Abstract
Introduction
The TIGER-3 (NCT02322281) study was initiated to compare the efficacy and safety of rociletinib, a third-generation EGFR tyrosine kinase inhibitor (TKI) that targets EGFR T790M and common EGFR-activating mutations, versus chemotherapy in patients with NSCLC who progressed on first- or second-generation EGFR TKIs.
Methods
Patients with advanced or metastatic EGFR-mutated NSCLC with disease progression on standard therapy (previous EGFR TKI and platinum-based chemotherapy) were randomized to oral rociletinib (500 or 625 mg twice daily) or single-agent chemotherapy (pemetrexed, gemcitabine, docetaxel, or paclitaxel).
Results
Enrollment was halted when rociletinib development was discontinued in 2016. Of 149 enrolled patients, 75 were randomized to rociletinib (n = 53: 500 mg twice daily; n = 22: 625 mg twice daily) and 74 to chemotherapy. The median investigator-assessed progression-free survival (PFS) was 4.1 months (95% confidence interval [CI]: 2.6–5.4) in the rociletinib 500-mg group and 5.5 months (95% CI: 1.8–8.1) in the 625-mg group versus 2.5 months (95% CI: 1.4–2.9) in the chemotherapy group. An improved PFS was observed in patients with T790M-positive NSCLC treated with rociletinib (n = 25; 500 mg and 625 mg twice daily) versus chemotherapy (n = 20; 6.8 versus 2.7 mo; hazard ratio = 0.55, 95% CI: 0.28–1.07, p = 0.074). Grade 3 or higher hyperglycemia (24.0%), corrected QT prolongation (6.7%), diarrhea (2.7%), and vomiting (1.3%) were more frequent with rociletinib than chemotherapy (0%, 0%, 1.4%, and 0%, respectively).
Conclusions
Rociletinib had a more favorable median PFS versus chemotherapy but had higher rates of hyperglycemia and corrected QT prolongation in patients with advanced EGFR-mutated NSCLC who progressed on previous EGFR TKI. Incomplete enrollment prevented evaluation of the primary efficacy end point.
Keywords: EGFR tyrosine kinase inhibitor, Epidermal growth factor receptor mutations, Non–small cell lung cancer, Phase III randomized clinical trial, Rociletinib
Introduction
Activating EGFR mutations (exon 21 L858R and deletions in exon 19) have been detected in approximately 30% of patients of East Asian descent and 10% to 15% of patients of Northern or Western European descent with NSCLC.1 Patients whose tumors carry these mutations typically have good responses to therapy with a first-generation (e.g., gefitinib, erlotinib) or second-generation (e.g., afatinib, dacomitinib) EGFR tyrosine kinase inhibitor (TKI) as assessed by progression-free survival (PFS).2, 3, 4 However, after a median of 8 to 16 months of EGFR TKI therapy, the emergence of resistance, which is driven by a mutation in exon 20 (T790M, the “gatekeeper mutation”) in 50% to 60% of cases, results in disease progression.4, 5, 6, 7 This led to the development of third-generation EGFR TKIs, including rociletinib and osimertinib, which added activity against T790M.
Rociletinib is a third-generation, orally-bioavailable, irreversible EGFR TKI that selectively targets common EGFR-activating mutations, such as L858R and deletions in exon 19, and the resistance T790M gatekeeper mutation with minimal activity toward wild-type EGFR.10, 8, 9 In a phase 1 and 2 trial (TIGER-X, NCT01526928), rociletinib was investigated in patients with EGFR-mutated, T790M-positive, and T790M-negative NSCLC previously treated with a first- or second-generation EGFR TKI.11 In the phase 1 portion of the study, 57 patients received a free-base formulation of rociletinib 150 to 900 mg twice daily. During the phase 2 portion, 548 patients received rociletinib (hydrogen bromide salt formulation) at 500, 625, or 750 mg twice daily. In the final TIGER-X analysis that included 443 patients who received at least one dose of rociletinib (500, 625, or 750 mg twice daily) and had centrally confirmed T790M-positive tumors, the confirmed objective response rate (ORR) was 33.9%.12 Across all three dosing groups, the most common treatment-emergent adverse events (AEs) included hyperglycemia, diarrhea, nausea, fatigue, and decreased appetite.
TIGER-3 (NCT02322281) was a phase 3 randomized trial initiated in 2014 to assess the efficacy and safety of rociletinib versus chemotherapy in patients with EGFR-mutated NSCLC who progressed on first- or second-generation EGFR TKIs. To be eligible for inclusion, patients in TIGER-3 had to have been previously treated with platinum-doublet chemotherapy.13 No third-generation EGFR TKIs were available at the time of initiation of the trial; however, in 2016, osimertinib received approval from the U.S. Food and Drug Administration. The clinical development of rociletinib was halted in 2016 per sponsor decision. Here, we report the final results of TIGER-3.
Materials and Methods
Study Design
TIGER-3 was an international, phase 3, randomized, open-label study. Eligible patients were aged 18 years or older with metastatic or unresectable, locally advanced EGFR-mutated NSCLC (excluding exon 20 insertion–activating mutation) with radiological progression after at least one first- or second-generation EGFR TKI and one line of platinum-based doublet chemotherapy for advanced or metastatic NSCLC. Biopsy or surgical resection of either primary or metastatic tumor tissue within 60 days before study treatment was required for the central determination of T790M mutation status; however, results were not required before randomization. Central genotyping of the collected tissues was performed with the therascreen EGFR RGQ PCR Kit (Qiagen, Germantown, MD).14 Patients who received previous treatment with rociletinib or other T790M-positive EGFR-specific medications (including osimertinib [AZD9291], olmutinib [HM61713], and TAS-121) were excluded. Patients with brain metastases were eligible if lesions were treated, asymptomatic, and stable. The full inclusion and exclusion criteria are listed in the Supplementary Data.
