Abstract
Introduction
Patients with early-stage NSCLC typically must choose between a surgery with superior local control (lobectomy) or one that preserves lung parenchyma (wedge). Recognizing that many patients with cancer have competing mortality risks unrelated to cancer, we investigated whether an established model of predicting life expectancy could be used to identify patients with stage I NSCLC for whom survival after wedge is not different from lobectomy.
Methods
A retrospective cohort study using the National Cancer Institute’s Surveillance Epidemiology and End Results—Medicare was performed to evaluate survival among treatment-naive patients, diagnosed 2005–2015, who underwent lobectomy or wedge for stage I (≤2 cm tumors) NSCLC. Comorbidity-related life expectancy (CR-LE) was estimated using a standard life-table approach based on comorbid conditions, sex, and age. Cox models and perioperative complications were stratified by 5-year CR-LE.
Results
A total of 4560 patients (median age 74, interquartile range 70–78) were identified. CR-LE was greater than or equal to 5 years for 4016 patients (wedge = 23%). CR-LE was less than 5 years for 544 patients (wedge = 41%). Among patients with CR-LE greater than or equal to 5, wedge resection was associated with higher risk of mortality than lobectomy (hazard ratio: 1.68, 95% confidence interval: 1.52–1.86, p < 0.001). For those with CR-LE less than 5, there was no significant difference in mortality risk between lobectomy and wedge (hazard ratio: 1.19, 95% confidence interval: 0.96–1.47; p = 0.11). CR-LE less than five patients who underwent a lobectomy had higher 90-day mortality compared with wedge (9% versus 4%, p = 0.04).
Conclusion
The survival advantage of lobectomy over wedge for stage I NSCLC seems to dissipate among patients with shorter life expectancy owing to age and comorbidities. Wedge resection may be a reasonable option for patients at high risk of dying from non–cancer-related causes.
Keywords: Non–small cell lung cancer, Stage I, Lobectomy, Wedge, Mortality
Introduction
For patients with early-stage– lung cancer, surgical resection has historically been associated with the greatest survival.1,2 For localized tumors, pulmonary lobectomy has been considered the standard of care in large part owing to a randomized trial by the Lung Cancer Study Group.3 The trial revealed superior survival among patients with early-stage NSCLC (≤3 cm) managed by lobectomy compared with sublobar resection (i.e., wedge or segmentectomy). This finding has been supported by numerous4, 5, 6 (but not all7, 8, 9) observational studies.
Although lobectomy may offer a long-term survival advantage in some patients with early-stage NSCLC, others might not tolerate the greater loss of lung tissue, both perioperatively and long term. More specifically, a pulmonary lobectomy entails removing more lung parenchyma than a wedge resection (60%–70% more tissue). Given the high prevalence of smoking-related lung injury and other lung diseases (e.g., interstitial lung disease) in patients with NSCLC, most have some baseline degree of pulmonary dysfunction before surgery, which may be worsened by a large parenchymal resection (lobectomy). By preserving a greater amount of lung tissue, sublobar may have a less negative impact on postoperative pulmonary function, although this is still debatable.10, 11, 12 Furthermore, patients with a compromised life expectancy owing to other comorbidities might want to avoid a more extensive resection that can be associated with higher prevalence of perioperative complications.13, 14, 15 Finally, a limited literature suggests that for patients with significant lung disease, quality of life after sublobar is superior to that following lobectomy.16, 17, 18, 19, 20 As a result, many patients with early-stage NSCLC must strike a balance between procedures that may maximize how long they will live, with procedures that maximize how well they recover.
Not all patients with lung cancer die of lung cancer. In fact, between 15% and 20% of patients with early-stage NSCLC will die from unrelated causes.21 In this more fragile population, the oncologic advantage of taking more lung tissue (lobectomy) may not be as important as diminishing the risk of perioperative death and preserving lung function (wedge).22, 23, 24 We hypothesized that the long-term survival advantage of lobectomy over wedge for early-stage NSCLC would lessen in the setting of competing mortality risks. To test this, we compared survival and complication rates of surgically managed early-stage NSCLC stratified according to their predicted life expectancy from causes unrelated to cancer. The objective was to determine if a subset of these patients whose health is most tenuous could potentially choose a less extensive resection without compromising their outcomes.
Materials and Methods
Data Source
The study was conducted using the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER)—Medicare 2018 linked database.25 The linked deidentified data from these two sources include clinical, demographic information, and outcomes for patients with cancer from SEER and Medicare claims for health care services. The institutional review board of the Yale School of Medicine approved this study with consent waived.
Study Sample
The SEER—Medicare linked database (2018) was queried for treatment-naive patients 67 years or older, diagnosed with having stage I NSCLC (bronchoalveolar carcinoma excluded) who underwent lobectomy or wedge in the 6 months after diagnosis. Included patients were diagnosed with having invasive NSCLC as their first malignancy from January 2005 to December 2015. To capture comorbidity claims adequately, only patients continuously enrolled in Medicare part A and B-fee for service for 2 years before diagnosis and 1 year after diagnosis were included. Patients with tumors greater than 2 cm, incomplete staging, treatment or follow-up data and those without microscopic or histologic confirmation were excluded (Supplementary Fig. 1 for consort diagram).
