Abstract
Introduction
Optimal management of people with advanced NSCLC depends on accurate identification of predictive markers. Yet, real-world data in this setting are limited. We describe the impact, timeliness, and outcomes of molecular testing for patients with advanced NSCLC and good performance status in England.
Methods
In collaboration with Public Health England, patients with stages IIIB to IV NSCLC, with an Eastern Cooperative Oncology Group performance status of 0 to 2, in England, between June 2017 and December 2017, were identified. All English hospitals were invited to record information.
Results
A total of 60 of 142 invited hospitals in England participated in this study and submitted data on 1157 patients. During the study period, 83% of patients with advanced adenocarcinoma underwent molecular testing for three recommended predictive biomarkers (EGFR, ALK, and programmed death-ligand 1). A total of 80% of patients with nonsquamous carcinomas on whom biomarker testing was performed had adequate tissue for analysis on initial sampling. First-line treatment with a tyrosine kinase inhibitor was received by 71% of patients with adenocarcinoma and a sensitizing EGFR mutation and by 59% of those with an ALK translocation. Of patients with no driver mutation and a programmed death-ligand 1 expression of greater than or equal to 50%, 47% received immunotherapy.
Conclusions
We present a comprehensive data set for molecular testing in England. Although molecular testing is well established in England, timeliness and uptake of targeted therapies should be improved.
Keywords: Non–small cell lung cancer, Mutation testing, Biomarkers, Targeted therapy, Personalized medicine
Introduction
Until recently, treatment options for patients with advanced NSCLC were limited to platinum doublet chemotherapy combinations. Nevertheless, the discovery of drugs targeted at tumors with single genetic drivers, such as mutations, insertions, and deletions in the EGFR gene and rearrangements of the ALK gene, has become pivotal to the management of such patients.1 Clinical trials have revealed improved progression-free survival with targeted therapy compared with chemotherapy, and such therapies are generally better tolerated than conventional chemotherapy.2, 3, 4 The newest class of approved drugs for patients with advanced NSCLC is immune checkpoint inhibitors. Important improvement in overall survival has been revealed with pembrolizumab in patients whose tumors have a programmed death-ligand 1 (PD-L1) expression level greater than 50%, but without EGFR or ALK alteration compared with chemotherapy alone.5 On the basis of this evidence, current U.K. and international guidelines recommend that all patients with nonsquamous NSCLC undergo testing at diagnosis for EGFR “mutations,” ALK, ROS-1, NTRK rearrangements, and PD-L1 expression as a minimum to identify patients suitable for first-line targeted therapy.6
Data from the National Lung Cancer Audit (NLCA) in 2019 reveal that the 1-year survival of those with advanced NSCLC is 17% in England and Wales.7 Despite major advances in treatment, this figure is unchanged from 2015. Contributing factors include the recognized gap between clinical guidelines and their real-world implementation with observational data revealing molecular testing rates as low as 30%.8 Although there is a wealth of clinical trial data in this area, information from the real-world setting is limited. Moreover, the most real-world evidence studies have been based on claims data with inherent problems of accuracy of clinical coding and lack of clinical detail, such as performance status (PS). Progress for patients with advanced NSCLC requires establishing robust predictive biomarker testing. We report a national study investigating the efficacy and outcomes of biomarker testing in patients with advanced NSCLC in England.
Materials and Methods
Data Source and Patient Selection
This observational cross-sectional study included patients diagnosed with lung cancer (International Classification of Disease code C34) pathologically and clinically confirmed as stages IIIB to IVB NSCLC (TNM version 8), with an Eastern Cooperative Oncology Group PS of 0 to 2, in England, between June 2017 and December 2017. Cases were identified by the National Cancer Registration and Analysis Service, Public Health England. A secure dedicated portal created by the NLCA team in collaboration with Public Health England was prepopulated with the identified cases and allocated to each of the 142 NHS trusts in England that see patients with lung cancer on the basis of where they began their lung cancer diagnostic pathway (“place first seen”). All these trusts were invited to record information on at least 15 patients in their allocation. The portal was open from October 2018 to January 2019 for data collection.
Outcomes
Five important outcomes were defined before data collection by the research team to evaluate the efficacy, timeliness, and outcomes of molecular testing in England.
-
1.
