Abstract
Introduction
We compared NSCLC treatment and survival within and outside a multidisciplinary model of care from a large community health care system.
Methods
We implemented a rigorously benchmarked “enhanced” Multidisciplinary Thoracic Oncology Conference (eMTOC) and used Tumor Registry data (2011–2017) to evaluate guideline-concordant care. Because eMTOC was located in metropolitan Memphis, we separated non-MTOC patient by metropolitan and regional location. We categorized National Comprehensive Cancer Network guideline-concordant treatment as “preferred,” or “appropriate” (allowable under certain circumstances). We compared demographic and clinical characteristics across cohorts using chi-square tests and survival using Cox regression, adjusted for multiple testing. We also performed propensity-matched and adjusted survival analyses.
Results
Of 6259 patients, 14% were in eMTOC, 55% metropolitan non-MTOC, and 31% regional non-MTOC cohorts. eMTOC had the highest rates of African Americans (34% versus 28% versus 22%), stages I to IIIB (63 versus 40 versus 50), urban residents (81 versus 78 versus 20), stage-preferred treatment (66 versus 57 versus 48), guideline-concordant treatment (78 versus 70 versus 63), and lowest percentage of nontreatment (6 versus 21 versus 28); all p values were less than 0.001. Compared with eMTOC, hazard for death was higher in metropolitan (1.5, 95% confidence interval: 1.4–1.7) and regional (1.7, 1.5–1.9) non-MTOC; hazards were higher in regional non-MTOC versus metropolitan (1.1, 1.0–1.2); all p values were less than 0.05 after adjustment. Results were generally similar after propensity analysis with and without adjusting for guideline-concordant treatment.
Conclusions
Multidisciplinary NSCLC care planning was associated with significantly higher rates of guideline-concordant care and survival, providing evidence for rigorous implementation of this model of care.
Keywords: Multidisciplinary care, Multidisciplinary Thoracic Oncology Conference, Outcomes, Quality of care, Guideline-concordant treatment, Survival
Introduction
With a widening array of diagnostic, staging, and treatment options, controlled by clinicians from different specialties, the complexity of lung cancer care suggests a need for structured multidisciplinary interaction.1 Nevertheless, the multidisciplinary care model is infrequently practiced in the United States, especially in community health care systems where up to 85% of patients with lung cancer receive care.2 Its usefulness has been questioned in countries where it has been implemented by regulatory fiat.3 Given that multidisciplinary care delivery disrupts conventional referral processes, requires manpower and infrastructure investments, and demands clinical specialists, the paucity of supportive data is a major barrier to widespread adoption.4
wIn the United States, “multidisciplinary care” is poorly defined and lacks implementation know-how and high-quality evidence to quantify its presumed benefits.2,5 The published evidence of benefit is mixed, mostly limited to intermediary outcomes, such as more timely care, improved staging, increased surgery referrals, and clinical trial enrollment.5, 6, 7, 8, 9 There is scant, contradictory evidence of survival benefit.10, 11, 12, 13 The combination of implementation barriers and a dearth of evidence accounts for shallow penetration, despite strong advocacy for the model.14,15 Evidence from community-level care environments might stimulate wider dissemination in the United States and other countries.
We implemented a rigorously benchmarked multidisciplinary thoracic oncology program within a large community-based health care system using team- and implementation-science principles16 and evaluated its impact by comparing the processes and outcomes of NSCLC care within the greater health care system.
Materials and Methods
The Baptist Memorial Healthcare Corporation (BMHCC) is a community-based health care system covering 107 counties in Mississippi, Arkansas, Tennessee, Kentucky, Alabama, and Missouri, states with some of the highest per-capita U.S. lung cancer incidence and mortality.17 This service area includes approximately 40% of persistent poverty “Delta Regional Authority” counties, congressionally identified as the most socioeconomically challenged region in the United States. In 2011, we started a weekly Multidisciplinary Thoracic Oncology Conference (MTOC) involving all key lung cancer specialists—thoracic surgeons, medical and radiation oncologists, pulmonologists, pathologists, radiologists, nurse navigators, and a palliative care nurse; in 2012, we started a colocated weekly clinic involving thoracic surgeons, medical and radiation oncologists, pulmonologists, nurse navigators, and radiologists.16
Colocated clinic patients were discussed in the MTOC, which was “enhanced” by prospective data collection and rigorous benchmarking, on the basis of team science “coordinating mechanisms,” including closed-loop communication, shared mental models, mutual trust, and mutual performance monitoring.16,18 The key performance benchmarks were the concordance between recommendations and care delivered, timeliness of closed-loop communication with all responsible clinicians (defined as acknowledgment by the recipient of receipt of recommendations within 48 hours of the multidisciplinary clinical encounter), rates of stage confirmation, and timeliness of care delivery.18 Providers could also refer patients in for enhanced MTOC (eMTOC) review, without clinic evaluation. Patients were re-presented in the eMTOC until a consensus treatment plan was decided.
The multidisciplinary forum was used for decision-making only. All clinical care procedures were performed within the same locations as other health care system patients with NSCLC. With the permission of the Institutional Review Board of the BMHCC (institutional review board #14–15), including a waiver of the informed consent requirement for this low-risk, institutional quality improvement program, we constructed a prospective database of all patients seen in the clinic and discussed in the eMTOC.
To compare the care and outcomes of patients with NSCLC who received or did not receive multidisciplinary case planning, we conducted a retrospective observational study identifying NSCLC cases from the eMTOC within the institutional Tumor Registry from 2011 to 2017. We compared patient demographic, clinical, treatment characteristics, use of guideline-concordant care, and survival between eMTOC and non-MTOC cases.
The eMTOC cohort included all BMHCC Tumor Registry patients with NSCLC who were presented in the eMTOC from 2011 to 2017. The non-MTOC cohort consisted of all other tumor registry patients with NSCLC in the same time period. Because the eMTOC was based in metropolitan Memphis, we separated the non-MTOC cohort into “metropolitan” and “regional” subcohorts on the basis of the Rural-Urban Commuting Area code of the institution where each patient’s care was registered. Codes 1 to 3 are considered urban, 4 or higher, rural.19 We also identified patient-level rurality on the basis of each patient’s zip code of residence at the time of lung cancer diagnosis.20
Using National Comprehensive Cancer Network guidelines, we categorized treatment as “stage-preferred” when the primary treatment for patients with that clinical stage was administered and “stage-appropriate” to include alternative evidence-based treatment modalities which might be received under certain circumstances (Supplementary Table A.1).21 Stage was based on the seventh edition of the American Joint Committee on Cancer definitions.
