Abstract
Introduction
This study explored the use, safety, and efficacy of initial use of an ALK-inhibiting targeted therapy (ALK tyrosine kinase inhibitor [TKI]) in patients with ALK-rearranged NSCLC in a population-based, real-world clinical population within the province of Alberta, Canada.
Methods
Demographic, clinical, treatment, and outcome data of the patients with advanced or metastatic ALK-rearranged NSCLC receiving their first ALK TKI between 2014 and 2019 were included in the analysis.
Results
A total of 92 patients with ALK-rearranged NSCLC treated with ALK TKI (78% crizotinib, 22% alectinib) were identified. In the ALK-rearranged cohort, 1-year survival rate was 73% and median overall survival (OS) and progression-free survival (PFS) were 48.5 months and 17.0 months, respectively. An objective response rate of 49% was observed, and adverse events were reported in 70% of the patients, primarily of low grade (84%). Case-matched comparison to patients with ALK-wildtype disease treated with cytotoxic chemotherapy revealed the benefit of ALK TKI in the context of an ALK rearrangement (ALK-rearranged versus ALK-wildtype) (median post-treatment initiation OS: 46.8 versus 14.2 mo, p < 0.001). Outcomes, measured from the time of ALK TKI initiation, differed by Eastern Cooperative Oncology Group (ECOG) (ECOG < 2 versus ECOG ≥ 2) (median OS: not reached versus 6.8 mo, p < 0.001; median PFS 17.6 versus 7.4 mo, p = 0.02), disease presentation (relapsed versus de novo) (median PFS: 30.8 versus 15.0 mo, p = 0.04), and brain metastasis onset (brain metastases development during ALK TKI versus baseline brain metastases) (not reached versus 12.8 mo, p = 0.04).
Conclusions
Clinical trials have firmly established that ALK TKIs are safe, well tolerated, and effective; these findings reveal that their impact in a real-world setting is just as profound. The availability and use of ALK TKI therapies contribute to the impressive gains in survival experienced by contemporary patients with ALK-rearranged disease, rendering patients with this oncodriven form of NSCLC among the longest surviving patients with lung cancer.
Keywords: ALK-rearranged NSCLC, Targeted therapy, Real-world outcomes, Adverse events
Introduction
ALK-rearranged NSCLC, present in 3% to 5% of patients with advanced NSCLC, represents the second most common oncogene-driven NSCLC.1 Oncogenic ALK fusions are amenable to targeted therapy by means of three generations of ALK-receptor tyrosine kinase inhibitors (ALK tyrosine kinase inhibitor [TKI]) that act to block the signaling cascades that promote tumor growth and progression.2 Clinical trial data clearly reveal the safety and efficacy of ALK TKI, but within the context of highly selected trial populations. Given the evidence for benefit in clinical trial populations, evaluation of real-world populations with ALK-rearranged NSCLC to determine whether clinical trial data are reflective of the real-world clinical setting is of critical importance.
Although the need for real-world evidence is widely understood, the ability to provide comprehensive data on true, unbiased real-world populations that capture the full heterogeneity (demographic, geographic, clinical, and socioeconomic) of patients encountered in clinical practice has proven challenging. The low incidence of ALK rearrangements among patients with NSCLC often results in scarce data in real-world cohorts. Compounding these issues is the loss of diversity which occurs when retrospective reviews are conducted by means of single-center studies that may limit analysis to patients from a single region or be restricted to specific health care insurance providers.
This study sought to retrospectively review the outcome, safety, efficacy, and experiences of ALK inhibition in a real-world clinical population by means of the use of a province-wide cohort, situated within a single payer, universal health care system, representing all patients with ALK-rearranged disease at the time of their initial exposure to ALK TKI therapy within Alberta, Canada, from 2014 to 2019.
Materials and Methods
This study used the Glans-Look Lung Cancer Research (GLR) database, an institutional database that captures patient-level demographic, clinical, treatment, response, and outcome data by means of chart reviews of electronic medical records for all patients with a diagnosis of lung cancer who present for diagnosis and treatment within the Canadian province of Alberta. The data in the GLR used for this analysis were collected under ongoing institutional review board–approved protocol at our institution (HREBA.CC-16-0574_REN4), and as a retrospective review, no consent is required. Study data within the GLR database are collected and managed using the Research Electronic Data Capture data capture tools hosted at the University of Calgary.3,4
Patients with unresectable locally advanced or metastatic NSCLC were identified. Patient selection was refined to include only those positive for an ALK translocation, identified by a combination of immunohistochemistry with fluorescence in situ hybridization confirmation (from 2014 to 2018) or by upfront immunohistochemistry testing with fluorescence in situ hybridization confirmation for equivocal results only (2018–current), as per the International Association for the Study of Lung Cancer and Association for Molecular Pathology biomarker guidelines established in 2013 and revised in 2018,5,6 and receiving an initial ALK TKI therapy (first exposure to ALK TKI), as confirmed by Alberta Health Services pharmacy records, between January 2014 and December 2019. At the time of this study, two ALK TKIs were approved for use in Canada: crizotinib (approved for use after progression on cytotoxic chemotherapy in May 2013 and as a first-line therapy for patients with treatment-naive ALK-rearranged disease in July 2015)7 and alectinib (approved for as a first-line therapy for patients with treatment-naive ALK-rearranged disease in July 2018).8 Patients in receipt of ALK TKI therapies accessed outside of the centralized Alberta Health Services pharmacy-dispensing system are not reflected in this study. In addition, a comparator cohort of patients with ALK-wildtype disease, tested and confirmed negative for detectable ALK rearrangements, who underwent standard-of-care cytotoxic chemotherapy for their advanced or metastatic NSCLC was identified and data were extracted from the GLR. Patients with ALK-wildtype disease diagnosed from 2014 to 2017 were matched (without replacement) on the American Joint Committee on Cancer eighth edition M-stage, histological subtype, sex, Eastern Cooperative Oncology Group (ECOG), smoking history, and 5-year age group to the ALK-rearranged cohort for survival analysis comparisons.
