Abstract
Introduction
The International Association for the Study of Lung Cancer proposed a new grading criteria for invasive adenocarcinoma. However, its utility has not been validated.
Methods
Patients who underwent complete resection of lung adenocarcinoma were included in this study. Then, they were divided into the following three groups on the basis of the criteria recently proposed by the International Association for the Study of Lung Cancer: grade 1, lepidic predominant tumor, with less than 20% of high-grade patterns; grade 2, acinar or papillary predominant tumor, with less than 20% of high-grade patterns; and grade 3, any tumor with greater than or equal to 20% of high-grade patterns.
Results
Recurrence-free survival (RFS) was significantly different among the proposed grades (p < 0.001). The RFS of patients upgrading from current grade 2 (papillary or acinar predominant tumor) to proposed grade 3 (5-y RFS, 65.2%) was significantly worse than that of patients with proposed grade 2 (77.1%, hazard ratio = 1.882, 95% confidence interval: 1.236–2.866) but not significantly different from that of patients with grade 3 in both the current (micropapillary or solid predominant tumor) and proposed criteria (53.2%, hazard ratio = 0.761, 95% confidence interval: 0.456–1.269). Among patients with pathologic stage 0 or I, RFS was well stratified by the new grading system (p < 0.001) but not among patients with stage II or III (p = 0.334). In the multivariable analysis, the new grading was not a predictive factor of RFS.
Conclusions
Although the proposed grading system well stratified RFS in patients with pathologic stage 0 or I lung adenocarcinoma, there is room for improvement.
Keywords: Lung adenocarcinoma, Grade, Pathological grade, Lung cancer
Introduction
Tumor grading is an important component in the pathologic diagnosis of several types of cancer.1, 2, 3, 4 However, there has been no consensus in the grading system of lung adenocarcinoma. Currently, the grading of lung adenocarcinoma is performed on the basis of the predominant subtype: low grade (grade 1), lepidic predominant; intermediate grade (grade 2), acinar or papillary predominant; and high grade (grade 3), solid or micropapillary predominant.5 Grading is a significant predictor and is widely used. However, the current grading seems to be insufficient because the second predominant subtype is also a significant prognostic factor.6,7 In addition, even if it is not a predominant subtype, the presence of a high-grade subtype, such as micropapillary, is a predictor of worse prognosis.5,8,9 Spread through air spaces (STAS),10, 11, 12 nuclear grade,13, 14, 15 and mitotic grade14, 15, 16 are also prognostic factors. However, no study has investigated on all these factors. Recently, the International Association for the Study of Lung Cancer (IASLC) proposed a grading system.17 In the study of IASLC, the best models for classifying prognosis on the basis of histology, STAS, nuclear grade, and mitotic grade were assessed. As a result, the model using histology and its proportions was considered the best. In the proposed grading criteria, lung adenocarcinoma is classified into three grades, which are as follows: grade 1, lepidic predominant tumor, with less than 20% of high-grade patterns; grade 2, acinar or papillary predominant tumor, with less than 20% of high-grade patterns; and grade 3, any tumor with greater than or equal to 20% of high-grade patterns (solid, micropapillary, or complex gland). However, other than the initial research, no study has used this grading system. In the study of IASLC, these criteria were validated using the training, validation, and testing cohorts but the number of participants in each cohort was restricted. Hence, we investigated the utility of these criteria in patients with completely resected lung adenocarcinoma at our hospital.
Materials and Methods
Patients
This retrospective study was approved by the Institutional Review Board of Hiroshima University Hospital. However, the need for informed consent was waived. Patients who underwent curative intent resection between April 2007 and March 2020 at Hiroshima University Hospital for lung adenocarcinoma but did not receive neoadjuvant therapy were included in this study. The variants of adenocarcinoma were excluded from this research.
