Abstract
Introduction
Given the concern for cardiopulmonary toxicity in patients with NSCLC undergoing postoperative radiation therapy (PORT), the purpose of this study was to evaluate the association between heart dose and overall survival (OS) in patients undergoing PORT with modern techniques.
Methods
This is a retrospective study of consecutive patients with NSCLC treated with PORT between May 2004 and January 2017. Clinical records were reviewed and radiation dose distributions were analyzed for association with OS.
Results
A total of 284 patients were analyzed. At the time of surgery, most patients had pathologic American Joint Committee on Cancer seventh edition stage III disease (91.2 %) and received either preoperative or adjuvant chemotherapy (92.3 %). Most patients underwent a lobectomy (81.3 %) and had R0 (80.6 %) or R1 (19.4 %) resection. PORT was delivered with a median radiation dose of 54 Gy, and 70.4 % of patients were treated with intensity-modulated radiation therapy. Dosimetric variables across a large range of doses to the heart were highly significant (p < 0.05) for OS. The volume of the heart receiving 8 Gy (HV8) was the most significant dosimetric variable (p < 0.001), and the median HV8 was 35.5 %. The median OS was 33.2 versus 53.6 months (p < 0.005) for patients with HV8 above or below 35.5 %, respectively. On multivariable analysis accounting for other potential prognostic confounders, HV8 remained highly significant (p < 0.001).
Conclusions
The data reveal a strong correlation between increasing heart dose and OS in patients with NSCLC undergoing PORT. Taken together with the recently presented LungART trial, lowering heart dose in PORT patients may help to decrease the risk of morbidity and mortality and improve the therapeutic ratio of PORT.
Keywords: Postoperative radiation therapy, Cardiac toxicity, Lung cancer, Heart dose
Introduction
For patients who undergo surgical resection for locally advanced NSCLC, the role of postoperative radiation therapy (PORT) is controversial. The PORT meta-analysis, which used antiquated radiation techniques, indicated that PORT was associated with significant toxicity resulting in poorer overall survival (OS) in patients with N0-N1 disease and lack of significant benefit for N2 disease.1 PORT using more modern, linear accelerator–based radiation techniques have been found to lower recurrence rates, and some studies have indicated an improvement in OS.2, 3, 4, 5
Most recently, the LungART trial (Phase III study comparing post-operative conformal radiotherapy to no post-operative radiotherapy in patients with completely resected non-small cell lung cancer and mediastinal N2 involvement, NCT00410683), which randomized 501 patients with completely resected NSCLC with pathologic N2 nodal involvement to PORT versus no PORT, was presented at the 2020 European Society for Medical Oncology Annual Meeting.6 Whereas we are still awaiting the trial publication, the presented outcomes failed to exhibit a statistically significant improvement in the primary end point, 3-year disease-free survival, with PORT. Although PORT lowered the mediastinal relapse rate from 46.1 % to 25 %, this benefit was offset by an increase in cardiopulmonary toxicity and cardiopulmonary deaths.
Heart dose and cardiac toxicity have been of greater concern recently with the publication of RTOG 0617 (Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study), which revealed that high dose definitive radiation (74 Gy versus 60 Gy) concurrent with chemotherapy in locally advanced NSCLC results in poorer OS, potentially owing to higher heart doses.7 Multiple additional studies have revealed that higher heart dose worsens OS in patients with NSCLC treated with definitive radiation.7, 8, 9
The impact of heart dose on OS has not been extensively studied in patients undergoing PORT. The PORT patient population differs from the definitive radiation patient population as these patients must all be medically fit enough to undergo a thoracic operation. A previous study of 43 patients reported that heart dose did not correlate with OS, although these findings are limited owing to the small sample size.10 The purpose of this analysis was to study a large patient population undergoing PORT using modern techniques to evaluate the association between heart dose and OS.
Materials and Methods
We reviewed the clinical records and dosimetry of consecutively treated patients with NSCLC at a single academic institution receiving PORT with computed tomography-based treatment plans, image-guided radiation therapy, and detailed dose distributions between May 2004 and January 2017 to allow adequate time for follow-up. This study was completed under an institutional review board–approved protocol and as a retrospective review, no consent is required.