After screening, patients were randomized in a 1:1 ratio to receive oral rociletinib or investigator’s choice of single-agent cytotoxic chemotherapy (pemetrexed, gemcitabine, docetaxel, or paclitaxel). Initially, the starting dose of rociletinib was 625 mg twice daily, but after a protocol amendment, it was decreased to 500 mg twice daily in an effort to improve tolerability. Randomization was stratified on the presence of brain metastases (yes versus no), Eastern Cooperative Oncology Group performance status (0 versus 1), and region (East Asian versus non-East Asian).
Ethical Considerations
TIGER-3 was conducted in compliance with Good Clinical Practices, including the International Conference on Harmonization’s Technical Requirements for Registration of Pharmaceuticals for Human Use Guidelines, the U.S. Food and Drug Administration regulatory requirements, and the ethical principles of the Declaration of Helsinki. All patients provided written informed consent, which was reviewed and approved by local ethics committees.
Treatments and Dosing
Depending on the protocol version in place at the time of study entry, patients were treated with rociletinib 625 or 500 mg twice daily in a 21-day continuous cycle. Two dose reduction steps were allowed for each patient (in decrements of 125 mg) for grade 3 or 4 hematologic and nonhematologic toxicities.
The investigators’ choices of chemotherapy included any one of the following: (1) pemetrexed 500 mg/m2 intravenously (IV) on day 1 of each 21-day cycle; (2) gemcitabine 1250 mg/m2 IV on days 1 and 8 of each 21-day cycle; (3) docetaxel 75 mg/m2 (60 mg/m2 for East Asian patients) IV on day 1 of each 21-day cycle or 35 mg/m2 IV weekly as part of a continuous 21-day cycle (d 1, 8, and 15 of each 21-d cycle); or (4) paclitaxel 80 mg/m2 IV weekly as part of a continuous 21-day cycle (d 1, 8, and 15 of each 21-d cycle).
Patients were treated until radiographically confirmed disease progression, unacceptable toxicity, or other withdrawal criteria were met. Patients could choose to continue rociletinib therapy after radiographic progression if the patient provided consent and the investigator and sponsor approved. Patients who progressed while taking chemotherapy could cross over to receive rociletinib until disease progression, unacceptable toxicity, or other withdrawal criteria were met.
End Points
The primary end point was investigator-assessed PFS according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The PFS was calculated as one plus the number of days from the date of randomization to documented radiographic progression as determined by the investigator, or death owing to any cause, whichever occurred first.
The planned secondary end points included ORR, duration of response (DOR), overall survival, and pharmacokinetics. ORR was defined as the proportion of patients with a confirmed complete response or confirmed partial response (PR) in the efficacy population. The DOR for a complete response or PR was measured from the date that a response (per RECIST) was first recorded until the first date that progressive disease (PD) was objectively documented. The overall survival, pharmacokinetics, and planned exploratory end points were not analyzed owing to the early termination of the study.
Efficacy and Safety Evaluations
Tumor scans were performed at screening, every 6 (±1) weeks until tumor progression or other withdrawal criteria were met, and at the end-of-treatment visit. Patients who discontinued rociletinib or chemotherapy without disease progression were scanned every 6 weeks until tumor progression occurred. Tumor assessments involved clinical examination and appropriate imaging (usually computed tomography scans of the chest and abdomen with appropriate slice thickness, per RECIST); other scans (magnetic resonance imaging and radiograph) were performed if necessary. Brain imaging (computed tomography or magnetic resonance imaging) was required at baseline; follow-up scans were conducted throughout the study for patients with brain lesions at enrollment. A central laboratory assessed the presence or absence of the T790M mutation in formalin-fixed paraffin-embedded tumor tissues. The details of additional planned evaluations are available in the Supplementary Data.
Safety evaluations included the following: (1) AEs; (2) clinical laboratory evaluations (hematology, serum chemistry, and urinalysis); (3) 12-lead electrocardiograms; (4) physical examination; (5) vital sign measurements; (6) body weight; (7) concomitant medications or procedures; and (8) Eastern Cooperative Oncology Group performance status. Patients were monitored for AEs from the first dose of rociletinib or chemotherapy until 28 days after the last dose of protocol-specified treatment. AEs were classified according to the U.S. National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.15 Safety assessments included the following: (1) study drug exposure AEs; (2) shift tables of changes in clinical laboratory parameters; (3) previous and concomitant hyperglycemia medications; (4) vital signs; (5) glucose elevations; and (6) changes in the corrected QT (QTc) interval.
Statistical Considerations
The target enrollment was 600 patients on the basis of a minimum anticipated treatment effect of a 4-month (chemotherapy) versus 6-month (rociletinib) median PFS in all patients. A total of 600 patients was predicted to result in 400 progression events, providing approximately 90% power to detect a hazard ratio [HR] of 0.70 at a two-sided 0.025 significance level.
The intention-to-treat population included all randomized patients. The efficacy and safety population included patients who had received at least one dose of rociletinib or single-agent cytotoxic chemotherapy. Kaplan-Meier methodology was used to summarize time-to-event variables.
The testing of primary and key secondary end points among the centrally confirmed T790M-positive and all randomized patients using an ordered, stepdown, multiple comparisons procedure was planned. Owing to the early termination of the study, this procedure was not undertaken. Stratified and unstratified log-rank tests and HRs were used to compare the PFS distributions among the rociletinib-treated (500 mg twice daily, 625 mg twice daily) and single-agent cytotoxic chemotherapy-treated patients in the efficacy population and according to centrally confirmed T790M mutation status. Investigator-assessed ORR was analyzed in the efficacy population and by T790M mutation status. DOR was analyzed in the efficacy population.
