Abstract
Introduction
In patients with relapsed SCLC, amrubicin (AMR) is the current standard treatment in Japan. Nevertheless, its efficacy is not satisfactory and prognosis is poor. Preclinical study suggested that anthracycline agent might induce immunogenic cell death and work synergistically with immune checkpoint inhibitors.
Methods
Patients with relapsed SCLC who relapsed after completion of platinum-containing regimen were registered. Patients were treated with pembrolizumab (200 mg, flat dose on d 1, every 3 wk for 2 y) plus AMR (40 mg/m2 on d 1–3, every 3 wk until progression). Primary end point was overall response rate (ORR). Secondary end points consisted of progression-free survival (PFS), overall survival, and safety. On the basis of the hypothesis that this treatment will improve ORR from 20% to 40% (0.1 of one-sided α and power of 0.8), 25 patients are required (trial identifier: NCT03253068).
Results
Between November 2017 and October 2019, a total of 25 patients were enrolled. Most participants (88%) relapsed within 90 days after platinum-containing therapy and all patients were immune checkpoint inhibitor-naive. ORR, the primary end point, was 52.0% (95% confidence interval [CI]: 31.3%–72.2%). Median PFS was 4.0 months (95% CI: 2.8–7.0 mo), and PFS rate at 1 year was 14.4%. Median overall survival was 10.6 months (95% CI: 7.3–21.3 mo). Common adverse events greater than or equal to grade 3 were neutropenia (64%), leukopenia (40%), and febrile neutropenia (16%). No treatment-related deaths occurred.
Conclusions
Among patients with relapsed SCLC, pembrolizumab plus AMR was effective and tolerable.
Keywords: Small cell lung cancer, Pembrolizumab, Amrubicin, Refractory
Introduction
SCLC comprises approximately 15% of newly diagnosed lung cancer cases, and they are mostly extended disease at diagnosis.1 SCLC is basically sensitive to first-line chemotherapy; however, most patients acquire resistance within 6 months. Prognosis of relapsed SCLC is poor, and there is no established standard regimen. National Comprehensive Cancer Network guideline recommends cytotoxic chemotherapy monotherapy, such as topotecan or lurbinectin, for patients who relapsed more than or equal to 6 months after first-line chemotherapy, whereas rechallenge with other regimen or lurbinectin is recommended for patients who relapsed less than 6 months after first-line chemotherapy.2 Their median progression-free survival (mPFS) was only several months.3, 4, 5
Amrubicin (AMR) is an anthracycline agent that was developed in Japan. In a phase 3 trial comparing AMR with topotecan in relapsed SCLC, AMR significantly improved overall response rate (ORR) and PFS and revealed similar overall survival (OS), despite failing to reveal superiority.6 In a Japanese phase 2 trial for patients with refractory SCLC, AMR revealed 33% of ORR and 3.5 months of mPFS.7 Considering its convenient dosing schedule (d 1–3, every 3 wk), AMR is currently the only drug that Japanese guideline recommends for administration in patients with relapsed SCLC.
Immune checkpoint inhibitor (ICI) monotherapy has revealed modest activity in patients with relapsed SCLC. In a phase 2 trial (KEYNOTE 028), pembrolizumab (Pembro) alone had 33% of ORR, but mPFS was only 1.9 months.8 A recent phase 3 trial (CheckMate 331) compared nivolumab with NGT or AMR and revealed 13.7% of ORR and 1.4 months of mPFS.9 Thus, to enhance the efficacy of ICI, combination treatment could be a reasonable approach. Although recent phase 3 studies that combined platinum-doublet chemotherapy plus ICI in treatment-naive patients with extensive disease SCLC already revealed extension of OS,10,11 development of an active treatment option for relapsed SCLC is still worth challenging. In addition, preclinical data suggested the synergistic effect of ICI plus anthracycline agent.12 Here, we report the result of an open-label, multi-institutional, single-arm phase 2 study that tested Pembro plus AMR in patients with pretreated SCLC.