Segmentectomy patients (an anatomical form of sublobar resection) were excluded because this procedure tends to be performed far less commonly than wedge and by a smaller cohort of surgeons (perhaps relating to the additional complexity that segmentectomies entail).26 Furthermore, the objective of the study was to establish a cohort that could have maximal sparing of lung parenchyma while not sacrificing prognosis. Although segmentectomy does remove considerably less parenchyma than lobectomy in most settings, the greatest differential in tissue removal is between lobectomy and wedge.12
Data Elements
The International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM), and Current Procedural Terminology-Healthcare Common Procedure Coding System (HCPCS) were used to extract claims for treatments (Supplementary Table1) and comorbid conditions.27 The transition to ICD-10-CM occurred in October 2015. Therefore, claims before October 2015 were based solely on ICD-9-CM, whereas claims from that point forward incorporated ICD-9-CM and ICD-10-CM codes (except surgery type because ICD-10-CM does not allow for differentiation between wedge and segmentectomy).28 Surgery type was classified as wedge or lobectomy. Independent variables included the following: age, sex, race, area of residence (according to ZIP code), income (according to ZIP code), Elixhauser comorbidity, year of diagnosis, tumor grade, tumor histology and laterality, and receipt of adjuvant chemotherapy or radiation. The American Joint Committee on Cancer staging 6th (<2010) and 7th (≥2010) editions were used. Survival time was calculated from day of surgery to death or December 31, 2017 (overall survival [OS]) or December 31, 2015 (cancer-specific survival [CSS]).
Indicators of Adversity in the Postoperative Period
Previous work suggests that surgical complications might not be adequately captured in claims data.29 Therefore, surrogates of complications were studied including prolonged length of hospital stay (categorized as >14 d30), 30-day readmission, and 90-day postoperative mortality. In addition, a new indication for the use of supplemental oxygen (≥45 d post discharge) was derived using claims as previously described.31 Patients with claims for supplemental oxygen during the year before surgery (i.e., chronic oxygen need) were excluded from this analysis.
Comorbidity-Related Life Expectancy
For each patient, the comorbidity-related life expectancy (CR-LE) was estimated using predictors unrelated to cancer (i.e., comorbidities, age, and sex) as a mechanism to understand the competing risk to each patient’s survival posed by health- and age-related factors. The ICD-9-CM, ICD-10-CM, and HCPCS codes were used to identify claims in the 24 through 3 months before cancer diagnosis to generate a modified list of comorbidities as described by Elixhauser et al.27 Elixhauser captures 31 comorbidities which has been found to perform better than other measures in predicting mortality beyond 30 days, in particular, in administrative data.32 CR-LE was calculated using a standard life-table approach.33,34 Age, sex, and Elixhauser comorbidities of each patient in our cohort were used to derive what would have been their annual mortality rates according to those characteristics if they were not to have cancer (using a sample of patients without cancer). A predicted life expectancy was generated from these characteristics, as previously described.34 Patients were stratified into two groups based on whether their CR-LE was less than 5 or greater than or equal to 5 years.
When stratifying patients by life expectancy, 5 years was chosen as cutoff because it is comparable to the traditionally calculated metric of long-term (estimated 5-year) survival for patients with cancer.35 In the shared decision-making process, patients and families are typically counseled using predicted 5-year survival estimates. However, after preliminary selection, the 5-year cutoff was more rigorously evaluated as a moderator of the comparative effectiveness by moderation analysis described subsequently.
Statistical Analysis
CR-LE as a Moderator of the Relationship Between Surgery Type and Long-Term OS. Moderation models are used to test whether the effect of an independent variable on a dependent variable differ across levels of a third variable.36 To evaluate if CR-LE moderates the relationship between surgery type (independent variable) and long-term OS (dependent variable), a multivariate Cox proportional hazards model was built including an interaction effect of CR-LE (categorical, <5, or ≥5 years) and surgery type. Additional covariates included age, sex, race, area of residence, income, Elixhauser comorbidity, year of diagnosis, tumor grade, tumor histology, laterality, and receipt of adjuvant chemotherapy. The inclusion of covariates in the model was based on clinical relevance and known impact on survival. Given the quality of tumor resection between the two interventions is one of the aspects that we were indirectly aiming to compare, adjuvant radiotherapy was not adjusted for in the model as it could be an indication of positive margins (i.e., patients included, but “adjuvant radiation” was not a covariate).
Once 5-year CR-LE was found to moderate the relationship between surgery type and long-term OS (i.e., the interaction effect was significant), the population was stratified in CR-LE levels. The Kaplan-Meier method was used to calculate 5-year OS. Differences between the OS of lobectomy and wedge were assessed with the log-rank test. An adjusted survival analysis was performed using Cox models within strata, adjusting for the same set of covariates as previously mentioned.
CSS. SEER captures a cause-of-death variable derived from death certificates. More specifically, cancer-specific death is coded as an event if the cause of death is attributable to the cancer of interest, in this case lung cancer; deaths from other causes are treated as censored observations.37 Five-year lung CSS was assessed using Kaplan-Meier for CR-LE strata. Given that most of the 90-day perioperative mortality was likely secondary to surgical complications (and not to the cancer progression per se), survival analyses were landmarked at postoperative day 90. Cox models were built using the same methodology as the OS analysis. Patients with missing cancer-specific mortality were excluded from these analyses.
Bivariate analyses were performed using the chi-square test or Fisher’s exact test. A two-tailed p value less than 0.05 was considered statistically significant. A power analysis was calculated. Scaled Schoenfeld and Martingale residuals were used to test for violations of the proportional hazards assumption. Missing values for examined variables were less than 3%, so a complete case analysis was done. The distribution of characteristics in the sample did not change upon exclusion of patients with missing data. All statistical analyses were done using SAS 9.4 and STATA 14.1. STROBE guidelines for observational studies were followed.