Pathologic Diagnosis and Grading Criteria
Pathologic staging was determined according to the eighth edition of the TNM classification of malignant tumors.18 All patients underwent pathologic examination using the WHO classification.19 The current pathologic grading was based on the predominant subtype, which are as follows: grade 1, lepidic predominant; grade 2, acinar or papillary predominant; and grade 3, solid or micropapillary predominant.5 The proposed pathologic grading was based on the following grading criteria, which was recently proposed by the IASLC: grade 1, lepidic predominant tumor, with less than 20A–E.% of high-grade patterns; grade 2, acinar or papillary predominant tumor, with less than 20% of high-grade patterns; and grade 3, any tumor with greater than or equal to 20% of high-grade patterns (solid, micropapillary, and/or complex gland).17 All patients were evaluated for lymphatic invasion (LY), vascular invasion (V), and pleural invasion (PL). The diagnosis of LY was based on the immunostaining results for D2-40 to validate the location of the lymphatic duct. To determine the degree of tumor invasion above the elastic layer of the vessels and the visceral pleura, the presence of PL and V is evaluated by means of elastic van Gieson staining. These pathologic diagnoses were made by experienced pathologists, which also included the authors (TK, KK, and YT). Representative images of each subtype are revealed in Supplementary Figure 1A–E.
Statistical Analysis
Data are presented as median and interquartile range for continuous variables and n (%) for categorical variables. Categorical variables were compared using the chi-square test. Continuous variables were analyzed using the unpaired t test. Recurrence-free survival (RFS) was defined as the time interval from the date of surgery until the date of recurrence, death, or last follow-up visit. Overall survival (OS) was defined as the time interval from the date of surgery until the date of death owing to any cause or until the last follow-up visit. Survival data were calculated using the Kaplan-Meier method and were compared using the log-rank test. Multivariable analysis using Cox proportional hazards regression analysis for RFS and OS was also performed. In the multivariable analysis, age (continuous variable), sex (male or female), invasive tumor size (continuous variable), lymphovascular invasion (presence of LY or V), PL, lymph node metastasis, and proposed grade were included as variables. The JMP software version 14 (SAS Institute, Cary, NC) was used for all statistical analyses. A p value less than 0.05 is considered statistically significant for all analyses.
Results
In total, 1059 patients were included in this study. The characteristics of the patients are illustrated in Table 1. The number of patients with proposed grade 1, grade 2, and grade 3 was 382 (36.1%), 490 (46.3%), and 187 (17.7%), respectively.
Table 1.
Patient Characteristics
| Variables | All Patients n = 1059 | Proposed Grade 1 n = 382 | Proposed Grade 2 n = 490 | Proposed Grade 3 n =187 | p Value |
|---|---|---|---|---|---|
| Age (IQR) | 69 (63–75) | 68 (62–74) | 69 (63–75) | 71 (64–76) | 0.042 |
| Sex, male, n (%) | 570 (54.7) | 174 (45.4) | 269 (54.9) | 137 (73.2) | <0.001 |
| Type of surgery, n (%) | 0.047 | ||||
| Lobectomy | 570 (53.8) | 140 (36.6) | 306 (62.4) | 124 (66.7) | |
| Segmentectomy | 326 (30.8) | 152 (39.8) | 135 (27.6) | 39 (21.0) | |
| Wedge resection | 161 (15.2) | 89 (23.2) | 49 (10.0) | 23 (12.3) | |
| Pneumonectomy | 2 (0.2) | 1 (0.3) | 0 (0) | 1 (0.5) | |