Materials and Methods
Eligibility Criteria
Eligible patients were as follows: (1) those with pathologically proven SCLC; (2) those with confirmed radiological relapse with first-line chemotherapy; (3) those with adequate tumor tissue sample to test programmed death-ligand 1 (PD-L1) immunohistochemistry; (4) those with Eastern Cooperative Oncology Group performance status 0 to 1; (5) those with adequate organ function within 7 days before registration; (6) those with measurable lesion per Response Evaluation Criteria in Solid Tumors version 1.1; and (7) those with informed, documented consent to participate in the study.
Patients were excluded if they had the following: (1) history of noninfectious pneumonitis that required steroids, currently active pneumonitis, or any evidence of interstitial lung disease on computed tomography (CT); (2) history of previous anticancer therapy with an anti–programmed cell death protein-1, anti–PD-L1, anti–PD-L2, anti-CD137, anti–CTLA-4 antibody, or other drugs specifically targeting T-cell co-stimulation or checkpoint pathways; (3) concomitant systemic steroid therapy less than or equal to 3 days from registration or receiving any other form of immunosuppressive medication; (4) autoimmune disease that has required systemic treatment in the previous 2 years; (5) symptomatic central nervous system metastases or carcinomatous meningitis; or (6) active hepatitis type B or C.
Outcomes
Primary end point was ORR by investigators. Secondary end points were PFS, duration of response, OS, and safety.
Treatment
AMR (40 mg/m2) was administered intravenously every 3weeks on days 1 to 3. Pembro (200 mg, flat dose) was administered intravenously every 3 weeks on day 1. AMR was continued until disease progression or unacceptable toxicity. Pembrolizumab was continued until disease progression, unacceptable toxicity or up to 2 years.
Assessment
Before registration, all patients had to received enhanced chest-abdominal CT and enhanced magnetic resonance imaging of the brain. To evaluate the efficacy, enhanced chest-abdominal CT was taken every six weeks. Enhanced magnetic resonance imaging of the brain was taken every six weeks if the patients had brain metastases. Tumor assessments were based on Response Evaluation Criteria in Solid Tumors, version 1.1. Adverse events (AEs) were graded using Common Terminology Criteria for Adverse Events, version 4.0.
PD-L1 expression was evaluated in formalin-fixed, paraffin-embedded tumor samples acquired by surgical resection or biopsy. As the timing of sample collection was not defined in the protocol, most samples were obtained at diagnosis. Procedures were centrally processed using the 22C3 anti–PD-L1 antibody (proprietary mouse monoclonal antibody, Merck & Co., Inc., Kenilworth, NJ) at QualTek Molecular Laboratories (Goleta, CA). Samples were considered to be PD-L1 positive if they exhibited membranous PD-L1 expression on tumor cells, inflammatory cells (lymphocytes or macrophages), or the stroma. The combined positive score (CPS) was the ratio of PD-L1–positive cells (tumor cells, lymphocytes, and macrophages) to the total number of tumor cells multiplied by 100. PD-L1 expression on tumor-infiltrating lymphocytes (TILs) was evaluated in four levels (0, 1+, 2+, and 3+). These processes and assessments basically followed the previous report.13
Statistical Analysis
On the basis of the pivotal phase 3 study of AMR in patients with refractory SCLC,6 the response rate threshold and expected response rate were set as 20% and 40%, respectively. Under this assumption, the required sample size to test the difference in the population proportions is 22 (α = 0.10, one-sided and 1-β = 0.80). Taking into account the assumption that 10% of subjects will be ineligible, the sample size in this study was estimated as 25.
Ethical Considerations
The study is conducted in accordance with the principles of the Declaration of Helsinki, and the protocol was approved by the institutional review board of each participating institution. Written informed consent was obtained from all patients before any screening or inclusion procedures. This protocol was registered at ClinicalTrials.gov (identifier: NCT03253068).
