Abstract
Background
The precise mechanisms underlying the aetiology of post-stroke fatigue remain poorly understood. Inflammation has been associated with clinically significant fatigue across a number of neurological disorders; however, at present there is a lack of evidence regarding the association of fatigue and inflammation in the chronic phase of stroke recovery.
Aims
The aim of this study was to examine fatigue in a cohort of stroke survivors in the chronic phase of stroke, compared with matched controls, and to explore associations between the pro-inflammatory cytokine interleukin-6, high-sensitivity C-reactive Protein and fatigue.
Methods
We performed an exploratory cross-sectional study of 70 people in the chronic phase of stroke recovery, and 70 age matched controls. Fatigue was assessed using the Fatigue Assessment Scale. Interleukin-6 was measured in serum using a commercially available enzyme immunoassay kit. Both outcome measures were assessed contemporaneously.
Results
Clinically significant fatigue, defined as a score ≥24 on the Fatigue Assessment Scale, was reported by 60% of stroke survivors, and 15.7% of controls. The odds of experiencing clinically significant fatigue was 8.04 times higher among stroke survivors compared to control participants (odds ratio 8.045; 95% CI: 3.608, 17.939; P < 0.001). The fatigue score was significantly correlated with the level of both interleukin-6 and high-sensitivity c-reactive protein, however once entered into a linear regression model with cardiovascular covariables, this relationship was no longer statistically significant.
Conclusions
This study shows that fatigue may be associated with systemic inflammation in the chronic phase of stroke. The pathological mechanisms underlying post-stroke fatigue and its clinical implications require further study.
Keywords: C-reactive protein, Fatigue, Inflammation, Interleukin-6, Stroke, Recovery
1. Introduction
Over the last decade, the treatment of stroke has improved substantially, with the introduction of second generation thrombolytics and thrombectomy. These advances in stroke care have vastly improved survival rates, which although a welcome development, means that increasing numbers of people are living with significant stroke induced impairment within the community. Effectively managing recovery and rehabilitation after stroke has proven challenging (Rose et al., 2014; Teasell, 2012). Many symptoms that patients report experiencing have proven to be very difficult to remediate, even in a modest way. Fatigue, classically described by a feeling of tiredness that cannot be improved by rest, is often reported as a major problem by stroke survivors (de Groot et al., 2003; White et al., 2012; Morley et al., 2005). Current reports indicate that the pooled prevalence of fatigue in stroke survivors is up to 50% (95% CI: 43–57%), and is relatively consistent in severity across time after stroke (Cumming et al., 2016). Apart from the impact on the patient’s lived experience, fatigue is also associated with a number of unfavourable outcomes including increased mortality (Naess et al., 2012), depression (van de Port et al., 2007a), delayed return to work (Andersen et al., 2012), increased dependency (Maaijwee et al., 2015) and poorer quality of life (van de Port et al., 2007b).
The precise mechanisms underlying the aetiology of post-stroke fatigue remain poorly understood.8, 9 While a number of potential predisposing factors such as stroke subtype (Naess et al., 2010), lesion site (Christensen et al., 2008; Snaphaan et al., 2011), stroke severity at admission (Radman et al., 2012; Wu et al., 2014a), cognitive dysfunction (Naess et al., 2010; Radman et al., 2012) and behavioural patterns (Duncan et al., 2012) have emerged, findings across studies have been variable. Radman et al. (2012), and Christensen et al. (2008), for instance, did not find an association between lesion site and post-stroke fatigue, in contrast to the study of Snaphaan et al. (2011), who associated infratentorial infarctions with an increased risk of post-stroke fatigue. To date, no clear evidence exists for any characteristic of stroke to be predictive of the development of fatigue. It is also uncertain whether socio-demographic factors including age, gender, marital status, living situation, education, and returning to paid work are associated with fatigue. However, fatigue does appear to be associated with other chronic affective symptoms, with depression being most widely reported (Wu et al., 2014b). Despite this, other evidence suggests that fatigue has been reported to be present even in the absence of depression (van der Werf et al., 2001). Thus, the exact determinants and underlying cause of fatigue post-stroke are unclear at present. The underlying causal mechanisms are likely to be complex and multifactorial, and probably overlap with chronic affective disorders.
An increasing body of evidence has linked post-stroke fatigue to pro-inflammatory markers. Certainly, this evidence has not ascribed any causality to these findings although the relationship is intriguing and has been reported in an increasing number of texts. (Ponchel et al., 2015; Kutlubaev et al., 2012). While not representing a mechanistic explanation per se, it is recognised that ischaemic insults, such as stroke, can trigger an inflammatory cascade in the brain leading to activation of inflammatory cells and the generation of inflammatory cytokines and reactive oxygen species (Jin et al., 2010; Nakajima and Kohsaka, 2001; Amantea et al., 2009; Schilling et al., 2003) into brain tissue. Activation of inflammatory cells within the brain appears also to be associated with the release of inflammatory cytokines into peripheral circulation (Shichita et al., 2012). Several studies have shown an association between poor stroke outcome and an increased level of inflammatory biomarkers (Whiteley et al., 2009; Capuron and Miller, 2011).