Survival metrics—median post-advanced/metastatic disease discovery survival (median overall survival [mOS]), median post-ALK TKI initiation survival, and median progression-free survival (mPFS)—along with treatment patterns, treatment events, response, and outcomes were calculated using data elements contained in the GLR. For determination of best response and progressive disease, serial diagnostic imaging reports were compared with a baseline computed tomography scan taken before initiation of ALK TKI therapy. Response was determined using the Response Evaluation Criteria in Solid Tumours version 1.1 criteria; if actual measurements were not reported within the diagnostic imaging reports, then response was recorded on the basis of the documented opinion of the reviewing radiologist.
Occurrence and management of adverse events (AEs) noted in the GLR were derived from clinical progress notes, urgent care/emergency room reports, hospital discharge reports, pharmacist contact notes, oncology clinic nursing notes, and laboratory testing reports. AEs were recorded using Common Terminology Criteria for Adverse Events version 5.0 codes, descriptors, and grades, as standardized and grouped according to Medical Dictionaries for Regulatory Activities’ Primary System Organ Class terms and hierarchy.
Demographic and clinical characteristics of the study cohort were summarized using descriptive statistics, and distributions of features were compared across different subgroups using univariate methods including time-to-event models that were evaluated using the Kaplan-Meier approach. A p value less than 0.05 was considered a priori as statistically significant. All statistical analysis and data manipulation were performed using Stata Statistics/Data Analysis version 12.9
Results
A total of 92 patients with ALK-rearranged NSCLC treated with ALK TKI were identified, in a 6-year (2014–2019 inclusive) study period, yielding a median of 17 patients per year for analysis. 53% of the patients were alive at the time of the analysis. Patients initially received either crizotinib (78%) or alectinib (22%) ALK TKI therapy. Demographic and clinical characteristics are summarized in Table 1, and clinical response and outcome are summarized in Supplementary Data 1. Patients treated with alectinib and crizotinib did not exhibit significantly different demographic characteristics.
Table 1.
Demographic and Clinical Features for Patients With ALK-Rearranged NSCLC on Initial ALK TKI
| Total Cohort |
Initial ALK TKI |
|||
|---|---|---|---|---|
| Total Cohort (N = 92), n (%) | Crizotinib (n = 72) | Alectinib (n = 20) | p Value | |
| Demographics | ||||
| Age at treatment initiation | ||||
| Median (y), (IQR) | 58.4 (51.6–70.6) | 58.7 (51.6–71.2) | 57.3 (51.7–62.3) | Z = −1.14; p = 0.3 |
| <70 y | 67 (73) | 51 (71) | 16 (80) | X2, df(1), p = 0.4 |
| ≥70 y | 25 (27) | 21 (29) | 4 (20) | |
| Sex | ||||
| Male | 47 (51) | 37 (51) | 10 (50) | X2, df(1), p = 0.9 |
| Female | 45 (49) | 35 (49) | 10 (50) | |
| Smoking status | ||||
| Never smoker | 51 (55) | 37 (51) | 14 (70) | X2, df(1), p = 0.1 |
| Ever smoker | 41 (45) | 35 (49) | 6 (30) | |
| Body mass index (kg/m2) | ||||
| Median, (IQR) | 25.9 (22.5–29.1) | 25.0 (22.5–29.3) | 24.4 (22.2–27.8) | Z = −0.5; p = 0.6 |
| <18.5 (underweight) | 3 (3) | 2 (3) | 1 (5) | |
| 18.5–24.8 (normal) | 42 (46) | 32 (44) | 10 (50) | X2, df(4), p = 0.8 |
| 24.9–29.9 (overweight) | 27 (29) | 22 (31) | 5 (25) | |
| >29.9 (obese) | 18 (20) | 14 (19) | 4 (20) | |
| Missing data | 2 (2) | 2 (3) | 0 (0) | |
| Income (Canadian dollars) | ||||
| Median, (IQR) | 50,422 (45,361–64,063) | 49,607 (45,361–63,203) | 58,456 (50,192–67,316) | Z = 1.5, p = 0.1 |
| Below median | 51 (55) | 35 (49) | 4 (20) | |
| Above median | 41 (45) | 37 (51) | 16 (80) | |
| Geographic location of residence | ||||
| Urban | 76 (83) | 58 (81) | 18 (90) | X2, df(1), p = 0.3 |
| Rural | 16 (17) | 14 (19) | 2 (10) | |
| Clinical characteristics | ||||
| Initial ALK TKI | —- | |||
| Crizotinib | 72 (78) | —- | —- | |
| Alectinib | 20 (22) | |||
| 2015–2017 | 67 (73) | 65 (90) | 2 (10) | X2, df(1), p < 0.001a |
| 2018–2019 | 25 (27) | 7 (10) | 18 (90) | |
| Cancer treatment center type | X2, df(1), p < 0.6 | |||
| Academic | 74 (80) | 57 (79) | 17 (85) | |
| Community/regional | 18 (20) | 15 (21) | 3 (15) | |
| ECOG PS | ||||
| Good (0 or 1) | 72 (78) | 58 (81) | 16 (75) | X2, df(1), p = 0.9 |
| Poor (2 or 3) | 18 (22) | 14 (19) | 4 (25) | |
| Stage at initiation of initial ALK TKI | ||||
| M0 (advanced) | 10 (11) | 7 (10) | 3 (15) | X2, df(4), p = 0.4 |
| M1a | 24 (26) | 19 (26) | 5 (25) | |
| M1b | 32 (35) | 28 (39) | 4 (20) | |
| M1c | 26 (28) | 18 (25) | 8 (40) | |
| Previous cytotoxic chemotherapy exposure | ||||
| Curative-intent | 8 (9) | 8 (11) | 0 (0) | X2, df(2), p = 0.002a |
| Palliative-intent | 14 (15) | 14 (20) | 0 (0) | |
| None | 70 (76) | 50 (69) | 20 (100) | |
| Metastatic disease presentation | X2, df(1), p = 0.06 | |||
| Relapsed early stage | 16 (17) | 15 (21) | 1 (5) | |
| De novo stage IV | 66 (72) | 50 (69) | 17 (80) | |
| Advanced disease | 10 (11) | 7 (10) | 3 (15) | |
| Brain metastases development | X2, df(4), p = 0.01a | |||
| None (to date) | 44 (48) | 30 (42) | 14 (70) | |
| At baseline | 19 (21) | 14 (19) | 5 (25) | |
| During initial ALK TKI | 16 (17) | 16 (22) | 0 (0) | |
| After initial ALK TKI | 11 (12) | 10 (14) | 1 (5) | |
| Unknown | 2 (2) | 2 (3) | 0 (0) | |
| Baseline brain metastases management (before initiation of initial ALK TKI) | (N = 19) | (n = 14) | (n = 5) | X2, df(3), p = 0.004a |
| No brain metastases management | 7 (37) | 2 (14) | 5 (100) | |
| Whole-brain radiotherapy | 9 (47) | 9 (64) | 0 (0) | |
| Stereotactic radiosurgery | 1 (5) | 1 (7) | 0 (0) | |
| Resection of brain lesions | 1 (5) | 1 (7) | 0 (0) | |
| Multimodal treatment (resection and radiotherapy) | 1 (5) | 1 (7) | 0 (0) | |
ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; PS, performance status; TKI, tyrosine kinase inhibitor.