Our methods for radiation therapy planning, target definition, and anatomical volumes for this specific patient population were previously published.11 Of note, as compared with the LungART study (which included more elective nodal coverage), for the patients in this study, the target volume included the involved nodal stations, bronchial stump, and ipsilateral hilum extending into the ipsilateral lower paratracheal and subcarinal spaces at the treating physician’s discretion, as per our current institutional standard. In addition, heart volumes were retrospectively recontoured according to the RTOG 1106 (Randomized Phase II Trial of Individualized Adaptive Radiotherapy Using During-Treatment FDG-PET/CT and Modern Technology in Locally Advanced Non-Small Cell Lung Cancer [NSCLC]) Atlas for Organs at Risk.
Statistical Analysis
Dosimetric and clinical variables were correlated with OS using univariate and multivariate Cox proportional hazards models with significance defined as a p value less than 0.05. OS was assessed using the Kaplan-Meier method and was calculated from the start of radiation therapy. For lungs and heart, the dosimetric variables tested were the following: (1) the minimum dose to the hottest x% volume with volumes ranging from 0 % to 100 % in increments of 5 %; (2) the percentage volume that received at least dose x with doses ranging from 2 to 60 Gy in increments of 2 Gy (Vx); (3) the maximum, minimum and mean dose; and (4) the total volume. Clinical variables listed in Table 1 were tested. Clinical variables found to be significant (p < 0.05) on univariate analysis were used in a multivariate analysis (MVA) along with the most significant (lowest p value) dosimetric variable.
Table 1.
Patient and Treatment Characteristics and Their Univariate Correlations (HR [95 % CI] and p Value) With Overall Survival
| Variables | n (%) | Univariate Analysis |
|
|---|---|---|---|
| HR [95 % CI] | p Value | ||
| Age, median (range), y | 67 (28–87) | 1.025 [1.008–1.043] | 0.004a |
| Sex | |||
| Female | 170 (59.6) | ||
| Male | 115 (40.4) | 1.49 [1.08–2.06] | 0.016a |
| KPS, median (range) | 90 (70–100) | 1.010 [0.992–1.030] | 0.230 |
| Pathologic stage | |||
| I–II | 25 (9) | 1 | |
| III | 259 (91) | 1.21 [0.71–2.06] | 0.490 |
| Clinical stage (before surgery) | |||
| I–II | 106 (37.3) | ||
| III | 179 (62.7) | 1.36 [0.97–1.92] | 0.075 |
| Smoking status | |||
| Never (a) | 50 (17.6) | 1 | |
| Former (b) | 212 (74.3) | 1.337 [0.860–2.080] | 0.200 |
| Current (c) | 23 (8.1) | 0.943 [0.436–2.040] | 0.880 |
| Smokers (b and c), pack years, median (range) | 30 (0–165) | 1.000 [0.993–1.001] | 0.910 |
| Smokers (b), y since quitting, median (range) | 11 (0–55) | 1.010 [0.995–1.020] | 0.260 |
| Chemotherapy use | 263 (92.3) | ||
| Preoperative | 150 (57.0) | 1 | |
| Postoperative | 113 (43.0) | 0.609 [0.430–0.862] | 0.005a |
| Increasing extent of surgery by ordinal categorical variables | 1.44 [1.06–1.95] | 0.019a | |
| 1 = Wedge resection | 29 (10.2) | ||
| 2 = Segmentectomy | 5 (1.8) | ||
| 3 = Lobectomy | 232 (81.3) | ||
| 4 = Pneumectomy | 19 (6.7) | ||
| Surgical resection margin status | |||
| R0 | 230 (80.6) | ||
| R1 | 55 (19.4) | 1 | 0.890 |
| R2 | 0 (0) | 0.986 [0.811–1.2] | |
| Primary tumor size, median (range), cm | 3.2 (0.8–15) | 1.15 [1.08–1.23] | <0.001a |
| No. positive lymph nodes | 1.069 [1.034–1.105] | <0.001a | |
| 0 | 23 (8) | ||
| 1 | 58 (20) | ||
| 2 | 44 (15) | ||
| 3 | 46 (16) | ||
| 4 | 26 (9) | ||
| ≥ 5 | 87 (31) | ||
| Positive subcarinal node (pathologic) | 104 (36) | 1.69 [1.22–2.36] | 0.002a |
| RT start date | |||
| before 2010 | 61 (21) | 1 | |
| after 2010 | 223 (79) | 1.42 [0.96–2.09] | 0.080 |
| Radiation technique | |||
| IMRT | 201 (70.4) | 0.912 [0.643–1.290] | 0.610 |
| 3DCRT | 84 (29.6) | 1 | |
| Radiation prescription dose, median (range), Gy | 54 (45–70) | 1.021 [0.979–1.064] | 0.320 |
| 45–50 | 40 (14) | ||
| 50–54 | 202 (71) | ||
| 56–66 | 42 (15) | ||
| 70 | 1 (0) | ||
| Heart volume, median (range), cm3 | 570 (242–1406) | 1.001 [1.000–1.001] | 0.001a |
| HV8 | Median = 35.5 % | 1.015 [1.008–1.021] | <0.001a |
3DCRT, three-dimensional conformal radiation therapy; CI, confidence interval; HR, hazard ratio; HV8, volume of the heart receiving 8 Gy; IMRT, intensity-modulated radiation therapy; KPS, Karnofsky Performance Score; RT, radiation therapy.
