Abstract
Prenatal environment has long-lasting effects on offspring development and health. Research on prenatal stress identified various mechanisms of these effects, from changes in epigenetic and gene expression profiles to Maternal-Placental-Fetal (MPF) stress biology. There is also evidence for the role of additional risk and protective factors influencing the impact of prenatal stress on maternal and infant outcomes. Considering these findings, we present the study protocol of BABIP, a prospective birth cohort from Turkey. The aim of the project is to investigate the effect of prenatal stress on MPF stress biology (i.e. neuroendocrine, immune and metabolic systems), differential DNA methylation and gene expression patterns, and infant birth and developmental outcomes. We are recruiting 150 pregnant women and their babies for a longitudinal project with 4 time points: 20–24 (T1) and 30–34 (T2) weeks of pregnancy, and 1-month (T3) and 4-months (T4) after giving birth. Maternal early and prenatal environment (prenatal stress, early life stress, psychosocial resources, and health-related behaviors) are assessed during pregnancy with MPF stress biology, DNA methylation and gene expression measures. Infant birth outcomes, DNA methylation and development are assessed postpartum. BABIP is the first prospective birth cohort from Turkey with extensive measures on prenatal environment and health. Through investigating the multilevel impact of prenatal stress and related risk and protective factors during and after pregnancy, BABIP will contribute to our understanding of the mechanisms by which prenatal environment influences infant development and health. Being the first such cohort from Turkey, it may also allow identification of prenatal risk and protective factors specific to the context and population in Turkey.
Keywords: Birth cohort, Prenatal stress, Stress biology, Gene expression, Epigenetics, Infant development
Epidemiological research in the last few decades established the importance of prenatal environment on offspring’s life-long health and disease risk (Halfon et al., 2018). Studies on the role of various psychosocial and behavioral prenatal factors reported persistent changes in offspring health, observed as early as in birth outcomes (e.g. birth weight, gestation length) to risk for later cardiovascular, metabolic, immune, and psychological disorders (Gluckman et al., 2008; Langley-Evans and McMullen, 2010). These findings led to the development of fetal programming approaches to health and disease across the life span (e.g. Developmental Origins of Health and Disease; Wadhwa et al., 2009).
In this context, prenatal stress has been the most widely examined risk factor that in humans primarily encompasses exposure to acute and chronic stressors, mood disorders or low socioeconomic status (SES) during pregnancy (Entringer et al., 2010). Prenatal stress has been associated with adverse health outcomes across the life span, starting from birth (e.g. preterm birth and low birth weight), to infancy and childhood (e.g. increased allergies, socioemotional and cognitive problems, and higher risk for metabolic and psychological disorders), and adulthood (e.g. higher risk for cardiovascular, neuropsychiatric, metabolic disorders (Entringer et al., 2015; Glover, 2014; Halfon et al., 2018; Wadhwa et al., 2001). The way prenatal stress leads to these health problems is suggested to be through changes in Maternal-Placental-Fetal (MPF) stress biology (Entringer et al., 2010; Osborne et al., 2018; Van den Bergh et al., 2017; Wadhwa, 2005). So far, prenatal stress has been linked primarily with neuroendocrine and immune system markers of MPF stress biology, such as markers of the Hypothalamic-Pituitary-Adrenal (HPA) axis (e.g. placental Corticotropin Releasing Hormone (pCRH), adrenocorticotropic hormone (ACTH) and cortisol) and inflammation (e.g. C-reactive protein (CRP), cytokines like interleukin (IL) 6, 10, 1β, and Tumor Necrosis Factor-α; Entringer et al., 2015; Entringer and Wadhwa, 2013; Hantsoo et al., 2019; Plant et al., 2016; Rakers et al., 2017; Sandman, 2018; Van den Bergh et al., 2017; Wadhwa, 2005). In addition to these markers, there is emerging evidence for changes by prenatal stress at the molecular level, such as in epigenetic (mostly DNA methylation) and gene expression patterns of mothers and newborns (e.g. Braithwaite et al., 2015; Capron et al., 2018; Kertes et al., 2016; McGowan and Matthews, 2018; Miller et al., 2017; Sosnowski et al., 2018). These results from different levels of analysis emphasize the importance attaining a multilevel perspective in understanding the impact of prenatal stress.