All analyses were conducted using Statistical Analysis System software (SAS Institute, Cary, NC) version 9.1 or higher.
Results
Patient Enrollment and Demographics
From May 2015 to May 2016, a total of 149 patients were enrolled in the TIGER-3 study at 53 sites in 10 countries (Australia, France, Germany, Italy, The Netherlands, Republic of Korea, Spain, Republic of China, United Kingdom, and the United States). Enrollment was halted when rociletinib development for patients with NSCLC was discontinued in 2016. However, patients who continued to derive clinical benefit from study treatment were allowed to remain in the study at the discretion of the investigator as part of an extension phase. Target enrollment was not achieved. Therefore, hypothesis testing as per protocol was not feasible; p values are provided for descriptive purposes only.
Of the 149 patients enrolled, 75 were randomized to rociletinib (n = 53: 500 mg twice daily; n = 22: 625 mg twice daily) and 74 to chemotherapy (Table 1). The treatment groups were generally well-balanced. In the combined rociletinib group (500-mg and 625-mg doses) and chemotherapy group, 25 (33.3%) and 20 (27.0%) patients were T790M-positive, respectively; 36 (48.0%) and 42 (56.8%) were T790M-negative, and 14 (18.7%) and 12 (16.2%) had nonevaluable T790M status, respectively. A total of 148 patients received at least one dose of the study drug (75 patients in the rociletinib group and 73 patients in the chemotherapy group) and were included in the safety and efficacy populations. One patient discontinued owing to PD before receiving a single dose of chemotherapy. Of the patients assigned to chemotherapy, 39 (52.7%) crossed over to the rociletinib group at the time of progression.
Table 1.
Patient Demographics and Baseline Clinical Characteristics: ITT Population
| Characteristic | Rociletinib |
Chemotherapy (n = 74) | ||
|---|---|---|---|---|
| 500 mg Twice Daily (n = 53) | 625 mg Twice Daily (n = 22) | Overall (n = 75) | ||
| Age, y | ||||
| Median (min, max) | 62.0 (37.0, 86.0) | 63.5 (43.0, 90.0) | 62.0 (37.0, 90.0) | 62.5 (40.0, 85.0) |
| Female, n (%) | 35 (66.0) | 13 (59.1) | 48 (64.0) | 39 (52.7) |
| Region, n (%) | ||||
| North America | 11 (20.8) | 12 (54.5) | 23 (30.7) | 26 (35.1) |
| Europe | 20 (37.7) | 8 (36.4) | 28 (37.3) | 27 (36.5) |
| Asia | 21 (39.6) | 1 (4.5) | 22 (29.3) | 20 (27.0) |
| Other | 1 (1.9) | 1 (4.5) | 2 (2.7) | 1 (1.4) |
| Baseline ECOG PS, n (%) | ||||
| 0 | 15 (28.3) | 9 (40.9) | 24 (32.0) | 21 (28.4) |
| 1 | 38 (71.7) | 13 (59.1) | 51 (68.0) | 52 (70.3) |
| 2 | 0 | 0 | 0 | 1 (1.4) |
| Smoking status, n (%) | ||||
| Current smoker | 2 (3.8) | 0 | 2 (2.7) | 3 (4.1) |
| Former smoker | 14 (26.4) | 9 (40.9) | 23 (30.7) | 28 (37.8) |
| Never smoked | 37 (69.8) | 13 (59.1) | 50 (66.7) | 43 (58.1) |
| Time since NSCLC diagnosis, mo | ||||
| Median (min, max) | 35.2 (8.8, 212.0) | 39.4 (12.6, 69.0) | 36.2 (8.8, 212.0) | 29.5 (7.4, 105.3) |
| History of CNS metastases, n (%) | 23 (43.4) | 9 (40.9) | 32 (42.7) | 31 (41.9) |
| History of hyperglycemia, n (%) | 8 (15.1) | 4 (18.2) | 12 (16.0) | 8 (10.8) |
| No. of previous therapies | ||||
| Median (min, max) | 3 (1, 8) | 3 (2, 6) | 3 (1, 8) | 3 (0, 13) |
| T790M status by central test, n (%) | ||||
| Positive | 16 (30.2) | 9 (40.9) | 25 (33.3) | 20 (27.0) |
| Negative | 26 (49.1) | 10 (45.5) | 36 (48.0) | 42 (56.8) |
| Unknown | 11 (20.8) | 3 (13.6) | 14 (18.7) | 12 (16.2) |
| Activating EGFR mutations at randomization,a n (%) | ||||
| Exon 19 deletion | 21 (39.6) | 11 (50.0) | 32 (42.7) | 35 (47.3) |
| Exon 20 insertionb | 1 (1.9) | 2 (9.1) | 3 (4.0) | 1 (1.4) |
| L858R | 18 (34.0) | 6 (27.3) | 24 (30.4) | 29 (39.2) |
| G719X | 1 (1.9) | 0 | 1 (1.3) | 3 (4.1) |
| L861Q | 5 (9.4) | 0 | 5 (6.7) | 2 (2.7) |
| S768I | 1 (1.9) | 1 (4.5) | 2 (2.7) | 3 (4.1) |
| Otherc | 1 (1.9) | 0 | 1 (1.3) | 4 (5.4) |
CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; ITT, intention-to-treat; max, maximum; min, minimum.
Patients may be counted in more than one category.
Patients with an exon 20 insertion were eligible for inclusion if they also had another activating EGFR mutation.
Other activating mutations included E709A in the rociletinib 500-mg twice-daily group and E709A, E709G, G724S, and G729A in the chemotherapy group.