Many studies that have investigated the relationship between inflammation and post-stroke fatigue have focused on the acute stage of stroke recovery (Ormstad et al., 2011; Wen et al., 2018). We have little information on whether fatigue present in the later stages of the recovery process is also associated with enhanced peripheral pro-inflammatory activity (Wu et al., 2015; Becker, 2016). In the present study, we undertook an exploratory study in a previously described cohort (Gyawali et al., 2020), to consider if there may be a relationship between self-reported fatigue and the inflammatory biomarkers high sensitivity C-reactive protein (hsCRP) and cytokine interleukin-6 (IL-6) during the chronic phase of stroke recovery. Several large-scale prospective epidemiological studies have identified high sensitivity hsCRP and IL-6 as reliable markers of low-grade chronic systemic inflammation (Ridker et al., 1997; Koenig et al., 1999; Bruunsgaard et al., 2003). These inflammatory biomarkers have shown strong predictive power for cardiovascular mortality and morbidity (Ridker et al., 1997; Koenig et al., 1999; Bruunsgaard et al., 2003). Furthermore, these biomarkers are routinely measured by standard commercial clinical laboratories, thus offering easy translation of the study in clinical settings. We measured both IL-6 and hsCRP levels in a cohort of chronic stroke survivors and compared this to group of age-matched controls.
2. Materials and methods
2.1. Participants
Full details of the study population have been described in a related publication (Gyawali et al., 2020). Briefly, participants were recruited between November 2017 and February 2019, and included 70 stroke survivors, and 70 age-matched controls. Community-dwelling stroke survivors in the chronic phase of stroke recovery (>5 months post-stroke) were recruited via the Hunter Stroke Research Volunteer Register based at the Hunter Medical Research Institute (HMRI). Stroke survivors who provided informed consent visited the study site, either independently or with assistance from community workers or family members, for a single study visit. Age-matched control participants were recruited from either the HMRI control registry, or via social media advertisements. Exclusion criteria included a history of pituitary and adrenal gland diseases for both groups, and the history of stroke for the control group. Participant’s history of cardiovascular risk factors was not a part of inclusion/exclusion criteria but were collected as described in covariables section. Ethics approval for this study was obtained from the Hunter New England Local Health District Human Research Ethics Committee (17/06/21/4.02). Written informed consent was obtained from all participants prior to the study.
2.2. Measures
Fatigue: Fatigue was measured using the Fatigue Assessment Scale (FAS) (Michielsen et al., 2004). The FAS is a 10-item scale evaluating both mental and physical symptoms of chronic fatigue in a 5-point likert-type response scale of 1 = never to 5 = always. The total possible score ranges from 10 to 50, where higher score represents higher level of fatigue. The FAS scale has acceptable psychometric properties, having good test-retest reliability and face validity (Mead et al., 2007; Smith et al., 2008). The FAS has previously been used to measure fatigue in stroke survivors, with one recent publication proposing a cut-off of ≥24 for identifying the presence of post-stroke fatigue (Cumming and Mead, 2017). This cut-off was applied in the present study to dichotomise fatigue for analysis.
Inflammatory markers: Approximately 10 mLs of blood was collected from all participants in EDTA and plain vials after fatigue assessment. The blood sample was centrifuged (1917 G for 10 min) within 60 min at room temperature. Aliquots (500 μL each) of plasma and serum samples were stored in Eppendorf tubes at -80 °C. IL-6 and hsCRP were measured from stored serum samples using commercially available ELISA assay kits (Cusabio®).
The distributions of hsCRP and IL-6 were both highly skewed, and in order to better fit a linear relationship between variables and stabilise the variance for analysis, values were log transformed. Log-transformed hsCRP and IL-6 were used for the analysis of cross-sectional associations between inflammatory markers and fatigue.
Covariables: Data were collected on demographic characteristics (age, sex), anthropometrics (height, weight, waist circumference, and blood pressure), self-reported clinical history of comorbid conditions (history of mental illness, diabetes mellitus, dyslipidemia and hypertension), and self-reported level of physical activity. Self-reported type and date of last stroke was collected from stroke survivors.
2.3. Statistical analyses
As this was an exploratory analysis no formal sample size calculation was performed. All analyses were conducted using SPSS version 25.0 software (IBM Corp., 2017). Linear regression analysis was used to compare FAS score and level of peripheral IL-6 and hsCRP between stroke survivors and age-matched controls. Pearson correlations were used to examine crude associations between fatigue and inflammatory markers in stroke survivors and controls. Chi-square tests were performed to compare the odds of being fatigued among stroke survivors compared to controls. Cross sectional associations between inflammatory markers and fatigue were examined using multivariate linear regression analysis, using these variables as continuous. To facilitate comparison across models, standardized regression coefficients (β) were calculated, which express the change in standardised fatigue score per one standard deviation in log-transformed hsCRP or IL-6 concentration.
Covariates were selected based on previous literature around variables considered to be associated with systemic inflammation. Regression models were adjusted for demographics (age and sex), biomedical factors (BMI, systolic blood pressure, history of medical conditions), and health behaviours (physical activity) that may affect inflammatory status. Where models included stroke survivors only, adjustment was performed for time since stroke and stroke type.