Results
Patient Characteristics
In total, 4560 patients were included, of which 3398 (75%) underwent a lobectomy and 1162 (25%) a wedge. Patients who underwent a wedge tended to be older (80–84: 19% versus 13%, p < 0.001) and have a higher Elixhauser comorbidity score (3+: 40% versus 26%, p < 0.001) than patients who underwent lobectomy. Patients in the wedge group were also more likely to be white (93% versus 90%, p < 0.001), have a CR-LE less than 5 years (19% versus. 10%, p < 0.001), have a tumor located in the left lung (46% versus 40%, p < 0.001), and receive adjuvant radiotherapy (4% versus 1%, p < 0.001) (Table1).
Table 1.
Patient Characteristics of the Entire Study Population
| Characteristic | Lobectomy (n = 3398) No. (%)a |
Wedge (n = 1162) No. (%)a |
p Value |
|---|---|---|---|
| Age | <0.001 | ||
| 67–69 | 765 (23) | 192 (17) | |
| 70–74 | 1194 (35) | 360 (31) | |
| 75–79 | 905 (27) | 334 (29) | |
| 80–84 | 435 (13) | 216 (19) | |
| 85+ | 99 (3) | 60 (5) | |
| Sex | 0.19 | ||
| Male | 1437 (42) | 466 (40) | |
| Female | 1961 (58) | 696 (60) | |
| Race | <0.001 | ||
| White | 3073 (90) | 1080 (93) | |
| Black | 163 (5) | 56 (5) | |
| Other | 162 (5) | 26 (2) | |
| Non-metropolitan area of residenceb | 0.73 | ||
| No | 2842 (84) | 972 (84) | |
| Yes | 556 (16) | 190 (16) | |
| Income quintileb | 0.13 | ||
| Q1 | 680 (20) | 220 (19) | |
| Q2 | 497 (15) | 159 (14) | |
| Q3 | 683 (20) | 266 (23) | |
| Q4 | 660 (19) | 244 (21) | |
| Q5 | 878 (26) | 273 (23) | |
| Elixhauser comorbidity | <0.001 | ||
| None | 944 (28) | 188 (16) | |
| 1 to 2 | 1585 (47) | 508 (44) | |
| 3+ | 869 (26) | 466 (40) | |
| Year of diagnosis | 0.61 | ||
| 2005 | 331 (10) | 95 (8) | |
| 2006 | 329 (10) | 112 (10) | |
| 2007 | 336 (10) | 120 (10) | |
| 2008 | 320 (9) | 115 (10) | |
| 2009 | 323 (10) | 91 (8) | |
| 2010 | 308 (9) | 108 (9) | |
| 2011 | 321 (9) | 108 (9) | |
| 2012 | 241 (7) | 97 (8) | |
| 2013 | 294 (9) | 112 (10) | |
| 2014 | 296 (9) | 102 (9) | |
| 2015 | 299 (9) | 102 (9) | |
| Tumor grade | 0.03 | ||
| 1 | 592 (17) | 202 (17) | |
| 2 | 1681 (49) | 527 (45) | |
| 3 | 889 (26) | 327 (28) | |
| 4 | 38 (1) | 21 (2) | |
| Not determined | 198 (6) | 85 (7) | |
| Tumor histology | 0.27 | ||
| Adenocarcinoma | 2231 (66) | 729 (63) | |
| Squamous cell carcinoma | 962 (28) | 355 (31) | |
| Large cell | 80 (2) | 34 (3) | |
| Other | 125 (4) | 44 (4) | |
| Tumor laterality | <0.001 | ||
| Right | 2045 (60) | 622 (54) | |
| Left | 1353 (40) | 540 (46) | |
| Adjuvant chemotherapy | 0.20 | ||
| No | 3256 (96) | 1103 (95) | |
| Yes | 142 (4) | 59 (5) | |
| Adjuvant radiotherapy | <0.001 | ||
| No | 3354 (99) | 1114 (96) | |
| Yes | 44 (1) | 48 (4) | |
| Comorbidity-related predicted life expectancy | <0.001 | ||
| <5 years | 323 (10) | 221 (19) | |
| ≥5 years | 3075 (90) | 941 (81) |
Percentages might not add up to 100% owing to approximation.
Based on patients’ ZIP code area.
Overall, 4016 patients had a CR-LE greater than or equal to 5 years (77% lobectomy; 23% wedge). Patient characteristics can be found in Supplementary Table 2. A total of 544 patients had a CR-LE less than 5 years (59% lobectomy; 41% wedge). In the CR-LE less than five stratum, patients who underwent wedge and lobectomy were largely similar; however, the wedge subset had a higher proportion of left-sided tumors (Supplementary Table 3).