Statistical Analysis
With three exclusive cohorts, eMTOC, metropolitan non-MTOC, and regional non-MTOC, we summarized demographic characteristics, clinical stage, and guideline-concordant care using appropriate summary statistics. We compared characteristics across groups with chi-square or Kruskal-Wallis tests. We used Kaplan-Meier plots and log-rank tests to compare overall and stage-stratified survival and calculated hazard ratios (HRs) with 95% confidence intervals (CIs) using Cox proportional regression. Proportional hazard assumption was inspected visually. We evaluated the unadjusted univariable association between the hazards of death and our cohorts and then further adjusted for potential confounders or effect modifiers.
Confounding (when a factor not in the causal pathway influences the primary association of interest) was identified when the crude and adjusted bivariable-level HR differed by more than 10%. Effect modification (when a variable not in the causal pathway influences the primary association of interest to varying degrees across its levels) was identified when a significant, bivariable, interaction term existed between the potential effect modifier and primary predictor (p < 0.05). The fully adjusted model included all confounders and significant effect modifiers. Variables considered for confounding and effect modification included age, sex, race, insurance, and clinical stage.
Rural patients are more likely to attend rural institutions, metropolitan patients to attend metropolitan institutions, and the eMTOC was located in a metropolitan part of the health care system. Therefore, it was questionable whether patient-level rurality (defined by the Rural-Urban Commuting Area code of the patient’s zip code of residence at the time of cancer diagnosis) is in the causal pathway or associated with our exposure groups (institution-level rurality). For this reason, we excluded patient-level rurality from the primary analysis. We adjusted for multiple comparisons among the groups using Tukey’s adjustments, or the false discovery rate where indicated.22
Additional Sensitivity Analyses
To minimize the impact of the asymmetric distribution of demographic and clinical characteristics between cohorts, we performed stabilized inverse probability weighting (SIPW) and propensity-matched analysis.23 Both approaches aim to balance baseline covariates that might bias our primary association. Propensity-matched analyses force balance between exposure groups but can greatly reduce sample size and power. Therefore, we also used the SIPW approach, weighting for age, race, sex, insurance, and clinical stage. We excluded patients with unknown clinical stage in our primary analysis.
After propensity weighting or matching, we stratified by receipt of guideline-concordant treatment and re-evaluated HRs. Because guideline-concordant care can influence survival, stratification enables comparison of patients with similar care. For additional rigor, we repeated both propensity analyses, including patient-level rurality as an adjuster.
The asymmetrical distribution of missing clinical stage raised concern for misclassification bias and the absence of performance status and comorbidity data raised the possibility of differences in the prevalence of competing causes of mortality between cohorts. To evaluate this, we made four independent assumptions to encapsulate the “best case” and “worst case” scenarios: first, we assumed that patients with a reported stage but no treatment received stage-preferred treatment (assuming they were all moribund patients for whom end-of-life care is preferable); second, we assumed that those missing a stage-appropriate indicator (owing to missing clinical stage) all received guideline-concordant care; third, we assumed that these same patients did not receive guideline-concordant care; fourth, we assumed that patients who were missing clinical stage information but received some treatment had received guideline-concordant care and those missing clinical stage who received no treatment had not received guideline-concordant care. For each of these settings, we reran our propensity analyses outlined previously. This allowed us to evaluate the impact of “missingness” and possible misclassification across the groups. We also reconstructed our Kaplan-Meier plots after eliminating those who received no treatment to control missingness.
Results
Distribution of Patient Demographic Characteristics
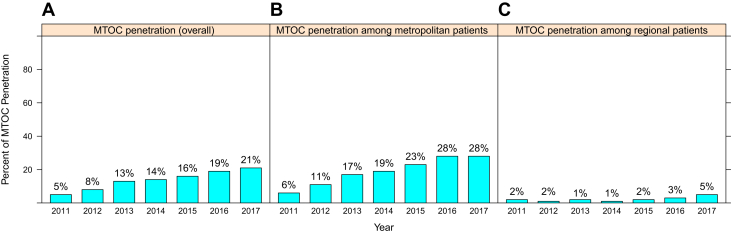
From 2011 to 2017, a total of 6259 patients with NSCLC were registered among the five hospital registries, of which 864 (14%) were discussed in eMTOC, 3464 (55%) received non-MTOC care through metropolitan hospitals, and 1931 (31%) received non-MTOC care in regional hospitals (Table 1). The eMTOC proportion of all patients in the study timespan evolved from 5% in 2011 to 21% in 2017 (Fig. 1A–C). The eMTOC proportion of all metropolitan and regional patients was 19.1% and 2.3%, respectively.
Table 1.