Significant result.
The data are less mature for the alectinib-treated cohort, as evidenced by a shift over time from the predominant use of crizotinib (the initial ALK TKI prescribed to 97% of the cohort before 2018) to alectinib (the initial ALK TKI prescribed to 73% of the cohort after 2018). Nevertheless, some differences in clinical characteristics were noted on the basis of the initial ALK TKI treatment. Crizotinib-treated patients were more often given palliative-intent cytotoxic chemotherapy before initial ALK TKI treatment (20% versus 0%, p = 0.002), and, compared with alectinib, exhibited a higher overall rate of brain metastases development up to the time of analysis (70% versus 42%, p = 0.01). Furthermore, crizotinib-treated patients with brain metastases at baseline were more likely to receive non-systemic means of brain lesion control (stereotactic radiosurgery or whole-brain radiation) than alectinib-treated patients with baseline brain metastases (63% versus 0%, p = 0.004).
Although crizotinib- and alectinib-treated patients exhibited similar response to treatment, crizotinib-treated patients experienced higher rates of treatment changes (26% versus 0%, p < 0.001) owing to treatment breaks (19% of crizotinib-treated cohort) with or without dose modifications (15% of crizotinib-treated cohort). Increased rates of treatment changes were likely owing to a higher likelihood of AEs with crizotinib therapy, compared with alectinib-treated patients, particularly those affecting vision (31% versus 0%, p < 0.001), the gastrointestinal system (38% versus 15%, p = 0.04), or AEs resulting in abnormal laboratory values (29% versus 5%, p = 0.01). Alectinib-treated patients were more likely to experience skin-related AEs (20% versus 3%, p = 0.01). Overall, for the entire cohort, AEs accounted for 16% of all reasons for ALK TKI termination.
Initial ALK TKI had been discontinued in 76% of the cohort at the time of analysis (11% of alectinib-treated, 92% of crizotinib-treated); of these patients who had completed initial ALK TKI, 81% were given additional systemic and non-systemic modalities to control their NSCLC: 77% underwent subsequent therapy with next-generation ALK TKI and 19% underwent radiotherapy, primarily stereotactic radiotherapy to the brain, before any additional systemic therapy. Initiation of systemic therapies post-initial ALK TKI was associated with significant gains in survival, with the general trend of increasing survival in association with increasing subsequent lines of systemic therapy, although as a contemporary cohort these data are not yet mature.
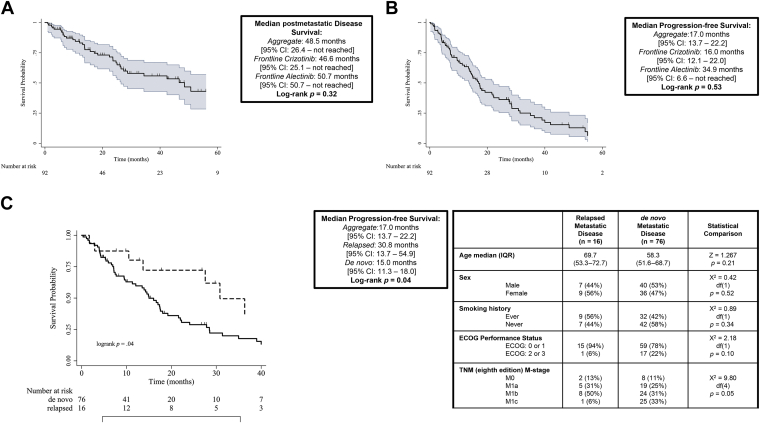
The mOS of the entire cohort was 48.5 months, and the mPFS was 17.0 months at the time of analysis; neither mOS nor mPFS differed significantly between the two different initial ALK TKIs (Fig. 1A and B), although it should be noted that the alectinib-treated cohort has less mature data. The mPFS was found to significantly vary by initial disease presentation; patients receiving ALK TKI for relapsed early stage disease (recurrence with metastatic disease after curative-intent surgical resection [stage I, II, or III]) had significantly longer time to progression than did ALK TKI-treated patients presenting with de novo advanced NSCLC (mPFS: 30.8 versus 15.0 mo; p = 0.04). These differences in mPFS were observed in the absence of significant demographic or clinical characteristics between relapsed and de novo patients (Fig. 1C).
Figure 1.
Survival outcomes for ALK-rearranged cohort. (A) Median postmetastatic disease survival. (B) Median progression-free survival. (C) Median progression-free survival relapsed versus de novo. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range.