Results
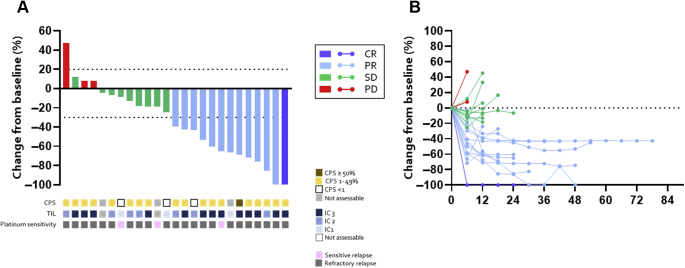
Between November 2017 and October 2019, a total of 25 patients were registered from three Japanese institutions. At the time of data cutoff, 23 patients discontinued the study treatment and median follow-up time was 9.4 months (range: 4.7–24.2 mo). Baseline patient characteristics are found in Table 1. Median age was 66 years (range: 36–80), and 16 patients (64%) were of male sex. Most patients (22 of 25, 88%) relapsed within 90 days of the last administration of previous platinum-doublet chemotherapy (= refractory relapse). CPS was positive in 19 patients (76%). Regarding PD-L1 expression on TIL, 13 patients (52%) were assessed as 3+, seven patients (28%) were 2+, and five patients (20%) were as 1+ or not assessable, respectively. At the data cutoff time, the median number of administration of the study drugs was four each (range: 1–27). Regarding AMR, eight patients required dose reduction. Three patients discontinued study treatment for reasons other than disease progression.
Table 1.
Baseline Characteristics
| Characteristics | N = 25 |
|---|---|
| Age, median (range) | 66 (36–80) |
| Male/female | 16/9 |
| Smoker/unknown | 24/1 |
| ECOG performance status 0/1 | 8/17 |
| Previous chemotherapeutic regimen, n (%) | |
| Platinum + CPT-11 | 14 (56) |
| Platinum + VP-16 | 13 (52) |
| CDDP + CPT-11 followed by experimental drug (ADC) | 1 (4) |
| CBDCA + VP-16 followed by experimental drug (ADC) | 1 (4) |
| Sensitivity of previous platinum therapy, n (%) | |
| Sensitive (relapsed >90 d of last chemotherapy) | 3 (12) |
| Refractory (relapsed ≤90 d of last chemotherapy) | 22 (88) |
| Previous radiotherapy, yes/no | 3/22 |
| PD-L1 expression on tumor cell or lymphocytes CPS, n (%) | |
| ≥1% | 19 (76) |
| <1% or not assessable | 6 (24) |
| PD-L1 expression on lymphocytes TIL, n (%) | |
| 3+ | 13 (52) |
| 2+ | 7 (28) |
| 1 or not assessable | 5 (20) |
ADC, antibody-drug conjugate; CBDCA, carboplatin; CDDP, cisplatin; CPS, combined positive score; CPT-11, irinotecan; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed-death-ligand 1; TIL, tumor-infiltrating lymphocyte; VP-16, etoposide.
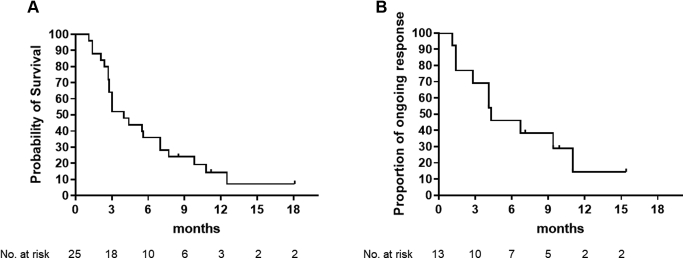
Waterfall and spider plots are found in Figure 1. One patient had complete response and 12 had partial response as their best response. ORR was therefore 52.0% (95% confidence interval [95% CI]: 31.3%–72.2%), which met the primary end point. mPFS was 4.0 months (95% CI: 2.8–7.0 mo), and PFS rate at 1 year was 14.4% (Fig. 2A). Duration of response was 4.3 months (95% CI: 1.4–11.0 mo) (Fig. 2B). Median OS was 10.6 months (95% CI: 7.3–21.3 mo), and OS rate at 1 year was 45.2%.