| Pathologic stage, n (%) | <0.001 | ||||
| 0 | 124 (11.7) | 124 (32.4) | 0 (0) | 0 (0) | |
| IA1 | 275 (26.0) | 184 (48.0) | 75 (15.3) | 16 (8.6) | |
| IA2 | 253 (23.9) | 43 (11.2) | 171 (34.9) | 39 (21.0) | |
| IA3 | 94 (8.9) | 5 (1.3) | 74 (15.1) | 15 (8.1) | |
| IB | 136 (12.8) | 14 (3.7) | 81 (16.5) | 41 (22.0) | |
| IIA | 22 (2.1) | 2 (0.5) | 11 (2.2) | 9 (4.8) | |
| IIB | 93 (8.8) | 2 (0.5) | 48 (9.8) | 43 (23.1) | |
| IIIA | 57 (5.4) | 8 (2.1) | 27 (5.5) | 22 (11.8) | |
| IIIB | 5 (0.5) | 0 (0) | 3 (0.6) | 2 (1.1) | |
| Invasive characteristics, n (%) | |||||
| LY | 198 (18.7) | 11 (2.9) | 102 (20.8) | 85 (45.5) | <0.001 |
| V | 235 (22.2) | 17 (4.5) | 118 (24.1) | 100 (53.5) | <0.001 |
| PL | 174 (16.4) | 17 (4.5) | 96 (19.6) | 61 (32.6) | <0.001 |
| Predominant subtype, n (%) | <0.001 | ||||
| Lepidic | 368 (34.8) | 365 (95.6) | 0 (0) | 3 (1.6) | |
| Papillary | 537 (50.8) | 0 (0) | 456 (93.1) | 81 (43.3) | |
| Acinar | 48 (4.5) | 0 (0) | 34 (6.9) | 14 (7.5) | |
| Solid | 66 (6.2) | 0 (0) | 0 (0) | 66 (35.3) | |
| Micropapillary | 23 (2.2) | 0 (0) | 0 (0) | 23 (12.3) | |
| Mucinus | 17 (1.6) | 17 (4.4) | 0 (0) | 0 (0) |
IQR, interquartile range; LY, lymphatic invasion; PL, pleural invasion; V, vascular invasion.
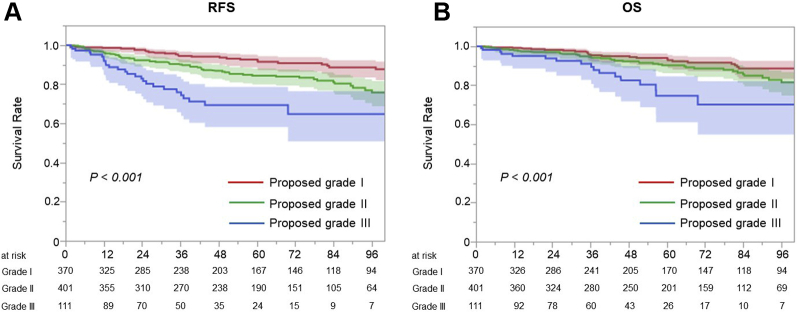
The RFS and OS of all participants were analyzed, with a median follow-up of 49 (24–85) months. There was a significant difference in terms of RFS (p < 0.001, Fig. 1A) and OS among the proposed grades (p < 0.001, Fig. 1B).
Figure 1.
Prognosis of all participants. (A) There was a significant difference in terms of RFS among the proposed grades (p < 0.001). (B) There was a significant difference in terms of OS among the proposed grades (p < 0.001). OS, overall survival; RFS, recurrence-free survival.
Table 2 reveals the differences between the grades in the current and proposed criteria. Most patients with grade 1 disease in the current criteria (n = 382, 99.2%) presented with grade 1 disease in the proposed criteria. All patients with grade 3 disease in the current criteria presented with grade 3 disease in the proposed criteria. Among patients with current grade 2, 95 patients (16.2%) were upgraded to grade 3 in the proposed criteria and their characteristics are illustrated in Supplementary Table 1. We compared the prognosis among patients who were classified as grade 2 in both the current and proposed criteria, grade 3 in both the current and proposed criteria, and upgraded from current grade 2 to grade 3 in the proposed criteria. The RFS of patients upgraded to the proposed grade 3 in the proposed criteria (5-y RFS rate = 65.2%, 95% confidence interval [CI]: 53.2–75.5) was significantly worse than that of patients with grade 2 in both the current and proposed criteria (5-y RFS rate = 77.1%, 95% CI: 72.7–81.0, hazard ratio [HR] = 1.882, 95% CI: 1.236–2.866). The RFS did not significantly differ between patients with upgraded and original grade 3 disease (5-y RFS rate = 53.2%, 95% CI: 40.0–63.0, HR = 0.761, 95% CI: 0.456–1.269) (Fig. 2A). Similarly, the OS of patients with grade 2 in the current criteria and upgraded to grade 3 in the proposed grade (5-y OS rate = 75.1%, 95% CI: 61.1–85.3) was significantly worse than that of patients with grade 2 in both the current and proposed criteria (5-y OS rate = 85.9%, 95% CI: 82.0–89.1; HR = 2.055, 95% CI: 1.216–3.473). The OS did not significantly differ between patients with upgraded and original grade 3 (5-y OS rate = 68.4%, 95% CI: 53.3%–80.5%, HR = 0.968, 95% CI: 0.498–1.880) (Fig. 2B).