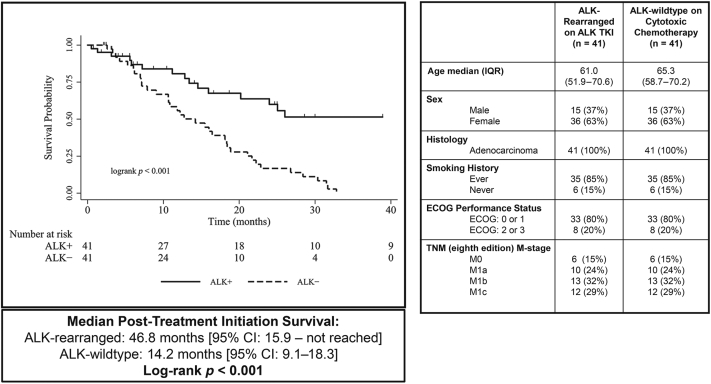
A case-matching protocol identified a cohort of 41 matched cases, on which Kaplan-Meier analysis was performed to determine post-treatment initiation survival, measured as the time (in mo) from the start of initial ALK TKI therapy (ALK-rearranged group) or the start date of cytotoxic chemotherapy (ALK-wildtype group). Patients with ALK-mutant NSCLC who received initial ALK TKI had significantly longer mOS from the time of first-line systemic therapy initiation than those with ALK-wildtype NSCLC initially treated with cytotoxic chemotherapy (46.8 versus 14.2 mo, log-rank p < 0.001) (Fig. 2).
Figure 2.
Case-matched cohort survival analysis and demographic features. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; TKI, tyrosine kinase inhibitor.
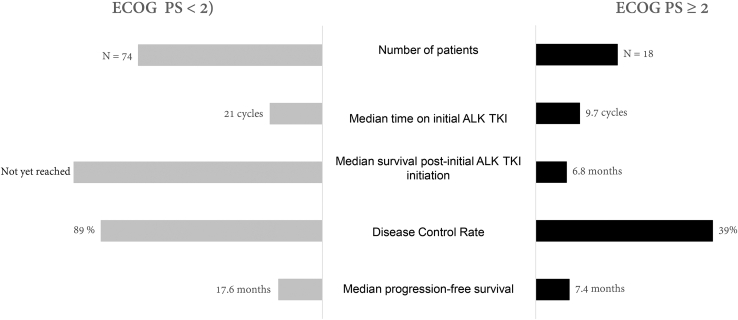
ECOG performance status (PS) at the time of initial ALK TKI initiation, representing the treating oncologist’s assessment of the patient’s functional status in relation to their NSCLC diagnosis or any other significant concurrent comorbidities, was associated with significant influence on ALK TKI treatment outcomes. A total of 20% of the cohort presenting with ECOG PS greater than or equal to 2 had less time on an initial ALK TKI treatment (21 versus 9.7 cycles, p < 0.001) and had a lower disease control rate (DCR), when compared with those with ECOG PS less than 2 (DCR: 39% versus 89%, p < 0.001). In addition, an ECOG PS greater than or equal to 2 was associated with reduced mPFS (7.4 versus 17.6 mo, log-rank p = 0.02) and median post-ALK TKI initiation survival survival (6.8 versus not yet reached, log-rank p < 0.001) (Fig. 3).
Figure 3.
Impact of ECOG PS on treatment outcome. ECOG, Eastern Cooperative Oncology Group; PS, performance status; TKI, tyrosine kinase inhibitor.
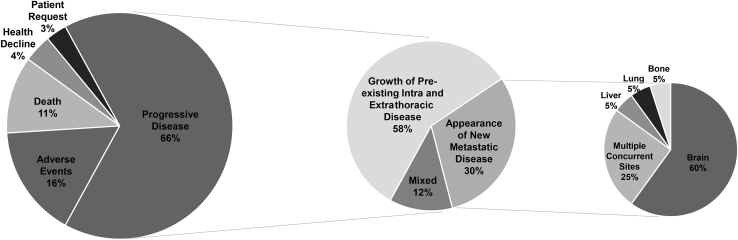
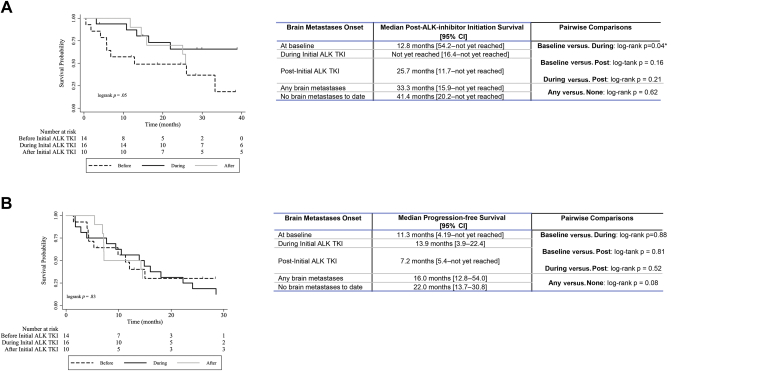
The most common reason for discontinuation of initial ALK TKI therapy at the time of analysis was progressive disease. A total of 58% of the patients with progressive disease were noted to have advancement of their preexisting lesions, with a further 30% exhibiting new metastatic disease, with brain metastasis the most common site of metastatic spread (Fig. 4). It should be noted that with no brain metastasis development during initial ALK TKI treatment with alectinib at the time of analysis, less mature data within the alectinib-treated cohort, and a lower rate of alectinib discontinuation at the time of analysis, investigation into sites of progressive disease and brain metastases was restricted to the crizotinib-treated cohort. Within the crizotinib-treated cohort, 50% of the patients had brain metastases at the time of analysis: 21% before, 17% developing during, and 12% developing post-initial ALK TKI. For the 36% of the crizotinib-treated cohort developing brain metastases during or after initial ALK TKI, median time to brain metastases onset was 16.4 months. Neither presence nor absence of brain metastases significantly affected median post-ALK TKI initiation survival or mPFS in the crizotinib-treated cohort. Improved median post-ALK TKI initiation survival was observed in patients developing brain metastases during initial treatment with crizotinib, compared with those with brain metastases at baseline (Fig. 5A and B).