Characteristics of Baptist Memorial Healthcare Corporation NSCLC Tumor Registry Cohort
| Demographics | Tumor Registry Cohort | eMTOC | Metropolitana | Regionalb |
|---|---|---|---|---|
| N (%) | 6259 | 864 (14) | 3464 (55) | 1931 (31) |
| Racec | ||||
| White | 4503 (71.9) | 564 (65.3) | 2446 (70.6) | 1493 (77.3) |
| Black or African American | 1688 (27) | 291 (33.7) | 966 (27.9) | 431 (22.3) |
| Other/unknown | 68 (1.1) | 9 (1) | 52 (1.5) | 7 (0.4) |
| Sexc | ||||
| Female | 2857 (45.7) | 435 (50.3) | 1613 (46.6) | 809 (41.9) |
| Male | 3400 (54.3) | 429 (49.7) | 1849 (53.4) | 1122 (58.1) |
| Age in y, median (IQR) | 68 (61–75) | 68 (61–75) | 68 (61–75) | 69 (61–76) |
| Insurancec | ||||
| Medicare | 1444 (23.1) | 197 (22.8) | 872 (25.2) | 375 (19.4) |
| Medicaid | 953 (15.2) | 119 (13.8) | 445 (12.8) | 389 (20.1) |
| Commercial | 3157 (50.4) | 454 (52.5) | 1726 (49.8) | 977 (50.6) |
| Uninsured | 705 (11.3) | 94 (10.9) | 421 (12.2) | 190 (9.8) |
| Clinical stage groupingc,d | ||||
| Early: I/II/IIIA(T3/N1) | 1741 (27.8) | 352 (40.7) | 823 (23.8) | 566 (29.3) |
| Locally-advanced: IIIA(T4/N2)/IIIB | 1128 (18) | 189 (21.9) | 540 (15.6) | 399 (20.7) |
| Advanced: IV | 2745 (43.9) | 246 (28.5) | 1606 (46.4) | 893 (46.2) |
| Unknown | 645 (10.3) | 77 (8.9) | 495 (14.3) | 73 (3.8) |
| Patient ruralityc | ||||
| Not rural | 3760 (60.1) | 693 (80.2) | 2687 (77.6) | 380 (19.7) |
| Rural | 2499 (39.9) | 171 (19.8) | 777 (22.4) | 1551 (80.3) |
| Treatment | ||||
| Stage-preferred treatment ratec,e | 3087 (55) | 520 (66.1) | 1675 (56.4) | 892 (48) |
| Guideline-concordant treatment ratec,e,f | 3839 (68.4) | 618 (78.5) | 2051 (69.1) | 1170 (63) |
| No treatment ratec | 1376 (22.0) | 58 (6.7) | 755 (21.8) | 563 (29.2) |
eMTOC, enhanced Multidisciplinary Thoracic Oncology Conference; IQR, interquartile range.
Metropolitan: patients without care planning through the eMTOC, who received care within metropolitan Memphis.
Regional: patients without care planning through the eMTOC, who received care within institutions outside greater metropolitan Memphis.
Indicates statistically significant differences across the three cohorts (all p < 0.05).
Grouped according to guideline-concordant treatment approach-surgery for early stage, concurrent chemoradiation for locally advanced, palliative systemic therapy for advanced.
According to National Comprehensive Cancer Network guidelines.
Including alternative treatment recommendations acceptable under certain circumstances.
Figure 1.
Enhanced MTOC penetration over time: (A) within the tumor registry overall; (B) among metropolitan patients; (C) among regional patients. MTOC, Multidisciplinary Thoracic Oncology Conference.
Key demographic and clinical characteristics were unevenly distributed among the three cohorts (Table 1). Notably, eMTOC had the highest proportions of black (34% eMTOC versus 28% metropolitan versus 22% regional), female (50%, 47%, and 42%, respectively), stages I to IIIB (63%, 39%, 50%, respectively), and urban-residing patients (80%, 78%, and 20%, respectively). The non-MTOC regional cohort had the highest percentage of Medicaid insurance (14%, 13%, and 20%, respectively), and the non-MTOC metropolitan cohort had the highest proportion of Medicare insurance (23%, 25%, 19%, respectively). The frequency of commercial insurance was similar across all groups.
Treatment Patterns According to Cohort
In the whole cohort, 3087 (55%) received stage-preferred treatment and 752 patients (12%) received stage-appropriate alternative treatment modalities, for a total of 3839 (68%) who received guideline-concordant treatment. eMTOC patients most often received stage-preferred treatment (66% versus 56% versus 48%, p < 0.001) and guideline-concordant treatment (79% versus 69% versus 63%, p < 0.001). They also had the lowest proportion without treatment (7% versus 22% versus 29%, p < 0.001).
Survival
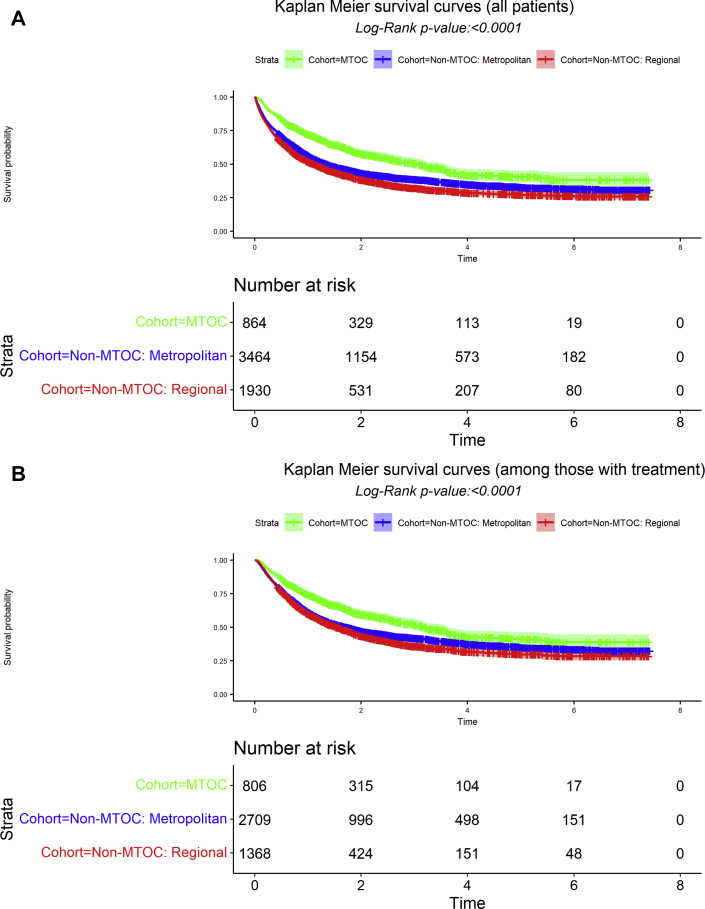
Among the 6259 patients, 60% had died as of December 31, 2017, with a median survival time of 16.8 months from the date of diagnosis. The aggregate survival probability was 57% (95% CI: 56%–58%) at one year, 38% (37%–40%) at 3 years, and 32% (31%–34%) at 5 years. The median 1-, 3-, and 5-year survival probabilities of the eMTOC, non-MTOC metropolitan, and non-MTOC regional cohorts were as follows: median 36.7 months (95% CI: 30.4–42.8) versus 16.2 months (14.6–17.8) versus 13.3 months (11.8–14.8), log-rank p value less than 0.001; 1-year survival 72% (69–75) versus 56% (55–58) versus 52% (49–54); 3-year survival 51% (47–54) versus 39% (37–40) versus 32% (30–34); 5-year 40% (36–45) versus 33% (31–35) versus 27% (25–30) (Fig. 2A).