Patient Disposition and Drug Exposure
The median duration of therapy was 4.2 months in the rociletinib 500-mg group, 4.2 months in the rociletinib 625-mg group, and 1.2 months in the chemotherapy group. Two patients (2.7%) were treated with chemotherapy for more than 12 months, whereas 15 patients (20.0%) received rociletinib for more than 12 months. Most patients in the combined rociletinib group (500-mg and 625-mg doses, 56 of 75; 74.7%) and chemotherapy group (49 of 74; 66.2%) discontinued the study drug owing to PD (Table 2).
Table 2.
Reasons for Study Drug Discontinuation
| Reason | Rociletinib |
Chemotherapy (n = 74)b | ||
|---|---|---|---|---|
| 500 mg Twice Daily (n = 53)a | 625 mg Twice Daily (n = 22) | Overall (n = 75)a | ||
| Progressive disease | 42 (79.2) | 14 (63.6) | 56 (74.7) | 49 (66.2) |
| AE | 3 (5.7) | 4 (18.2) | 7 (9.3) | 6 (8.1) |
| Patient choice | 1 (1.9) | 1 (4.5) | 2 (2.7) | 6 (8.1) |
| Physician decision | 2 (3.8) | 0 | 2 (2.7) | 5 (6.8) |
| Death (excluding disease progression) | 3 (5.7) | 3 (13.6) | 6 (8.0) | 3 (4.1) |
AE, adverse event.
A total of 2 patients discontinued because of study termination.
A total of 4 patients discontinued because of other reasons, and one patient had missing data.
Efficacy
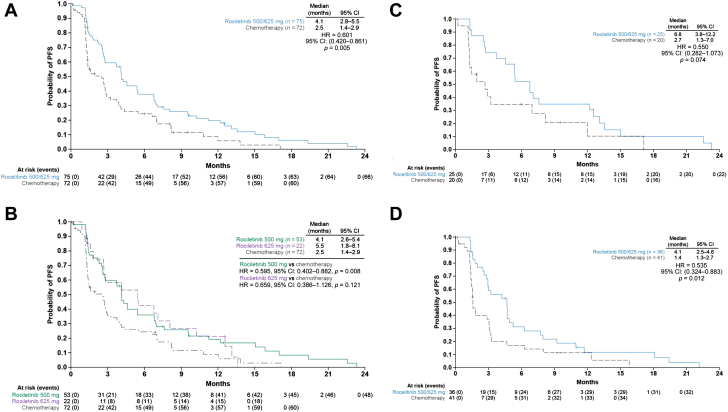
In the efficacy population (n = 148), the median investigator-assessed PFS was 4.1 months (95% CI: 2.8–5.5 mo) in the combined rociletinib group (500-mg and 625-mg doses) versus 2.5 months (95% CI: 1.4–2.9, HR = 0.60 [95% CI: 0.42–0.86], p = 0.005) in the chemotherapy group (Fig. 1A). The median PFS was 4.1 months (95% CI: 2.6–5.4) in the rociletinib 500-mg group and 5.5 months (95% CI: 1.8–8.1) in the rociletinib 625-mg group (Fig. 1B).
Figure 1.
Investigator-assessed PFS. (A) Rociletinib (500 mg and 625 mg twice daily doses) versus chemotherapy (efficacy population, n = 148).a(B) Rociletinib 625 mg twice daily or 500 mg twice daily versus chemotherapy (efficacy population). (C) Rociletinib (500-mg and 625-mg twice-daily doses) versus chemotherapy in T790M-positive patients; and (D) Rociletinib (500-mg and 625-mg twice-daily doses) versus chemotherapy in T790M-negative patients. aOne patient discontinued because of progressive disease before receiving a single dose of chemotherapy. One patient in the chemotherapy group was not followed up for PFS as the date of their death was not recorded. CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
PFS was also analyzed according to T790M-mutation status for the rociletinib (pooled 500 mg and 625 mg) and chemotherapy groups. For the T790M-positive population, the median PFS was 6.8 months (95% CI: 3.8–12.2) in the rociletinib group versus 2.7 months (95% CI: 1.3–7.0, HR = 0.55 [95% CI: 0.28–1.07], p = 0.074) in the chemotherapy group (Fig. 1C). In the T790M-negative population, the median PFS was 4.1 months (95% CI: 2.5–4.6) in the rociletinib group versus 1.4 months (95% CI: 1.3–2.7, HR = 0.54 [95% CI: 0.32–0.88], p = 0.012) in the chemotherapy group (Fig. 1D). In the T790M-unknown subgroup, the median PFS was 2.3 months with rociletinib (95% CI: 1.2–9.6) versus 4.4 months with chemotherapy (95% CI: 1.4–7.1), (HR = 0.87 [95% CI: 0.36–2.11], p = 0.759; Supplementary Fig. 1). The Kaplan-Meier analyses of PFS in the individual rociletinib dose groups by T790M status are illustrated in the Supplementary Data (Supplementary Fig. 2A–F).
The investigator-assessed confirmed ORR was determined in the efficacy population. A confirmed ORR of 17.3% (95% CI: 9.6–27.8) was observed in the combined rociletinib group (500-mg + 625-mg doses) versus 8.2% (95% CI: 3.1–17.0) in the chemotherapy group (Table 3). All responses were PRs. Similar ORRs were seen for both doses of rociletinib: 17.0% (95% CI: 8.1–29.8) and 18.2% (95% CI: 5.2–40.3) for 500 mg and 625 mg, respectively.
Table 3.