Apart from the widespread effects of prenatal stress, previous research identified additional factors that may alter these effects. First of all, characteristics of prenatal stressors, such as their type, timing, and duration, are important to consider while measuring prenatal stress (reviewed in Wadhwa, 2005). Secondly, various studies emphasized the role of early life stress (ELS; e.g. childhood traumatic experiences, low family SES) on prenatal stress and programming of stress biology, possibly via epigenetic mechanisms (Anacker et al., 2014; Baumeister et al., 2016; Choi and Sikkema, 2016; Danese and J Lewis, 2017; Danese et al., 2007; Heim and Binder, 2012; Murgatroyd, 2014). Given the evidence on the intergenerational impact of maternal ELS on offspring development and health (Buss et al., 2017; Moog et al., 2018; Plant et al., 2013, 2018), it is necessary to consider maternal ELS while investigating prenatal stress. Thirdly, prenatal stress was reported to interact with other health-related behaviors, such as nutrition, sleep and smoking, which may further influence offspring health (Damron, 2017; Hux et al., 2017; Lindsay et al., 2017; Okun et al., 2014). For instance, various studies established associations between nutrition during pregnancy and metabolic outcomes of the mother and offspring, from lipid profiles (e.g. cholesterol, triglycerides) and adipokine levels (e.g. leptin, adiponectin) to BMI, weight gain and obesity measures, influencing maternal and offspring health (e.g. Gademan et al., 2014; Mudd et al., 2015; Valleau and Sullivan, 2014). Finally, although there is evidence on the protective effects of prenatal factors like psychosocial resources (e.g. social support, self-esteem) on maternal and offspring health during and after pregnancy (e.g. Franck et al., 2016; Katzow et al., 2019; Li et al., 2017; Stapleton et al., 2012; Tani and Castagna, 2017), very few studies so far considered their role in relation to prenatal stress and changes in stress biology (e.g. Hahn-Holbrook et al., 2013; Luecken et al., 2013; Ross et al., 2019). Considering these results, it is important to have comprehensive measures of prenatal stress, together with measures of ELS, health-related behaviors and psychosocial resources.
In light of the progress in the field summarized above, we initiated the first prospective birth cohort from Turkey (BABIP - Bogazici Mother-Baby Relationship Project) that focuses on the role of prenatal risk and protective factors on stress biology and infant development with the following aims:
Aim 1: a) To investigate the impact of prenatal stress on maternal MPF stress biology during pregnancy (i.e. neuroendocrine, immune and metabolic systems), and b) whether MPF stress biology mediates this impact on child birth and developmental outcomes.
Aim 2: To investigate the contributions of maternal ELS, psychosocial resources and health-related behaviors on the associations investigated at Aim 1.
Aim 3: To investigate differential whole genome blood DNA methylation and gene expression patterns of women at high and low quartiles of prenatal stress.
Aim 4: To investigate differential whole genome salivary DNA methylation patterns of babies born to women at high and low quartiles of prenatal stress, controlling for postnatal environment.