Figure 1.
(A) Waterfall plot (best percentage change in tumor burden from baseline) and (B) spider plot. Bars and lines colored blue, light blue, green, or red represent CR, PR, SD, and PD, respectively. CPS score, PD-L1 expression on TIL levels, and sensitivity to the previous platinum therapy are also described. CPS, combined positive score; CR, complete response; IC, immune checkpoint; PD, disease progression; PD-L1, programmed death-ligand 1; PR, partial response; SD, stable disease; TIL, tumor-infiltrating lymphocyte.
Figure 2.
Kaplan-Meier curves of (A) progression-free survival and (B) duration of response. No., number.
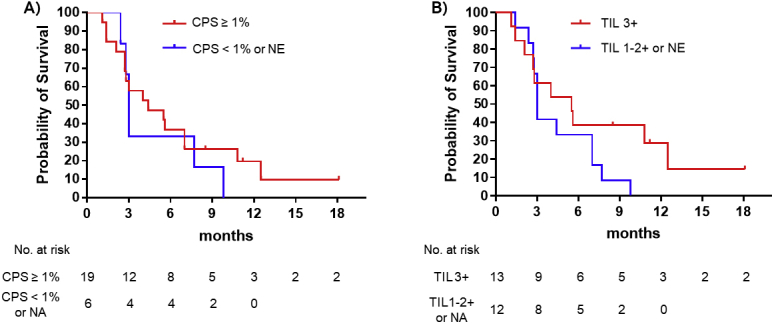
As post hoc analyses, ORR and PFS were evaluated on the basis of CPS or TIL level. Patients with CPS greater than or equal to 1% (n = 19) tended to have better efficacy outcomes than those with CPS less than 1% or not assessable (n = 6); ORR (58% versus 33%), and mPFS (4.4 mo versus 3.0 mo, hazard ratio [HR] = 0.73, 95% CI: 0.25–1.91) (Supplementary Fig. 1A). Similarly, patients with TIL 3+ (n = 13) had better ORR and mPFS than those with TIL 1+, 2+, or not assessable (n = 12); ORR (69% versus 33%); and mPFS (5.5 mo versus 3.0 mo, HR = 0.57, 95% CI: 0.24–1.34) (Supplementary Fig. 1B).
Hematologic and gastrointestinal AEs were often observed (Supplementary Table 1). Of 25 patients, 15 (60%) had greater than or equal to grade 3 neutropenia, three (12%) had grade 3 anemia, and three (12%) had greater than or equal to grade 3 platelet decrease. Four patients (16%) had febrile neutropenia. Three (12%) had pneumonitis, but all were grade 1 or 2. Regarding immune-related AEs, five patients (20%) had thyroid disorders and one (4%) had adrenal insufficiency; all were appropriately treated. There were no treatment-related deaths.
Discussion
Because of the aggressive phenotype of relapsed SCLC, development of novel therapeutic regimen has not been succeeded for a long time. Recently, lurbinectedin, a novel oncogenic transcriptional inhibitor, revealed 35.2% of ORR and 3.5 months of mPFS,5 which led to accelerated approval from the U.S. Food and Drug Administration. Nevertheless, the prognosis still remains poor and subset analysis revealed declined efficacy among patients with refractory relapse (22.2% of ORR and 2.6 mo of mPFS).
Therefore, both rapid and durable responses are required to develop novel regimen for refractory SCLC, and ICI plus another agent is a reasonable strategy. Fan et al.14 reported a single-arm phase 2 trial of camrelizumab (anti–programmed cell death protein-1 inhibitor) plus apatinib (anti-vascular epithelial growth factor receptor 2 inhibitor) that revealed 34.0% of ORR and 3.6 months of mPFS in 59 Chinese patients with relapsed SCLC. In their study, efficacy was not diminished among 31 platinum-refractory cases. Our study revealed 52% of ORR despite 88% of participants having refractory relapse. More importantly, 14.4% of patients were progression free at one year, which was superior to that found in the previous studies of lurbinectedin or chemotherapy alone (<5%). Similar combination strategy is also tested in another trial (E7389 liposomal formulation plus nivolumab, NCT04078295).