A time dependent analysis was also conducted to examine the potential interaction between time since stroke and inflammatory markers, and the potential relationship with fatigue. This was examined by modelling the interaction of each inflammatory marker by time, on the FAS score.
3. Results
A total of 70 stroke survivors ranging from 5 months to 28 years post-stroke (median 38.5 months), and 70 matched controls participated in the study. The demographic and clinical characteristics of this cohort have been previously published (Gyawali et al., 2020). Table 1 presents demographic and clinical characteristics for both stroke survivors and age-matched control participants.
Table 1.
Comparison of demographic and clinical data.
| Stroke survivors N = 70 |
Controls N = 70 |
P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, mean years (SD) | 61.9 (13.8) | 64.6 (10.0) | 0.192 |
| Gender, male N (%) | 38 (54.3) | 24 (34.3) | 0.027 |
| Clinical characteristics | |||
| BMI, mean kg/m2 (SD) | 29.01 (6.3) | 28.0 (5.7) | 0.332 |
| Waist Circumference, mean cm (SD) | 98.7 (21.5) | 95.4 (15.5) | 0.301 |
| Systolic BP, mean mmHg (SD) | 131 (17) | 131 (18) | 0.985 |
| Diastolic BP, mean mmHg (SD) | 78 (12) | 79 (6) | 0.724 |
| Self-reported history of: | |||
| - Diabetes, n (%) | 10 (14.3) | 6 (8.6) | 0.234 |
| - Hypertension, n (%) | 28 (40.0) | 21 (30.0) | 0.131 |
| - Dyslipidaemia, n (%) | 38 (54.3) | 16 (22.9) | < 0.001 |
| - History of mental illness, n (%) | 15 (21.4) | 11 (15.7) | 0.302 |
| Physical activity, mean sessions per week (SD) | 1.0 (0.9) | 1.2 (0.7) | 0.222 |
| Stroke type, ischemic/haemorrhagic/unknown | 41/26/3 | – | – |
| Time since stroke, median months (IQR) | 38.5(13.75, 117.50) | – | – |
Note: the demographic and clinical characteristics of this study cohort have been previously reported in Gyawali et al. (2020) (Gyawali et al., 2020).
Overall, the clinical and demographic characteristics of stroke survivors and matched controls were similar, except for gender distribution and dyslipidaemia.
Table 2 summarises the mean values and comparisons of the FAS and inflammatory markers for both stroke survivors and control participants. Samples collected from two stroke survivors were insufficient for analysis.
Table 2.
Comparison of mean (SD) fatigue level (FAS Score) and inflammatory markers (IL-6 and hsCRP) between stroke survivors and controls.
| Variables | Stroke survivors (n = 70) | Controls (n = 70) | P-Value |
|---|---|---|---|
| Fatigue assessment scale (FAS) scorea | 24.90 (7.88) | 18.56 (5.36) | < 0.001 |
| IL-6b | 4.70 (1.50) | 3.15 (2.16) | < 0.001 |
| hsCRPb | 2.82 (2.95) | 1.67 (1.99) | 0.022 |
The comparison was adjusted for age and cardiometabolic risk factors including diabetes mellitus, hypertension, dyslipidaemia and waist circumference.
The comparison was adjusted for cardiometabolic risk factors including diabetes mellitus, hypertension, dyslipidaemia, and waist circumference.
Stroke survivors reported statistically significantly greater levels of fatigue compared with age-matched controls. There were also statistically significantly greater levels of peripheral IL-6 and hsCRP in stroke survivors relative to controls.
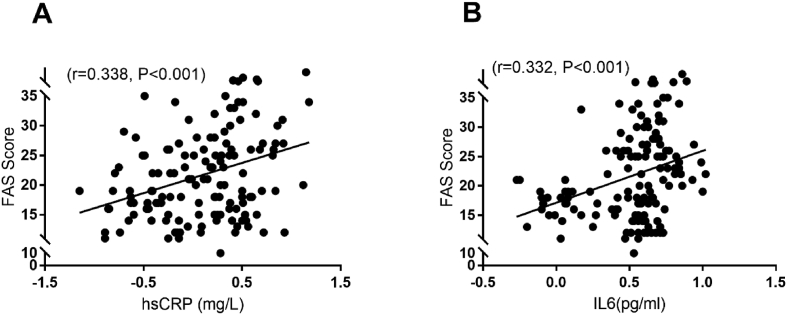
Correlations between inflammatory markers and FAS score for the entire study population, stroke survivors, and controls, are summarised in Fig. 1, and Table 3. In stroke survivors, inflammatory markers and FAS score were weakly to moderately correlated. There was no correlation between inflammatory markers and FAS score in the control group.
Fig. 1.
Scatter plot showing correlation between inflammatory markers (A) hsCRP and (B) IL-6; and FAS score. The values of hsCRP and IL-6 were log transformed.
Table 3.
