., Montalban X., Peluffo H., Martín M., Comabella M., Sayós J. Effect of specific mutations in Cd300 complexes formation; potential implication of Cd300f in multiple sclerosis. Sci. Rep. 2017;7:13544. doi: 10.1038/s41598-017-12881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa T., Izawa K., Isobe M., Takahashi M., Maehara A., Yamanishi Y., Kaitani A., Okumura K., Teshima T., Kitamura T., Kitaura J. Ceramide-CD300f binding suppresses experimental colitis by inhibiting ATP-mediated mast cell activation. Gut. 2015;65:777–787. doi: 10.1136/gutjnl-2014-308900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim D.B., Weber M.D., Niraula A., Sawicki C.M., Liu X., Jarrett B.L., Ramirez-Chan K., Wang Y., Roeth R.M., Sucaldito A.D., Sobol C.G., Quan N., Sheridan J.F., Godbout J.P. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatr. 2018;23:1421–1431. doi: 10.1038/mp.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.J., Vos T., Lozano R., Naghavi M., Flaxman A.D., Michaud C. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Peluffo H., Solari-Saquieres P., Negro-Demontel M.L., Francos-Quijorna I., Navarro X., López-Vales R., Sayós J., Lago N. CD300f immunoreceptor contributes to peripheral nerve regeneration by the modulation of macrophage inflammatory phenotype. J. Neuroinflammation. 2015;12:145. doi: 10.1186/s12974-015-0364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman T.J.C., Benyamin B., de Leeuw C.A., Sullivan P.F., van Bochoven A., Visscher P.M., Posthuma D. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 2015;47:702–709. doi: 10.1038/ng.3285. [DOI] [PubMed] [Google Scholar]
- Ramirez K., Fornaguera-Trías J., Sheridan J.F. Stress-Induced microglia activation and monocyte trafficking to the brain underlie the development of anxiety and depression. Curr. Top. Behav. Neurosci. 2017;31:155–172. doi: 10.1007/7854_2016_25. [DOI] [PubMed] [Google Scholar]
- Rojas-Carvajal M., Fornaguera J., Mora-Gallegos A., Brenes J.C. Testing experience and environmental enrichment potentiated open-field habituation and grooming behaviour in rats. Anim. Behav. 2018;137 doi: 10.1016/j.anbehav.2018.01.018. [DOI] [Google Scholar]
- Rubio G., López-Ibor J.J. Generalized anxiety disorder: a 40-year follow-up study. Acta Psychiatr. Scand. 2007;115:372–379. doi: 10.1111/j.1600-0447.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- Samuels B.A., Hen R. In: Mood and Anxiety Related Phenotypes in Mice. Gould T., editor. Humana Press; 2011. Novelty-suppressed feeding in the mouse; pp. 107–121. [DOI] [Google Scholar]
- Shiba E., Izawa K., Kaitani A., Isobe M., Maehara A., Uchida K., Maeda K., Nakano N., Ogawa H., Okumura K., Kitamura T., Shimizu T., Kitaura J. Ceramide-CD300f binding inhibits lipopolysaccharide-induced skin inflammation. J. Biol. Chem. 2017;292:2924–2932. doi: 10.1074/jbc.M116.768366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D.J., Vasconcelos M.F., Albrechet-Souza L., Ceresér K.M.M., de Almeida R.M.M. Microglial Over-Activation by Social Defeat Stress Contributes to Anxiety- and Depressive-Like Behaviors. Front Behav. Neurosci. 2017 Oct 24;11:207. doi: 10.3389/fnbeh.2017.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman O., Germain P.L., Bohacek J. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress. 2018;21:443–452. doi: 10.1080/10253890.2018.1438405. [DOI] [PubMed] [Google Scholar]
- Vesga-López O., Schneier F.R., Wang S., Heimberg R.G., Liu S.-M., Hasin D.S., Blanco C. Gender differences in generalized anxiety disorder: results from the national epidemiologic survey on alcohol and related conditions (NESARC) J. Clin. Psychiatr. 2008;69:1606–1616. [PMC free article] [PubMed] [Google Scholar]
- Vieira M.M., Ferreira T.B., Pacheco P.A., Barros P.O., Almeida C.R., Araújo-Lima C.F., Silva-Filho R.G., Hygino J., Andrade R.M., Linhares U.C., Andrade A.F., Bento C.A. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J. Neuroimmunol. 2010;229:212–218. doi: 10.1016/j.jneuroim.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Wang Y.L., Han Q.Q., Gong W.Q., Pan D.H., Wang L.Z., Hu W., Yang M., Li B., Yu J., Liu Q. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflammation. 2018;15:21. doi: 10.1186/s12974-018-1054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo A.P., Gibson G. Blood gene expression profiles suggest altered immune function associated with symptoms of generalized anxiety disorder. Brain Behav. Immun. 2015;43:184–191. doi: 10.1016/j.bbi.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H.U. Generalized anxiety disorder: prevalence, burden, and cost to society. Depress. Anxiety. 2002;16:162–171. doi: 10.1002/da.10065. [DOI] [PubMed] [Google Scholar]
- Xi H., Katschke K.J., Jr., Helmy K.Y., Wark P.A., Kljavin N., Clark H., Eastham-Anderson J., Shek T., Roose-Girma M., Ghilardi N., van Lookeren Campagne M. Negative regulation of autoimmune demyelination by the inhibitory receptor CLM-1. J. Exp. Med. 2010;207:7–16. doi: 10.1084/jem.20091508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaqueu Lima T., Roberto Sardinha L., Sayos J., Eugenio Mello L., Peluffo H. Astrocytic expression of the immunoreceptor CD300f protects hippocampal neurons from amyloid-β oligomer toxicity in vitro. Curr. Alzheimer Res. 2017;14:778–783. doi: 10.2174/1567205014666170202121709. [DOI] [PubMed] [Google Scholar]
- Zimmerman G., Shaltiel G., Barbash S., Cohen J., Gasho C.J., Shenhar-Tsarfaty S., Shalev H., Berliner S.A., Shelef I., Shoham S., Friedman A., Cohen H., Soreq H. Post-traumatic anxiety associates with failure of the innate immune receptor TLR9 to evade the pro-inflammatory NFκB pathway. Transl. Psychiatry. 2012;2:e78. doi: 10.1038/tp.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.