The proportion of patients with lung adenocarcinoma that underwent molecular testing from the point of diagnosis to data input. Testing for EGFR, ALK, and PD-L1 was chosen in line with guidance current at the time of the study. The incidence of a positive test was calculated by dividing the number of positive tests by the total number of successful tests. Molecular tests refer to mutations, deletions, and insertions of the EGFR gene. A sensitizing EGFR mutation was defined as those occurring in exon 19 or 21. Exon 20 mutations and other rare mutations were excluded from this group. Rearrangement or fusion of the ALK gene was detected by immunohistochemistry for a fusion protein with or without confirmatory fluorescence in situ hybridization technique. The evaluation of the extent of PD-L1 ligand was by immunohistochemistry. All tests recorded had been performed as per local standard of care.
-
2.
Tissue acquisition technique and sample adequacy. The first specimen obtained for initial diagnosis and profiling was evaluated for adequacy as defined by the necessity for a second biopsy.
-
3.
Timeliness of testing. This was the time in calendar days between tissue sampling and reporting of the results of molecular testing.
-
4.
First-line systemic therapy of patients with successful molecular testing. Treatment was categorized by pathologic and molecular subtypes.
-
5.
Median survival of patients with stages IIIB to IVB adenocarcinoma. Survival was defined from the date of first sample to date of death and was analyzed according to the results of molecular testing. The median follow-up time from the first sample was 225 days with an interquartile range (IQR) of 87 to 438 days.
Statistical Analysis
Management of data and statistical analysis were performed using STATA version 15 (StataCorp). Multivariate logistic regression was used to evaluate the predictive factors for requiring a second biopsy. Regression analyses were also used to evaluate whether the method of tissue sampling, or whether EGFR testing occurred on site, influenced the odds of the time between biopsy and availability of results of molecular testing being more than three weeks. Results were presented as OR and adjusted for age, PS, and stage. Kaplan-Meier survival estimates were obtained for patients diagnosed with adenocarcinoma categorized by molecular test result.
Results
A total of 60 English trusts submitted data on 1157 patients that met the inclusion criteria. Patient demographics are summarized in Table 1. More than half of the cohort were 65 to 80 years old with approximately one-third less than 65 years old. There was a higher proportion of male (55.8%) to female (44.3%) patients. In addition, 17.3% had stage IIIB NSCLC and 82.7% had stage IV. Furthermore, two-thirds of the patients had a PS of 0 to 1 (76%) leaving 24% with a PS of 2. A total of 66% had a histologic diagnosis of adenocarcinoma.
Table 1.
Characteristics of Patients With Advanced Lung Cancer
| Category | N | % |
|---|---|---|
| Sex | ||
| Female | 512 | 44.3 |
| Male | 645 | 55.8 |
| Age, y | ||
| <65 | 353 | 30.5 |
| 65–80 | 674 | 58.3 |
| >80 | 130 | 11.2 |
| Performance status | ||
| 0 | 276 | 23.9 |
| 1 | 597 | 51.6 |
| 2 | 284 | 24.6 |
| Stage | ||
| IIIB | 200 | 17.3 |
| IV | 957 | 82.7 |
| NSCLC subtype | ||
| Adenocarcinoma | 758 | 65.5 |
| Squamous cell carcinoma | 268 | 23.2 |
| Adenosquamous carcinoma | 11 | 1.0 |
| Large cell neuroendocrine carcinoma | 26 | 2.3 |
| Other | 8 | 0.7 |
| Not otherwise specified | 44 | 3.8 |
| Unknown | 42 | 3.6 |
Molecular Testing
Excluding missing data, 558 of 758 patients (74%) with advanced lung adenocarcinoma received testing for all three recommended biomarkers (EGFR, ALK, and PD-L1). The most common molecular test performed in 701 of 758 patients (92%) was for EGFR. Testing was successful in most patients from the time of diagnosis to data input into the portal (98% for EGFR, 99% for ALK, and 95% for PD-L1). Of patients tested with lung adenocarcinoma, 10% (76 of 701) had an activating EGFR mutation, 4% (24 of 606) were ALK positive, and PD-L1 expression was greater than or equal to 50% in 38% (252 of 659) of patients (Table 2). Of patients whose tumors did not have a genetic driver (EGFR wild-type and ALK negative), PD-L1 expression was evaluated in 80% (846 of 1054). PD-L1 expression according to EGFR and ALK results is found in Table 3. A total of 15% of patients with a sensitizing EGFR mutation had PD-L1 expression greater than or equal to 50% compared with 43% of EGFR wild-type patients with high PD-L1 (p < 0.001).