Pearson correlations between FAS and inflammatory markers: within group and combined.
| FAS |
|||
|---|---|---|---|
| R | P-value | ||
| IL-6 | Whole population (n = 138) | 0.332 | < 0.001 |
| Stroke survivors (n = 68) | 0.272 | 0.025 | |
| Control (n = 70) | 0.156 | 0.197 | |
| Whole population (n = 138) | 0.335 | < 0.001 | |
| hsCRP | Stroke survivors (n = 68) | 0.310 | 0.010 |
| Control (n = 70) | 0.118 | 0.119 | |
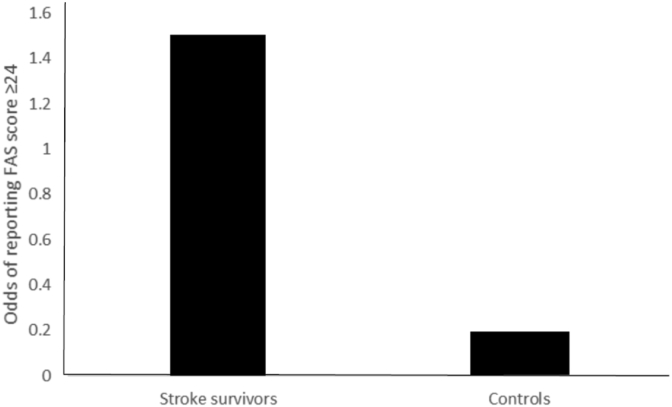
A clinical level of fatigue, defined as a score ≥24 on the FAS, was reported by 60% of stroke survivors, and 15.7% of controls. Table 4 and Fig. 2 summarises the odds of experiencing fatigue (cut-off ≥24) in stroke survivors compared with controls.
Table 4.
Odds of being fatigued in stroke survivors group compared to controls.
| Stroke (n = 70) | Control (n = 70) | Odds ratio (95% CI), P-value |
||
|---|---|---|---|---|
| FAS | ≥24 | 42 | 11 | 8.045 (3.61, 17.94), < 0.001 |
| <24 | 28 | 59 | ||
Fig. 2.
Odds of reporting significant fatigue (FAS score ≥24) in stroke survivors and controls.
The odds of experiencing significant levels of fatigue was 8.04 times higher among stroke survivors compared to control participants.
Multivariate linear regression models examining the cross-sectional relationship between peripheral inflammatory markers (IL-6 and hsCRP) and FAS score are summarised in Table 5. There were statistically significant cross-sectional associations between both IL-6 and hsCRP and fatigue in the stroke survivor group. Specifically, higher levels of inflammatory markers were associated with higher levels of fatigue after adjusting for age, sex, stroke type, and time since stroke (baseline model). These associations remained statistically significant in a subsequent model including physical activity (marker of health behaviour). When biomedical variables (BMI, blood pressure, and comorbidities) were added into these models, the relationship between circulating inflammatory markers and fatigue was no longer statistically significant.
Table 5.
Cross-sectional association between inflammatory markers (hsCRP and IL-6) and fatigue.
| IL-6 | hsCRP | |||||
| β | t | P | β | t | P | |
| Stroke survivors only (n = 70) | ||||||
| Unadjusted model | 0.278 | 2.353 | 0.022 | 0.283 | 2.398 | 0.019 |
| Age, sex, stroke type, time since stroke (Baseline model) | 0.283 | 2.247 | 0.028 | 0.255 | 2.043 | 0.045 |
| Baseline + biomedical factors | 0.204 | 1.457 | 0.151 | 0.264 | 1.863 | 0.068 |
| Baseline + health behaviour | 0.307 | 2.389 | 0.020 | 0.252 | 2.009 | 0.049 |
| Fully adjusted model | 0.222 | 1.579 | 0.120 | 0.252 | 1.759 | 0.085 |
| Total study population (n = 140) | ||||||
| Unadjusted model | 0.332 | 4.103 | < 0.001 | 0.337 | 4.181 | < 0.001 |
| Age and sex (Baseline model) | 0.338 | 4.078 | < 0.001 | 0.341 | 4.161 | < 0.001 |
| Baseline + biomedical factors | 0.221 | 2.526 | 0.013 | 0.321 | 3.769 | < 0.001 |
| Baseline + health behaviour | 0.341 | 4.082 | < 0.001 | 0.343 | 4.161 | < 0.001 |
| Fully adjusted model | 0.223 | 2.533 | 0.013 | 0.325 | 3.780 | < 0.001 |
Biomedical factors used in adjustment set included BMI, systolic blood pressure, and history of diabetes mellitus, hypertension, dyslipidemia, and mental illness.
Health behavior used in adjustment set was physical activity.
Statistically significant (p,0.05) results are bolded.
For the stroke group, there was no statistically significant interaction between time post-stroke and IL-6 on the FAS (P = 0.322), and between time post stroke and hsCRP on the FAS (P = 0.679). This suggests that the relationship between these inflammatory markers and fatigue, as indexed by the FAS, does not appear to vary with length of time post stroke.