Response Rates in the Efficacy Population
| End Point | Overalla |
T790M Mutation–Positive |
T790M Mutation–Negative |
T790 Mutation Unknown |
||||
|---|---|---|---|---|---|---|---|---|
| Rociletinibb (n = 75) | Chemotherapy (n = 73) | Rociletinibb (n = 25) | Chemotherapy (n = 20) | Rociletinibb (n = 36) | Chemotherapy (n = 41) | Rociletinibb (n = 14) | Chemotherapy (n = 12) | |
| Confirmed ORR,c n (%) [95% CI] | 13 (17.3) [9.6–27.8] | 6 (8.2) [3.1–17.0] | 9 (36.0) [18.0–57.5] | 3 (15.0) [3.2–37.9] | 3 (8.3) [1.8–22.5] | 2 (4.9) [0.6–16.5] | 1 (7.1) [0.2–33.9] | 1 (8.3) [0.2–38.5] |
| Best overall confirmed response, n (%) | ||||||||
| CR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PR | 13 (17.3) | 6 (8.2) | 9 (36.0) | 3 (15.0) | 3 (8.3) | 2 (4.9) | 1 (7.1) | 1 (8.3) |
| SD | 44 (58.7) | 28 (38.4) | 12 (48.0) | 7 (35.0) | 25 (69.4) | 12 (29.3) | 7 (50.0) | 9 (75.0) |
| PD | 11 (14.7) | 31 (42.5) | 3 (12.0) | 8 (40.0) | 4 (11.1) | 21 (51.2) | 4 (28.6) | 2 (16.7) |
| NE | 7 (9.3) | 8 (11.0) | 1 (4.0) | 2 (10.0) | 4 (11.1) | 6 (14.6) | 2 (14.3) | 0 |
| Median duration of response (95% CI), mo | 11.0 (4.3–13.7) | 6.8 (4.5–NA) | 12.3 (2.5–22.1) | NA (4.5–NA) | 5.5 (2.8–13.1) | NA (5.8–NA) | 4.3d | 6.8d |
CI, confidence interval; CR, complete response; NA, not assessable; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors version 1.1; SD, stable disease.
Includes patients with unevaluable T790M mutation status.
Rociletinib 500-mg twice daily and 625-mg twice-daily dosage groups were pooled for this analysis.
Assessed according to RECIST.
No 95% CI interval as n = 1.
Response data were also analyzed according to T790M mutation status. For the T790M-positive population, the ORR was 36.0% (95% CI: 18.0–57.5) in the rociletinib group and 15.0% (95% CI: 3.2–37.9) in the chemotherapy group. In the T790M-negative population, the ORR in the rociletinib group was 8.3% (95% CI: 1.8–22.5) versus 4.9% (95% CI: 0.6–16.5) in the chemotherapy group. In the small T790M-unknown subgroup, the ORR was 7.1% (95% CI: 0.2–33.9) for the combined rociletinib group versus 8.3% (95% CI: 0.2–38.5) for the chemotherapy group.
The median confirmed DOR was 11.0 months (95% CI: 4.3–13.7) in the rociletinib group versus 6.8 months (95% CI: 4.5–not assessable [NA]) in the chemotherapy group (HR = 0.91, 95% CI: 0.22–3.81, p = 0.895) (Table 3). For patients with T790M-positive tumors treated with rociletinib, the median DOR was 12.3 months (95% CI: 2.5–22.1) versus NA (95% CI: 4.5–NA) for chemotherapy-treated patients. For rociletinib-treated patients with T790M-negative tumors, the median DOR was 5.5 months (95% CI: 2.8–13.1) versus NA (95% CI: 5.8–NA) for patients treated with chemotherapy.
Adverse Events
Nearly all patients in the safety population (n = 148) experienced at least one treatment-emergent AE (TEAE) (74 of 75 [98.7%] in the combined rociletinib group; 71 of 73 [97.3%] in the chemotherapy group) (Table 4). A summary of TEAEs by rociletinib dose is illustrated in the Supplementary Table 1.
Table 4.
Most Common TEAEs
| Eventa | Rociletinibb (n = 75) |
Chemotherapy (n = 73) |
||
|---|---|---|---|---|
| Any Grade, n (%) | Grade ≥3, n (%) | Any Grade, n (%) | Grade ≥3, n (%) | |
| Patients with ≥1 TEAE | 74 (98.7) | 49 (65.3) | 71 (97.3) | 42 (57.5) |
| Diarrhea | 48 (64.0) | 2 (2.7) | 12 (16.4) | 1 (1.4) |
| Hyperglycemia | 44 (58.7) | 18 (24.0) | 6 (8.2) | 0 |
| Nausea | 28 (37.3) | 3 (4.0) | 20 (27.4) | 4 (5.5) |
| Fatigue | 28 (37.3) | 6 (8.0) | 18 (24.7) | 7 (9.6) |
| Decreased appetite | 28 (37.3) | 0 | 10 (13.7) | 2 (2.7) |
| Cough | 21 (28.0) | 0 | 14 (19.2) | 0 |
| QTc prolongation | 20 (26.7) | 5 (6.7) | 0 | 0 |
| Vomiting | 18 (24.0) | 1 (1.3) | 6 (8.2) | 0 |
| Anemia | 9 (12.0) | 2 (2.7) | 18 (24.7) | 2 (2.7) |
QTc, corrected QT interval; TEAE, treatment-emergent adverse event.
TEAEs of greater than or equal to 20% incidence in either group are illustrated, sorted by descending incidence in rociletinib-treated patients.
Rociletinib 500-mg twice daily and 625-mg twice-daily dosage groups were pooled for this analysis.
Grade 3 or higher TEAEs were reported by 49 of 75 (65.3%) rociletinib-treated patients and 42 of 73 (57.5%) chemotherapy-treated patients. The most frequently reported grade 3 or higher TEAE for rociletinib was hyperglycemia (18 of 75 [24.0%] versus 0 with chemotherapy); five of 75 (6.7%) rociletinib-treated patients experienced grade 3 or higher QTc prolongation versus none with chemotherapy. Neutropenia and neutrophil count decrease were the most common grade 3 or higher TEAEs in the chemotherapy group (both eight of 73 [11.0%]) and were observed more often in chemotherapy-treated patients than rociletinib-treated patients (both one of 75 [1.3%] in rociletinib-treated patients).