Figure 2.
Survival of patients with NSCLC receiving care within the Baptist Memorial Healthcare Corporation from 2011 to 2017, stratified by model and location of care delivery. Multidisciplinary case planning (eMTOC) versus nonmultidisciplinary case planning before care within metropolitan Memphis hospitals (non-MTOC: metropolitan) versus nonmultidisciplinary case planning before care within regional hospitals (non-MTOC: regional). (A) Includes patients who received no treatment. (B) Excludes patients who received no treatment. eMTOC, enhanced Multidisciplinary Thoracic Oncology Conference; MTOC, Multidisciplinary Thoracic Oncology Conference.
When the cohort was further stratified by stage, there remained significant differences in overall survival among early stage patients, defined as clinical stages I to IIIA (T3N1), for whom primary surgical resection is the preferred treatment (Supplementary Fig. A.1, log-rank p < 0.001), and clinical stage IV (Supplementary Fig. A.2, p < 0.001). The difference in patients with locally advanced disease, clinical stages IIIA with T4 or N2 and IIIB, was not statistically significant (Supplementary Fig. A.3, p = 0.2098). In an analysis restricted to persons who received treatment, there remained significant differences in overall survival (Fig. 2B, log-rank p < 0.001).
In a crude Cox proportional hazards model (Table 2), eMTOC patients had significantly lower hazard of death than metropolitan non-MTOC patients (HR = 0.65, 95% CI: 0.58–0.73) and regional non-MTOC patients (0.58 [0.52–0.66]); regional patients had significantly higher hazards than metropolitan patients (1.11 [1.04–1.20]). We identified no confounders. Insurance and clinical stage were the only effect modifiers. We therefore conducted additional analysis stratifying the cohort by insurance and treatment-related stage groupings (early, locally advanced, and advanced) before evaluating the survival impact of the model and location of care delivery.
Table 2.
Cox Proportional Hazard Model With Stabilized Inverse Probability Weighting Propensity and Propensity-Matched Analyses
| Care Delivery | Hazard Ratio (95% CI) | Unadjusted p Value | Adjusted p Valuea |
|---|---|---|---|
| Crude (no adjustments) | |||
| eMTOC vs. non-MTOC: metropolitan | 0.65 (0.58–0.73) | <0.0001 | <0.0001 |
| eMTOC vs. non-MTOC: regional | 0.58 (0.52–0.66) | <0.0001 | <0.0001 |
| Non-MTOC: regional vs. metropolitan | 1.11 (1.04–1.20) | 0.0038 | 0.0106 |
| Stabilized inverse probability weightingb | |||
| eMTOC vs. non-MTOC: metropolitan | 0.83 (0.74–0.92) | 0.0005 | 0.0014 |
| eMTOC vs. non-MTOC: regional | 0.73 (0.65–0.81) | <0.0001 | <0.0001 |
| Non-MTOC: regional vs. metropolitan | 1.14 (1.06–1.23) | 0.0004 | 0.0012 |
| Propensity-matchedb | |||
| eMTOC vs. non-MTOC: metropolitan | 0.63 (0.5–0.81) | 0.0002 | 0.0007 |
| eMTOC vs. non-MTOC: regional | 0.63 (0.49–0.82) | 0.0005 | 0.0016 |
| Non-MTOC: regional vs. metropolitan | 1.00 (0.85–1.17) | 0.9700 | 0.9992 |
Note: Excluding patients with unknown stage. Non-MTOC metropolitan: patients without care planning through the eMTOC, who received care within metropolitan Memphis; non-MTOC regional: patients without care planning through the eMTOC, who received care within institutions outside greater metropolitan Memphis.
CI, confidence interval; eMTOC, enhanced Multidisciplinary Thoracic Oncology Conference; MTOC, Multidisciplinary Thoracic Oncology Conference.
Multiple comparisons adjusted using Tukey’s correction.
Propensity adjusting for age, race, sex, insurance, and clinical stage.
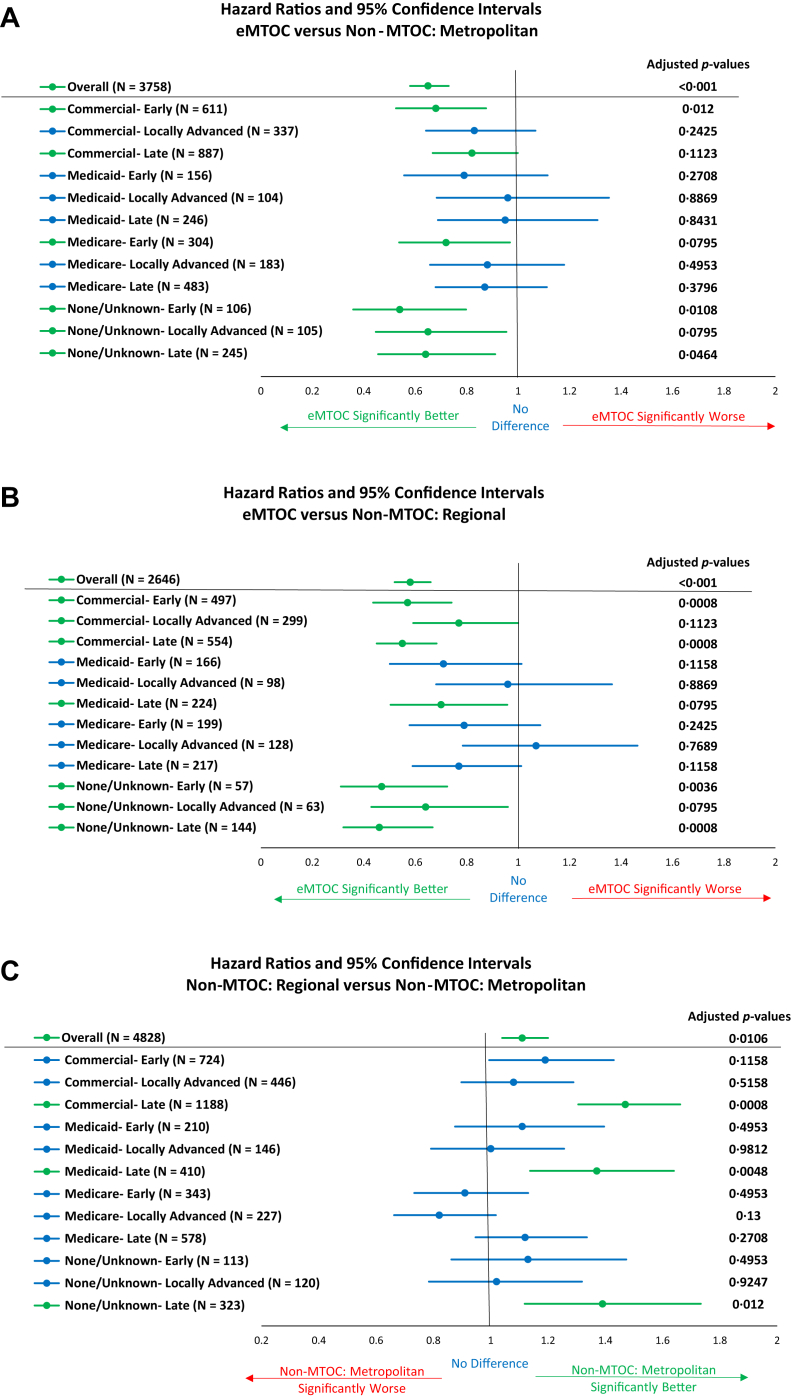
eMTOC patients had significantly lower hazards of death than metropolitan patients among early or advanced stage patients with commercial insurance, Medicare patients, and patients with no, or unknown, insurance regardless of stage grouping (Fig. 3A). Compared with regional patients, hazards were also significantly lower for commercially insured eMTOC patients with early or late stage and for those with no or unknown insurance who had late or early stage compared (Fig. 3B). There were no subsets of patients in whom non-MTOC care was associated with superior survival. Regional patients had higher hazards than late-stage metropolitan patients with commercial, Medicaid, or patients with no or unknown insurance (Fig. 3C).
Figure 3.