4. Discussion
There is widespread recognition that fatigue is a frequent and significant symptom of concern for patients that have suffered a stroke (van der Werf et al., 2001; van Eijsden et al., 2012). Currently, while there have been some promising developments in the symptomatic control of fatigue in context of stroke, such as the use of modafinil (Lillicrap et al., 2018), very little is known about the modulatory or causal biological mechanisms. While acknowledging this paucity of knowledge in the context of stroke, there has been a considerable amount of research into fatigue in the context of other pathological conditions (Bower and Lamkin, 2013; Lasselin et al., 2012). In these other contexts fatigue is frequently observed to co-occur with elevated levels of pro-inflammatory signalling molecules. Somewhat surprisingly, investigation of inflammatory status in stroke survivors has received little attention in the research literature, particularly in the context of chronic recovery. Our research team, interested in the biophysical and psychological features of stroke survivors in the longer term, have been investigating a cohort of stroke survivors with a median post-stroke interval of 38 months. Given the linkages that other research groups have identified in stroke and other long-term conditions, we thought it would be of interest to undertake an exploratory study of two well recognised pro-inflammatory molecules, namely IL-6 and hsCRP, and their possible relationship with symptoms of fatigue. This study therefore has been very much undertaken from a discovery and hypothesis generation perspective, rather than in an effort to establish causality.
Firstly, in the present study, we considered fatigue status using the ten item FAS. The FAS is considered to be unidimensional, has been extensively validated, and is recognised for its robust psychometric properties (Mead et al., 2007; Smith et al., 2008). We identified that stroke survivors had significantly higher FAS scores relative to control participants (~33% higher, with a mean of 24.90 (7.88) vs 18.56 (5.36) for controls). We also used a cutoff proposed by Cumming et al, to define clinically significant fatigue, equal to a score of 24 or higher on the FAS (Cumming and Mead, 2017). Using this criteria, we observed that the majority of stroke survivors reported significant fatigue (60% of stroke survivors compared with 15.7% of control participants). This finding is consistent with previous reports by Ingles et al. (1999), who identified 68% of stroke survivors presented with significant fatigue relative to 35% of age-matched controls, and Van Der Werf et al (van der Werf et al., 2001), (51% in stroke survivors vs 16% in controls).
With respect to the inflammatory biomarkers, we analysed circulating levels of both IL-6 and hsCRP, two of the most highly investigated cytokines in cardiovascular and neuroimmunological research. Levels of both markers were found to be significantly elevated in the stroke group (4.7 pg/mL vs 3.15 for controls for IL-6 and 2.82 pg/mL vs 1.67 for controls for hsCRP). The elevation of both markers is interesting as it suggests that irrespective of the cause, there is evidence for a significant inflammatory disturbance in stroke survivors. Clearly, based on this data no inferences can be made about the origin source of these inflammatory markers (central or peripheral origin), nor can we make inferences about whether these markers are simply epiphenomena, or whether they may be mechanistically involved with modulating fatigue status. Of note, Pearson correlation analysis of the relationship between IL-6, hsCRP and FAS indicated a significant positive correlation, indicating that higher levels of IL-6 and hsCRP were correlated with higher levels of self-reported fatigue on the FAS. An interaction analysis between inflammatory markers and time post stroke on fatigue was not statistically significant, which suggests that the relationship between inflammation and fatigue was preserved regardless of time post-stroke, which was variable in our stroke survivor group.
In an attempt to further understand the relationship between pro-inflammatory cytokines and fatigue in the stroke cohort, we analysed the influence of a number of potentially modulatory variables using linear regression. Our initial (baseline) model considered age, sex, stroke type and time since stroke as covariates. We next added physical activity as a covariate to the baseline model, given that this is a health behaviour known to modify systematic inflammation, and the significant relationship between IL-6/hsCRP and fatigue remained. This suggests that the inclusion of these covariates did not alter the positive and significant relationship between either IL-6 or hsCRP and fatigue. Although several studies have shown that regular physical activity reduces systemic inflammation, this appears to be dependent on the intensity. As we did not collect this information, we could not assess this potential relationship. Further, although physical activity has been shown in some studies to reduce cardiovascular risk via its anti-inflammatory effects, this relationship is presently less clear for fatigue outcomes and should be investigated in future studies.
Significant systemic inflammation has been observed in association with conditions including diabetes (Pitsavos et al., 2007), hypertension (Joppa et al., 2006), dyslipidaemia (Esteve et al., 2005), obesity (Ellulu et al., 2017), and psychiatric disorders (Osimo et al., 2018). When these comorbidities were entered into the regression model, the relationship between IL-6/hsCRP and fatigue among stroke survivors was no longer statistically significant. This result is notable as it suggests that the relationship between stroke and fatigue may not directly be the result of elevated pro-inflammatory signalling molecules but may also be driven by an interaction with other peripheral disease processes and cardiovascular risk factors. People with high systemic inflammation due to a condition such as hypertension or diabetes have a high prevalence of psychiatric comorbidity, however less is known about symptoms of fatigue (Gold et al., 2020). The level of inflammation may be important for determining distressing symptoms that occur alongside these conditions, including depression, anxiety, and fatigue, however a lot of prior research has focused on single conditions and outcomes only. Our results suggest that there may be interactions between inflammatory conditions, which may increase the risk of experiencing distressing symptoms such as fatigue. Much of the research on systemic inflammation and outcomes such as depression or fatigue has considered disease conditions individually and not collectively, and as such, studies have not directly addressed common causes of fatigue across inflammatory diseases. Future research should consider these potential relationships, examining comorbid conditions associated with systemic inflammation, across a range of outcome variables.