Table 2.
Frequency and Outcomes of Predictive Marker Testing in Patients With Advanced Lung Adenocarcinoma (N = 758)
| EGFR | ALK | PD-L1 | ||
|---|---|---|---|---|
| Number tested, n (%) | 701 (92) | 606 (80) | 659 (87) | |
| % with successful testing | 98 | 99 | 95 | |
| Results | 10% with sensitizing mutation | 4.0% with rearrangement present | TPS < 1% | 33% |
| TPS 1%–49% | 24% | |||
| TPS ≥ 50% | 38% | |||
PD-L1, programmed death-ligand 1; TPS, tumor proportion score
Table 3.
PD-L1 Status by EGFR and ALK Test Status
| EGFR and ALK Status | PD-L1 Status |
||||
|---|---|---|---|---|---|
| PD-L1 Failed | <1% | 1%–49% | ≥50% | PD-L1 Unknown | |
| Sensitizing EGFR mutation | 4 | 25 | 28 | 9 | 10 |
| ALK positive | 1 | 8 | 5 | 10 | 3 |
| EGFR wild-type and ALK negative | 18 | 161 | 115 | 207 | 28 |
| EGFR wild-type and ALK unknown | 6 | 30 | 21 | 45 | 18 |
| ALK negative and EGFR unknown | 3 | 6 | 4 | 4 | 0 |
PD-L1, programmed death-ligand 1.
Tissue Acquisition Technique and Diagnostic Sample Adequacy
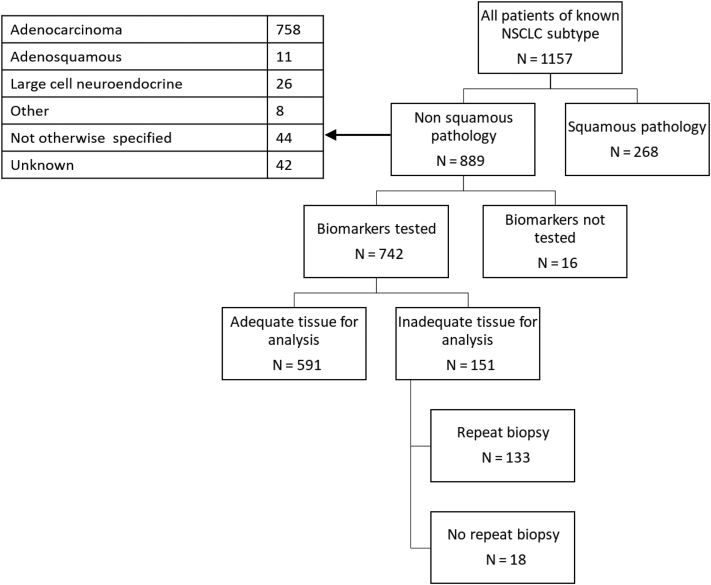
The methods of sample acquisition for patients with advanced NSCLC are illustrated in Table 4. In approximately one-third, tissue was obtained by means of radiologically guided biopsy of lung or supraclavicular lymph nodes, with the next most common method being endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) (24%). Sampling by means of pleural biopsy or fluid aspiration was conducted in 11% of patients, the vast majority by means of the latter (74%). Of 742 patients on whom molecular testing was performed, 591 (80%) had adequate tissue for analysis on initial sampling. A total of 133 patients (18%) required a second biopsy (Fig. 1).
Table 4.
Methods of Sample Acquisition
| Sample Acquisition Technique | Number (N = 1157) | % |
|---|---|---|
| Radiology-guided biopsy of lung or supraclavicular lymph nodes | 356 | 30.8 |
| EBUS-TBNA | 273 | 23.6 |
| Bronchoscopic biopsy | 212 | 18.3 |
| Mediastinoscopy, surgical lung resection, biopsy of other metastatic site, blood sample, and other | 155 | 13.4 |
| Thoracoscopy pleural biopsy, image-guided pleural biopsy, pleural fluid aspiration | 127 | 11.0 |
| Unknown | 34 | 2.9 |
EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration.