In contrast, attempts to explore efficient predictive biomarkers of ICI have been warranted among SCLC. Unlike other malignancies, the value of PD-L1 expression or TMB in SCLC has not been clarified. Prospective trials revealed that CPS score did not predict PFS with Pembro or nivolumab.9,13 In this study, patients with CPS greater than or equal to 1% or TIL 3+ tended to have slightly better outcomes, but not statistically significant. Gay et al.15 recently proposed four subtypes of SCLC on the basis of transcription factors and the inflamed subtype revealed higher sensitivity to ICI plus chemotherapy. Future prospective studies should consider the validation of such patient selection.
Hematologic toxicity revealed in this study seemed to be similar to that with AMR monotherapy conducted in Japan7; however, it is slightly more severe compared with pivotal trial conducted in the United States.6 As found in a previous international study, some racial differences may have an effect here.16 AEs observed in this study were basically from either AMR or Pembro alone, not from additives.
This study has several limitations. On the basis of the study design, several biases and confounding factors cannot be eliminated. Nevertheless, the rarity of the study population allowed us to conduct this study as a single-arm trial similar to previous trials,5,14 and it was helpful to evaluate the activity and safety of this regimen without requiring a lot of patient resources and time. Second, unavailability of AMR among countries outside of Japan makes it difficult to expand these results. Nevertheless, the result encouraged to support the previous findings of paclitaxel plus Pembro (33% of ORR and 5.0 mo of mPFS).17 Third, owing to the timing of the trial planned, we excluded those who received previous ICI. Thus, we do not know whether Pembro plus AMR is effective in patients who become resistant to chemotherapy plus ICI in their first-line treatment. Although platinum-doublet plus ICI has become a new first-line treatment in SCLC,10,11 the actual benefits have been unsatisfactory. Our data clearly revealed that Pembro plus AMR is active and tolerable in refractory SCLC, so this approach should be explored further, for example, ICI plus another cytotoxic drug combination, such as topotecan or lurbinectedin, or rechallenge after platinum-doublet plus ICI.
In conclusion, Pembro plus AMR was effective and tolerable among patients with relapsed SCLC.
CRediT Authorship Contribution Statement
Hiroaki Akamatsu: Conceptualization, Methodology, Data analyzation, Writing—original draft preparation.
Shunsuke Teraoka: Methodology, Patient accrual, Revise manuscript.
Hidetoshi Hayashi and Daichi Fujimoto: Patient accrual, Manuscript review, Site coordinator.
Atsushi Hayata, Koji Haratani, Yuichi Ozawa, Takeshi Yoshida, and Tsutomu Iwasa: Patient accrual, Manuscript review.
Toshio Shimokawa: Statistical planning, Data cleaning, Analyzation.
Keisuke Tomii and Kazuhiko Nakagawa: Patient accrual, Manuscript review, Site principal investigator.
Nobuyuki Yamamoto: Patient accrual, Manuscript review, Head of this study.
Acknowledgments
This work was supported by Merck Sharp & Dohme, USA. The authors are grateful to data managers and other support staff (especially, Ms. Hana Nishida) at the Data Center at Wakayama Medical University. The authors also acknowledge proofreading and editing by Benjamin Phillis at the Clinical Study Support Center at Wakayama Medical University Hospital. Dr. Akamatsu, the principal investigator, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Merck Sharp & Dohme, USA provided pembrolizumab for this study and was also involved in review of the manuscript and its approval. Merck Sharp & Dohme, USA was not involved in the design or conduct of the study (collection, management, analysis, and interpretation of the data and decision to submit the manuscript for publication).