In terms of interpreting the possible relationship (or non-relationship) between elevated levels of pro-inflammatory signalling molecules and fatigue, it is important to consider the potential power of the current study. As we undertook this as an exploratory study and there are limited studies considering the relationship between pro-inflammatory signalling and stroke survivors in the chronic phase of recovery, we chose a sample size of seventy. This may not have been sufficient to allow for the discrimination of a modest modulatory role of pro-inflammatory signalling on fatigue status. To investigate whether the issue of sample size may have influenced the modelling within the stroke cohort, we considered it of interest to collapse the results from both stroke and healthy controls. In the full regression model, considering all the potential covariates, the relationship between pro-inflammatory signalling molecules (IL-6 and hsCRP) remained significant in the entire study population. Given this finding, we would anticipate that future studies should use our results as a basis for estimating effect size.
In terms of considering the findings from the current study, we see that it is important to recognise the exploratory nature of the investigation, which was done very much in the spirt of stimulating hypotheses to guide future investigations. We recognise there are a number of limitations, including the fact that the study was cross-sectional. It is also of note that the there was no external verification of the stroke diagnosis or linking to medical records. Stroke was self-reported in this study, as such the severity of stroke could therefore not be included in statistical analysis. The occlusion and infarct size might moderate the relationship of IL-6/hsCRP and fatigue, however, previous studies have shown only a limited association between chronic fatigue and stroke severity, suggesting the importance of exploring additional contributory variables (Chen and Marsh, 2018). It also possible that the level of IL-6/hsCRP in the chronic phase of stroke recovery may be related to secondary neurodegenerative changes or alternatively other peripheral mechanisms such as cardiovascular disease. Ideally, future studies will be able to undertake a more detailed longitudinal investigation into the relationship between pro-inflammatory cytokines, peripheral risk factors, and clinically significant fatigue with the inclusion of late phase MRI.
5. Conclusions
Fatigue is among the most frustrating symptoms experienced by stroke survivors. While the specific mechanisms responsible for fatigue remain elusive, the potential involvement of inflammation is intriguing. There is robust evidence from other fields that pro-inflammatory cytokines can by themselves elicit sickness-like behaviour, including symptoms of fatigue. Therefore, it does not seem unreasonable to consider that at least a component of the fatigue experienced by stroke survivors may be driven by elevated levels of pro-inflammatory cytokines such as IL-6. Fortunately, this hypothesis is eminently testable, and our results suggest that exploring pharmacological strategies to limit inflammation and reduce fatigue in stroke survivors may be worthwhile.
Author contributions
FRW, MN, PG and MH conceived and designed the study. PG, WZC, LKO and MK were involved in protocol development, gaining ethical approval, patient recruitment and data collection. PG and MH were involved in data analysis and PG, MH and FRW wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Funding
This study was supported by the School of Medicine and Public Health and the Faculty of Health, University of Newcastle, Australia; the Hunter Medical Research Institute; the John Hunter Charitable Trust; the Priority Research Centre for Stroke and Brain Injury Research Centre; and the NHMRC Centre for Research Excellence in Stroke Recovery and Rehabilitation.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Amantea D., Nappi G., Bernardi G., Bagetta G., Corasaniti M.T. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- Andersen G., Christensen D., Kirkevold M., Johnsen S. Post-stroke fatigue and return to work: a 2-year follow-up. Acta Neurol. Scand. 2012;125:248–253. doi: 10.1111/j.1600-0404.2011.01557.x. [DOI] [PubMed] [Google Scholar]
- Becker K.J. Inflammation and the silent sequelae of stroke. Neurotherapeutics. 2016;13:801–810. doi: 10.1007/s13311-016-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J.E., Lamkin D.M. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav. Immun. 2013;30:S48–S57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H., Ladelund S., Pedersen A.N., Schroll M., Jørgensen T., Pedersen B. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin. Exp. Immunol. 2003;132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L., Miller A.H. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Marsh E.B. Chronic post-stroke fatigue: it may no longer be about the stroke itself. Clin. Neurol. Neurosurg. 2018;174:192–197. doi: 10.1016/j.clineuro.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Christensen D., Johnsen S.P., Watt T., Harder I., Kirkevold M., Andersen G. Dimensions of post-stroke fatigue: a two-year follow-up study. Cerebrovasc. Dis. 2008;26:134–141. doi: 10.1159/000139660. [DOI] [PubMed] [Google Scholar]