Figure 4.
ALK TKI termination reasons and sites of failure. TKI, tyrosine kinase inhibitor.
Figure 5.
Impact of brain metastases on outcome. (A) Median survival after ALK TKI initiation. (B) Median progression-free survival. CI, confidence interval; TKI, tyrosine kinase inhibitor.
Discussion
This study explored the real-world clinical experience and outcomes of patients with ALK-rearranged advanced NSCLC at the time of their first treatment exposure to ALK TKI therapy in the Canadian province of Alberta. Our analysis of a cohort of 92 patients was able to capture the broad heterogeneity inherent in real-world clinical populations, representing patients with a range of disease presentations, functional status, treatment histories, socioeconomic status, and treatments with different generations of ALK TKI according to standard-of-care recommendations at the time of diagnosis. The cohort described in this study was comparable to those of previous studies which identified patients with ALK-rearranged NSCLC as predominantly never-smokers, had good PS, predisposed to brain metastases development, and younger than those patients with ALK-rearrangement negative NSCLC.10
Notably, this review reveals mOS of 48.4 months (41.4 mo median post-ALK TKI initiation survival) and mPFS of 17.0 months within this real-world cohort. This observation exceeds the range of overall outcome estimates found in published real-world retrospective and foundational clinical trial studies and is comparable with those outcome estimates within contemporary clinical trial publications to date1,10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 (Supplementary Data 2: outcome estimates for ALK TKI use in real-world and clinical trial ALK-rearranged populations). The combined cohort reinforces previously observed distinctions between the outcomes of patients with NSCLC with relapsed versus de novo metastatic disease. These findings suggest that differences in overall disease burden at diagnosis in relapsed versus de novo metastatic patients may affect treatment efficacy.22 Compared with the crizotinib treatment cohort in the ALEX clinical trial, the crizotinib cohort in this study had a lower 1-year survival rate (71% versus 83%). This is likely reflective of the fact that 20% of our real-world clinical patients were unable to access crizotinib without first having cytotoxic chemotherapy, now known to be substandard in ALK-rearranged populations. In both the ALEX trial and in this study, alectinib was offered to treatment-naive patients and yielded similar 1-year survival rates (84% versus 86%, respectively).23
Further supporting the superior outcomes of use of ALK TKI in ALK-rearranged populations, and a strength of this current study, was the ability to quantify the dramatic improvement in prognosis derived from the appropriate inhibition of ALK-positive NSCLC, by means of the use of a one-to-one case-match analysis, matched on several potentially confounding factors. Similar to the outcomes observed among the ALK-wildtype matched cohort, in biomarker agnostic populations, use of cytotoxic platinum-doublet chemotherapy in advanced NSCLC yields mOS times less than 12 months, in the era before immunotherapy use.24 In addition, retrospective analysis on randomized clinical trials has verified that survival outcomes do not differ significantly between ALK-mutant and ALK-wildtype patients when both were treated with non-ALK TKI therapeutic regimens.25 This suggests that ALK rearrangement in itself is not a significant prognostic factor in advanced NSCLC, in which patients with ALK-wildtype NSCLC can serve as a prognostic proxy for patients with ALK-mutant NSCLC if denied ALK TKI therapy. Taken with the results of this matched cohort study, this reinforces the conclusion that the improved prognosis comes only in the context of both ALK rearrangement and ALK-inhibiting therapy.
First-time exposure to ALK TKI was observed to produce both a rapid and durable objective response rate (ORR) and DCR without significant differences between alectinib and crizotinib. The aggregate ORR (48%) within this study is lower than the greater than 60% ORR observed in recent phase 3 trials investigating crizotinib and/or alectinib,23,26 and among other real-world cohorts.10,12,13,21 However, the observed ORR does align with additional real-world cohorts that were comprised all-comers meeting the study criteria,11 or phase 2 clinical trials using multicenter and previously treated populations.16 More consistent with both clinical trial and other real-world populations was the DCR (76%) within this study, suggesting a higher proportion of patients were assessed with stable disease as their best response rather than a “positive” response of partial/complete response. Critically, this study identified a proportion of patients (21%) which did not respond to ALK TKI, primarily by virtue of death or ALK TKI discontinuation before response assessment. The remaining nonresponders (∼10%) exhibited refractory disease (progressive disease as best response) to initial exposure to ALK TKI, suggesting the potential of undetected preexisting resistance mechanisms in addition to the detected ALK fusion, such as KIT, IGF1R, HER3, or MET amplification. These concomitant mutations result in ALK-inhibitor resistance in ALK TKI-naive patients, occurring in the absence of ALK TKI-mediated selection of secondary resistance mutations, and pose a significant challenge in treating patients with complex tumor mutational profiles.27,28
Significantly, among patients in this study with ECOG PS greater than or equal to 2, we observed a reduction in post-treatment initiation and progression-free survival, alongside lower DCRs, when compared with patients with ECOG PS less than 2, as has previously been observed within other oncogene-driven NSCLC populations on targeted therapy.11,29,30 This is congruent with previous findings that PS is a key predictor of outcome in advanced cancer in which, regardless of tumor molecular characteristics, patients with poor PS experience inferior outcomes to those with better ECOG PS.29,31 However, given that ECOG greater than or equal to 2 generally precludes cytotoxic chemotherapy, the observed DCR of 39% and safe ALK TKI tolerability, as noted by a median of 9.7 cycle duration of initial ALK TKI treatment found among patients with ECOG PS greater than or equal to 2 in this cohort, reinforces the benefit of ALK TKI use in patients with poor PS (albeit with lower efficacy and tolerability).32, 33, 34 Indeed, availability of well-tolerated–targeted therapies for advanced NSCLC remains a key factor in improving the extremely poor prognosis for those ineligible to receive cytotoxic chemotherapy.35
As orally available small molecule inhibitors of ALK, both crizotinib and alectinib were found to have ease of use and efficacy but did present with notably different side effect profiles. This study found higher rates of AEs in crizotinib-treated patients and a previously recognized propensity toward gastrointestinal, visual, and laboratory value abnormalities, compared with alectinib.20,21,36 Supporting the impression that both crizotinib and alectinib are tolerable agents with manageable treatment-associated AEs,35 this study revealed that treatment modification and/or treatment breaks effectively mitigated most documented AEs. Paradoxically, this study found lower rates of serious AEs (11% compared with a median of 26% and 38% in a meta-analysis of clinical trials using alectinib and crizotinib, respectively),35 yet a much higher rate of AE-mediated treatment discontinuation (16%) than previously published real-world and clinical trial studies (range: 3.2%–7.8%).1,7,37 Discontinuation owing to AE was often because of intolerability (patient perspective) rather than toxicity (medical perspective). Furthermore, the onset of availability of the better-tolerated and effective alectinib may have encouraged the termination of crizotinib in the context of drug intolerability.