1. Methods
1.1. Participants and study design
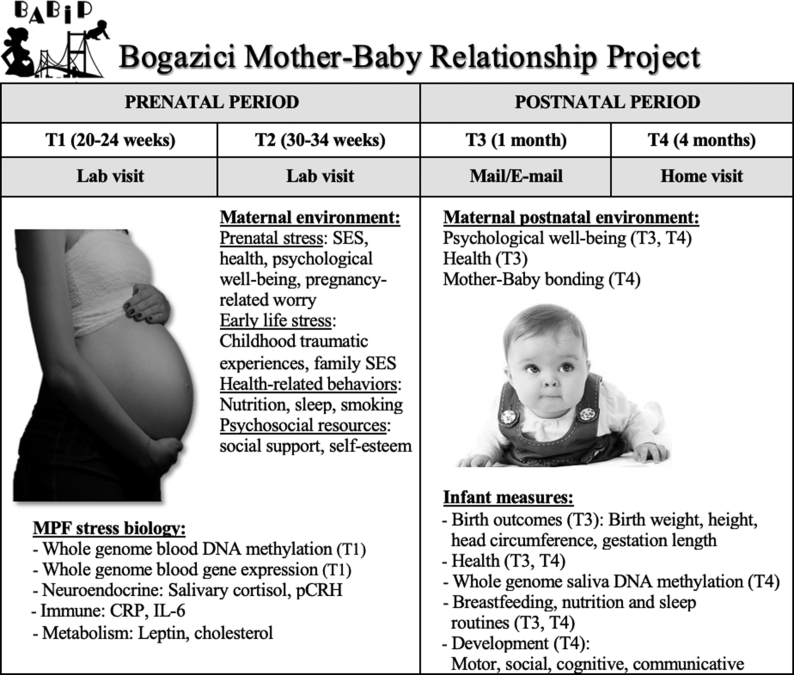
The project is approved by the Human Research and Ethics Committee of Bogazici University. We plan to recruit a total of 150 pregnant women and their babies from Istanbul, Turkey, through doctors’ offices, flyers and online advertisements. Data collection has already started in 2018 and is expected to continue until April 2020. Women interested in participation are checked for eligibility via phone interviews and are included in the study if they are from Turkey, older than 18 years of age, within 24 weeks of pregnancy, have a singleton intrauterine pregnancy and no current diagnosis of chronic disorders or severe pregnancy complications. The timeline and procedure of the study is summarized as the following (Fig. 1): Eligible participants are scheduled for their first visit at Biruni Laboratories, our collaborating private laboratory near Bogazici University, during 20–24 weeks of pregnancy (T1). During this visit, oral and written consents are obtained by trained graduate assistants. Afterwards, participants complete questionnaires, give saliva samples and have blood their blood samples collected by nurses. They are also instructed on the saliva sampling procedure at home and provided with saliva collection kits (including Salivettes, sleep diaries, sampling time sheets). The whole visit lasts about 1.5–2 hours. The second visit (T2) is during 30–34 weeks of pregnancy and follows a similar procedure as T1, except consenting. All the questionnaire and biological samples collected are transferred immediately to our Psychoepigenetics Laboratory at Bogazici University and processed and stored there under appropriate conditions until further use. One-month after giving birth (T3), a paper or an online questionnaire is sent to mothers assessing maternal health and psychological well-being as well as infant birth outcomes and health. The final assessment is conducted 4-months after giving birth (T4) as a home visit, collecting measures on mothers’ health, psychological well-being, and relationship with the infant, as well as infant’s saliva and development. For their participation, mothers are compensated with information booklets and 4 online expert seminars about health during pregnancy and child development. In addition, they receive a detailed report of their baby’s development at 4 months together with children books.
Fig. 1.
Project timeline and summary of maternal and infant measures.
1.2. Maternal early and perinatal environmental measures
Participants complete standardized questionnaires in Turkish about demographics, health history (e.g. diagnoses, medications), health-related behaviors (e.g. nutrition, sleep, smoking), psychosocial resources (e.g. social support, self-esteem), ELS (e.g. childhood traumatic experiences, family SES) and prenatal stress (e.g. SES, health, psychological well-being, pregnancy-related worry). For state measures, such as psychological well-being (i.e. current depression and anxiety symptoms), the same measures are completed at multiple time points. At T3 & T4, mothers also report on any birth complications and health problems.
1.3. MPF stress biology measures
MPF stress biology markers of the neuroendocrine (blood pCRH, salivary cortisol across two days), immune (CRP, IL-6) and metabolic systems (leptin, cholesterol (HDL & LDL)) will be measured at T1 and T2. For salivary cortisol, measures of cortisol awakening response, diurnal slope and total cortisol output will be calculated from samples collected across the day.
1.4. Molecular measures
At the molecular level, whole genome DNA methylation and gene expression arrays will be performed from blood samples collected at T1 by PAXgene DNA and RNA tubes, respectively. Differential DNA methylation and gene expression patterns of individuals at high and low quartiles of prenatal stress will be compared, controlling for demographics, ELS, health and health-related behaviors and psychosocial resources.
