Before the recent approval of capmatinib in the United States, crizotinib was recommended by the National Comprehensive Cancer Network guidelines for the treatment of advanced MET exon 14 mutant (METex14) NSCLC. The role of capmatinib in patients exhibiting disease progression after treatment with crizotinib is not well established. Here, we describe a patient with metastatic METex14 NSCLC who, after an initial systemic response to crizotinib, experienced isolated central nervous system (CNS) progression followed by a leptomeningeal response to capmatinib, highlighting the CNS activity of capmatinib.
A 75-year-old never-smoker male patient was diagnosed as having metastatic lung adenocarcinoma harboring a MET exon 14 mutation (c.3028G>C, p.D1010H) with concurrent amplification of the mutant MET allele. There were no other targetable oncogenic mutations, and the programmed death-ligand 1 tumor proportion score was 80%. No definitive CNS metastases were found in the initial brain magnetic resonance imaging (MRI). The patient began off-label crizotinib 250 mg twice daily and experienced clinical improvement and a radiographic response to therapy (Fig. 1). After 9 months of treatment, he developed dehydration, hyponatremia, and confusion. A brain and spine MRI revealed an enhancing nodule in the left frontal sulcus, consistent with leptomeningeal disease, and an enhancing lesion in the cauda equina. A positron emission tomography–computed tomography scan did not reveal any evidence of systemic disease progression (Fig. 1). Plasma genotyping (Guardant360) revealed the MET c.3028G>C mutation at a very low allele fraction (0.06%) and no other apparent resistance mutations.
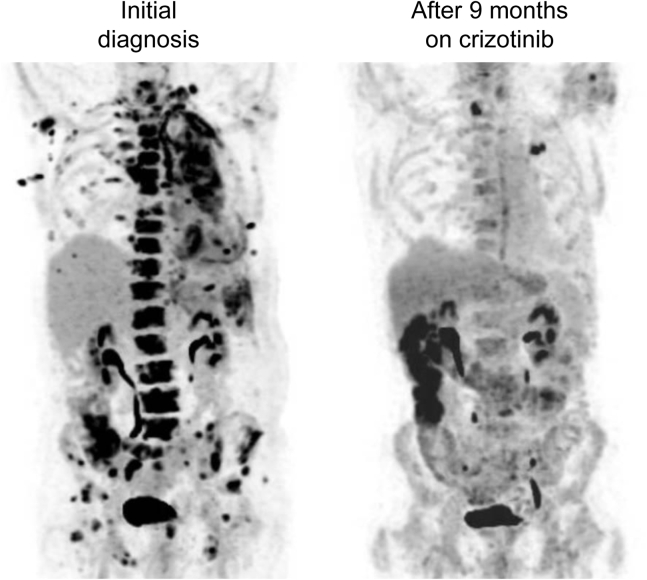
Figure 1.
Maximum intensity projection coronal image of 18F-fluorodeoxyglucose positron emission tomography study at the time of the initial diagnosis revealed extensive disease in the left lung, pleura, lymph nodes, bone, and soft tissues (left). After 9 months of treatment with crizotinib (right), the overall marked response was noted in the disease sites in the chest, abdomen, and pelvis.
Crizotinib was discontinued, and the patient received one dose of carboplatin, pemetrexed, and pembrolizumab. He subsequently developed hearing loss, headaches, photophobia, diplopia, facial numbness, and gait instability. Brain and spine imaging revealed two new 4- to 5-mm parenchymal metastases and also multiple foci of leptomeningeal disease along the right parieto-occipital region, left frontal sulcus, cerebellar folia, second, third, fourth, seventh, and eighth cranial nerves, and along spinal cord (Fig. 2A–D, left).
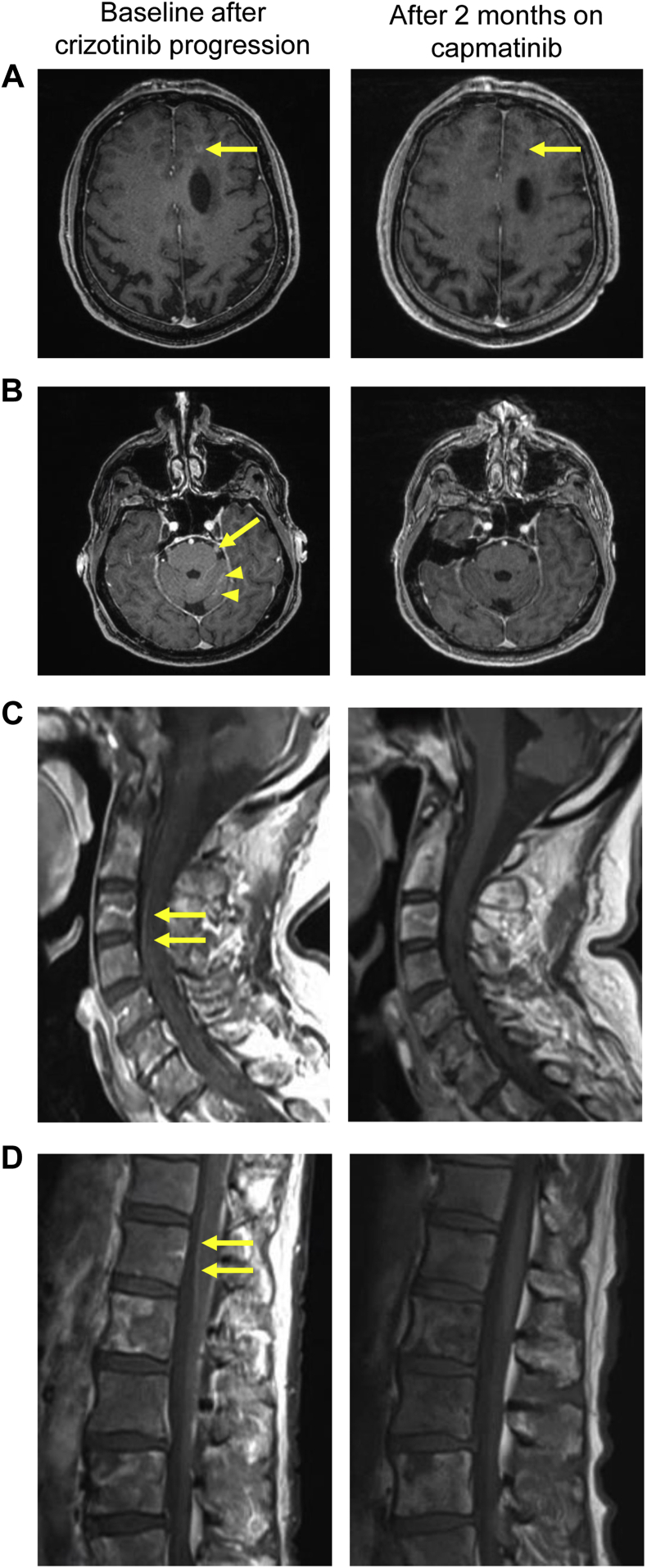
Figure 2.