The central nervous system (CNS) is a common location of both baseline metastatic disease and site of subsequent metastatic spread for patients with ALK-rearranged NSCLC. The brain is the most common site of disease progression when initial ALK TKI was discontinued.
Median time-to-onset of brain metastases was 16.4 months after initiation of initial ALK TKI, a longer interval than that found in previous real-world cohorts (range: 7–11 mo).15,38 Interestingly, this study revealed that neither the presence/absence of brain metastasis had significant impact on either mPFS or median post-ALK TKI initiation survival, although patients with baseline brain metastasis had decreased survival times compared with those developing brain metastases during initial ALK TKI therapy. Patients with baseline brain metastasis often underwent whole-brain radiotherapy before initial exposure to crizotinib, which theoretically may allow for a period of control over brain-based disease in parallel with an interval of crizotinib-mediated systemic control. Despite the potential for baseline brain metastasis to negatively affect prognosis, the observed mOS among ALK TKI–treated patients with baseline brain metastases well exceeds that known for patients with brain metastasis treated solely with stereotactic radiosurgery, whole-brain radiotherapy,39 or conventional cytotoxic chemotherapy.40 These findings add to a body of literature suggesting that, despite poor blood-brain barrier penetration, crizotinib may have some efficacy in patients with brain metastases.41 This efficacy is likely limited as a long-term systemic treatment modality owing to its inferior CNS control in relation to the availability of next-generation ALK inhibitors with proven CNS efficacy (i.e., alectinib).42 Nevertheless, crizotinib may contribute to the extended mOS observed in contemporary ALK-mutant cohorts (such as the mOS of 57.4 mo in the ALEX trial),19 particularly in the context of patients without baseline CNS involvement by providing an interval of systemic control. Of particular note, the use of crizotinib, even with acknowledged low CNS penetration and control, does seem to benefit patients with ALK-rearranged NSCLC with brain metastases at baseline, allowing them to achieve similar PFS to those developing brain metastases on initial ALK TKI therapy or those whose brain metastases are not detected until after the conclusion of the initial ALK TKI.
As evidenced in the frequency of CNS involvement during initial ALK TKI therapy, progressive disease, owing to the inevitable development of ALK TKI resistance,43 was the singular most common reason for discontinuation of the initial ALK TKI. Subsequently, this study cohort reveals that most patients (77%) terminating initial ALK TKI go on to subsequent lines of systemic therapy, with a small proportion opting for radiotherapy (primarily for the control of brain metastasis) before commencing second-line systemic options. All patients accessing subsequent lines of systemic therapy used one or more additional ALK TKI over the course of their treatment. The observed rate of radiotherapy treatment (19%) immediately following initial use of ALK TKI in this study’s cohort was lower than that found within other real-world cohorts,44 as was the use of cytotoxic chemotherapy in subsequent treatment lines,37 with less than one-tenth of patients accessing cytotoxic chemotherapy post-initial ALK TKI. Likely, the availability of next-generation ALK inhibitors with better CNS penetration may be displacing the need for radiotherapy (particularly in metastatic disease in the brain) and decreasing the reliance on cytotoxic chemotherapy in subsequent lines of treatment.
Predominant use of second- and third-generation ALK TKI through successive lines of systemic therapy suggests that continuation of treatment with targeted ALK inhibitors, which may be used continually until resistance or toxicity develops, contributes to both the high mOS observed in this cohort and serves to decrease the number of treatment lines required to produce such results. The extended mOS found in contemporary ALK-mutant cohorts, and observed within this study cohort, are a result of the incremental additions to disease control offered by the availability of a spectrum of systemic treatment options, including multiple generations of ALK TKI therapy and pemetrexed-based cytotoxic chemotherapy.45 Unfortunately, immune checkpoint inhibition seems to lack effectiveness in ALK-rearranged NSCLC.46
This study should be viewed within the context of the inherent limitations of retrospective studies and relative immaturity of data for the alectinib-treated cohort. Despite these acknowledged limitations, this study does exhibit some particular and unique strengths: specifically the ability to provide population-level investigation into treatment and outcomes of patients with ALK-mutant disease on initial ALK TKI derived from a regional population of 4.3 million, accessing single-payer, universal health care lending equality in terms of treatment and care. Furthermore, on the basis of Alberta’s population size, incidence of ALK rearrangements, proportion of patients with advanced disease, and systemic therapy uptake rates,1,35,47, 48, 49 the cohort size of this study well reflects expected incidence and is an indication that use of centralized pharmacy-dispensing records within a publicly funded health care system has comprehensively identified the population of interest for this study.