1.5. Infant measures
After giving birth (T3 & T4), mothers complete questionnaires related to infant’s birth outcomes (e.g. birth weight, height and head circumference, gestation length), health, and breastfeeding, nutrition and sleep routines. During the visit at T4, trained graduate assistants collect saliva samples from the infants via Oragene OG-250 kits for whole genome DNA methylation analysis and measure infants’ motor, social, cognitive and communicative development via standardized developmental tests. Differential salivary DNA methylation patterns of infants will be compared between those born to mothers from high and low quartiles of prenatal stress, controlling for demographics, ELS, health and health-related behaviors, and psychosocial resources, and postnatal maternal and infant environment (e.g. health, routines, psychological well-being).
1.6. Data privacy, management and analysis plan
All participants are assigned with ID numbers throughout the study and no personal information is utilized on any of the measures collected. Only the principal investigator and graduate project assistants are provided access to the online password-protected file with identifying information. All of the questionnaires and biological samples collected are immediately transferred to and stored under lock at our laboratory at Bogazici University until further analysis. Data is entered into files in Bogazici University’s secured network. In case of withdrawal from the study, all of participants’ questionnaires, biological samples and data are destroyed according to appropriate guidelines.
Composite scores of prenatal stress, ELS, and psychosocial behavior will be created from the different questionnaires used for each variable. For testing Aims 1 & 2, mixed-effects regression models will be utilized to test the effects on MPF stress biology markers at T1 and T2. For whole genome DNA methylation and gene expression arrays, samples of women at the high and low quartiles of prenatal stress and their infants will be processed. Differential DNA methylation and gene expression patterns will be calculated between high and low prenatal stress groups, controlling for aforementioned covariates. Differentially expressed genes will be confirmed by qPCRs. Further bioinformatic analysis will be conducted for determining gene networks influenced by prenatal stress.
1.7. Descriptive characteristics
Currently, 65 pregnant women participated in the study (T1) and 20 are scheduled to participate starting from their 20th week of pregnancy. Six participants withdrew from the study after T1 due to moving to other cities, unwillingness to participate, and time and logistic constraints. The descriptive characteristics of the remaining women and the babies assessed so far (N = 6) are summarized in Table 1.
Table 1.
Descriptive characteristics of maternal and infant outcomes.
| Maternal outcomes | M (SD) or % |
|---|---|
| Age | 32.80 (3.7) |
| Married | 100% |
| Pre-pregnancy BMI | 22.95 (5.17) |
| Planned pregnancy | 85% |
| Nulliparous | 71% |
| Education (higher education %) | 83% |
| Satisfaction with housing conditions | 75% |
| Smoking before pregnancy | 30% |
| Smoking during pregnancy |
12% |
|
Infant birth outcomes |
|
| Sex (% girls) | 33% |
| Gestation length (weeks) | 39.67 (0.9) |
| Birth weight (kg) | 3.33 (0.3) |
| Birth height (cm) | 48.83 (1.3) |
| Birth head circumference (cm) | 35.40 (1.4) |
2. Discussion
BABIP is the first prospective birth cohort from Turkey investigating the impact of prenatal stress on infant development and health through multiple MPF stress biology and molecular measures. It also has a significance by considering the role of ELS, psychosocial resources and health-related behaviors together with prenatal stress. Through this project, we aim to contribute to our understanding of the underlying pathways and mother-infant outcomes influenced by prenatal stress. Considering that most of the existing studies of prenatal stress in Turkey so far focused on maternal health (e.g. Bolak Boratav et al. (2016) on postpartum depression) and infant birth outcomes (e.g. Uguz et al. (2013) on birth weight and gestation length) rather than biological mechanisms, this cohort will also allow determining any aspects of the prenatal environment and stress biology that is important for the context and population of Turkey.