Survival of Lobectomy Versus Wedge Across Strata of Life Expectancy
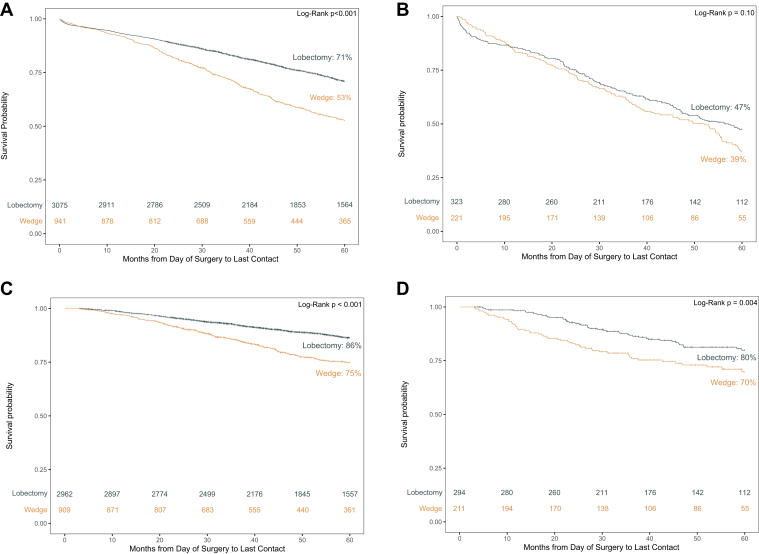
Unadjusted Kaplan-Meier analyses revealed that for patients in the CR-LE greater than or equal to five stratum, wedge had an inferior 5-year OS (53%) compared with lobectomy (71%) (log-rank p < 0.001) (Fig. 1A). However, the 5-year OS in the CR-LE less than five stratum was not observed to be significantly different between those who underwent wedge (39%) and lobectomy (47%) (p = 0.10) (Fig. 1B).
Figure 1.
Kaplan-Meier survival curves across CR-LE levels. (A) CR-LE ≥5. (B) CR-LE <5. (C) CR-LE ≥5. (D) CR-LE <5. CR-LE, comorbidity-related life expectancy.
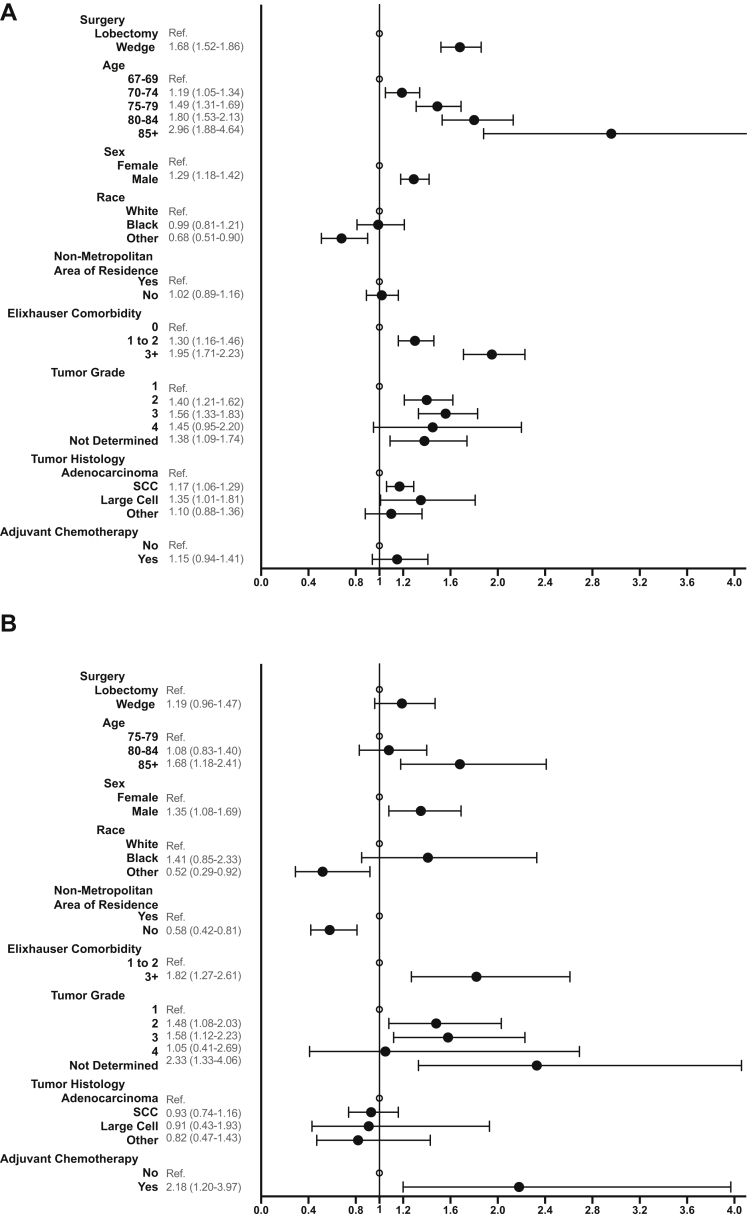
Adjusted survival analysis using Cox models indicated that in the CR-LE greater than or equal to five stratum, wedge was associated with a higher risk of mortality than lobectomy (hazard ratio [HR]:1.68; 95% confidence interval [CI]: 1.52–1.86, p < 0.001). However, among patients with CR-LE less than 5 years, the mortality differential narrowed between wedge and lobectomy (HR: 1.19; 95% CI 0.96–1.47) and was no longer significant (p = 0.11) (Fig. 2A and B).
Figure 2.
Forest plots of the overall mortality multivariate Cox models. (A) CR-LE ≥ 5. (B) CR-LE < 5. List of covariates is abbreviated for figure clarity, complete Cox Models can be found in Supplementary Tables 4 and 5. CR-LE, comorbidity-related life expectancy.