Five patients (6.7%) in the rociletinib group experienced a grade 4 TEAE. One patient each (1.3%) had lymphopenia, lymphocyte count decreased, and hypophosphatemia, all of which were assessed as not related to study drug; two patients (2.7%) had grade 4 hyperglycemia assessed as being related to study drug. Eleven patients (15.1%) in the chemotherapy group experienced at least one grade 4 TEAE (neutropenia: three of 73 [4.1%]; γ1 γ-glutamyltransferase increase: one of 73 [1.4%]; lymphocyte count decreased: one of 73 [1.4%]; neutrophil count decreased: four of 73 [5.5%]; aspiration: one of 73 [1.4%]; hypercalcaemia: one of 73 [1.4%]; white blood cell count decreased: one of 73 [1.4%]); the events in eight patients (11.0%) were assessed as related to study drug.
Treatment interruption owing to a TEAE occurred in 37 of 75 patients (49.3%) in the combined rociletinib group and 19 of 73 (26.0%) in the chemotherapy group. Dose reduction owing to a TEAE occurred in 16 of 75 patients (21.3%) in the combined rociletinib group and 11 of 73 (15.1%) in the chemotherapy group (Supplementary Table 1). The most common TEAE leading to dose reduction was fatigue in the rociletinib group (6.7%) and neutropenia in the chemotherapy group (4.1%). Excluding disease progression, 12 of 75 patients (16.0%) in the combined rociletinib group and 11 of 73 (15.1%) in the chemotherapy group discontinued treatment owing to a TEAE, most often owing to diarrhea (4.0%) in rociletinib-treated patients and pleural effusion (2.7%) in chemotherapy-treated patients.
There were seven (9.3%) and two (2.7%) deaths owing to disease progression in the combined rociletinib group and chemotherapy group, respectively. Deaths because of a TEAE (excluding disease progression) were reported in six patients (8.0%) in the combined rociletinib group (two cases of pneumonia and one case each of cardiopulmonary arrest, dehydration, subdural hematoma, and sudden death) and one (1.4%) patient in the chemotherapy group (owing to an infection). The sudden death was the only TEAE considered by the investigator to be related to the study drug.
Other TEAEs of interest included pneumonitis and cataract. Four of 75 patients (5.3%) in the combined rociletinib group had pneumonitis versus none in the chemotherapy group. Cataract was reported in eight of 75 (10.7%) rociletinib-treated patients and one of 73 (1.4%) chemotherapy-treated patient. Three patients in the rociletinib group had grade 3 cataract, which were all considered to be related to the study drug.
Clinical Laboratory Assessments
Grade 3 or higher postbaseline glucose values (>250 mg/dL/13.9 mmol/liter) were observed in 17 of 75 (22.7%) of rociletinib-treated patients, five (6.7%) of whom had at least two incidences of grade 3 or higher postbaseline glucose levels. Hyperglycemia was managed with antihyperglycemic therapy and dose reductions. The frequencies of previous hyperglycemia medications in the safety population were 9.3% and 9.6% in the rociletinib and chemotherapy groups, respectively. After study treatment, 50.7% and 5.5% of patients in the rociletinib and chemotherapy groups, respectively, required hyperglycemia medication.
QTc Findings
QTc prolongation was observed in 20 of 75 (26.7%) of rociletinib-treated patients; five of 75 (6.7%) experienced a grade 3 QTc prolongation (prolonged QTc >500 msec [Common Terminology Criteria for Adverse Events version 4.03]) (Table 4). All events were assessed as related to the study drug. Nine rociletinib-treated patients (12.0%) experienced a QTc prolongation of greater than or equal to 501 msec using the Fridericia correction method. Of these, five patients (9.4%) were in the rociletinib 500 mg, and four (18.2%) were in the 625-mg group. One rociletinib-treated patient, who subsequently had a sudden death, experienced a serious QTc prolongation (559 ms) 2 weeks after the initiation of treatment, which was assessed as related to the study drug (as previously mentioned above). No patients in the chemotherapy group experienced QTc prolongation.
Discussion
The unmet need for novel therapies to treat patients with EGFR-mutated NSCLC, especially T790M-positive NSCLC, stimulated the discovery and clinical evaluation of rociletinib. Development of rociletinib for the treatment of patients with EGFR-mutant NSCLC who had been previously treated with an EGFR-targeted therapy and whose tumor carried the T790M mutation was halted in 2016 per sponsor decision. After this decision, regulatory submissions to the United States and European authorities were withdrawn. Beginning in late 2016, Clovis Oncology continued to provide rociletinib to patients who elected to continue receiving rociletinib therapy.
In the TIGER-3 study, a comparable number of patients in the rociletinib and chemotherapy groups experienced TEAEs; however, the types of TEAEs varied between the two groups. In the rociletinib group, we reported grade 3 or higher hyperglycemia and QTc prolongation—these TEAEs were not observed or reported in the chemotherapy group. In addition, grade 3 diarrhea and vomiting occurred at a higher rate in the rociletinib group. The incidence of pneumonitis (5.3%) in patients receiving rociletinib was higher than the reported incidence rate (1.3%–2.6%) in patients with advanced NSCLC who were treated with a first- (gefitinib) or second-generation (afatinib) EGFR TKI.3,4 Discontinuation and dose reduction of study drug owing to TEAEs were more frequent in the rociletinib group than the chemotherapy group. One death in the rociletinib group was considered related to the study drug by the investigator; no drug-related deaths were reported in the chemotherapy group.
Treatment-related hyperglycemia is known to occur after the initiation of rociletinib treatment.16 In humans, rociletinib has three major metabolites: M460, M502, and M544. M460 and M502 were found to exhibit inhibitory activity against the insulin growth factor receptor 1 and insulin receptor.16 In the TIGER-3 study, these metabolites also likely contributed to treatment-emergent hyperglycemia after initiation of rociletinib therapy.