Statistical significance (p < 0.05).
Results
Patient, Treatment, and Dosimetric Characteristics
A total of 284 consecutive patients were reviewed. The patient demographics and treatment factors are listed in Table 1. At diagnosis, most patients had clinical stage III disease (62.7 %). At the time of surgery, most patients had pathologic American Joint Committee on Cancer seventh edition stage III disease (91.2 %). Most patients received either preoperative or adjuvant chemotherapy (92.3 %). A wide variety of platinum-based chemotherapy regimens were used. Cisplatin-based chemotherapy was administered most often (63.1 %). The most often used carboplatin-based regimens included carboplatin and pemetrexed (59.8 %) and carboplatin and taxane (paclitaxel or docetaxel, 18.6 %). No patients received concurrent chemotherapy. Most patients underwent a lobectomy (81.3 %), and all patients had either an R0 (80.6 %) or R1 (19.4 %) resection.
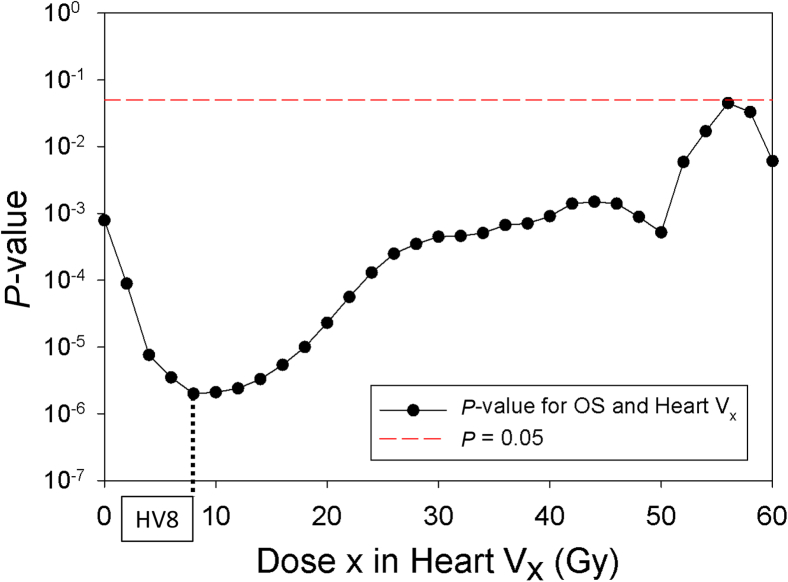
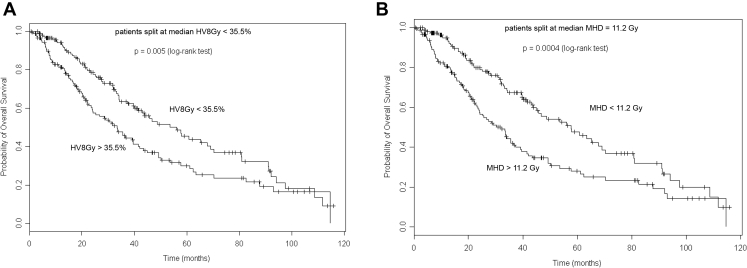
With a median follow-up time of 64.4 (range: 0.7–115.9) months, the median, 3-year, and 5-year OS were 41.5 months, 53 %, and 37 %, respectively. Lung dosimetric variables were not predictive of OS. Dosimetric variables across a large range of doses to the heart were highly significant (p < 0.05) for OS (Fig. 1). The volume of the heart receiving 8 Gy (HV8) was the most significant dosimetric variable (p < 0.0001), and the median HV8 was 35.5 %. The median OS was 33.2 versus 53.6 months (p < 0.005), for patients with HV8 above or below 35.5 % (Fig. 2A). The median mean heart dose (MHD) for the population was 11.2 Gy. The median OS for patients with MHD above versus below 11.2 Gy was 31.7 versus 57.5 months (p < 0.001), respectively (Fig. 2B).