Figure 1.
Biomarker testing in advanced NSCLC.
The results of a multivariate analysis exploring predictive factors for the requirement of a second biopsy are found in Table 5. The likelihood of requiring a second biopsy was not associated with sex, PS, or stage. Older patients were significantly less likely to have a second biopsy (adjusted OR for >80 y compared with <65 y: 0.20 [95% confidence interval: 0.07–0.59]). There was a significantly increased likelihood of requiring a second biopsy if initial sampling was by means of pleural biopsy or aspiration (most being by means of aspiration) with an adjusted OR of 2.37 (95% confidence interval: 1.20–4.70).
Table 5.
Multivariate Model of Predictors for Requiring a Second Biopsy
| Model Parameter | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Sex | ||
| Female | 1 | 1 |
| Male | 0.99 (0.67–1.46) | 1.10 (0.73–1.64) |
| Age, y | ||
| <65 | 1 | 1 |
| 65–80 | 0.80 (0.53–1.21) | 0.76 (0.50–1.16) |
| >80 | 0.19 (0.06–0.56) | 0.20 (0.07–0.59) |
| Performance status | ||
| 0 | 1 | 1 |
| 1 | 0.83 (0.53–1.31) | 0.88 (0.55–1.40) |
| 2 | 0.58 (0.32–1.05) | 0.68 (0.36–1.27) |
| Stage | ||
| IIIB | 1 | 1 |
| IV | 1.08 (0.65–1.81) | 0.92 (0.53–1.59) |
| Sample method | ||
| EBUS-TBNA | 1 | 1 |
| Radiology-guided lung biopsy | 0.91 (0.54–1.53) | 0.96 (0.56–1.62) |
| Bronchoscopic biopsy | 0.65 (0.33–1.26) | 0.71 (0.36–1.39) |
| Thoracoscopic pleural biopsy, image-guided pleural biopsy, pleural aspiration | 1.99 (1.04–3.80) | 2.37 (1.20–4.70) |
| Mediastinoscopy, surgical lung resection, biopsy of other metastatic site, blood sample, and other | 1.11 (0.58–2.13) | 1.27 (0.64–2.50) |