Although the development of rociletinib was halted, other third-generation EGFR TKIs have been developed. In 2015, the third-generation EGFR TKI osimertinib was first approved for the treatment of patients with metastatic EGFR T790M-positive NSCLC who have progressed after treatment with a first-generation EGFR TKI,17,18 based on the outcome of the phase 2 AURA study19 and the phase 3 AURA3 study.20 More recently, osimertinib was approved for first-line treatment of patients with EGFR mutation–positive NSCLC, on the basis of results from the phase 3 FLAURA study.21, 22, 23
Other third-generation EGFR TKIs in development include nazartinib24 and lazertinib.25,26 Nazartinib is being evaluated in combination with gefitinib in patients with recurrent or Stage IIIB to IV EGFR-mutated NSCLC. A dose-finding study of lazertinib is in progress among patients with EGFR-mutated advanced NSCLC.26 Similar to rociletinib, the sponsors have halted the development of other EGFR TKIs. Although olmutinib is approved in South Korea for the treatment of patients with EGFR T790M mutation–positive lung cancer, it is not approved for other indications or in other territories, and its development was discontinued by Boehringer Ingelheim in 2016.27 Other agents (including ASP8273 or PF-06747775) are no longer being developed for the treatment of patients with EGFR-mutated NSCLC.28,29
In the TIGER-3 study, although there was a trend toward improved PFS with rociletinib versus second-line chemotherapy in the T790M-positive and T790M-negative patient populations, early termination of the study precluded formal hypothesis testing of the primary end point. In patients who received rociletinib, 15 (20%) received treatment for more than 12 months. Nevertheless, rociletinib had unacceptable toxicities, including a higher incidence of hyperglycemia and QTc prolongation compared with chemotherapy. Whereas no conclusion can be drawn because of the early termination of the trial, rociletinib activity in patients with T790M-negative tumors could potentially be explained by the heterogeneity of the tumor cells or limitation in the sensitivity of the assay that detects T790M mutations. This brings into question whether these tumors were truly T790M negative. To our knowledge, TIGER-3 is the only randomized study that has compared second-line chemotherapy with an EGFR TKI after patients failed both a first- or second-generation EGFR TKI and platinum-based chemotherapy in an unselected patient population. In addition to osimertinib, we hope that novel agents will be developed to provide patients with EGFR mutation–positive advanced NSCLC with new treatment options that have a favorable benefit-risk profile in this setting.
Acknowledgments
This work was supported by Clovis Oncology, Inc. The company designed and provided funding for the phase 3 TIGER-3 study (CO-1686-020; NCT02322281; EudraCT 2014-003437-26). The company collected and analyzed the data, which were provided to all authors on request. Writing and editorial assistance was funded by Clovis Oncology, Inc., and was provided by Wendy Smith, PhD, Stephen Mason, PhD, and Frederique H. Evans, MBS (Ashfield Healthcare Communications, Middletown, CT). All authors were involved in the interpretation of the data and drafting of the manuscript. The authors made the final decision to submit the manuscript. The authors thank all of the patients and their families and caregivers for their participation in the TIGER-3 study.
Footnotes
Disclosure: Dr. Yang reports receiving personal fees from Boehringer Ingelheim, Eli Lilly, Bayer Pharmaceuticals, Roche/Genentech, Chugai Pharmaceutical, Merck Sharp & Dohme, Pfizer, Novartis, Bristol-Myers Squibb, Ono Pharmaceuticals, AstraZeneca, ACTGenomics, Merck Serono, Celgene, Yuhan Pharmaceuticals, Daiichi Sankyo, Hansoh Pharmaceuticals, Takeda Pharmaceuticals, Blueprint Medicines, and G1 Therapeutics outside of the submitted work. Dr. Reckamp reports receiving grants and personal fees from Loxo Oncology, Guardant, Boehringer Ingelheim, Takeda Pharmaceuticals, and Genentech; grants from Bristol-Myers Squibb, Exelixis, Pfizer, Xcovery, Janssen, Zeno, Adaptimmune, Acea Biosciences, and GlaxoSmithKline; and personal fees from Eli Lilly, Tesaro, and AstraZeneca outside of the submitted work. Dr. Shih reports receiving personal fees and other fees from Clovis Oncology, Inc. during the conduct of the study and Clovis Oncology, Inc. outside of the submitted work. Dr. Popat reports receiving personal fees from Bristol-Myers Squibb, Roche, Takeda Pharmaceuticals, AstraZeneca, Pfizer, Merck Sharp & Dohme, EMD Serono, Guardant Health, AbbVie, Boehringer Ingelheim, OncLive, Medscape, Incyte, Paradox Pharmaceuticals, Eli Lilly outside of the submitted work. Dr. Novello reports receiving personal fees (as advisor/speaker bureau) from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Bristol-Myers Squibb, Bayer Pharmaceuticals, Pfizer, Roche, Merck Sharp & Dohme, Takeda outside of the submitted work. Dr. Groen reports receiving grants from Boehringer Ingelheim and other fees from Roche outside of the submitted work. Dr. Golsorkhi reports receiving personal fees and other fees from Clovis Oncology, Inc. during the conduct of the study and outside of the submitted work. Dr. Despain reports receiving personal fees and other fees from Clovis Oncology, Inc. during the conduct of the study and personal fees from Clovis Oncology, Inc. outside the of submitted work. Dr. Blakely reports receiving grants from Clovis Oncology during the conduct of the study and grants from AstraZeneca outside of the submitted work. Dr. Bazhenova reports receiving personal fees from Genentech, Novartis, Boehringer Ingelheim, Blueprint, Takeda Pharmaceuticals, AstraZeneca, BeyondSpring, G1 Therapeutics, Bayer Pharmaceuticals, AbbVie, Loxo Oncology, Eli Lilly, Pfizer, Boston Bio outside of the submitted work. Dr. Wakelee reports receiving personal fees (for consulting) from AstraZeneca, Xcovery, Janssen, Daiichi Sankyo, Inc., Helsinn, Mirati and Blueprint; and grants to institution for research conduct from Acea Biosciences, Arrys Therapuetics, AstraZeneca/Med Immune, Bristol-Myers Squibb, Celgene, Clovis Oncology, Exelixis, Eli Lilly, Pfizer, and Pharmacyclics outside of the submitted work. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100114.