- Cumming T.B., Mead G. Classifying post-stroke fatigue: optimal cut-off on the fatigue assessment scale. J. Psychosom. Res. 2017;103:147–149. doi: 10.1016/j.jpsychores.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Cumming T.B., Packer M., Kramer S.F., English C. The prevalence of fatigue after stroke: a systematic review and meta-analysis. Int. J. Stroke. 2016;11:968–977. doi: 10.1177/1747493016669861. [DOI] [PubMed] [Google Scholar]
- Duncan F., Mead G., Dennis M., Creig C., Lewis S. Fatigue is associated with reduced physical activity one month after stroke. Cerebrovasc. Dis. 2012;33:61. [Google Scholar]
- van Eijsden H.M., van de Port I.G.L., Visser-Meily J.M.A., Kwakkel G. 2012. Poststroke Fatigue: Who Is at Risk for an Increase in Fatigue? Stroke Research and Treatment 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellulu M.S., Patimah I., Khaza’ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci.: AMS. 2017;13:851. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve E., Ricart W., Fernández-Real J.M. Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin. Nutr. 2005;24:16–31. doi: 10.1016/j.clnu.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Gold S.M., Köhler-Forsberg O., Moss-Morris R., Mehnert A., Miranda J.J., Bullinger M., Steptoe A., Whooley M.A., Otte C. Comorbid depression in medical diseases. Nat. Rev. Dis. Primers. 2020;6:1–22. doi: 10.1038/s41572-020-0200-2. [DOI] [PubMed] [Google Scholar]
- de Groot M.H., Phillips S.J., Eskes G.A. Fatigue associated with stroke and other neurologic conditions: implications for stroke rehabilitation. Arch. Phys. Med. Rehabil. 2003;84:1714–1720. doi: 10.1053/s0003-9993(03)00346-0. [DOI] [PubMed] [Google Scholar]
- Gyawali P., Chow W.Z., Hinwood M., Kluge M., English C., Ong L.K., Nilsson M., Walker F.R. Opposing associations of stress and resilience with functional outcomes in stroke survivors in the chronic phase of stroke: a cross-sectional study. Front. Neurol. 2020;11:230. doi: 10.3389/fneur.2020.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles J.L., Eskes G.A., Phillips S.J. Fatigue after stroke. Arch. Phys. Med. Rehabil. 1999;80:173–178. doi: 10.1016/s0003-9993(99)90116-8. [DOI] [PubMed] [Google Scholar]
- Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joppa P., Petrasova D., Stancak B., Tkacova R. Systemic inflammation in patients with COPD and pulmonary hypertension. Chest. 2006;130:326–333. doi: 10.1378/chest.130.2.326. [DOI] [PubMed] [Google Scholar]
- Koenig W., Sund M., Fröhlich M., Fischer H.-G.n., Löwel H., Döring A., Hutchinson W.L., Pepys M.B. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- Kutlubaev M., Duncan F., Mead G. Biological correlates of post-stroke fatigue: a systematic review. Acta Neurol. Scand. 2012;125:219–227. doi: 10.1111/j.1600-0404.2011.01618.x. [DOI] [PubMed] [Google Scholar]
- Lasselin J., Layé S., Dexpert S., Aubert A., Gonzalez C., Gin H., Capuron L. Fatigue symptoms relate to systemic inflammation in patients with type 2 diabetes. Brain Behav. Immun. 2012;26:1211–1219. doi: 10.1016/j.bbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Lillicrap T.P., Levi C.R., Holliday E., Parsons M.W., Bivard A. Short- and long-term efficacy of modafinil at improving quality of life in stroke survivors: a post hoc sub study of the modafinil in debilitating fatigue after stroke trial. Front. Neurol. 2018;9 doi: 10.3389/fneur.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaijwee N.A., Arntz R.M., Rutten-Jacobs L.C., Schaapsmeerders P., Schoonderwaldt H.C., van Dijk E.J., de Leeuw F.-E. Post-stroke fatigue and its association with poor functional outcome after stroke in young adults. J. Neurol. Neurosurg. Psychiatry. 2015;86:1120–1126. doi: 10.1136/jnnp-2014-308784. [DOI] [PubMed] [Google Scholar]
- Mead G., Lynch J., Greig C., Young A., Lewis S., Sharpe M. Evaluation of fatigue scales in stroke patients. Stroke. 2007;38:2090–2095. doi: 10.1161/STROKEAHA.106.478941. [DOI] [PubMed] [Google Scholar]
- Michielsen H.J., De Vries J., Van Heck G.L., Van de Vijver F.J., Sijtsma K. Examination of the dimensionality of fatigue. Eur. J. Psychol. Assess. 2004;20:39–48. [Google Scholar]
- Morley W., Jackson K., Mead G. Post-stroke fatigue: an important yet neglected symptom. Age Ageing. 2005;34 doi: 10.1093/ageing/afi082. 313-313. [DOI] [PubMed] [Google Scholar]
- Naess H., Lunde L., Brogger J., Waje-Andreassen U. Post-stroke pain on long-term follow-up: the Bergen stroke study. J. Neurol. 2010;257:1446–1452. doi: 10.1007/s00415-010-5539-y. [DOI] [PubMed] [Google Scholar]