CI, confidence interval; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration.
Timeliness of Testing
The median time from tissue acquisition to results of EGFR “mutational” analysis being available was 18 days (IQR: 14–21), for ALK rearrangement 17 days (IQR: 13–24), and for assessment of PD-L1 expression also 17 days. Using a multivariable logistic regression model, the likelihood of time from biopsy to availability of molecular results being more than 3 weeks was not significantly associated with age, PS, stage, sampling technique, or whether EGFR testing was on site.
First-Line Treatment for Patients With Advanced Lung Adenocarcinoma
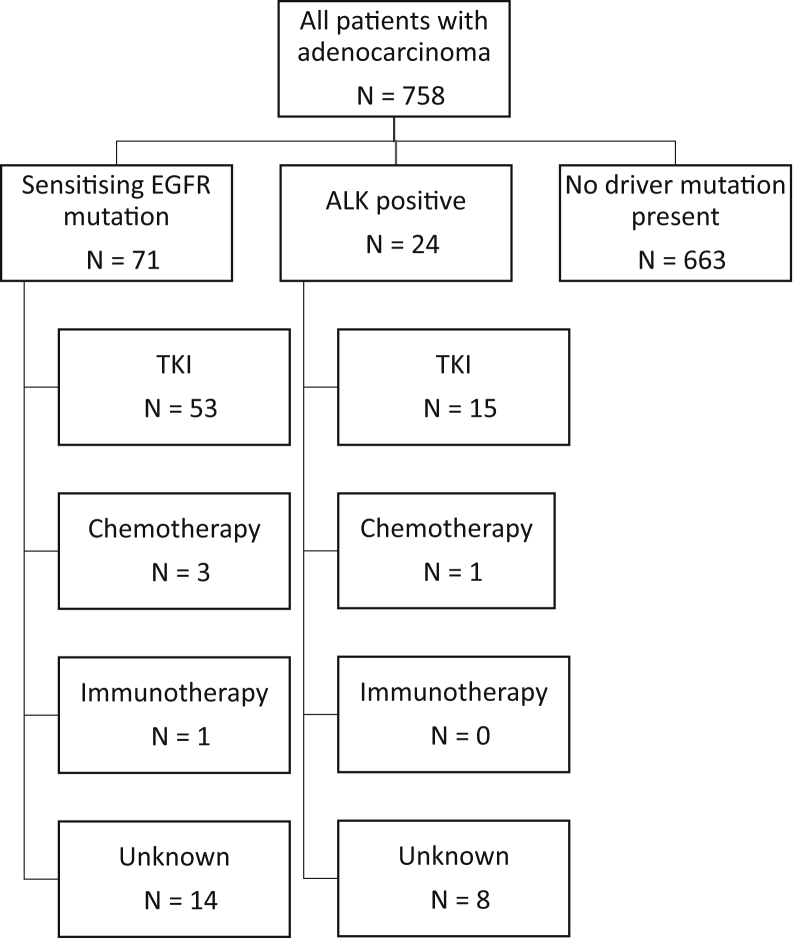
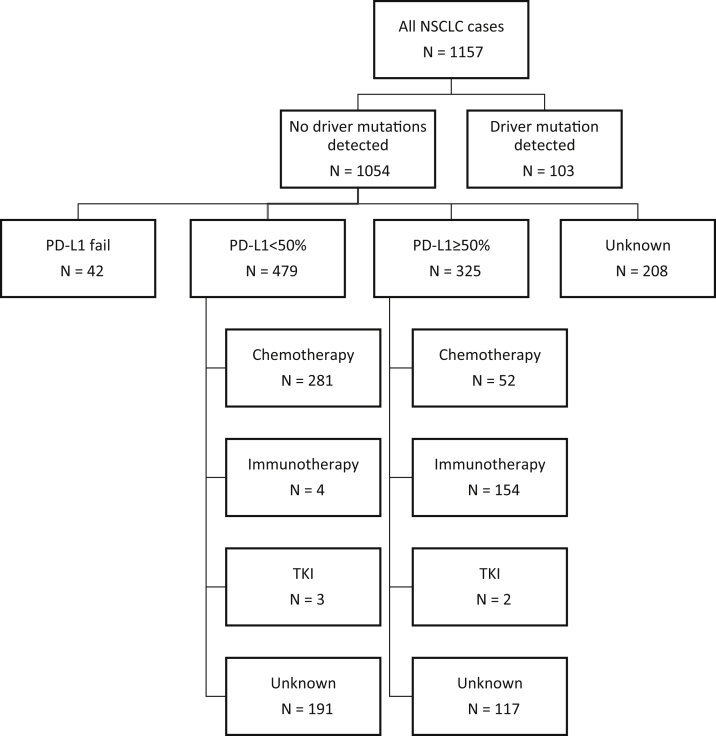
The treatments received by patients with advanced lung adenocarcinoma are summarized in Figure 2. Of patients with a sensitizing EGFR mutation, 54 of 76 (71%) received a first-line tyrosine kinase inhibitor (TKI) compared with 16 of 27 (59%) of those that were ALK positive. No genetic driver was detected in 1054 patients; the treatments received by these are found in Figure 3. In those whose tumors had a PD-L1 expression level of greater than or equal to 50%, 52 of 325 (16%) received first-line chemotherapy and 154 of 325 (47%) immunotherapy.
Figure 2.
Treatment patterns for biomarker-positive patients with advanced lung adenocarcinoma. TKI, tyrosine kinase inhibitor.
Figure 3.
Treatment patterns for patients with no driver mutations detected. PD-L1, programmed death-ligand 1; TKI, tyrosine kinase inhibitor.
