Introduction
The small-cell transformation of EGFR tyrosine kinase inhibitor (TKI)–treated NSCLC is known, but de novo occurrence of synchronous SCLC and lung adenocarcinoma is very rare and has been reported in only 0.03%1 of patients with NSCLC. Here, we report a case of synchronous EGFR-mutant SCLC and lung adenocarcinoma in a patient who responded well to osimertinib.
Case Report
A 39-year-old male patient, never-smoker, with a long-term cough was diagnosed as having a lung mass through endobronchial ultrasound and bronchoscopy, which revealed a solid pattern of adenocarcinoma with paratracheal lymph node involvement (Fig. 1A and B) depicting small-cell carcinoma along with multiple liver, bone, and brain metastases. Next-generation sequencing-based testing done on both components revealed several alterations (Table 1). Liquid biopsy on a droplet digital polymerase chain reaction assay revealed an inframe deletion in exon 19 of EGFR with 33.4 copies/mL and T790M mutation at 0.2 copies/mL.
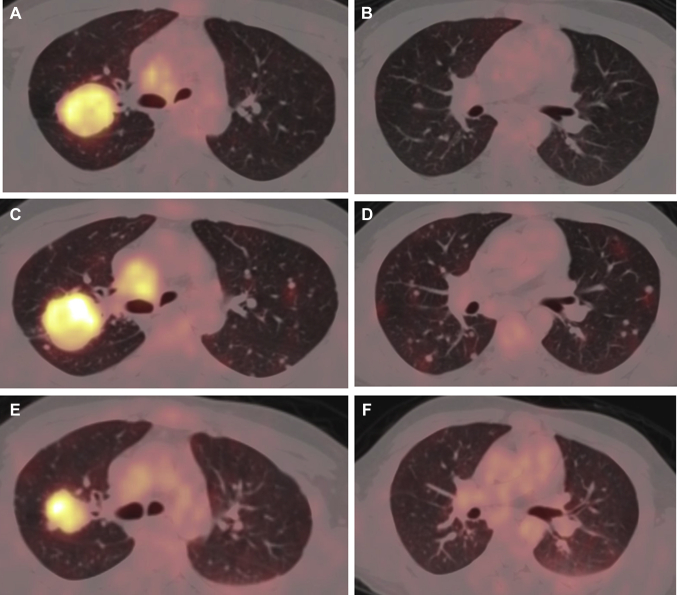
Figure 1.
(A, B) Fused FDG PET/CT axial images of the baseline scan (lung window) illustrating metabolically active soft tissue mass lesion in the upper lobe of the right lung. (C, D) Fused FDG PET/CT scan axial images after three cycles of chemotherapy (lung window) exhibiting an increase in the metabolic activity of the mass lesion in the upper lobe of the right lung, along with an increase in the size, number, and metabolic activity of the bilateral lung nodules. (E, F) Fused FDG PET/CT scan axial images after the start of osimertinib treatment (lung window) illustrating a decrease in size and metabolic activity of the mass lesion in the upper lobe of the right lung along with a considerable decrease in the size, number, and metabolic activity of bilateral lung nodules. CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography.
Table 1.
Molecular Alterations Detected in Both of the Components on Next-Generation Sequencing
| Variants | Adenocarcinoma (%VAF) | Small Cell (%VAF) | ACMG |
|---|---|---|---|
| EGFR | p.Glu746_Ala750del (43.7) | p.Glu746_Ala750del (40.2) | Pathogenic |
| TP53 | p.Arg175His (79.52) | p.Arg175His (77.45) | Pathogenic |
| MYC amplification | Copy number: 6.32 | Copy number: 5 | — |
| RICTOR amplification | Copy number: 5.82 | Copy number: 6.38 | — |
| TERT amplification | Copy number 4.93 | Copy number:5.65 | — |
| PIK3CB amplification | Copy number: 19.95 | Not detected | — |
| ARID1A | Not detected | p.Gln1631fs (9.40) | Pathogenic |
ACMG, American College of Medical Genetics and Genomics; VAF, variant allele fraction.