Magnetic resonance imaging (MRI) of brain parenchyma and leptomeningeal disease before treatment with capmatinib (left panels), and at 2 months of capmatinib therapy exhibiting treatment response (right panels). (A) Axial enhanced T1-weighted MRI revealing a 5-mm rim-enhancing lesion in the left frontal lobe (left, arrow) and residual punctate T1 hypointense lesion after treatment (right, arrow). (B) Leptomeningeal enhancement along the cisternal segment of the left trigeminal nerve (left, arrow) and along the folia of the left cerebellar hemisphere (left, arrowheads), the enhancement was no more detectable after 2 months of treatment (right panel). (C, D) Sagittal enhanced T1-weighted MRI revealing leptomeningeal enhancement along the spinal cord (arrows), which was no longer seen after 2 months (right panels).
Carboplatin, pemetrexed, and pembrolizumab were discontinued, and capmatinib 400 mg twice daily was started after obtaining signed informed consent for an institutional review board–approved investigational new drug application through Novartis’s Managed Access Program (Dana-Farber Harvard Cancer Center protocol #20-085). The patient noted a rapid clinical improvement in his headaches, nausea, energy level, appetite, and gait, though he continued to experience some persistent facial numbness, hearing loss, and diplopia. MRI imaging 2 months later revealed a decrease in the intraparenchymal brain metastases and leptomeningeal enhancement of the brain and spinal cord (Fig. 2A–D, right).
In METex14 cases of disease progression caused by acquired resistance mutations in the MET kinase domain, such as at positions D1228 or Y1230,1 switching therapy from one type I MET tyrosine kinase inhibitor (TKI) to another (e.g., crizotinib, capmatinib, tepotinib, savolitinib) is unlikely to be beneficial since these alterations confer high-level resistance to this drug class.2 However, disease progression owing to limited drug CNS penetration, a known liability of crizotinib,3,4 may be responsive to other type I MET TKIs that have better CNS activity, such as capmatinib.5 Additional prospective studies (such as NCT02750215) may be informative in understanding the clinical scenarios in which capmatinib might be most effective in patients who have received previous MET TKIs, including those with previous CNS-only disease progression.
Footnotes
Disclosure: Dr. Nishino reports receiving personal fees from Daiichi Sankyo, AstraZeneca, and Roche and grants from Merck, AstraZeneca, Daiichi Sankyo, Canon Medical Systems, and National Institutes of Health, outside of the submitted work. Dr. Awad reports receiving grants and personal fees from Genentech, AstraZeneca, and Bristol-Myers Squibb; grants from Eli Lilly; and personal fees from Merck, Maverick, Blueprint Medicine, Syndax, Ariad, Nektar, and Gritstone, outside of the submitted work. The remaining authors declare no conflict of interest.
References
- 1.Recondo G., Bahcall M., Spurr L.F. Molecular mechanisms of acquired resistance to MET tyrosine kinase inhibitors in patients with MET exon 14-mutant NSCLC. Clin Cancer Res. 2020;26:2615–2625. doi: 10.1158/1078-0432.CCR-19-3608. [DOI] [PubMed] [Google Scholar]
- 2.Fujino T., Kobayashi Y., Suda K. Sensitivity and resistance of MET exon 14 mutations in lung cancer to eight MET tyrosine kinase inhibitors in vitro. J Thorac Oncol. 2019;14:1753–1765. doi: 10.1016/j.jtho.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T., Oya Y., Tanaka K. Clinical impact of crizotinib on central nervous system progression in ALK-positive non-small lung cancer. Lung Cancer. 2016;97:43–47. doi: 10.1016/j.lungcan.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Costa D.B., Shaw A.T., Ou S.H. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garon EB, Heist RS, Seto T, et al. Capmatinib in METex14-mutated (mut) advanced non-small cell lung cancer (NSCLC): results from the phase II GEOMETRY mono-1 study, including efficacy in patients (pts) with brain metastases (BM). Paper presented at: American Association for Cancer Research Virtual Annual Meeting I: Virtual. April 27–28, 2020; Philadelphia, PA.