Table 2.
Differences Between the Current and Proposed Grades
| Current Grade | Proposed Grade | n (%) |
|---|---|---|
| Grade 1 (n = 385) | Grade 1 | 382 (99.2) |
| Grade 2 | 0 (0) | |
| Grade 3 | 3 (0.8) | |
| Grade 2 (n = 585) | Grade 1 | 0 (0) |
| Grade 2 | 490 (83.8) | |
| Grade 3 | 95 (16.2) | |
| Grade 3 (n = 89) | Grade 1 | 0 (0) |
| Grade 2 | 0 (0) | |
| Grade 3 | 89 (100) |
Figure 2.
Prognosis of patients who were upgraded from grade 2 to proposed grade 3 in the proposed criteria. (A) The RFS of patients upgraded from grade 2 to grade 3 in the proposed criteria (5-y RFS rate = 65.2%, 95% CI: 53.2–75.5) was significantly worse than that of patients with grade 2 in both the current and proposed criteria (5-y RFS rate = 77.1%, 95% CI: 72.7–81.0, HR = 1.882, 95% CI: 1.236–2.866). The RFS did not significantly differ between patients upgraded from grade 2 to grade 3 in the proposed criteria and patients with grade 3 in both the current and proposed criteria (5-y RFS rate = 53.2%, 95% CI: 40.0–63.0, HR = 0.761, 95% CI: 0.456–1.269). (B) The OS of patients upgraded from grade 2 to grade 3 in the proposed criteria (5-y OS rate = 75.1%, 95% CI: 61.1–85.3) was significantly worse than that of patients with grade 2 in both the current and proposed criteria (5-y OS rate = 85.9%, 95% CI: 81.9–89.1, HR = 2.055, 95% CI: 1.216–3.473). The OS did not significantly differ between patients upgraded from grade 2 to grade 3 in the proposed criteria and patients with grade 3 in both the current and proposed criteria (5-y OS rate = 68.4%, 95% CI: 53.3–80.5, HR = 0.968, 95% CI: 0.498–1.880). CI, confidence interval; HR, hazard ratio; OS, overall survival; RFS, recurrence-free survival.
Furthermore, we analyzed the prognosis of patients with pathologic stage I. The characteristics of the participants are presented in Supplementary Table 2. There were significant differences in terms of RFS (p < 0.001, Fig. 3A) and OS (p < 0.001, Fig. 3B) among the proposed grades. In contrast, among patients with pathologic stage II or III, there were no significant differences in RFS (p = 0.334, Supplementary Fig. 2A) and OS (p = 0.223, Supplementary Fig. 2B).
Figure 3.
Prognosis of patients with pathologic stage 0 or I. (A) A higher grade in the proposed criteria was associated with a worse RFS (p < 0.001). (B) A higher grade in the proposed criteria was associated with a worse OS (p < 0.001). OS, overall survival; RFS, recurrence-free survival.