Forest plots illustrating hazard ratios and 95% confidence intervals of cohorts stratified by insurance and treatment-related stage groupings (early, locally advanced, and advanced). (A) Multidisciplinary case planning (eMTOC) versus nonmultidisciplinary case planning before care within metropolitan Memphis hospitals (non-MTOC: metropolitan). (B) eMTOC versus nonmultidisciplinary case planning before care within regional hospitals (non-MTOC: regional). (C) Non-MTOC: regional versus non-MTOC: Metropolitan. Because of software limitations, the adjusted p values presented use the false discovery rate for multiple comparison adjustments. eMTOC, enhanced Multidisciplinary Thoracic Oncology Conference; MTOC, Multidisciplinary Thoracic Oncology Conference.
Propensity Analysis
In both propensity analyses, eMTOC patients had significantly lower hazards compared with metropolitan and regional patients (Table 2). The SIPW approach indicated that regional patients had significantly higher hazards than metropolitan patients, but the matched approach revealed no differences.
There was no significant difference in hazard for death between metropolitan patients managed within or outside the eMTOC (HR = 0.95, 95% CI: 0.83–1.09, adjusted p = 0.7608 by SIPW; HR = 0.8 [0.59–1.09], adjusted p = 0.3237 in matched analysis, Table 3) among those with stage-preferred treatment. Care within the eMTOC remained significantly less hazardous for non-recipients of stage-preferred treatment (HR = 0.73 [0.61–0.86], adjusted p = 0.0008 in SIPW analysis; HR = 0.48 [0.31–0.73], adjusted p = 0.0018 by propensity matching).
Table 3.
Cox Proportional Hazard Model With Stabilized Inverse Probability Weighting Propensity and Propensity-Matched Analyses Stratified by Stage-Preferred Treatment Status
| Care Delivery | Hazard Ratio (95% CI) | Unadjusted p Value | Adjusted p Valuea |
|---|---|---|---|
| Stratified by stage-preferred treatment | |||
| Stabilized inverse probability weightingb | |||
| Among those with stage-preferred treatment | |||
| eMTOC vs. non-MTOC: metropolitan | 0.95 (0.83, 1.09) | 0.4811 | 0.7608 |
| eMTOC vs. non-MTOC: regional | 0.81 (0.7–0.93) | 0.0040 | 0.0112 |
| Non-MTOC: regional vs. metropolitan | 1.18 (1.06–1.31) | 0.0022 | 0.0064 |
| Among those without stage-preferred treatment | |||
| eMTOC vs. non-MTOC: metropolitan | 0.73 (0.61–0.86) | 0.0003 | 0.0008 |
| eMTOC vs. non-MTOC: regional | 0.71 (0.59–0.84) | 0.0001 | 0.0003 |
| Non-MTOC: regional vs. metropolitan | 1.03 (0.93–1.14) | 0.5943 | 0.8553 |
| Propensity matchedb | |||
| Among those with stage-preferred treatment | |||
| eMTOC vs. non-MTOC: metropolitan | 0.8 (0.59–1.09) | 0.1518 | 0.3237 |
| eMTOC vs. non-MTOC: regional | 0.76 (0.54–1.06) | 0.1002 | 0.2272 |
| Non-MTOC: regional vs. metropolitan | 1.06 (0.83–1.34) | 0.6486 | 0.8919 |
| Among those without stage-preferred treatment | |||
| eMTOC vs. non-MTOC: metropolitan | 0.48 (0.31–0.73) | 0.0006 | 0.0018 |
| eMTOC vs. non-MTOC: regional | 0.53 (0.34–0.82) | 0.0042 | 0.0117 |
| Non-MTOC: regional vs. metropolitan | 0.9 (0.73–1.13) | 0.3705 | 0.6431 |
| Stratified by stage-appropriate treatment | |||
| Stabilized inverse probability weightingb | |||
| Among those with stage-appropriate treatment | |||
| eMTOC vs. non-MTOC: metropolitan | 0.91 (0.8–1.03) | 0.1244 | 0.2739 |
| eMTOC vs. non-MTOC: regional | 0.83 (0.73–0.95) | 0.0061 | 0.0168 |
| Non-MTOC: regional vs. metropolitan | 1.09 (1–1.2) | 0.0651 | 0.1552 |
| Among those without stage-appropriate treatment | |||
| eMTOC vs. non-MTOC: metropolitan | 0.69 (0.56–0.86) | 0.0007 | 0.002 |
| eMTOC vs. non-MTOC: regional | 0.6 (0.48–0.74) | <0.0001 | <0.0001 |
| Non-MTOC: regional vs. metropolitan | 1.15 (1.03–1.3) | 0.0171 | 0.045 |
| Propensity matchedb | |||
| Among those with stage-appropriate treatment | |||
| eMTOC vs. non-MTOC: metropolitan | 0.71 (0.54–0.94) | 0.0167 | 0.0440 |
| eMTOC vs. non-MTOC: regional | 0.75 (0.55–1.02) | 0.0633 | 0.1514 |
| Non-MTOC: regional vs. metropolitan | 0.95 (0.77–1.17) | 0.6187 | 0.8724 |
| Among those without stage-appropriate treatment | |||
| eMTOC vs. non-MTOC: metropolitan | 0.48 (0.28–0.8) | 0.0049 | 0.0136 |
| eMTOC vs. non-MTOC: regional | 0.46 (0.27–0.78) | 0.0041 | 0.0114 |
| Non-MTOC: regional vs. metropolitan | 1.03 (0.8–1.34) | 0.7979 | 0.9645 |
Note: Excluding patients with unknown stage. Non-MTOC metropolitan: patients without care planning through the eMTOC, who received care within metropolitan Memphis; non-MTOC regional: patients without care planning through the eMTOC, who received care within institutions outside greater metropolitan Memphis.