Footnotes
Cite this article as: Akamatsu H, et al. Pembrolizumab Plus Amrubicin in Patients With Relapsed SCLC: Multi-Institutional, Single-Arm Phase 2 Study. JTO Clin Res Rep 2021;2:100184
Disclosure: Dr. Akamatsu reports receiving honoraria from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Merck Sharp & Dohme K.K., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. Ltd. and research funding from Chugai Pharmaceutical Co. Ltd. and Merck Sharp & Dohme K.K. Dr. Teraoka reports receiving honoraria from Chugai Pharmaceutical Co. Ltd., Novartis Pharma K.K., AstraZeneca K.K., Taiho Pharmaceutical Co. Ltd., Ono Pharmaceutical Co., Ltd., and Boehringer Ingelheim Japan Inc. Dr. Hayashi reports receiving honoraria from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Merck Sharp & Dohme K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Shanghai Haihe Biopharm, Kyorin Pharmaceutical Co. Ltd., Novartis Pharma K.K., and Taiho Pharmaceutical Co. Ltd. and research funding from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., and Ono Pharmaceutical Co. Ltd. Dr. Fujimoto reports receiving honoraria from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Merck Sharp & Dohme K.K., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. Ltd. and research funding from AstraZeneca K.K. Dr. Haratani reports receiving honoraria from AS ONE Corporation, AstraZeneca K.K., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Merck Sharp & Dohme K.K., and Pfizer Japan Inc. and research funding from AstraZeneca K.K. and Merck Sharp & Dohme K.K. Dr. Ozawa reports receiving honoraria from Ono Pharmaceutical Co., Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co., Eli Lilly, Merck Sharp & Dohme K.K., Novartis, AstraZeneca, and Boehringer Ingelheim. Dr. Tomii reports receiving honoraria from Astellas Pharma Inc., AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo, Eli Lilly Japan K.K., GlaxoSmithKline Pharmaceuticals Ltd., Kyorin Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co., Ltd., Merck Sharp & Dohme K.K., Novartis Pharma K.K, Sanofi K.K., Shionogi & Co., Ltd., Taiho Pharmaceutical Co. Ltd., and Teijin Pharma, Ltd. and having an advisory role at Eli Lilly Japan K.K. Prof. Nakagawa reports receiving honoraria from AstraZeneca K.K., Nichi-Iko Pharmaceutical Co., Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Merck Sharp & Dohme K.K., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb Company, Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Novartis Pharma K.K., SymBio Pharmaceuticals Limited., Pfizer Japan Inc., Chugai Pharmaceutical Co., Ltd., Clinical Trial Co., Ltd., Nanzando Co., Ltd., Medicus Shuppan Publishers Co., Ltd., Yodosha Co., Ltd., Care Net, Inc., Nikkei Business Publications, Inc., Reno Medical K.K., Daiichi Sankyo Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Thermo Fisher Scientific K.K., Medical Review Co., Ltd., Yomiuri Telecasting Corp., Roche Diagnostics K.K., Nippon Kayaku Co., Ltd., Bayer Yakuhin, Ltd., Merck Biopharma Co., Ltd., Medical Mobile Communications Co., Ltd., AbbVie Inc., and 3H Clinical Trial Inc. and research funding from Merck Sharp & Dohme K.K., A2 Healthcare Corp., inVentiv Health Japan, Astellas Pharma Inc., Daiichi Sankyo Co., Ltd., Novartis Pharma K.K., AbbVie Inc., Quintiles Inc./Iqvia Services Japan K.K., Icon Japan K.K., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd. EP-CRSU Co., Ltd., GRITSONE ONCOLOGY Inc., Linical Co., Ltd., Eli Lilly Japan K.K., Eisai Co., Ltd., Bristol-Myers Squibb Company, Nippon Boehringer Ingelheim Co., Ltd., Taiho Pharmaceutical Co., Ltd., Pfizer Japan Inc., PAREXEL International Corp., SymBio Pharmaceuticals Limited, Ono Pharmaceutical Co., Ltd., Merck Serono Co., Ltd./Merck Biopharma Co., Ltd., AstraZeneca K.K., CMIC Shift Zero K.K., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., EPS Corp., Bayer Yakuhin, Ltd., Syneos Health, EPS