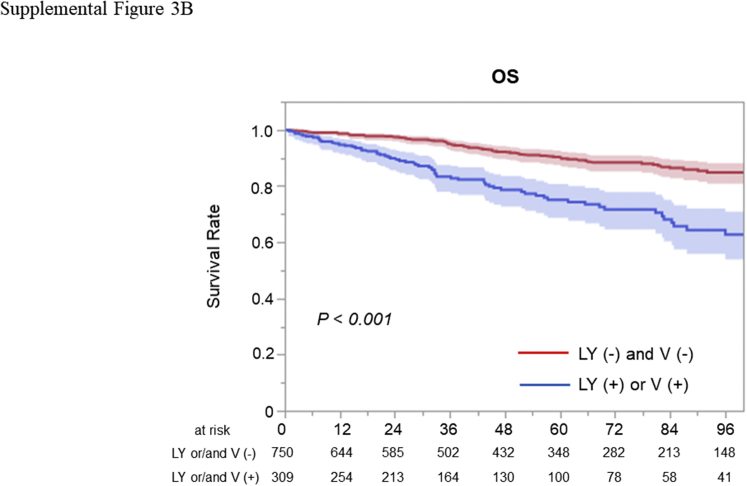
In the multivariable analysis for all included patients, proposed grade was not a significant predictor of RFS (HR = 1.065, 95% CI: 0.838–1.354, p = 0.607) and OS (HR = 1.079, 95% CI: 0.808–1.440, p = 0.524). In contrast, lymphovascular invasion was a significant predictor of RFS (HR = 2.014, 95% CI: 1.401–2.895, p < 0.001) and OS (HR = 1.561, 95% CI: 1.019–2.391, p = 0.041) (Supplementary Table 3). In the multivariable analysis for patients with stage 0 or I, proposed grade was not a significant predictor of RFS (HR = 1.061, 95% CI: 0.753–1.488, p = 0.733) and OS (HR = 1.078, 95% CI: 0.717–1.612, p = 0.715). In contrary, lymphovascular invasion was a significant predictor of RFS (HR = 2.229, 95% CI: 1.436–3.461, p < 0.001) and a marginally significant predictor for OS (HR = 1.602, 95% CI: 0.964–2.664, p = 0.070) (Table 3). Among all included patients, RFS (p < 0.001, Supplementary Fig. 3A) and OS (p < 0.001, Supplementary Fig. 3B) were significantly shorter in patients with lymphovascular invasion. Among patients with pathologic stage 0 or I, RFS (p < 0.001, Supplemental Fig. 4A) and OS (p < 0.001, Supplementary Fig. 4B) were also significantly shorter in patients with lymphovascular invasion.
Table 3.
Multivariable Analysis for RFS and OS (Patients With Stage 0 or I)
| Variables | RFS |
OS |
||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 1.075 (1.052–1.100) | <0.001 | 1.103 (1.074–1.135) | <0.001 |
| Sex (male) | 1.827 (1.213–2.753) | 0.004 | 2.780 (1.684–4.588) | <0.001 |
| Invasive size | 1.390 (1.098–1.744) | 0.005 | 1.388 (1.046–1.819) | 0.020 |
| Lymphovascular invasion | 2.229 (1.436–3.461) | <0.001 | 1.602 (0.964–2.664) | 0.070 |
| PL | 1.918 (1.228–2.995) | 0.004 | 1.249 (0.728–2.144) | 0.420 |
| Proposed grade | 1.061 (0.753–1.488) | 0.733 | 1.078 (0.717–1.612) | 0.715 |
CI, confidence interval; HR, hazard ratio; OS, overall survival; PL, pleural invasion; RFS, recurrence-free survival.