Until now, we have encountered some challenges in our study that needs to be acknowledged together with other limitations. Our first challenge is the representativeness of the population in terms of SES. Majority of our participants are from middle to high SES. We identified the main reasons for less participation from lower SES groups as time and logistic difficulties (e.g. working and transportation conditions) and bias for providing biological samples. In order to overcome this challenge, we are now communicating with local municipalities and family health centers for reaching out to women from lower SES neighborhoods. In addition, we are developing strategies for supporting their time and logistic constraints. Secondly, although we implemented some measures of compliance (e.g. log-sheets, text messages), we have not used objective measures of compliance for the daily saliva sampling, such as sleep-monitoring watches or time-recording collection bottles. We plan to use these measures for a subset of our remaining participants to compare with their self-reports. Thirdly, we do not have any physiological measures from the babies that would complement our understanding of the impact of prenatal environment on the offspring’s physiology. We currently plan to extend our baby assessments to include salivary neuroendocrine markers. Fourthly, we have not integrated the role of genetic factors into our analysis yet, although they are known to moderate the effects of environment (Bagot and Meaney, 2010). Future projects may include addition of genomic measures to account for the effects of any genetic polymorphisms. Finally, although we collect indirect measures of fathers’ environment from the mothers, we do not have paternal self-reports or biological measures. Considering the evidence from animal and limited human studies on the role of paternal environment and biology on the offspring development and health (e.g. Chan et al., 2018; Kinnally and Capitanio, 2015; Korja et al., 2018; Mychasiuk et al., 2013; Ramchandani et al., 2008; Rodgers et al., 2013), future studies should benefit from considering paternal measures as well.
In order to translate the topics of our project and raise public awareness on prenatal care and health in Turkey, we implemented several public education components into BABIP. In addition to the information booklets and seminars provided to the participants, we organized a public symposium on “Living Healthy during and after Pregnancy” with the support of many researchers and medical professionals from different disciplines. Furthermore, in the context of Maternal Mental Health Week 2019, we organized a public symposium on “Maternal Mental Health” in collaboration with Turkey Maternal Mental Health Platform. We also joined the representation of Turkey in the Postpartum Support International network that aims to support and raise awareness for the importance of maternal mental health during and after pregnancy. We plan to continue growing these public education components even further with including new topics related to prenatal environment and health.
Currently, we are applying for additional funding with national and international collaborators to continue incorporating additional maternal measures during pregnancy and following up babies beyond 4 months. Our primary goals are to include mother’s ultrasound measures during pregnancy, and baby’s attachment, sleep monitoring, neuroendocrine and microbiome measures at 6–12 months. We also have an ongoing collaboration with researchers from Germany, Drs. Sonja Entringer (Charite Universitatmedizin, Berlin) and Jacob Spallek (Brandenburg University of Technology, Cottbus-Senftenberg), as part of a multi-cohort project on the intergenerational transmission of health disparities in Turkish immigrants in Germany (Spallek et al., under review). We will share part of our data from BABIP to serve as a Turkish comparison group from Turkey, while investigating health disparities between Turkish-origin immigrants and Germans living in Germany. Inclusion of results from a Turkish population living in Turkey will be important to identify any changes in stress biology specific to genetic background and/or environmental factors independent of migration background. Through these collaborations, we aim to compare our results with different birth cohorts around the world and collectively increase our understanding of the impact of prenatal environment that would aid in developing prenatal care policies for enhancing mother and infant health.
Declaration of competing interest
Authors declare no conflicting interests.
Acknowledgements
We thank all Psychoepigenetics Lab members, Biruni Laboratories, and Drs. Sureyya Sahinoglu, Semra Levent, Zeki Sahinoglu and Nazan Aydin for their valuable contributions while conducting our project. We thank Drs. Carmine Pariante, Sonja Entringer, Jacob Spallek, Claudia Buss, Emma Adam and Alina Rodriguez for their theoretical and methodological feedback during the initiation of this project. We also thank all mothers and babies of the BABIP cohort for their participation. This project is funded by Bogazici University Research Foundation Grant #11662 awarded to EAD.