CSS
Unadjusted Kaplan-Meier survival analyses for lung (CSS were completed for CR-LE strata. In both strata, wedge had an inferior 5-year CSS compared with lobectomy (CR-LE ≥5: 75% versus. 86%, log-rank p < 0.001; CR-LE <5: 70% versus 80%, p = 0.004) (Fig. 1C and D). Cox models confirmed that in both strata, patients who had undergone wedge had a higher risk of mortality than those who had a lobectomy (CR-LE ≥5 HR: 1.84, 95% CI: 1.56–2.16, p < 0.001; CR-LE <5 HR: 1.65, 95% CI: 1.12–2.43; p = 0.01) (Fig. 3A and B).
Figure 3.
Forest plots of the cancer-specific mortality multivariate Cox models. (A) CR-LE ≥ 5. (B) CR-LE < 5. List of covariates is abbreviated for figure clarity, complete Cox Models can be found in Supplementary Tables 6 and 7. CR-LE, comorbidity-related life expectancy.
Indicators of Adversity in the Postoperative Period
Several established indicators of adversity in the postoperative period or prolonged recovery were evaluated. Patients in the CR-LE less than five stratum who underwent a lobectomy had a significantly higher proportion of prolonged length of stay (13% versus 7% for wedge, p = 0.03) and 90-day mortality than those who underwent wedge (9% versus 4% for wedge, p = 0.04) but no significant difference in hospital readmissions or use of supplemental oxygen greater than or equal to 45 days post discharge. Among patients with CR-LE greater than or equal to 5 years, the only significant difference was that 14% of those who underwent wedge still required supplemental oxygen greater than or equal to 45 days post discharge compared with 11% of patients who underwent lobectomy (p < 0.001) (Table2).
Table 2.
Prevalence of Surrogates for Complications in the Perioperative Period Across CR-LE Levels
| Surrogates for Complications | <5 Years of Comorbidity-Related Life Expectancy |
≥5 Years of Comorbidity-Related Life Expectancy |
||||
|---|---|---|---|---|---|---|
| Lobectomy (n = 323) No. (%)a |
Wedge (n = 221) No. (%)a |
p Value | Lobectomy (n = 3075) No. (%)a |
Wedge (n = 941) No. (%)a |
p Value | |
| Length of hospital stay | 0.03 | 0.35 | ||||
| ≤14 d | 282 (87) | 206 (93) | 2824 (92) | 873 (93) | ||
| >14 d | 41 (13) | 15 (7) | 251 (8) | 68 (7) | ||
| 30-d hospital readmission | 0.19 | 0.24 | ||||
| No | 282 (87) | 184 (83) | 2822 (92) | 852 (91) | ||
| Yes | 41 (13) | 37 (17) | 253 (8) | 89 (9) | ||
| 90-d mortality | 0.04 | 0.41 | ||||
| No | 294 (91) | >210 (>95) | 2977 (97) | 916 (97) | ||
| Yes | 29 (9) | <11 (<5) | 98 (3) | 25 (3) | ||
| Supplemental oxygen ≥ 45 d after dischargeb | 0.19 | <0.001 | ||||
| No | 254 (86) | 148 (81) | 2562 (89) | 664 (86) | ||
| Yes | 42 (14) | 34 (19) | 313 (11) | 111 (14) | ||
Note. Frequencies less than 11 not reported per SEER—Medicare guidelines.
CR-LE, comorbidity-related life expectancy; SEER, Surveillance Epidemiology and End Results.
Percentages might not add up to 100% owing to approximation.
Total for this category does not add up to the total number of patients in the table heading because patients that had claims for oxygen during the year before surgery were excluded from this analysis.
Discussion
The survival advantage of lobectomy over wedge for Medicare recipients with early- stage NSCLC varies depending on competing risks of mortality. Patients who had a CR-LE greater than or equal to five and underwent a wedge had inferior 5-year survival compared with patients who had a lobectomy. However, patients who had a CR-LE less than 5 and underwent a wedge seemed to have no difference in overall mortality when compared with those who underwent lobectomy. This finding is in line with previous studies that have challenged the survival benefit of lobectomy over wedge in older adults or patients with more comorbid conditions.38, 39, 40 The current study extends these findings by using CR-LE as a way to balance competing risks of mortality in older patients with cancer, therefore, informing the decision-making process.
Lobectomy was found to confer a CSS benefit over wedge across CR-LE strata. These findings were consistent with previous studies that have revealed sublobar to be associated with increased risk of recurrence and worse CSS.41,42 However, the difference between the OS and CSS in patients with CR-LE less than five supports that these patients had competing risks of survival indicating that a proportion of them were likely to die from causes other than cancer. Therefore, in this population, wedge might represent an alternative to lobectomy.
In patients at higher risk to die from etiologies other than cancer, lobectomy seemed to be a less safe procedure. Patients predicted to have a shorter CR-LE who underwent lobectomy had higher rates of prolonged hospital stay and 90-day mortality than those who underwent wedge. These findings agree with previous reports that have revealed that the risks attributed to lobectomy in the perioperative period can be higher than those of sublobar, especially for patients with a more significant comorbidity burden and older age.30,43 However, post hoc analyses looking at perioperative complications in the CALGB-140503 trial44 revealed no difference in perioperative morbidity and mortality between lobectomy and sublobar. Nevertheless, this trial included physically and functionally fit patients that do not match the CR-LE less than five cohort in our study.45 Interestingly, the percentage of patients requiring supplemental oxygen beyond 45 days post discharge was significantly higher for patients who underwent wedge than those who underwent lobectomy in the CR-LE greater than or equal to five group. This illustrates that patients undergoing wedge are more often deemed unfit for lobectomy owing to baseline comorbidities or poor pulmonary reserve.31 Overall, these findings suggest that individuals who seem to have less potential to benefit from lobectomy also have greater risk to be harmed by lobectomy.