Discussion
In this study, the newly proposed grading criteria were useful in stratifying the prognosis of resected lung cancer. There is no difference in prognosis between patients who were upgraded from grade 2 to grade 3 in the proposed criteria and those with grade 3 both in the current and proposed criteria. This result shows the efficacy of the new criteria. In our study, the prognosis of patients with pathologic stage 0 or I was well stratified by this grading but not in patients with pathologic stage II or III. The prognosis of completely resected stage I NSCLC is expected to be favorable. However, several patients experience recurrence after complete resection, such that the 5-year disease-free survival rates for clinical stage IA and stage IB disease are 84.3% and 65.8%, respectively.20 Therefore, some patients with stage I may require additional treatment, such as adjuvant therapy. In this study, patients with grade 3 in the proposed criteria were more likely to have invasive characteristics, such as large invasive component size, LY, V, and PL. These factors were not included in the original study of the proposed grading criteria and were not used to establish the proposed grading criteria. Large invasive component size, LY, V, and PL were high-risk factors for recurrence in stage I NSCLC.21, 22, 23 In fact, lymphovascular invasion was a significant prognostic factor in our study and prognosis was well stratified by presence or absence of lymphovascular invasion in analysis for patients with stage 0 or I and all included patients. In our cohort, the proportion of papillary predominant tumor was higher than that in the past study.10,11,14 Diagnosis of subtypes may more likely vary between pathologists and institutions, and this may be one of the challenges in the establishment of a grading system. In contrary, diagnosis of lymphovascular invasion is easy to perform and it does not vary between institutions and pathologists. Therefore, lymphovascular invasion may be more useful and convenient for predicting prognosis and selecting candidates for adjuvant therapy, and this needs to be further assessed.
This study had several strengths, that is, it compared the prognoses between patients upgraded from current grade 2 to grade 3 in the proposed criteria and others. The worse prognosis of patients upgraded from grade 2 to grade 3 in the proposed criteria than patients with grade 2 in both the current and proposed criteria strongly supported the appropriateness of the proposed grading system. These new grading criteria can be adapted similar to the current criteria if the subtypes and their proportions, which may have been used more often than the nuclear and cytologic grades, STAS, and mitotic grade, are identified.
Furthermore, this study had several limitations. First, this retrospective study was conducted at a single institution. Second, the follow-up period for the prognosis of patients with early stage NSCLC was short. However, the number of participants was sufficient because it was larger than those of the training, validation, and test cohorts of the original study. We believe that the validity of the new criteria was increased by this study.
In conclusion, upgrading from current grade 2 to proposed grade 3 was reasonable with similar survival as current grade 3. Especially, prognoses of patients with pathologic stage 0 or I were well stratified. However, the proposed grade was inferior to lymphovascular invasion as a prognostic factor and there is room for improvement.
Acknowledgment
This work was supported by the Japan Society for the Promotion of Science KAKENHI (grant number: JP20K17749). The authors thank Enago (www.enago.jp) for the English-language review.
Footnotes
Disclosure: The authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100126.
Supplementary Data
Supplementary Figure 1A.
Supplementary Figure 1B.
Supplementary Figure 1C.
Supplementary Figure 1D.
Supplementary Figure 1E.
Supplementary Figure 2A.
Supplementary Figure 2B.
Supplementary Figure 3A.
Supplementary Figure 3B.
Supplementary Figure 4A.
Supplementary Figure 4B.