Supplementary Data
References
- 1.Herbst R.S., Heymach J.V., Lippman S.M. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosell R., Moran T., Queralt C. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 3.Mok T.S., Wu Y.L., Thongprasert S. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Sequist L.V., Yang J.C., Yamamoto N. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 5.Pao W., Miller V.A., Politi K.A. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S.V., Bell D.W., Settleman J., Haber D.A. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 7.Yu H.A., Arcila M.E., Rekhtman N. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman J.W., Wakelee H., Gadgeel S. Dose optimization of rociletinib for EGFR mutated NSCLC. J Thorac Oncol. 2015;10(suppl 2):S318–S319. [Google Scholar]
- 9.Walter A.O., Sjin R.T., Haringsma H.J. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov. 2013;3:1404–1415. doi: 10.1158/2159-8290.CD-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sequist L.V., Soria J.C., Gadgeel S.M. First-in-human evaluation of CO-1686, an irreversible, highly selective tyrosine kinase inhibitor of mutations of EGFR (activating and T790M) J Clinical Oncol. 2014;32(suppl 15):8010. [Google Scholar]
- 11.Sequist L.V., Soria J.C., Camidge D.R. Update to rociletinib data with the RECIST confirmed response rate. N Engl J Med. 2016;374:2296–2297. doi: 10.1056/NEJMc1602688. [DOI] [PubMed] [Google Scholar]
- 12.Goldman J.W., Soria J.C., Wakelee H.A. Updated results from TIGER-X, a phase I/II open label study of rociletinib in patients (pts) with advanced, recurrent T790M-positive non-small cell lung cancer (NSCLC) J Clin Oncol. 2016;34(suppl 15):9045. [Google Scholar]
- 13.Yang J.C.H., Reckamp K.L., Kim Y.C. TIGER-3: A phase 3 randomized study of rociletinib vs chemotherapy in EGFR-mutated non-small cell lung cancer (NSCLC) J Thorac Oncol. 2017;12(suppl 2):S2397. [Google Scholar]
- 14.Goldman J.W., Karlovich C., Sequist L.V. EGFR genotyping of matched urine, plasma, and tumor tissue in patients with non–small-cell lung cancer treated with rociletinib, an EGFR tyrosine kinase inhibitor. JCO Precis Oncol. 2018;2:1–13. doi: 10.1200/PO.17.00116. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health. National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. http://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 16.Goldman J.W., Mendenhall M.A., Rettinger S.R. Hyperglycemia associated with targeted oncologic treatment: mechanisms and management. Oncologist. 2016;21:1326–1336. doi: 10.1634/theoncologist.2015-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skoulidis F., Papadimitrakopoulou V.A. Targeting the gatekeeper: osimertinib in EGFR T790M mutation-positive non-small cell lung cancer. Clin Cancer Res. 2017;23:618–622. doi: 10.1158/1078-0432.CCR-15-2815. [DOI] [PubMed] [Google Scholar]
- 18.Wang S., Cang S., Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9:34. doi: 10.1186/s13045-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J.C., Ahn M.J., Kim D.W. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017;35:1288–1296. doi: 10.1200/JCO.2016.70.3223. [DOI] [PubMed] [Google Scholar]
- 20.Mok T.S., Wu Y.L., Ahn M.J. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soria J.C., Ohe Y., Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 22.Ramalingam S.S., Gray J.E., Ohe Y. Osimertinib vs comparator EGFR-TKI as first-line treatment for EGFRm advanced NSCLC (FLAURA): final overall survival analysis. Ann Oncol. 2019;30(suppl 5):v914–v915. [Google Scholar]
- 23.Ohe Y., Imamura F., Nogami N. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol. 2019;49:29–36. doi: 10.1093/jjco/hyy179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institutes of Health. National Cancer Institute Clinical trials using nazartinib. https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/nazartinib?redirect=true
- 25.ClinicalTrials.gov Clinical trial of YH25448 in patients with EGFR mutation positive advanced NSCLC. https://clinicaltrials.gov/ct2/show/NCT03046992
- 26.Chui Cho B., Han J.Y., Lee K.H. YH25448, a 3rd generation EGFR-TKI, in patients with EGFR-TKI-resistant NSCLC: phase I/II study results. J Clin Oncol. 2018;36(suppl 15):9033. [Google Scholar]
- 27.Genetic Engineering & Biotechnology News Boehringer Ingelheim ends partnership with Hanmi to develop lung cancer candidate. https://www.genengnews.com/topics/translational-medicine/boehringer-ingelheim-ends-partnership-with-hanmi-to-develop-lung-cancer-candidate/
- 28.National Institutes of Health, National Cancer Institute Clinical trials using mavelertinib. https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/notrials?p1=mavelertinib
- 29.Astellas Astellas announces decision to discontinue ASP8273 treatment arm and close randomization for clinical study protocol 8273-CL-0302 - decision follows recommendation of independent data monitoring committee. https://newsroom.astellas.us/2017-05-10-Astellas-Announces-Decision-to-Discontinue-ASP8273-Treatment-and-Close-Randomization-for-Clinical-Study-Protocol-8273-CL-0302
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.