International Co., Ltd., Pfizer R&D Japan G.K., and Otsuka Pharmaceutical Co., Ltd.; and having consulting/advisory role for Takeda Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Eli Lilly Japan K.K. Prof. Yamamoto reports receiving honoraria from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo, Eli Lilly Japan K.K., Life Technologies, Merck Biopharma Co., Ltd., Merck Sharp & Dohme K.K., Nippon Kayaku Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Takeda Pharmaceutical, Taiho Pharmaceutical Co. Ltd., and Thermo Fisher Scientific K.K. and research funding from AbbVie G.K., Amgen, AstraZeneca K.K., Astellas Pharma Inc., Boehringer Ingelheim Japan Inc., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo, Eisai Co., Ltd., Eli Lilly Japan K.K., Kyorin Pharmaceutical Co. Ltd., Merck Sharp & Dohme K.K., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Takeda Pharmaceutical, Taiho Pharmaceutical Co. Ltd., Terumo Corp., Toppan Printing Co., Ltd., TOSO Co, Ltd., and Tsumura & Co. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100184.
Supplementary Data
Supplementary Figure 1.
References
- 1.Govindan R., Page N., Morgensztern D. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology, small cell lung cancer. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf Version 2. Available at: Accessed May 2, 2021.
- 3.O’Brien M.E., Ciuleanu T.E., Tsekov H. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 4.Baize N., Monnet I., Greillier L. Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2020;21:1224–1233. doi: 10.1016/S1470-2045(20)30461-7. [DOI] [PubMed] [Google Scholar]
- 5.Trigo J., Subbiah V., Besse B. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21:645–654. doi: 10.1016/S1470-2045(20)30068-1. [DOI] [PubMed] [Google Scholar]
- 6.von Pawel J., Jotte R., Spigel D.R. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32:4012–4019. doi: 10.1200/JCO.2013.54.5392. [DOI] [PubMed] [Google Scholar]
- 7.Murakami H., Yamamoto N., Shibata T. A single-arm confirmatory study of amrubicin therapy in patients with refractory small-cell lung cancer: Japan Clinical Oncology Group Study (JCOG0901) Lung Cancer. 2014;84:67–72. doi: 10.1016/j.lungcan.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Ott P.A., Elez E., Hiret S. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35:3823–3829. doi: 10.1200/JCO.2017.72.5069. [DOI] [PubMed] [Google Scholar]
- 9.Spigel D.R., Vicente D., Ciuleanu T.E. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate. Ann Oncol. 2021;32:631–641. doi: 10.1016/j.annonc.2021.01.071. [DOI] [PubMed] [Google Scholar]
- 10.Horn L., Mansfield A.S., Szczęsna A. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 11.Paz-Ares L., Dvorkin M., Chen Y. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 12.Fucikova J., Kralikova P., Fialova A. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- 13.Chung H.C., Piha-Paul S.A., Lopez-Martin J. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol. 2020;15:618–627. doi: 10.1016/j.jtho.2019.12.109. [DOI] [PubMed] [Google Scholar]
- 14.Fan Y., Zhao J., Wang Q. Camrelizumab plus apatinib in extensive-stage SCLC (PASSION): a multicenter, two-stage, phase 2 trial. J Thorac Oncol. 2021;16:299–309. doi: 10.1016/j.jtho.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Gay C.M., Stewart C.A., Park E.M. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39:346–360.e7. doi: 10.1016/j.ccell.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandara D.R., Kawaguchi T., Crowley J. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009;27:3540–3546. doi: 10.1200/JCO.2008.20.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y.J., Keam B., Ock C.Y. A phase II study of pembrolizumab and paclitaxel in patients with relapsed or refractory small-cell lung cancer. Lung Cancer. 2019;136:122–128. doi: 10.1016/j.lungcan.2019.08.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.