References
- 1.Rabe K., Snir O.L., Bossuyt V., Harigopal M., Celli R., Reisenbichler E.S. Interobserver variability in breast carcinoma grading results in prognostic stage differences. Hum Pathol. 2019;94:51–57. doi: 10.1016/j.humpath.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Helpap B., Ringli D., Tonhauser J. The significance of accurate determination of Gleason score for therapeutic options and prognosis of prostate cancer. Pathol Oncol Res. 2016;22:349–356. doi: 10.1007/s12253-015-0013-x. [DOI] [PubMed] [Google Scholar]
- 3.Tolonen T.T., Kujala P.M., Tammela T.L., Tuominen V.J., Isola J.J., Visakorpi T. Overall and worst Gleason scores are equally good predictors of prostate cancer progression. BMC Urol. 2011;11:21. doi: 10.1186/1471-2490-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice-Stitt T., Valencia-Guerrero A., Cornejo K.M., Wu C.L. Updates in histologic grading of urologic neoplasms. Arch Pathol Lab Med. 2020;144:335–343. doi: 10.5858/arpa.2019-0551-RA. [DOI] [PubMed] [Google Scholar]
- 5.Travis W.D., Brambilla E., Noguchi M. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sica G., Yoshizawa A., Sima C.S. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34:1155–1162. doi: 10.1097/PAS.0b013e3181e4ee32. [DOI] [PubMed] [Google Scholar]
- 7.Ito M., Miyata Y., Yoshiya T. Second predominant subtype predicts outcomes of intermediate-malignant invasive lung adenocarcinoma. Eur J Cardiothorac Surg. 2017;51:218–222. doi: 10.1093/ejcts/ezw318. [DOI] [PubMed] [Google Scholar]
- 8.Yoshizawa A., Motoi N., Riely G.J. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 9.Tsubokawa N., Mimae T., Sasada S. Negative prognostic influence of micropapillary pattern in stage IA lung adenocarcinoma. Eur J Cardiothorac Surg. 2016;49:293–299. doi: 10.1093/ejcts/ezv058. [DOI] [PubMed] [Google Scholar]
- 10.Warth A., Muley T., Kossakowski C.A. Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol. 2015;39:793–801. doi: 10.1097/PAS.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 11.Toyokawa G., Yamada Y., Tagawa T. Significance of spread through air spaces in resected pathological stage I lung adenocarcinoma. Ann Thorac Surg. 2018;105:1655–1663. doi: 10.1016/j.athoracsur.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Shiono S., Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg. 2016;23:567–572. doi: 10.1093/icvts/ivw211. [DOI] [PubMed] [Google Scholar]
- 13.Nakazato Y., Minami Y., Kobayashi H. Nuclear grading of primary pulmonary adenocarcinomas: correlation between nuclear size and prognosis. Cancer. 2010;116:2011–2019. doi: 10.1002/cncr.24948. [DOI] [PubMed] [Google Scholar]
- 14.von der Thusen J.H., Tham Y.S., Pattenden H. Prognostic significance of predominant histologic pattern and nuclear grade in resected adenocarcinoma of the lung: potential parameters for a grading system. J Thorac Oncol. 2013;8:37–44. doi: 10.1097/JTO.0b013e318276274e. [DOI] [PubMed] [Google Scholar]
- 15.Kadota K., Suzuki K., Kachala S.S. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25:1117–1127. doi: 10.1038/modpathol.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duhig E.E., Dettrick A., Godbolt D.B. Mitosis trumps T stage and proposed International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification for prognostic value in resected stage 1 lung adenocarcinoma. J Thorac Oncol. 2015;10:673–681. doi: 10.1097/JTO.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 17.Moreira A.L., Ocampo P.S., Xia Y. A Grading system for invasive pulmonary adenocarcinoma: a proposal from the IASLC pathology committee. J Thorac Oncol. 2020;15:1599–1610. doi: 10.1016/j.jtho.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstraw P., Chansky K., Crowley J. The IASLC Lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Travis W.D., Brambilla E., Nicholson A.G. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 20.Okami J., Shintani Y., Okumura M. Demographics, safety and quality, and prognostic information in both the seventh and eighth editions of the TNM classification in 18,973 surgical cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol. 2019;14:212–222. doi: 10.1016/j.jtho.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Tsutani Y., Miyata Y., Mimae T. The prognostic role of pathologic invasive component size, excluding lepidic growth, in stage I lung adenocarcinoma. J Thorac Cardiovasc Surg. 2013;146:580–585. doi: 10.1016/j.jtcvs.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 22.Tsutani Y., Miyata Y., Kushitani K., Takeshima Y., Yoshimura M., Okada M. Propensity score-matched analysis of adjuvant chemotherapy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2014;148:1179–1185. doi: 10.1016/j.jtcvs.2014.05.084. [DOI] [PubMed] [Google Scholar]
- 23.Tsutani Y., Suzuki K., Koike T. High-risk factors for recurrence of stage I lung adenocarcinoma: follow-up data from JCOG0201. Ann Thorac Surg. 2019;108:1484–1490. doi: 10.1016/j.athoracsur.2019.05.080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.