Figure 1.
The significance of heart dosimetric variables (Vx, on the x axis) on OS with p-values on the y axis, illustrating that all heart dosimetric variables are statistically significant with HV8 exhibiting the greatest significance. HV8, volume of the heart receiving 8 Gy; OS, overall survival; Vx, percentage volume that received at least dose x with doses ranging from 2 to 60 Gy in increments of 2 Gy.
Figure 2.
Overall survival by (A) HV8 and (B) MHD split at their median values. HV8, volume of the heart receiving 8 Gy; MHD, mean heart dose.
The clinical variables prognostic for OS were increasing age, male sex, preoperative (versus postoperative) chemotherapy, increasing extent of surgery, tumor size, number of positive lymph nodes, involvement of the subcarinal lymph node station at the time of surgery, and heart volume (Table 1). For MVA, because all heart dosimetric variables were strongly correlated with each other, HV8 was chosen. On MVA (HR [95 % confidence interval], p value), age (1.035 [1.016–1.054], p < 0.001), postoperative chemotherapy (0.66 [0.00–0.95], p = 0.023), extent of surgery (1.59 [1.10–2.28], p = 0.013), heart volume (1.0013 [1.0001–1.0024], p = 0.029), number of positive lymph nodes (1.038 [1.001–1.077], p = 0.041), and HV8 (1.013 [1.007–1.020], p < 0.001) remained significant for OS.
Discussion
Our data reveal that there is a very strong correlation between increasing heart dose and OS in patients with NSCLC undergoing PORT with modern radiation techniques. To our knowledge, this is the first study illustrating the association between heart dose and OS in the PORT setting. On MVA accounting for other prognostic factors, heart dose remained strongly predictive of OS.
Our patient population has some notable differences in baseline patient and treatment characteristics compared with the LungART trial patient population. For example, 70.4 % of our patients were treated with intensity-modulated radiation therapy (versus 11 % in LungART), our MHD was lower (11.2 Gy versus 13.4 Gy in LungART), and we treated patients with more advanced disease as 57 % of our patients received preoperative chemotherapy (versus 18 % in LungART). Despite these differences, increasing heart dose correlated with worse OS in our patient population as well. Of note, the most significant heart dose parameter was the heart volume receiving over 8 Gy, which is significantly lower than often used dosimetric guidelines to constrain radiation dose to the heart. Taken together, we can conclude that lowering heart dose in PORT patients has the potential to significantly decrease the risk of morbidity and mortality and improve the therapeutic ratio of PORT.
Progression of the disease continues to be the predominant cause of death in patients with NSCLC. Incorporating immunotherapy and targeted therapy into the treatment regimen of NSCLC patients who undergo resection may lower distant recurrence rates.12,13 As distant recurrence rates improve, reducing local-regional recurrence rates will become even more important. Therefore, delivering PORT to patients at the highest risk for mediastinal recurrence—provided it can be delivered safely—may be warranted. With improving radiation therapy treatment techniques, including proton therapy, it is becoming more feasible to lower heart dose, and early proton PORT data are encouraging.14,15 As treatment techniques improve and the thoracic radiation oncology field shifts to emphasizing lower heart doses, it may be possible to take advantage of the improvement in mediastinal recurrence rates with PORT while limiting toxicity.
Although the association between heart dose and OS is clear, the mechanisms driving this association are unclear. Because heart dose remained significant on MVA after accounting for other prognostic features, such as the number of positive lymph nodes and subcarinal nodal involvement, heart dose cannot simply be a reflection of more aggressive disease. Future directions of this study include correlating heart dose and dose to cardiac substructures with cardiac events. Limitations of this analysis include its retrospective nature and the possibility of unaccounted confounding variables. Despite these limitations, the strong correlation between heart dose and OS in the PORT patient population is clear. Lowering the heart dose, particularly the HV8, should be considered when planning for PORT to decrease the risk of morbidity and mortality of PORT.
CRediT Authorship Contribution Statement
Annemarie F. Shepherd: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing (original draft and review and editing).
Anthony F. Yu: Conceptualization, Data curation, Methodology, Writing (review and editing).
Michelle Iocolano, Jonathan E. Leeman, Aaron T. Wild, Brandon S. Imber: Data curation, Methodology, Writing (review and editing).
Jamie E. Chaft, Michael D. Offin: Conceptualization, Writing (review and editing), Validation.
James Huang, James M. Isbell: Writing (review and editing), Validation.