Patients with shorter life expectancy secondary to age and comorbidities must strike a balance between the best cancer operation and the operation that will allow them to recover better and potentially have a better quality of life. Although the advantage to taking less pulmonary parenchyma is largely theoretical, some studies have revealed that sublobar might offer a better postoperative pulmonary function than lobectomy.10, 11, 12 Furthermore, a recent study from the I-ELCART group revealed that older patients with NSCLC who had underwent wedge had better quality of life scores postoperatively compared with lobectomy.19 Therefore, patients who might have a compromised health should be counseled on the trade-offs associated with the different surgical options available for NSCLC. These concepts may potentially be extended to other forms of local therapy with lower potential for local control than lobectomy. For example, a patient of advanced age or considerable mortality risk from poor health may benefit from understanding the relationship between cancer-related and health-related risks when making a decision between lobectomy and stereotactic ablative radiosurgery for early-stage NSCLC.34
Limitations
In addition to those often associated with retrospective studies, our study had several limitations. First, we had low statistical power to evaluate wedge versus lobectomy in the CR-LE less than five sample. To detect the observed difference, one would need 1100 patients to achieve an 80% power (the CR-LE <5 sample had only 544 patients). However, even if the difference for CR-LE less than 5 had been significant, the HRs were still quite different across strata (1.19 versus 1.68) suggesting that the potential benefit from lobectomy seems to dissipate in patients with competing risks of mortality. Second, there may have been unmeasured confounders, such as comorbid conditions, functional status, and pulmonary function tests that could have affected the extent of resection patients ultimately underwent and their OS. However, we believe that these unmeasured confounders are likely biasing the outcomes against wedge (i.e., patients who underwent wedge are sicker than what we can capture), making the difference between wedge and lobectomy wider and therefore, minimizing our findings. Third, decisions on type of surgery offered can differ across institutions. Unfortunately, SEER had 12% missingness in hospital ID. Attempting to address this, sensitivity analyses were performed on a subgroup that had complete hospital ID information, clustering by hospital ID. These analyses revealed no meaningful change in the direction, magnitude, or significance of the findings. Fourth, not all surgery was necessarily performed by thoracic surgeons. Given that it has been found that thoracic surgeons have better surgical outcomes than general surgeons when performing lung resection, this could have affected differences in survival and complications.46 Finally, there may be important differences in the location, natural history, and lethality of tumors that are amenable to wedge (i.e., tend to be more peripheral, less likely to have radiographically occult nodal metastases)47,48 and those that require lobectomy (i.e., might be more centrally located, more likely to have occult nodal metastases). As a result, there may be some bias against the lobectomy cohort.
Our findings should not be interpreted to mean that CR-LE is a tool to consistently identify patients in whom wedge and lobectomy are equivalent. Rather, that as results from the CALGB-1450344 (lobectomy versus sublobar for stage I NSCLC), I-ELCART49 (practice-based prospective cohort of patients being diagnosed and treated for stage I NSCLC), and STEPS50 studies (lobectomy versus sublobar for stage I NSCLC in older adults) come out, using tools such as the CR-LE might be critical to understanding and ultimately incorporating the results into practice. In particular, given that the two randomized trials (CALGB-14503 and STEPS) will be composed of a largely functionally fit and relatively healthy population. Our results will offer the perspective of patients who have competing mortality risks and ultimately underscore that just because a procedure might be better in general (e.g., relatively healthy population), it does not mean that it is what is best for your patient (e.g., with a high comorbidity burden). Therefore, we believe that our study highlights the need for clinicians to weigh competing risks of survival in the treatment choice of older adults with stage I NSCLC.
In conclusion, the OS benefit of lobectomy over wedge seems to narrow in the presence of competing risks of mortality from older age or comorbid conditions in patients 67 year or older with stage I (≤2 cm) NSCLC in the SEER—Medicare database. Clinicians should consider competing risks of mortality when discussing treatment options for stage I NSCLC in the older adult population.
Acknowledgments
This project was made possible by the Yale National Clinician Scholars Program and by CTSA grant no. TL1 TR001864 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services, and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare Database. This study used the SEER-Medicare linked database.
Footnotes
Disclosure: Dr. Yu reports receiving speaking and consulting fees for Boston Scientific and is a member of the advisory board for Galera Pharmaceuticals, unrelated to the present work. Dr. Gross reports receiving funding, paid to Yale University, from the NCCN Foundation (partly funded by Pfizer/AstraZeneca); research funding paid to Yale University from Johnson and Johnson, funding paid to Yale University from Genentech, funding paid to Yale University and Flatiron and travel/speaking reimbursement fees. Dr. Boffa runs assays for free through Epic Sciences, unrelated to the present work. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100143.