References
- Anacker C., O’Donnell K.J., Meaney M.J. Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues Clin. Neurosci. 2014;16:321–333. doi: 10.31887/DCNS.2014.16.3/canacker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot R.C., Meaney M.J. Epigenetics and the biological basis of gene x environment interactions. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:752–771. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Baumeister D., Akhtar R., Ciufolini S., Pariante C.M., Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry. 2016;21:642–649. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolak Boratav H., Toker Ö., Küey L. Postpartum depression and its psychosocial correlates: a longitudinal study among a group of women in Turkey. Women Health. 2016;56:502–521. doi: 10.1080/03630242.2015.1101737. [DOI] [PubMed] [Google Scholar]
- Braithwaite E.C., Kundakovic M., Ramchandani P.G., Murphy S.E., Champagne F.A. Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics. 2015;10:408–417. doi: 10.1080/15592294.2015.1039221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Entringer S., Moog N.K., Toepfer P., Fair D.A., Simhan H.N., Heim C.M., Wadhwa P.D. Intergenerational transmission of maternal childhood maltreatment exposure: implications for fetal Brain development. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:373–382. doi: 10.1016/j.jaac.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron L.E., Ramchandani P.G., Glover V. Maternal prenatal stress and placental gene expression of NR3C1 and HSD11B2: the effects of maternal ethnicity. Psychoneuroendocrinology. 2018;87:166–172. doi: 10.1016/j.psyneuen.2017.10.019. [DOI] [PubMed] [Google Scholar]
- Chan J.C., Nugent B.M., Bale T.L. Parental advisory: maternal and paternal stress can impact offspring neurodevelopment. Biol. Psychiatry. 2018;83:886–894. doi: 10.1016/j.biopsych.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.W., Sikkema K.J. Childhood maltreatment and perinatal mood and anxiety disorders: a systematic review. Trauma Violence Abus. 2016;17:427–453. doi: 10.1177/1524838015584369. [DOI] [PubMed] [Google Scholar]
- Damron K.R. Review of the relationships among psychosocial stress, secondhand smoke, and perinatal smoking. J. Obstet. Gynecol. Neonatal Nurs. 2017;46:325–333. doi: 10.1016/j.jogn.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Danese A., J Lewis S. Psychoneuroimmunology of early-life stress: the hidden wounds of childhood trauma? Neuropsychopharmacology. 2017;42:99–114. doi: 10.1038/npp.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Pariante C.M., Caspi A., Taylor A., Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S., Wadhwa P.D. Developmental programming of obesity and metabolic dysfunction: role of prenatal stress and stress biology. Nestle Nutr Inst Workshop Ser. 2013;74:107–120. doi: 10.1159/000348454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S., Buss C., Wadhwa P.D. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr. Opin. Endocrinol. Diabetes Obes. 2010;17:507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S., Buss C., Wadhwa P.D. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology. 2015;62:366–375. doi: 10.1016/j.psyneuen.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck E., Vanderhasselt M.A., Goubert L., Loeys T., Temmerman M., De Raedt R. The role of self-esteem instability in the development of postnatal depression: a prospective study testing a diathesis-stress account. J. Behav. Ther. Exp. Psychiatry. 2016;50:15–22. doi: 10.1016/j.jbtep.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Gademan M.G., Vermeulen M., Oostvogels A.J., Roseboom T.J., Visscher T.L., van Eijsden M., Twickler M.T., Vrijkotte T.G. Maternal prepregancy BMI and lipid profile during early pregnancy are independently associated with offspring’s body composition at age 5-6 years: the ABCD study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Pract. Res. Clin. Obstet. Gynaecol. 2014;28:25–35. doi: 10.1016/j.bpobgyn.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A., Cooper C., Thornburg K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn-Holbrook J., Schetter C.D., Arora C., Hobel C.J. Placental corticotropin-releasing hormone mediates the association between prenatal social support and postpartum depression. Clin Psychol Sci. 2013;1:253–264. doi: 10.1177/2167702612470646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon N., Forrest C.B., Lerner R.M., Faustman E.M. In: Handbook of Life Course Health Development. Halfon N., Forrest C.B., Lerner R.M., Faustman E.M., editors. Springer; 2018. Handbook of life course health development. [Google Scholar]