CI, confidence interval; eMTOC, enhanced Multidisciplinary Thoracic Oncology Conference; MTOC, Multidisciplinary Thoracic Oncology Conference.
Multiple comparisons adjusted using Tukey’s correction.
Propensity adjusting for age, race, sex, insurance, and clinical stage.
eMTOC patients had lower hazard for death than regional patients, irrespective of receipt of stage-preferred treatment in the SIPW analysis (HR = 0.81 [ 0.7–0.93], adjusted p = 0.0112 with stage-preferred treatment; HR = 0.71 [0.59–0.84], adjusted p = 0.0003 without receipt of stage-preferred treatment). In the propensity-matched analysis, eMTOC patients who received stage-preferred treatment had a HR of 0.76 (0.54–1.06), p value equals to 0.2272 compared with regional patients. eMTOC patients who did not receive stage-preferred treatment had a HR of 0.53 (0.34–0.82), adjusted p value equals to 0.0117, compared with regional patients. Hazard for death was significantly higher in regional patients who received stage-preferred treatment, compared with metropolitan patients in the SIPW analysis, but not in patients who did not receive stage-preferred treatment, and there was no significant difference in either subset of patients in the propensity-matched analysis (Table 3). Essentially similar results were observed when stratified by stage-appropriate treatment (Table 3).
Sensitivity Analyses
Because we lacked data on patients’ performance status, which may influence treatment and to evaluate the impact of asymmetry in the distribution of missing clinical stage data, we repeated the propensity analyses under different hypothetical scenarios. In scenario 1, we assumed that patients with a clinical stage but no treatment had received guideline-concordant treatment, after excluding patients with missing clinical stage (Supplementary Table A.2). Scenario 2 included patients with missing clinical stage and assumed that they had received guideline-concordant treatment (Supplementary Table A.3) or (scenario 3) assumed that they had not received guideline-concordant treatment (Supplementary Table A.4). In scenario 4, we categorized those with a missing stage who received any treatment as having received guideline-concordant treatment and those who did not receive any treatment as not having received guideline-concordant treatment (Supplementary Table A.5). The hazard of eMTOC recipients was consistently lower (with or without statistical significance) than non-MTOC participants in all scenarios; and the hazard of regional non-MTOC patients was higher in comparison to metropolitan non-MTOC recipients in most scenarios. Adjustment for patient-level rurality did not change these relationships (data not found).
Discussion
From 2011 to 2017, although only 14% of patients with NSCLC received any form of structured multidisciplinary care planning, this trend increased over time, especially in the metropolitan health care system (Fig. 1). eMTOC patients were significantly more likely to receive any treatment and to receive guideline-concordant treatment. They also had significantly better survival, even after adjusting for potential confounding factors emanating from asymmetric distributions of demographic, clinical, and treatment characteristics. We designed the multidisciplinary program to have open access to indigent and racial minority patients, and through video conferencing, to patients and providers in more rural parts of the health care system. Although we excluded all patients with missing clinical stage information in the primary analysis, we included those who received no treatment, because one of the known attributes of multidisciplinary care is ensuring timely access to specialist treatment.5,6,24,25
The eMTOC was solely a decision-making forum. All patients received their diagnostic, staging, and treatment procedures within the same health care system, linking the survival benefit to the decision-making process. The survival benefit may be attributed to the greater likelihood of receiving any treatment, especially, guideline-concordant treatment. This is supported by the finding that metropolitan non-MTOC recipients of stage-preferred treatment had similar survival to the eMTOC patients. This was not the case in the regional non-MTOC patients, for whom rural cancer care delivery might pose additional challenges.20,26,27
We provide robust evidence for survival benefit from multidisciplinary decision-making. Reasons for the survival benefit of eMTOC decision-making may include improved staging, higher rates of delivery of guideline-concordant care, in particular, stage-preferred treatment (such as surgical resection for early stage and multimodality therapy for stage III NSCLC). For example, a recent analysis of the U.K.’s National Lung Cancer Audit revealed that fewer than 20% of patients with stage III NSCLC received multimodality treatment, 34% received only palliative treatment, 36% received no treatment, and aggregate 1-year survival was 32.9%.28 The proportion of patients who received no treatment was lowest in the eMTOC cohort. Although this might indicate a referral bias toward healthier patients in the eMTOC, it might also indicate the value of more appropriately timely care delivery within the multidisciplinary care structure.