Survival
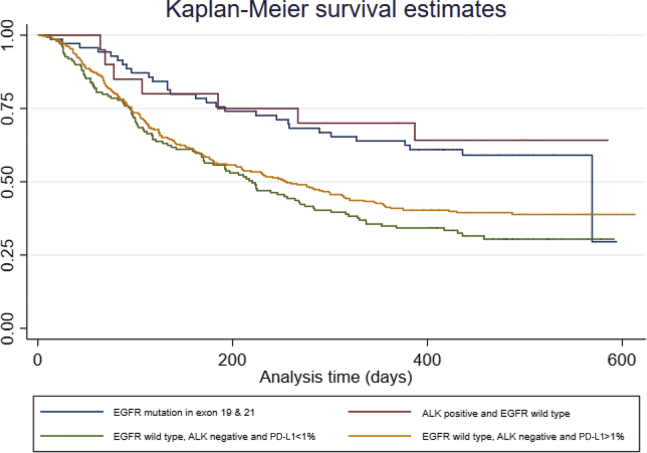
Of the 1088 patients for whom complete data were available, the median survival was 7.2 months. Kaplan-Meier estimates categorized by molecular testing reveal that survival was significantly improved for patients with an EGFR sensitizing mutation compared with those without a sensitizing mutation (12 mo versus 7 mo) (Fig. 4). As more than half of the patients with an ALK translocation were still alive at the time of data extraction, median survival estimates could not be calculated for this subgroup.
Figure 4.
Survival of patients with lung adenocarcinoma according to predictive biomarkers (n = 740), in which “analysis time” on the x-axis is found in days and survival probability on the y-axis is found as a number between 0 and 1 (in which, 1 = certain). Of 758 lung adenocarcinoma, 18 cases had missing survival data. PD-L1, programmed death-ligand 1.
Discussion
To our knowledge, this is the first national study of biomarker testing for patients with advanced NSCLC to include every treating unit. Often detected oncogenic drivers are present in approximately one-quarter of adenocarcinomas, and these tumors are targetable with approved drugs.9 Therefore, optimal management of this subgroup of patients is essential to achieving improved overall survival outcomes.
There are three key findings from this study. First, a high proportion of patients (83%) in this study cohort underwent testing for EGFR, ALK, and PD-L1 status, revealing testing is embedded in practice in England. In addition, the testing rates we report here compare favorably with previously published population-based studies. A retrospective study reported that testing rates in eight countries varied between 43% in Brazil and 85% in Taiwan, between 2011 and 2013.10 A more recent study conducted in the United States quoted rates of approximately 70%.11 The data we report here indicate that, in this population, only 4% of tests failed. This suggests that, when adequate tissue is acquired, diagnostic processes are appropriate. Nevertheless, it is likely that this rate is an underestimate. Genotyping technologies implemented by laboratories on the country vary in effectiveness at identifying mutant alleles at low tumor amounts. Laboratories that use next-generation sequencing techniques use more stringent metrics than those that use mutation-specific technologies, which may lead to false-negative results in the latter.
Second, even when a targetable genetic driver was present, only 68% of patients received appropriate, guideline-recommended first-line treatment. In addition, of those patients with tumors in which no such driver was present, but expression of PD-L1 was greater than 50%, less than half received immunotherapy. In the aforementioned multinational study, the rate of targeted first-line treatment of patients whose tumors tested positive for EGFR mutations or ALK rearrangements had varied from 30% in Germany to 89% in Japan.10 Nevertheless, a more recent study performed in Germany reported a targeted therapy treatment rate of up to 92%.8 It should be noted that these studies included all patients with advanced NSCLC unlike our cohort of patients with optimal PS of 0 to 2. Our findings equate to approximately 350 eligible patients per year in England missing out on the benefits of targeted/immune checkpoint therapies, including longer progression-free survival and avoidance of side effects.