- Naess H., Lunde L., Brogger J., Waje-Andreassen U. Fatigue among stroke patients on long-term follow-up. The Bergen Stroke Study. J. Neurol. Sci. 2012;312:138–141. doi: 10.1016/j.jns.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Kohsaka S. Microglia: activation and their significance in the central nervous system. J. Biochem. 2001;130:169–175. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- Ormstad H., Aass H.C.D., Amthor K.-F., Lund-Sørensen N., Sandvik L. Serum cytokine and glucose levels as predictors of poststroke fatigue in acute ischemic stroke patients. J. Neurol. 2011;258:670–676. doi: 10.1007/s00415-011-5962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo E.F., Cardinal R.N., Jones P.B., Khandaker G.M. Prevalence and correlates of low-grade systemic inflammation in adult psychiatric inpatients: an electronic health record-based study. Psychoneuroendocrinology. 2018;91:226–234. doi: 10.1016/j.psyneuen.2018.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsavos C., Tampourlou M., Panagiotakos D.B., Skoumas Y., Chrysohoou C., Nomikos T., Stefanadis C. Association between low-grade systemic inflammation and type 2 diabetes mellitus among men and women from the ATTICA study. Rev. Diabet. Stud.: Reg. Dev. Stud. 2007;4:98. doi: 10.1900/RDS.2007.4.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponchel A., Bombois S., Bordet R., Hénon H. 2015. Factors Associated with Poststroke Fatigue: a Systematic Review. Stroke Research and Treatment 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Port I.G.L., Kwakkel G., Bruin M., Lindeman E. Determinants of depression in chronic stroke: a prospective cohort study. Disabil. Rehabil. 2007;29:353–358. doi: 10.1080/09638280600787047. [DOI] [PubMed] [Google Scholar]
- van de Port I.G., Kwakkel G., Schepers V.P., Heinemans C.T., Lindeman E. Is fatigue an independent factor associated with activities of daily living, instrumental activities of daily living and health-related quality of life in chronic stroke? Cerebrovasc. Dis. 2007;23:40–45. doi: 10.1159/000095757. [DOI] [PubMed] [Google Scholar]
- Radman N., Staub F., Aboulafia-Brakha T., Berney A., Bogousslavsky J., Annoni J.-M. Poststroke fatigue following minor infarcts: a prospective study. Neurology. 2012;79:1422–1427. doi: 10.1212/WNL.0b013e31826d5f3a. [DOI] [PubMed] [Google Scholar]
- Ridker P.M., Cushman M., Stampfer M.J., Tracy R.P., Hennekens C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- Rose M., Ferguson A., Power E., Togher L., Worrall L. Aphasia rehabilitation in Australia: current practices, challenges and future directions. Int. J. Speech Lang. Pathol. 2014;16:169–180. doi: 10.3109/17549507.2013.794474. [DOI] [PubMed] [Google Scholar]
- Schilling M., Besselmann M., Leonhard C., Mueller M., Ringelstein E.B., Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Shichita T., Sakaguchi R., Suzuki M., Yoshimura A. Post-ischemic inflammation in the brain. Front. Immunol. 2012;3 doi: 10.3389/fimmu.2012.00132. 132-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith O.R.F., Van Den Broek K.C., Renkens M., Denollet J. Comparison of fatigue levels in patients with stroke and patients with end-stage heart failure: application of the fatigue assessment scale. J. Am. Geriatr. Soc. 2008;56:1915–1919. doi: 10.1111/j.1532-5415.2008.01925.x. [DOI] [PubMed] [Google Scholar]
- Snaphaan L., Van der Werf S., de Leeuw F.E. Time course and risk factors of post-stroke fatigue: a prospective cohort study. Eur. J. Neurol. 2011;18:611–617. doi: 10.1111/j.1468-1331.2010.03217.x. [DOI] [PubMed] [Google Scholar]
- Teasell R. Challenges in the implementation of evidence in stroke rehabilitation. Top. Stroke Rehabil. 2012;19:93–95. doi: 10.1310/tsr1902-93. [DOI] [PubMed] [Google Scholar]
- Wen H., Weymann K.B., Wood L., Wang Q.M. Inflammatory signaling in post-stroke fatigue and depression. Eur. Neurol. 2018;80:138–148. doi: 10.1159/000494988. [DOI] [PubMed] [Google Scholar]
- van der Werf S.P., van den Broek H.L., Anten H.W., Bleijenberg G. Experience of severe fatigue long after stroke and its relation to depressive symptoms and disease characteristics. Eur. Neurol. 2001;45:28–33. doi: 10.1159/000052085. [DOI] [PubMed] [Google Scholar]
- White J.H., Gray K.R., Magin P., Attia J., Sturm J., Carter G., Pollack M. Exploring the experience of post-stroke fatigue in community dwelling stroke survivors: a prospective qualitative study. Disabil. Rehabil. 2012;34:1376–1384. doi: 10.3109/09638288.2011.645111. [DOI] [PubMed] [Google Scholar]
- Whiteley W., Jackson C., Lewis S., Lowe G., Rumley A., Sandercock P., Wardlaw J., Dennis M., Sudlow C. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wang L., Teng W., Huang K., Shang X. Correlation of fatigue during the acute stage of stroke with serum uric acid and glucose levels, depression, and disability. Eur. Neurol. 2014;72:223–227. doi: 10.1159/000364902. [DOI] [PubMed] [Google Scholar]
- Wu S., Barugh A., Macleod M., Mead G. Psychological associations of poststroke fatigue: a systematic review and meta-analysis. Stroke. 2014;45:1778–1783. doi: 10.1161/STROKEAHA.113.004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Duncan F., Anderson N.H., Kuppuswamy A., Macloed M.R., Mead G.E. Exploratory cohort study of associations between serum C - reactive protein and fatigue after stroke. PloS One. 2015;10 doi: 10.1371/journal.pone.0143784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.