Existing studies of multidisciplinary care have been criticized for being small, noncomparative, noncontemporaneously comparative, or for not clearly defining “multidisciplinary.”3,5,6,29,30 Most did not attempt, or failed, to reveal a survival benefit.5,6,10,31,32 A large, multi-institutional retrospective analysis of tumor boards in the U.S. Veterans Affairs Health care system revealed no impact on receipt of guideline-concordant care or survival for lung cancer, among other cancers.10 Nevertheless, a few studies have revealed an association between multidisciplinary lung cancer care and survival. One was a 117-person, single-institutional, noncontemporaneously controlled, preimplementation versus postimplementation analysis,33 and another was a single-institutional retrospective analysis spanning 15 years.11 A retrospective cohort analysis of a Taiwanese national registry data also revealed a survival benefit, but it did not characterize the multidisciplinary structure nor specify how that cohort was identified.34 A retrospective analysis of 515 patients with stage III NSCLC treated at Taipei Veterans General Hospital identified multidisciplinary decision-making, T-category, performance status, and use of surgery as variables with independent association with survival.13 Recent analyses of the U.K.’s National Lung Cancer Audit also strongly support the association between the organization of services, receipt of guideline-concordant care, and lung cancer patient outcomes.12,28
Our analytical sample is large, within a relatively short time span, involves multiple institutions with different characteristics, includes a contemporaneous control, and involves a prospective multidisciplinary care cohort retrieved from a rigorously implemented, benchmarked, and monitored eMTOC. We used institutional tumor registries to homogenize the data collection and pragmatically evaluate the impact of the eMTOC. Conceptually, we connected Donabedian’s three domains for quality improvement, as follows: structure (eMTOC versus non-MTOC); process (delivery of guideline-concordant treatment); and outcome (survival).35 We provide real-world evidence of the value of multidisciplinary care within a large, diverse, community-based health care system.
Retrospective analysis of tumor registry data has inherent limitations, including missing information (e.g., performance status, comorbidities, patient-physician interaction, and decision-making), potential misclassification bias (given the uneven distribution of unstaged and untreated patients), and potential referral bias (e.g., preferential referral of healthier and more resource-replete patients to the eMTOC). Lower rates of non-treatment with eMTOC might indicate preferential referral of patients with better performance status, as might the smaller proportion of patients with advanced clinical stage. We used propensity and multiple sensitivity analyses, including imputation with extreme assumptions on missing data, to account for such biases with generally consistent results.
Using the Tumor Registry structure provides a pragmatic means of measurement, using the same lens to evaluate the eMTOC cohort in comparison to all other patients, without the selection bias that a prospective nonrandomly selected control group might introduce. In addition to a clearly defined and rigorously benchmarked multidisciplinary program, other strengths of our study include a prospectively identified eMTOC cohort and the evaluation of a large, “real-world” multi-institutional cohort. Because 85% of U.S. lung cancer patients receive care in similar community based health care systems, our findings are likely to be generalizable.
In summary, a rigorously implemented eMTOC improved the care and survival of NSCLC patients in one of the first studies to reveal a survival benefit from multidisciplinary planning. This should provide added impetus to promote adoption within diverse care delivery environments. Prospective care planning, evidence-based decision-making, incorporating team science principles to ensure optimal communication, transparent decision-making, and a high level of compliance with multidisciplinary recommendations are feasible in nonacademic health care systems. We are exploring the ability of this approach to overcome rurality associated disparities in lung cancer care.
CRediT Authorship Contribution Statement
Meredith Ray: Methodology, statistical analysis, Writer—review and editing.
Nicholas R. Faris: Project administration, Data curation, Writer—review and editing.
Carrie Fehnel: Data curation, Writer—review and editing.
Anna Derrick, Folabi Arigandoye, Alicia Pacheco, Robert Optican, Keith Tonkin, Jeffrey Wright, Roy Fox, Thomas Callahan, Edward T. Robbins, William Walsh, Philip Lammers, Shailesh Satpute: Writer—review and editing.
Matthew P. Smeltzer, Meghan Meadows-Taylor: Statistical consultant, Writer—review and editing.
Raymond U. Osarogiagbon: Conceptualization, Investigation, Funding acquisition, Writer—review and editing.
Acknowledgments
This work was partially supported by research grants from the Baptist Foundation, the National Institutes of Health (2R01CA172253), and a Patient-Centered Outcomes Research Institute Award (IH-1304-6147) to Dr. Osarogiagbon. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors, or Methodology Committee.
Footnotes
Disclosure: Dr. Osarogiagbon owns patents for a surgical lymph node specimen collection kit (US and China), owns stocks in Eli Lilly, Gilead Sciences, and Pfizer; has worked as a paid research consultant for the American Cancer Society, the Association of Community Cancer Centers, Astra Zeneca, Eli Lilly, Triptych Healthcare Partners, and Genentech/Roche; and is founder of Oncobox Device, Inc. Dr. Smeltzer has worked as a paid research consultant for the Association of Community Cancer Centers. The remaining authors declare no conflict of interest.
Cite this article as: Ray MA, Faris NR, Fehnel C, et al. Survival impact of an enhanced multidisciplinary thoracic oncology conference in a regional community health care system. JTO Clin Res Rep. 2021;2:100203.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100203.