The patient initially underwent whole-brain radiation therapy followed by three cycles of etoposide-cisplatin. Re-evaluation with positron emission tomography–computed tomography was suggestive of progressive disease. (Fig. 1C and D). He was subsequently treated with osimertinib and underwent another evaluation, which revealed a partial response. Serial evaluation was also done for the EGFR mutation status through liquid biopsy by droplet digital polymerase chain reaction after chemotherapy, which revealed 24.5 copies/mL of EGFR del19 mutation that subsequently fell to 8.5 copies/mL, indicating good response to treatment. The patient was initially diagnosed in November 2019; he received three cycles of chemotherapy until February 2020, but he did not respond to it and instead had progressive disease. He was started on osimertinib in February 2020, and re-evaluation in May 2020 revealed partial response (Figs. 1E and F) to treatment. He is currently on osimertinib (August 2020) and has tolerated the drug without any major adverse effects (grade 1 rash had been reported). He currently has good quality of life with Eastern Cooperative Oncology Group performance status of 1, and resolution of his cough, breathlessness, and backache.
Discussion
The morphological transformation of NSCLC to SCLC has been described as a rare mechanism of EGFR TKI resistance in 3% of cases.2 It was first described in 20063 in a 45-year-old woman with EGFR-mutant adenocarcinoma, after erlotinib treatment. There are varied hypotheses for the mechanism of this transformation. According to one theory, a clonal loss or mutation in TP53 and RB1 genes in type II alveolar cells4 results in the SCLC transformation of EGFR–mutated adenocarcinoma. However, according to another concept, given that the gene expression profiles of both components were similar, it may be hypothesized that these patients undergo a transformation of primary adenocarcinoma, as opposed to having two primary lung cancers arising from different clones.5
This case had two different histologies, with the molecular profile revealing the possible presence of two clones. In this case, the possible mechanisms leading to the small-cell transformation are the TP53 mutation, MYC amplification (a known oncogenic driver for SCLC4), and the PIK3CB amplification. The RICTOR and TERT amplification and the ARID1A mutation that were additionally detected may have contributed to genomic instability rather than the oncogenic process.
With respect to treatment, despite chemotherapy and TKIs, the outcome remains dismal. This patient responded well to osimertinib treatment, as evident on follow-up imaging and liquid biopsy studies. There is very scant literature available regarding osimertinib usage in this setting.
The uniqueness of this report rests on the de novo presentation of SCLC and NSCLC in a young nonsmoker, who has responded well to osimertinib treatment despite the various genomic alterations detected. Widespread usage of next-generation sequencing may help us move a step closer toward understanding the molecular biology of this complex disease.
Acknowledgments
Dr. Batra contributed to the study’s conceptualization, methodology, software, and the writing, reviewing, and editing of the article. Drs. Nathany, Sharma, and Jain contributed to data curation, writing and preparation of the original draft, software, and data validation. Dr. Bansal contributed to data visualization and study investigation. Dr. Mehta contributed to study supervision.
Footnotes
Disclosure: The authors declare no conflict of interest.
Informed consent has been sought from the patient, and no direct or indirect identifiers have been used in reporting this manuscript.
References
- 1.Ferrer L., Giaj Levra M., Brevet M. A brief report of transformation from NSCLC to SCLC: molecular and therapeutic characteristics. J Thorac Oncol. 2019;14:130–134. doi: 10.1016/j.jtho.2018.08.2028. [DOI] [PubMed] [Google Scholar]
- 2.Norkowski E., Ghigna M.R., Lacroix L. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol. 2013;8:1265–1271. doi: 10.1097/JTO.0b013e3182a407fa. [DOI] [PubMed] [Google Scholar]
- 3.Oser M.G., Niederst M.J., Sequist L.V., Engelman J.A. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–e172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J.K., Lee J., Kim S. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol. 2017;35:3065–3074. doi: 10.1200/JCO.2016.71.9096. [DOI] [PubMed] [Google Scholar]
- 5.Lin M.W., Su K.Y., Su T.J. Clinicopathological and genomic comparisons between different histologic components in combined small cell lung cancer and non-small cell lung cancer. Lung Cancer. 2018;125:282–290. doi: 10.1016/j.lungcan.2018.10.006. [DOI] [PubMed] [Google Scholar]