In summary, to the best of our knowledge, this represents the first single-payer, population-based review of North American patients with ALK-rearranged NSCLC on initial ALK TKI. Although clinical trials have firmly established that ALK TKI are safe, well-tolerated, and effective, these findings reveal that their impact in a real-world setting is just as profound. Case matching using patients with ALK-wildtype NSCLC receiving cytotoxic chemotherapy supports the conclusion that the improved prognosis comes only in the context of both ALK-rearrangement and ALK-inhibiting therapy. The availability and use of ALK TKI therapies contribute to the impressive gains in survival experienced by contemporary patients with ALK-rearranged disease, rendering patients with this driver mutation form of NSCLC among the longest surviving with lung cancer. The relative contribution of ALK TKI adoption into clinical practice to the overall improvement in outcomes of patients with NSCLC is a subject of ongoing study at our institutions.
Acknowledgments
Drs. Gibson, Dean, Elegbede, and Bebb declare research support for this study in the form of a Pfizer Global Medical grant [identification document #57104197] awarded to Dr. Bebb. The authors thank Pfizer for their financial support of this project, and specifically Dr. Martin Rupp and Dr. Andrew Ficzycz for useful discussions, and Dr. Robin Wiltshire for his review of this manuscript. We also thank supporters of the Glans-Look Lung Cancer Research database for their philanthropy.
Footnotes
Disclosure: Dr. Bebb received payment from Pfizer, Boehringer Ingelheim, AstraZeneca, Roche/Genentech, Bristol-Myers Squibb, Merck, Novartis, AbbVie, Takeda, and Lilly for participation in advisory boards. Dr. Box has received nonfinancial support from Amgen BioSciences and Novartis outside of the submitted work and honoraria for lectures from AstraZeneca. Dr. Sangha has received personal fees from Pfizer, Boehringer Ingelheim, AstraZeneca, Roche/Genentech, Bristol-Myers Squibb, Merck, Novartis, AbbVie, Takeda, and Teva Pharmaceuticals outside of the submitted work. Dr. Hao declares no conflict of interest.
Cite this article as: Gibson AJW, Box A, Dean ML, et al. Retrospective real-world outcomes for patients with ALK-rearranged lung cancer receiving ALK receptor tyrosine kinase inhibitors. JTO Clin Res Rep. 2021;2:100157.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100157.
Supplementary Data
References
- 1.Reynolds C., Masters E.T., Black-Shinn J. Real-world use and outcomes of ALK-positive crizotinib treated metastatic NSCLC in US community oncology practice: a retrospective observational study. J Clin Med. 2018;7:129. doi: 10.3390/jcm7060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xing P., Wang S., Hao X., Zhang T., Li J. Clinical data from the real world: efficacy of crizotinib in Chinese patients with advanced ALK-rearranged non-small cell lung cancer and brain metastases. Oncotarget. 2016;7:84666–84674. doi: 10.18632/oncotarget.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris P.A., Taylor R., Minor B.L. The REDCap consortium: building an international community of software partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindeman N.I., Cagle P.T., Beasley M.B. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137:828–860. doi: 10.5858/arpa.2012-0720-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindeman N.I., Cagle P.T., Aisner D.L. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018;142:321–346. doi: 10.5858/arpa.2017-0388-CP. [DOI] [PubMed] [Google Scholar]
- 7.Melosky B., Cheema P., Agulnik J. Canadian perspectives: update on inhibition of ALK-positive tumours in advanced non-small-cell lung cancer. Curr Oncol. 2018;25:317–328. doi: 10.3747/co.25.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cision ALECENSARO® (alectinib) approved by Health Canada for first-line treatment of ALK-positive lung cancer. https://www.newswire.ca/news-releases/alecensaro-alectinib-approved-by-health-canada-for-first-line-treatment-of-alk-positive-lung-cancer-685354061.html#:∼:text=MISSISSAUGA%2C%20ON%2C%20June%2013%2C,therapy)%20or%20metastatic%20non%2Dsmall
- 9.StataCorp . StataCorp LP; College Station, TX: 2011. Stata Statistical Software: Release 12. [Google Scholar]
- 10.Melosky B., Agulnik J., Albadine R. Canadian consensus: inhibition of ALK-positive tumours in advanced non-small-cell lung cancer. Curr Oncol. 2016;23:196–200. doi: 10.3747/co.23.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noronha V., Ramaswamy A., Patil V.M. ALK positive lung cancer: clinical profile, practice and outcomes in a developing country. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis K.L., Lenz C., Houghton K., Kaye J.A. Clinical outcomes of crizotinib in real-world practice settings for patients with advanced ALK-positive non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;98:238–239. [Google Scholar]
- 13.Davis K.L., Kaye J.A., Masters E.T., Iyer S. Real-world outcomes in patients with ALK-positive non-small cell lung cancer treated with crizotinib. Curr Oncol. 2018;25:e40–e49. doi: 10.3747/co.25.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J., Zheng J., Zhang X. Crizotinib in patients with anaplastic lymphoma kinase-positive advanced non-small cell lung cancer versus chemotherapy as a first-line treatment. BMC Cancer. 2018;10:18. doi: 10.1186/s12885-017-3720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Valle M.F.F., Chang A.Y. Real world experience on treatment, outcome and toxicity if crizotinib in patients with anaplastic lymphoma kinase positive advanced non-small cell lung cancer. J Thorac Dis. 2019;11:3864–3873. doi: 10.21037/jtd.2019.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackhall F., Camidge D.M., Shaw A.T. Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open. 2017;2 doi: 10.1136/esmoopen-2017-000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon B.J., Kim D.W., Wu Y.L. Final overall survival analysis from a story comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J Clin Oncol. 2018;36:2251–2258. doi: 10.1200/JCO.2017.77.4794. [DOI] [PubMed] [Google Scholar]
- 18.Davies J., Martinec M., Delmar P. Comparative effectiveness from a single-arm trial and real-world data: alectinib versus ceritinib. J Comp Eff Res. 2018;7:855–865. doi: 10.2217/cer-2018-0032. [DOI] [PubMed] [Google Scholar]