Supplementary Data
References
- 1.Raman V., Yang C.-F.J., Deng J.Z., D’Amico T.A. Surgical treatment for early stage non-small cell lung cancer. J Thorac Dis. 2018;10(suppl):S898–S904. doi: 10.21037/jtd.2018.01.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flehinger B.J., Kimmel M., Melamed M.R. The effect of surgical treatment on survival from early lung cancer. Implications for screening. Chest. 1992;101:1013–1018. doi: 10.1378/chest.101.4.1013. [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg R.J., Rubinstein L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 4.El-Sherif A., Gooding W.E., Santos R. Outcomes of sublobar resection versus lobectomy for stage I non–small cell lung cancer: a 13-year analysis. Ann Thorac Surg. 2006;82:408–416. doi: 10.1016/j.athoracsur.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Wolf A.S., Richards W.G., Jaklitsch M.T. Lobectomy versus sublobar resection for small (2 cm or Less) non–small cell lung cancers. Ann Thorac Surg. 2011;92:1819–1825. doi: 10.1016/j.athoracsur.2011.06.099. [DOI] [PubMed] [Google Scholar]
- 6.Shirvani S.M., Jiang J., Chang J.Y. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non–small cell lung cancers in the elderly. JAMA Surg. 2014;149:1244–1253. doi: 10.1001/jamasurg.2014.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wisnivesky J.P., Henschke C.I., Swanson S. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. 2010;251:550–554. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 8.Kilic A., Schuchert M.J., Pettiford B.L. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg. 2009;87:1662–1668. doi: 10.1016/j.athoracsur.2009.02.097. [DOI] [PubMed] [Google Scholar]
- 9.Altorki N.K., Yip R., Hanaoka T. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg. 2014;147:754–762. doi: 10.1016/j.jtcvs.2013.09.065. [DOI] [PubMed] [Google Scholar]
- 10.Keenan R.J., Landreneau R.J., Maley R.H., Jr. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78:228–233. doi: 10.1016/j.athoracsur.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Harada H., Okada M., Sakamoto T., Matsuoka H., Tsubota N. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg. 2005;80:2041–2045. doi: 10.1016/j.athoracsur.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Gu Z., Wang H., Mao T. Pulmonary function changes after different extent of pulmonary resection under video-assisted thoracic surgery. J Thorac Dis. 2018;10:2331–2337. doi: 10.21037/jtd.2018.03.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harpole D.H., Jr., Herndon J.E., 2nd, Young W.G., Jr., Wolfe W.G., Sabiston D.C. Stage I nonsmall cell lung cancer. A multivariate analysis of treatment methods and patterns of recurrence. Cancer. 1995;76:787–796. doi: 10.1002/1097-0142(19950901)76:5<787::aid-cncr2820760512>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.Landreneau R.J., Sugarbaker D.J., Mack M.J. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1997;113:691–700. doi: 10.1016/S0022-5223(97)70226-5. [DOI] [PubMed] [Google Scholar]
- 15.Dell’Amore A., Monteverde M., Martucci N. Lobar and sub-lobar lung resection in octogenarians with early stage non-small cell lung cancer: factors affecting surgical outcomes and long-term results. Gen Thorac Cardiovasc Surg. 2015;63:222–230. doi: 10.1007/s11748-014-0493-8. [DOI] [PubMed] [Google Scholar]
- 16.Balduyck B., Hendriks J., Lauwers P., Sardari Nia P., Van Schil P. Quality of life evolution after lung cancer surgery in septuagenarians: a prospective study. Eur J Cardio Thorac Surg. 2009;35:1070–1075. doi: 10.1016/j.ejcts.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 17.Schulte T., Schniewind B., Walter J., Dohrmann P., Küchler T., Kurdow R. Age-related impairment of quality of life after lung resection for non-small cell lung cancer. Lung Cancer. 2010;68:115–120. doi: 10.1016/j.lungcan.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz R.M., Yip R., Flores R.M. The impact of resection method and patient factors on quality of life among stage IA non-small cell lung cancer surgical patients. J Surg Oncol. 2017;115:173–180. doi: 10.1002/jso.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Février E., Yip R., Becker B.J. Change in quality of life of stage IA lung cancer patients after sublobar resection and lobectomy. J Thorac Dis. 2020;12:3488–3499. doi: 10.21037/jtd-20-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamatis G., Leschber G., Schwarz B. Perioperative course and quality of life in a prospective randomized multicenter phase III trial, comparing standard lobectomy versus anatomical segmentectomy in patients with non-small cell lung cancer up to 2 cm, stage IA (7th edition of TNM staging system) Lung Cancer. 2019;138:19–26. doi: 10.1016/j.lungcan.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Howlader N., Mariotto A.B., Woloshin S., Schwartz L.M. Providing clinicians and patients with actual prognosis: cancer in the context of competing causes of death. J Natl Cancer Inst Monogr. 2014;2014:255–264. doi: 10.1093/jncimonographs/lgu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z., Feng H., Zhao H. Sublobar resection is associated with better perioperative outcomes in elderly patients with clinical stage I non-small cell lung cancer: a multicenter retrospective cohort study. J Thorac Dis. 2019;11:1838–1848. doi: 10.21037/jtd.2019.05.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donington J., Ferguson M., Mazzone P. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012;142:1620–1635. doi: 10.1378/chest.12-0790. [DOI] [PubMed] [Google Scholar]
- 24.Gulack B.C., Yang C.J., Speicher P.J. A risk score to assist selecting lobectomy versus sublobar resection for early stage non-small cell lung cancer. Ann Thorac Surg. 2016;102:1814–1820. doi: 10.1016/j.athoracsur.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute. SEER-Medicare: Brief Description of the SEER-Medicare Database. https://healthcaredelivery.cancer.gov/seermedicare/overview/. Accessed May 15, 2020.