- Hantsoo L., Kornfield S., Anguera M.C., Epperson C.N. Inflammation: a proposed intermediary between maternal stress and offspring neuropsychiatric risk. Biol. Psychiatry. 2019;85:97–106. doi: 10.1016/j.biopsych.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Binder E.B. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hux V.J., Roberts J.M., Okun M.L. Allostatic load in early pregnancy is associated with poor sleep quality. Sleep Med. 2017;33:85–90. doi: 10.1016/j.sleep.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzow M., Messito M.J., Mendelsohn A.L., Scott M.A., Gross R.S. The protective effect of prenatal social support on infant adiposity in the first 18 Months of life. J. Pediatr. 2019;209:77–84. doi: 10.1016/j.jpeds.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertes D.A., Kamin H.S., Hughes D.A., Rodney N.C., Bhatt S., Mulligan C.J. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the democratic republic of Congo. Child Dev. 2016;87:61–72. doi: 10.1111/cdev.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally E.L., Capitanio J.P. Paternal early experiences influence infant development through non-social mechanisms in Rhesus Macaques. Front. Zool. 2015;12(Suppl. 1):S14. doi: 10.1186/1742-9994-12-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korja R., Nolvi S., Kataja E.L., Scheinin N., Junttila N., Lahtinen H., Saarni S., Karlsson L., Karlsson H. The courses of maternal and paternal depressive and anxiety symptoms during the prenatal period in the FinnBrain Birth Cohort study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley-Evans S.C., McMullen S. Developmental origins of adult disease. Med. Princ. Pract. 2010;19:87–98. doi: 10.1159/000273066. [DOI] [PubMed] [Google Scholar]
- Li Y., Long Z., Cao D., Cao F. Social support and depression across the perinatal period: a longitudinal study. J. Clin. Nurs. 2017;26:2776–2783. doi: 10.1111/jocn.13817. [DOI] [PubMed] [Google Scholar]
- Lindsay K.L., Buss C., Wadhwa P.D., Entringer S. The interplay between maternal nutrition and stress during pregnancy: issues and considerations. Ann. Nutr. Metab. 2017;70:191–200. doi: 10.1159/000457136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken L.J., Lin B., Coburn S.S., MacKinnon D.P., Gonzales N.A., Crnic K.A. Prenatal stress, partner support, and infant cortisol reactivity in low-income Mexican American families. Psychoneuroendocrinology. 2013;38:3092–3101. doi: 10.1016/j.psyneuen.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan P.O., Matthews S.G. Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology. 2018;159:69–82. doi: 10.1210/en.2017-00896. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Borders A.E., Crockett A.H., Ross K.M., Qadir S., Keenan-Devlin L., Leigh A.K., Ham P., Ma J., Arevalo J.M.G., Ernst L.M., Cole S.W. Maternal socioeconomic disadvantage is associated with transcriptional indications of greater immune activation and slower tissue maturation in placental biopsies and newborn cord blood. Brain Behav. Immun. 2017;64:276–284. doi: 10.1016/j.bbi.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog N.K., Entringer S., Rasmussen J.M., Styner M., Gilmore J.H., Kathmann N., Heim C.M., Wadhwa P.D., Buss C. Intergenerational effect of maternal exposure to childhood maltreatment on newborn Brain anatomy. Biol. Psychiatry. 2018;83:120–127. doi: 10.1016/j.biopsych.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd L.M., Holzman C.B., Evans R.W. Maternal mid-pregnancy lipids and birthweight. Acta Obstet. Gynecol. Scand. 2015;94:852–860. doi: 10.1111/aogs.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C. Epigenetic programming of neuroendocrine systems during early life. Exp. Physiol. 2014;99:62–65. doi: 10.1113/expphysiol.2013.076141. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R., Harker A., Ilnytskyy S., Gibb R. Paternal stress prior to conception alters DNA methylation and behavior of developing rat offspring. Neuroscience. 2013;241:100–105. doi: 10.1016/j.neuroscience.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Okun M.L., Tolge M., Hall M. Low socioeconomic status negatively affects sleep in pregnant women. J. Obstet. Gynecol. Neonatal Nurs. 2014;43:160–167. doi: 10.1111/1552-6909.