- 19.Peters S., Mok T.S.K., Gadgeel S.M. Updated overall survival (OS) and safety data from the randomized, phase III ALEX study of alectinib (ALC) versus crizotinib (CRZ) in untreated advanced ALK+ NSCLC. J Clin Oncol. 2020;38(suppl 15) 9518–9518. [Google Scholar]
- 20.Hida T., Nokihara H., Kondo M. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open label, randomized phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhou C., Kim S.W., Reungwetwattana T. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomized phase 3 study. Lancet Respir Med. 2019;7:437–466. doi: 10.1016/S2213-2600(19)30053-0. [DOI] [PubMed] [Google Scholar]
- 22.Gibson A.J.W., Li H., D’Silva A. Comparison of clinical characteristics and outcomes in relapsed versus de novo metastatic non-small cell lung cancer. Am J Clin Oncol. 2019;42:75–81. doi: 10.1097/COC.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 23.Peters S., Camidge D.R., Shaw A.T. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 24.Schad F., Thronicke A., Steele M.L. Overall survival of stage IV non-small cell lung cancer patients treated with Viscum album L. in addition to chemotherapy, a real-world observational multicenter analysis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw A.T., Yeap B.Y., Solomon B.J. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring alk gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon B.J., Mok T., Kim D.W. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 27.Isozaki H., Ichihara E., Takigawa N. Non-small cell lung cancer cells acquire resistance to the ALK inhibitor alectinib by activating alternate receptor tyrosine kinases. Cancer Res. 2016;76:1506–1516. doi: 10.1158/0008-5472.CAN-15-1010. [DOI] [PubMed] [Google Scholar]
- 28.Katayama R., Shaw A.T., Khan T.M. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malpelle U., Rossi A., Bria E. Relationship between performance status or younger age and osimertinib therapy in T790M-positive NSCLC: are the available data convincing? J Thorac Dis. 2019;11(suppl 15):S1837–S1840. doi: 10.21037/jtd.2019.08.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue A., Kobayashi K., Usui K. First-line gefitinib for patients with advanced non-small cell lung cancer harboring epidermal growth factor mutations without indication for chemotherapy. J Clin Oncol. 2009;27:1394–1400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 31.Kelly C.M., Shahrokni A. Moving beyond Karnofsky and ECOG performance assessments with new technologies. J Oncol. 2016;2016:6186543. doi: 10.1155/2016/6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmichael J.A., Wing-San Mak D., O’Brien M. A review of recent advances in the treatment of elderly and poor performance NSCLC. Cancers (Basel) 2018;10:236. doi: 10.3390/cancers10070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamai K., Nagata K., Otsuka K. Crizotinib administered via nasogastric and percutaneous endoscopic gastrostomy tubes for the successful treatment of ALK-rearranged lung cancer in a patient with poor performance status. Respir Investig. 2013;51:46–48. doi: 10.1016/j.resinv.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Ahn H.Y., Jeon K., Yoo H. Successful treatment with crizotinib in mechanically ventilated patients with ALK positive non-small cell lung cancer. J Thorac Oncol. 2013;8:250–253. doi: 10.1097/JTO.0b013e3182746772. [DOI] [PubMed] [Google Scholar]
- 35.Ko J.J., Tudor R., Li H. Reasons for lack of referral to medical oncology for systemic therapy in stage IV non-small-cell lung cancer: comparison of 2003–2006 with 2010–2011. Curr Oncol. 2017;24:e486–e493. doi: 10.3747/co.24.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou H., Sun D., Liu K. Safety and serious adverse events of approved ALK inhibitors in malignancies: a meta-analysis. Cancer Manag Res. 2019;11:4109–4118. doi: 10.2147/CMAR.S190098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies J., Martinec M., Coudert M., Delmar P., Crane G. Real-world anaplastic lymphoma kinase (ALK) rearrangement testing patterns, treatment sequences and survival of ALK inhibitor-treated patients. Curr Med Res Opin. 2019;35:535–542. doi: 10.1080/03007995.2018.1533458. [DOI] [PubMed] [Google Scholar]
- 38.Costa D.B., Shaw A.T., Ou S.-H.I. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenspoon J.N., Ellis P.M., Pond G., Caetano S., Broomfield J., Swaminath A. Comparative survival in patients with brain metastases from non-small cell lung cancer treated before and after implantation of radiosurgery. Curr Oncol. 2017;24:e146–e151. doi: 10.3747/co.24.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmerman S., Dziadziuszko R., Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014;40:716–722. doi: 10.1016/j.ctrv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Petrelli F., Lazzari C., Ardito R. Efficacy of ALK inhibitors on NSCLC brain metastasis: a systematic review and pooled analysis of 21 studies. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gadgeel S., Peters S., Mok T. Alectinib versus crizotinib in treatment-naïve anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29:2214–2222. doi: 10.1093/annonc/mdy405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dagogo-Jack I., Yoda S., Lennerz J.K. MET alternations are a recurring and actionable resistance mechanism in ALK-positive lung cancer. Clin Cancer Res. 2020;26:2535–2545. doi: 10.1158/1078-0432.CCR-19-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kayaniyil S., Hurry M., Wilson J. Treatment patterns and survival in patients with ALK-positive non-small-cell lung cancer: a Canadian retrospective study. Curr Oncol. 2016;23:e589–e597. doi: 10.3747/co.23.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zugazagoitia J., Molina-Pinelo S., Lopez-Rios F., Paz-Ares L. Biological therapies in nonsmall cell lung cancer. Eur Respir J. 2017;49:1601520. doi: 10.1183/13993003.01520-2016. [DOI] [PubMed] [Google Scholar]
- 46.Mazieres J., Drilon A., Lusque A. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver mutations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Statistics Canada Public data: population. https://www.google.com/publicdata/explore?ds=z8mqirbqgu9tsm_&met_y=population&idim=territory:CA01:CA02:CA11&hl=en&dl=en
- 48.Healthier Together, Alberta Health Services Lung cancer. https://www.healthiertogether.ca/health-conditions/cancer/lung-cancer/
- 49.Lung Cancer Canada Staging. https://www.lungcancercanada.ca/Lung-Cancer/Staging.aspx
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.