- 26.Llore N.P., Emerson D., Marshall M.B. Video-assisted thoracoscopic segmentectomy of the lower lobe: superior and basilar segmentectomy. Oper Tech Thorac Cardiovasc Surg. 2015;20:162–175. [Google Scholar]
- 27.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Clark J.M., Utter G.H., Nuño M., Romano P.S., Brown L.M., Cooke D.T. ICD-10-CM/PCS: potential methodologic strengths and challenges for thoracic surgery researchers and reviewers. J Thorac Dis. 2019;11(suppl 4):S585–S595. doi: 10.21037/jtd.2019.01.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson E.H., Louie R., Zingmond D.S. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256:973–981. doi: 10.1097/SLA.0b013e31826b4c4f. [DOI] [PubMed] [Google Scholar]
- 30.Wright C.D., Gaissert H.A., Grab J.D., O’Brien S.M., Peterson E.D., Allen M.S. Predictors of prolonged length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk-adjustment model. Ann Thorac Surg. 2008;85:1857–1865. doi: 10.1016/j.athoracsur.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Nicastri D.G., Alpert N., Liu B. Oxygen use after lung cancer surgery. Ann Thorac Surg. 2018;106:1548–1555. doi: 10.1016/j.athoracsur.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 32.Sharabiani M.T., Aylin P., Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50:1109–1118. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 33.Vaupel J.W., Zhang Z., van Raalte A.A. Life expectancy and disparity: an international comparison of life table data. BMJ Open. 2011;1 doi: 10.1136/bmjopen-2011-000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J.B., Soulos P.R., Cramer L.D., Decker R.H., Kim A.W., Gross C.P. Comparative effectiveness of surgery and radiosurgery for stage I non-small cell lung cancer. Cancer. 2015;121:2341–2349. doi: 10.1002/cncr.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Cancer Society. Cancer facts & figures 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf; 2019. Accessed May 5, 2020.
- 36.Fairchild A.J., MacKinnon D.P. A general model for testing mediation and moderation effects. Prev Sci. 2009;10:87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Cancer Institute. SEER cause-specific death classification. https://seer.cancer.gov/causespecific/. Accessed May 15, 2020.
- 38.Mery C.M., Pappas A.N., Bueno R. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest. 2005;128:237–245. doi: 10.1378/chest.128.1.237. [DOI] [PubMed] [Google Scholar]
- 39.Qiu C., Wang G., Xu J. Sublobectomy versus lobectomy for stage I non-small cell lung cancer in the elderly. Int J Surg. 2017;37:1–7. doi: 10.1016/j.ijsu.2016.11.090. [DOI] [PubMed] [Google Scholar]
- 40.Dong S., Roberts S.A., Chen S. Survival after lobectomy versus sub-lobar resection in elderly with stage I NSCLC: a meta-analysis. BMC Surg. 2019;19:38. doi: 10.1186/s12893-019-0500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramanian M., McMurry T., Meyers B.F., Puri V., Kozower B.D. Long-term results for clinical stage IA lung cancer: comparing lobectomy and sublobar resection. Ann Thorac Surg. 2018;106:375–381. doi: 10.1016/j.athoracsur.2018.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai C., Shen J., Ren Y. Choice of surgical procedure for patients with non-small-cell lung cancer ≤ 1 cm or > 1 to 2 cm among lobectomy, segmentectomy, and wedge resection: a population-based study. J Clin Oncol. 2016;34:3175–3182. doi: 10.1200/JCO.2015.64.6729. [DOI] [PubMed] [Google Scholar]
- 43.Linden P.A., D’Amico T.A., Perry Y. Quantifying the safety benefits of wedge resection: a society of thoracic surgery database propensity-matched analysis. Ann Thorac Surg. 2014;98:1705–1712. doi: 10.1016/j.athoracsur.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 44.ClinicalTrials.gov. Comparison of different types of surgery in treating patients with stage IA non-small cell lung cancer. NCT00499330. https://ClinicalTrials.gov/show/NCT00499330. Accessed July 9, 2020.
- 45.Altorki N.K., Wang X., Wigle D. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial. Lancet Respir Med (Alliance: CALGB. 2018;6(140503):915–924. doi: 10.1016/S2213-2600(18)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schipper P.H., Diggs B.S., Ungerleider R.M., Welke K.F. The influence of surgeon specialty on outcomes in general thoracic surgery: a national sample 1996 to 2005. Ann Thorac Surg. 2009;88:1566–1573. doi: 10.1016/j.athoracsur.2009.08.055. [DOI] [PubMed] [Google Scholar]
- 47.Casal R.F., Sepesi B., Sagar A.S. Centrally located lung cancer and risk of occult nodal disease: an objective evaluation of multiple definitions of tumour centrality with dedicated imaging software. Eur Respir J. 2019;53:1802220. doi: 10.1183/13993003.02220-2018. [DOI] [PubMed] [Google Scholar]
- 48.Decaluwé H., Petersen R.H., Brunelli A. Multicentric evaluation of the impact of central tumour location when comparing rates of N1 upstaging in patients undergoing video-assisted and open surgery for clinical Stage I non-small-cell lung cancer†. Eur J Cardio Thorac Surg. 2018;53:359–365. doi: 10.1093/ejcts/ezx338. [DOI] [PubMed] [Google Scholar]
- 49.Flores R., Henschke C., Taioli E. P2.06-045 initiative for early lung cancer Research on treatment (IELCART): topic: LAB, other. J Thorac Oncol. 2017;12:S1100. [Google Scholar]
- 50.Yang F., Sui X., Chen X. Sublobar resection versus lobectomy in surgical treatment of elderly patients with early-stage non-small cell lung cancer (STEPS): study protocol for a randomized controlled trial. Trials. 2016;17:191. doi: 10.1186/s13063-016-1312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.