Third, this study revealed that timeliness of testing in England also requires improvement. The median time from tissue acquisition to availability of results of EGFR testing was 18 days, a delay which might explain why some patients with a targetable mutation do not receive a first-line TKI. This delay is in keeping with the findings of the NLCA’s 2019 organizational audit. In this survey, 101 participating NHS trusts in England and Wales reported a turnaround time from biopsy to availability EGFR and ALK testing results of between 1 and 21 days and 93 centers reported a similar range for PD-L1 testing.12 Delays in PD-L1 testing may also reflect that only a subset of pathologists can perform the evaluation which also may not be available onsite. The data reveal that the 10-day “molecular testing standard” recommended by NHS England’s National Optimal Lung Cancer Pathway was achieved by only 37% of NHS trusts.12,13
There is evidence to reveal that, during this period, the PS of patients with advanced NSCLC deteriorates. Indeed, a study investigating challenges with enrolling patients requiring “biomarker-specific” data into clinical trials, found that declining PS led to most screen failures in those who did not require a biopsy.14 In addition, recent published data reveal that as many as 20% of mutation-positive patients eligible for TKI treatment receive conventional chemotherapy as a result of being unable to wait for results before commencing treatment, thereby missing out on optimal therapy.8
In addition to delays in biomarker results, deviations from guideline-recommended first-line treatment may also be attributed to delays in access to oncologists. Furthermore, clinician attitudes to prescribing novel therapies have been found to contribute to treatment rates. An online self-reported survey from oncologists in 10 countries suggested that testing occurred for 81% of patients with advanced NSCLC. Surprisingly, less than half responded that their treatment decisions were influenced by detected mutations and 23% stated that EGFR mutation status did not affect first-line therapy decisions.15
Strengths and Limitations
Before this study, there were limited national data on the management of patients with advanced NSCLC. The strength of this cohort study lies in the clinical data it has captured, particularly the assessment of PS and mode of tissue acquisition which other large databases often lack. Nevertheless, we acknowledge that only 60 of 142 hospitals uploaded data to the portal and participating trusts were asked to record data on a minimum of 15 patients. The patients selected by trusts may have introduced a selection bias. It is possible that data may differ in the trusts that did not respond. In addition, the retrospective nature of the audit may be subject to confounding and recall bias. Lastly, and similar to previous observational surveys of molecular testing, our results do not account for patient preference and contraindications to treatment. The data we present do, however, provide an important snapshot of current practice.
The results of our study are in keeping with a recently published international survey conducted by the International Association of the Study of Lung Cancer.16 This cited the following barriers to implementation of molecular testing: cost, quality of samples, access, awareness, and turnaround time. In that survey, one-third of the respondents were unaware of the most recent guidelines for testing and approximately a half stated that there was no policy to improve quality in their country. Probably the most often cited challenge to molecular testing is sufficiency of tissue.14 In 20% of patients in our cohort, the initial biopsy was inadequate for such testing. Previous studies have investigated whether samples procured by minimally invasive techniques such as EBUS-TBNA are sufficient to identify actionable genetic drivers. In our analysis, EBUS-TBNA was not significantly associated with the need for a second biopsy in adjusted regression analyses, providing further evidence that successful molecular testing can be undertaken on EBUS-derived specimens.17 Nevertheless, it is interesting to note that patients undergoing pleural aspiration are significantly more likely to require additional tissue. This finding is supported by a recent prospective study which revealed that negative pleural fluid cytology is often encountered in patients with malignant pleural effusion.18 These data question the value of pleural aspiration as a diagnostic tool in the lung cancer pathway.
Survival of patients with advanced NSCLC remains poor in real-world settings. Nevertheless, international guidelines now recommend testing for an increasing number of potentially targetable genetic alterations, and PD-L1 expression and progress in revealing efficacy of targeting new “molecular subgroups” is occurring rapidly.19 In May 2020 alone, the Food and Drug Administration approved six new systemic therapies for patients with advanced NSCLC on the basis of molecular testing, including capmatinib for MET exon 14 skip mutations and selpercatinib for RET-fusion–positive tumors. Next-generation sequencing, rather than single-gene testing, will therefore become the standard of care for the molecular testing of advanced NSCLC. To date, this has not been routinely established in England and it remains to be seen whether the excellent testing rates evident in this study can be maintained as more biomarkers are required to guide optimum management.
In conclusion, molecular testing and tailored therapies are central to the management of patients with advanced NSCLC. We highlight that, despite good molecular testing rates in England, there is much room for improvement in timeliness and access to treatment. These data provide an opportunity to make innovations to improve the availability and efficiency of molecular testing and, thereby, survival and quality of life for patients with advanced NSCLC.
CRediT Authorship Contribution Statement
Neal Navani, Paul Beckett, Susan V. Harden, and Judith Tweedie: Conception and design.
Jana B. Adizie, Aamir Khakwani, and Emily Peach: Statistical analysis.
Jana B. Adizie, Judith Tweedie, Aamir Khakwani, Emily Peach, Richard Hubbard, Natasha Wood, John R. Gosney, Susan V. Harden, Paul Beckett, Sanjay Popat, and Neal Navani: Drafting or revising the work.
Neal Navani: Guarantor of the article, Taking responsibility for the integrity of the work.