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne S., Biaggi A., Chua T.E., Du Preez A., Hazelgrove K., Nikkheslat N., Previti G., Zunszain P.A., Conroy S., Pariante C.M. Antenatal depression programs cortisol stress reactivity in offspring through increased maternal inflammation and cortisol in pregnancy: the Psychiatry Research and Motherhood - depression (PRAM-D) Study. Psychoneuroendocrinology. 2018;98:211–221. doi: 10.1016/j.psyneuen.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant D.T., Barker E.D., Waters C.S., Pawlby S., Pariante C.M. Intergenerational transmission of maltreatment and psychopathology: the role of antenatal depression. Psychol. Med. 2013;43:519–528. doi: 10.1017/S0033291712001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant D.T., Pawlby S., Sharp D., Zunszain P.A., Pariante C.M. Prenatal maternal depression is associated with offspring inflammation at 25 years: a prospective longitudinal cohort study. Transl. Psychiatry. 2016;6:e936. doi: 10.1038/tp.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant D.T., Pawlby S., Pariante C.M., Jones F.W. When one childhood meets another - maternal childhood trauma and offspring child psychopathology: a systematic review. Clin. Child Psychol. Psychiatry. 2018;23:483–500. doi: 10.1177/1359104517742186. [DOI] [PubMed] [Google Scholar]
- Rakers F., Rupprecht S., Dreiling M., Bergmeier C., Witte O.W., Schwab M. Transfer of maternal psychosocial stress to the fetus. Neurosci. Biobehav. Rev. 2017 doi: 10.1016/j.neubiorev.2017.02.019. [DOI] [PubMed] [Google Scholar]
- Ramchandani P.G., O’Connor T.G., Evans J., Heron J., Murray L., Stein A. The effects of pre- and postnatal depression in fathers: a natural experiment comparing the effects of exposure to depression on offspring. JCPP (J. Child Psychol. Psychiatry) 2008;49:1069–1078. doi: 10.1111/j.1469-7610.2008.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers A.B., Morgan C.P., Bronson S.L., Revello S., Bale T.L. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K.M., Thomas J.C., Letourneau N.L., Campbell T.S., Giesbrecht G.F., Team A.S. Partner social support during pregnancy and the postpartum period and inflammation in 3-month-old infants. Biol. Psychol. 2019;144:11–19. doi: 10.1016/j.biopsycho.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Sandman C.A. Prenatal CRH: an integrating signal of fetal distress. Dev. Psychopathol. 2018;30:941–952. doi: 10.1017/S0954579418000664. [DOI] [PubMed] [Google Scholar]
- Sosnowski D.W., Booth C., York T.P., Amstadter A.B., Kliewer W. Maternal prenatal stress and infant DNA methylation: a systematic review. Dev. Psychobiol. 2018;60:127–139. doi: 10.1002/dev.21604. [DOI] [PubMed] [Google Scholar]
- Stapleton L.R., Schetter C.D., Westling E., Rini C., Glynn L.M., Hobel C.J., Sandman C.A. Perceived partner support in pregnancy predicts lower maternal and infant distress. J. Fam. Psychol. 2012;26:453–463. doi: 10.1037/a0028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani F., Castagna V. Maternal social support, quality of birth experience, and post-partum depression in primiparous women. J. Matern. Fetal Neonatal Med. 2017;30:689–692. doi: 10.1080/14767058.2016.1182980. [DOI] [PubMed] [Google Scholar]
- Uguz F., Sahingoz M., Sonmez E.O., Karsidag C., Yuksel G., Annagur B.B., Annagur A. The effects of maternal major depression, generalized anxiety disorder, and panic disorder on birth weight and gestational age: a comparative study. J. Psychosom. Res. 2013;75:87–89. doi: 10.1016/j.jpsychores.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Valleau J.C., Sullivan E.L. The impact of leptin on perinatal development and psychopathology. J. Chem. Neuroanat. 2014;61–62:221–232. doi: 10.1016/j.jchemneu.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh B.R.H., van den Heuvel M.I., Lahti M., Braeken M., de Rooij S.R., Entringer S., Hoyer D., Roseboom T., Räikkönen K., King S., Schwab M. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2017 doi: 10.1016/j.neubiorev.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Wadhwa P.D. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wadhwa P.D., Sandman C.A., Garite T.J. The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Prog. Brain Res. 2001;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- Wadhwa P.D., Buss C., Entringer S., Swanson J.M. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]