Supplementary Data
References
- 1.Osarogiagbon R.U. In: Lung Cancer: A Practical Approach to Evidence-Based Clinical Evaluation and Management. Tanoue L., Detterbeck F., editors. Elsevier; St. Louis, MO: 2018. Achieving better quality of lung cancer care; pp. 167–182. [Google Scholar]
- 2.Osarogiagbon R.U. Overcoming the implementation gap in multidisciplinary oncology care programs. J Oncol Pract. 2016;12:888–891. doi: 10.1200/JOP.2016.014688. [DOI] [PubMed] [Google Scholar]
- 3.Tattersall M.H. Multidisciplinary team meetings: where is the value? Lancet Oncol. 2006;7:886–888. doi: 10.1016/S1470-2045(06)70916-0. [DOI] [PubMed] [Google Scholar]
- 4.Look Hong N.J., Gagliardi A.R., Bronskill S.E., Paszat L.F., Wright F.C. Multidisciplinary cancer conferences: exploring obstacles and facilitators to their implementation. J Oncol Pract. 2010;6:61–68. doi: 10.1200/JOP.091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhu Das I., Baker M., Altice C., Castro K.M., Brandys B., Mitchell S.A. Outcomes of multidisciplinary treatment planning in US cancer care settings. Cancer. 2018;124:3656–3667. doi: 10.1002/cncr.31394. [DOI] [PubMed] [Google Scholar]
- 6.Stone C.J.L., Vaid H.M., Selvam R., Ashworth A., Robinson A., Digby G.C. Multidisciplinary clinics in lung cancer care: a systematic review. Clin Lung Cancer. 2018;19:323–330.e3. doi: 10.1016/j.cllc.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Beckett P., Woolhouse I., Stanley R., Peake M.D. Exploring variations in lung cancer care across the UK--the ‘story so far’ for the National Lung Cancer Audit. Clin Med (Lond) 2012;12:14–18. doi: 10.7861/clinmedicine.12-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau K.K., Rathinam S., Waller D.A., Peake M.D. The effects of increased provision of thoracic surgical specialists on the variation in lung cancer resection rate in England. J Thorac Oncol. 2013;8:68–72. doi: 10.1097/JTO.0b013e3182762315. [DOI] [PubMed] [Google Scholar]
- 9.Khakwani A., Rich A.L., Powell H.A. The impact of the ‘hub and spoke’ model of care for lung cancer and equitable access to surgery. Thorax. 2015;70:146–151. doi: 10.1136/thoraxjnl-2014-205841. [DOI] [PubMed] [Google Scholar]
- 10.Keating N.L., Landrum M.B., Lamont E.B., Bozeman S.R., Shulman L.N., McNeil B.J. Tumor boards and the quality of cancer care. J Natl Cancer Inst. 2013;105:113–121. doi: 10.1093/jnci/djs502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilfinger T.V., Albano D., Perwaiz M., Keresztes R., Nemesure B. Survival outcomes among lung cancer patients treated using a multidisciplinary team approach. Clin Lung Cancer. 2018;19:346–351. doi: 10.1016/j.cllc.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Adizie J.B., Khakwani A., Beckett P. Impact of organisation and specialist service delivery on lung cancer outcomes. Thorax. 2019;74:546–550. doi: 10.1136/thoraxjnl-2018-212588. [DOI] [PubMed] [Google Scholar]
- 13.Hung H.Y., Tseng Y.H., Chao H.S. Multidisciplinary team discussion results in survival benefit for patients with stage III non-small-cell lung cancer. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ASCO-ESMO consensus statement on quality cancer care. J Clin Oncol. 2006;24:3498–3499. doi: 10.1200/JCO.2006.07.4021. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine (IOM) The National Academies Press; Washington, DC: 2013. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. [PubMed] [Google Scholar]
- 16.Smeltzer M.P., Rugless F.E., Jackson B.M. Pragmatic trial of a multidisciplinary lung cancer care model in a community healthcare setting: study design, implementation evaluation, and baseline clinical results. Transl Lung Cancer Res. 2018;7:88–102. doi: 10.21037/tlcr.2018.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mokdad A.H., Dwyer-Lindgren L., Fitzmaurice C. Trends and patterns of disparities in cancer mortality among US Counties, 1980–2014. JAMA. 2017;317:388–406. doi: 10.1001/jama.2016.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osarogiagbon R.U., Rodriguez H.P., Hicks D. Deploying team science principles to optimize interdisciplinary lung cancer care delivery: avoiding the long and winding road to optimal care. J Oncol Pract. 2016;12:983–991. doi: 10.1200/JOP.2016.013813. [DOI] [PubMed] [Google Scholar]
- 19.Economic Research Service, U.S. Department of Agriculture Rural-urban commuting area codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx Accessed August 25, 2018.
- 20.Ray M.A., Faris N.R., Derrick A., Smeltzer M.P., Osarogiagbon R.U. Rurality, stage-stratified use of treatment modalities and survival of non-small cell lung cancer. Chest. 2020;158:787–796. doi: 10.1016/j.chest.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network Non-small cell lung cancer, version 2.2019. https://www.nccn.org/professionals/physician_gls/ Accessed August 14, 2020.
- 22.Kramer C.Y. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics. 1956;12:307–310. [Google Scholar]
- 23.Robins J.M., Herman M.A., Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Moghissi K., Connolly C.K. Resection rates in lung cancer patients. Eur Respir J. 1996;9:5–6. doi: 10.1183/09031936.96.09010005. [DOI] [PubMed] [Google Scholar]
- 25.BTS recommendations to respiratory physicians for organizing the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998;53(suppl 1):S1–S8. doi: 10.1136/thx.53.suppl_1.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkins G.T., Kim T., Munson J. Residence in rural areas of the United States and lung cancer mortality. Disease incidence, treatment disparities, and stage-specific survival. Ann Am Thorac Soc. 2017;14:403–411. doi: 10.1513/AnnalsATS.201606-469OC. [DOI] [PubMed] [Google Scholar]
- 27.Johnson A.M., Hines R.B., Johnson J.A., 3rd, Bayakly A.R. Treatment and survival disparities in lung cancer: the effect of social environment and place of residence. Lung Cancer. 2014;83:401–407. doi: 10.1016/j.lungcan.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Adizie J.B., Khakwani A., Beckett P. Stage III non-small cell lung cancer management in England. Clin Oncol (R Coll Radiol) 2019;31:688–696. doi: 10.1016/j.clon.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Osarogiagbon R.U. “Like heart valve clinic, it probably saves lives, but… Who has time for that?” The challenge of disseminating multidisciplinary cancer care in the United States. Cancer. 2018;124:3634–3637. doi: 10.1002/cncr.31396. [DOI] [PubMed] [Google Scholar]
- 30.Osarogiagbon R.U. Making the evidentiary case for universal multidisciplinary thoracic oncologic care. Clin Lung Cancer. 2018;19:294–300. doi: 10.1016/j.cllc.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Riedel R.F., Wang X., McCormack M. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1:692–696. [PubMed] [Google Scholar]
- 32.Leo F., Venissac N., Poudenx M., Otto J., Mouroux J., Groupe d’Oncologie Thoracique Azuréen Multidisciplinary management of lung cancer: how to test its efficacy? J Thorac Oncol. 2007;2:69–72. doi: 10.1097/JTO.0b013e31802bff56. [DOI] [PubMed] [Google Scholar]
- 33.Forrest L.M., McMillan D.C., McArdle C.S., Dunlop D.J. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;93:977–978. doi: 10.1038/sj.bjc.6602825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan C.C., Kung P.T., Wang Y.H., Chang Y.C., Wang S.T., Tsai W.C. Effects of multidisciplinary team care on the survival of patients with different stages of non-small cell lung cancer: a national cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743–1748. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.