Data Availability
The data are collated, maintained, and quality assured by the National Cancer Registration and Analysis Service, which is part of Public Health England. Access to the data was facilitated by the Public Health England Office for Data Release (http://www.ndrs.nhs.uk/).
Acknowledgments
Neal Navani is supported by an MRC Clinical Academic Research Partnership (MR/T02481X/1). This work was partly undertaken at the University College London Hospitals/University College London which received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centre’s funding scheme. This work uses data that have been provided by patients and collected by the National Health Service as part of their care and support. This is a review of data from a national audit and individual patient consent was not required. All data were collated and maintained by the Cancer Registration and Analysis Service (NCRAS), which is part of Public Health England (PHE). This study was performed in accordance with the Declaration of Helsinki.
Footnotes
Cite this article as: Adizie JB, Tweedie J, Khakwani A, et al. Biomarker testing for people with advanced lung cancer in England. JTO Clin Res Rep. 2021;2:100176.
Disclosure: The authors declare no conflict of interest.
References
- 1.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R., Carcereny E., Gervais R. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Solomon B.J., Mok T., Kim D.-W. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 4.Yang J.C., Wu Y.L., Schuler M. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 5.Hiley C.T., Le Quesne J., Santis G. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet. 2016;388:1002–1011. doi: 10.1016/S0140-6736(16)31340-X. [DOI] [PubMed] [Google Scholar]
- 6.NICE (National Institute for Health and Care Excellence) Lung cancer: diagnosis and management: NICE guideline [NG122] https://www.nice.org.uk/guidance/ng122 Accessed.
- 7.Royal College of Physicians National Lung Cancer Audit (NLCA) annual report 2018. 2018. https://www.rcplondon.ac.uk/projects/outputs/national-lung-cancer-audit-nlca-annual-report-2018 Accessed.
- 8.Hardtstock F., Myers D., Li T. Real-world treatment and survival of patients with advanced non-small cell lung cancer: a German retrospective data analysis. BMC Cancer. 2020;20:1–14. doi: 10.1186/s12885-020-06738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D.H., Tsao M.S., Kambartel K.O. Molecular testing and treatment patterns for patients with advanced non-small cell lung cancer: PIvOTAL observational study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0202865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Appius A., Pattipaka T., Feyereislova A., Cassidy A., Ganti A.K. Real-world management of patients with epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer in the USA. PLoS One. 2019;14 doi: 10.1371/journal.pone.0209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royal College of Physicians Organisational audit report 2019. 2019. https://www.rcplondon.ac.uk/projects/outputs/organisational-audit-report-2019 Accessed.
- 13.National Health Service England National Optimal Lung Cancer Pathway for suspected and confirmed lung cancer: referral to treatment: UPDATE 2020. www.cancerresearchuk.org/sites/default/files/national_optimal_lung_pathway_aug_2017.pdf Version 3.0. Accessed May 17, 2020.
- 14.Spiegel M.L., Goldman J.W., Wolf B.R. Non–small cell lung cancer clinical trials requiring biopsies with biomarker-specific results for enrollment provide unique challenges. Cancer. 2017;123:4800–4807. doi: 10.1002/cncr.31056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spicer J., Tischer B., Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: global trends and differences. Ann Oncol. 2015;26(suppl 1):i57. [Google Scholar]
- 16.Smeltzer M.P., Wynes M.W., Lantuejoul S. The International Association for the Study of Lung Cancer (IASLC) global survey on molecular testing in lung cancer. J Thorac Oncol. 2020;15:1434–1448. doi: 10.1016/j.jtho.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Navani N., Brown J.M., Nankivell M. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med. 2012;185:1316–1322. doi: 10.1164/rccm.201202-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsim S., Paterson S., Cartwright D. Baseline predictors of negative and incomplete pleural cytology in patients with suspected pleural malignancy – data supporting ‘Direct to LAT’ in selected groups. Lung Cancer. 2019;133:123–129. doi: 10.1016/j.lungcan.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Kalemkerian G.P., Narula N., Kennedy E.B. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol. 2018;36:911–919. doi: 10.1200/JCO.2017.76.7293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are collated, maintained, and quality assured by the National Cancer Registration and Analysis Service, which is part of Public Health England. Access to the data was facilitated by the Public Health England Office for Data Release (http://www.ndrs.nhs.uk/).