Abraham J. Wu, Daphna Y. Gelblum: Writing (review and editing).
Narek Shaverdian: Conceptualization, Writing (review and editing).
Charles B. Simone II: Conceptualization, Investigation, Writing (review and editing).
Daniel R. Gomez: Project administration, Supervision, Writing (review and editing).
Ellen Yorke: Conceptualization, Data curation, Formal Analysis, Investigation, Writing (review and editing), Software.
Andrew Jackson: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing (review and editing), Software.
Andreas Rimner: Conceptualization, Investigation, Methodology, Supervision, Writing (review and editing).
Acknowledgments
This work received grants from Memorial Sloan Kettering Cancer Center Support Grant through the principal investigator (Dr. Craig Thompson, President and CEO of Memorial Sloan Kettering Cancer Center) from the National Institute of Health/National Cancer Institute (5 P30 CA008748-54, period: January 1, 2019 to December 31, 2023).
Footnotes
Drs. Jackson and Rimner contributed equally as senior authors to this work.
Disclosure: Dr. Offin reports serving as a consultant for PharmaMar, Novartis, Targeted Oncology, and Jazz Pharmaceuticals; and receiving funds for travel from Bristol-Myers Squibb and Merck Sharp & Dohme. Dr. Chaft reports serving as a consultant and receiving grants from Bristol-Myers Squibb, Merck, Genentech, and AstraZeneca. Dr. Rimner reports serving as a consultant for AstraZeneca, Varian Medical Systems, Merck, Cybrexa, and MoreHealth; receiving research grants from AstraZeneca, Varian Medical Systems, Boehringer Ingelheim, Pfizer, and Merck; and receiving travel reimbursement for Philips/Elekta. Dr. Wu reports receiving research grants from CivaTech Oncology; serving as a consultant for AstraZeneca; and receiving honoraria (travel grant) from AlphaTau Medical. Dr. Shaverdian reports receiving research funding from Novartis. Dr. Simone reports receiving an honorarium from Varian Medical Systems. The remaining authors declare no conflict of interest.
References
- 1.Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials PORT Meta-analysis Trialists Group. Lancet. 1998;352:257–263. [PubMed] [Google Scholar]
- 2.Billiet C., Decaluwe H., Peeters S. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: a meta-analysis. Radiother Oncol. 2014;110:3–8. doi: 10.1016/j.radonc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Douillard J.Y., Rosell R., De Lena M. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) randomized trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 4.Lally B.E., Zelterman D., Colasanto J.M. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;24:2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 5.Robinson C.G., Patel A.P., Bradley J.D. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol. 2015;33:870–876. doi: 10.1200/JCO.2014.58.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Pechoux C. LBA3_PR - An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement. Primary end-point analysis of Lung ART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Ann Oncol. 2020;31(suppl 4):S1142–S1215. [Google Scholar]
- 7.Bradley J.D., Hu C., Komaki R.R. Long-term results of NRG oncology RTOG 0617: Standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38:706–714. doi: 10.1200/JCO.19.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkins K.M., Chaunzwa T.L., Lamba N. Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with non-small cell lung cancer. JAMA Oncol. 2021;7:206–219. doi: 10.1001/jamaoncol.2020.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speirs C.K., DeWees T.A., Rehman S. Heart Dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12:293–301. doi: 10.1016/j.jtho.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 10.Lee C.C., Chua G.W.Y., Zheng H. Are heart doses associated with survival in patients with non-small cell lung cancer who received post-operative thoracic radiotherapy?: a national population-based study. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepherd A.F., Iocolano M., Leeman J. Clinical and Dosimetric predictors of radiation pneumonitis in patients with non-small cell lung cancer undergoing postoperative radiation therapy. Pract Radiat Oncol. 2021;11:e52–e62. doi: 10.1016/j.prro.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provencio M., Nadal E., Insa A. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–1422. doi: 10.1016/S1470-2045(20)30453-8. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y.L., Tsuboi M., He J. Osimertinib in resected EGFR-Mutated non-small-cell lung cancer. N Engl J Med. 2020;383:1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 14.Liao Z., Lee J.J., Komaki R. Bayesian Adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:1813–1822. doi: 10.1200/JCO.2017.74.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remick J.S., Schonewolf C., Gabriel P. First clinical report of proton beam therapy for postoperative radiotherapy for non-small-cell lung cancer. Clin Lung Cancer. 2017;18:364–371. doi: 10.1016/j.cllc.2016.12.009. [DOI] [PubMed] [Google Scholar]