Abstract
Background
Exercise training reduces inflammation in breast cancer survivors; however, the mechanism is not fully understood.
Objectives
The effects of acute and chronic exercise on monocyte toll-like receptor (TLR2 and 4) expression and intracellular cytokine production were examined in sedentary breast cancer survivors.
Methods
Eleven women with stage I, II, or III breast cancer within one year of treatment completion performed an acute, intermittent aerobic exercise trial. Blood samples were obtained before, immediately, and 1 h after a 45-min acute exercise trial that was performed before and after 16 weeks of combined aerobic and resistance. LPS-stimulated intracellular IL-1ß, TNF, and IL-6 production, and TLR2 and TLR4 expression were evaluated in CD14+CD16- and CD14+CD16+ monocytes using flow cytometry.
Results
Exercise training decreased IL-1ß+CD14+CD16- proportion (24.6%, p=0.016), IL-1ß+CD14+CD16- mean fluorescence intensity (MFI) (−9989, p=0.014), IL-1ß+CD14+CD16+ MFI (−11101, p=0.02), and IL-6+CD14+CD16- proportion (16.9%, P=0.04). TLR2 and TLR4 expression did not change following exercise training but decreased 1 h after acute exercise in CD14+CD16- (−63, p=0.002) and CD14+CD16+ (−18, p=0.006) monocytes, respectively. Immediately after the acute exercise, both monocyte subgroup cell concentration increased, with CD14+CD16+ concentrations being decreased at 1 h post without changes in intracellular cytokine production.
Conclusions
Exercise training reduced monocyte intracellular pro-inflammatory cytokine production, especially IL-1ß, although these markers did not change acutely. While acute exercise downregulated the expression of TLR2 and TLR4 on monocytes, this was not sustained over the course of training. These results suggest that the anti-inflammatory effect of combined aerobic and resistance exercise training in breast cancer survivors may be, in part, due to reducing resting monocyte pro-inflammatory cytokine production.
Keywords: Monocyte, Exercise, Breast cancer, Inflammation, Cytokines, Toll like receptor
Highlights
-
•
Acute exercise increased monocyte concentrations at 0 h after exercise, with a decrease in CD14+CD16+ monocytes only at 1 h.
-
•
Exercise training decreased IL-1β and IL-6 proportions while acute exercise reduced the TLR2 and TLR4 expression.
-
•
Anti-inflammatory effect of exercise may be partly explained by reduced pro-inflammatory cytokine production by monocytes.
1. Introduction
Breast cancer treatments create an inflammatory status which leads to several side effects such as heart disease (Hooning et al., 2007) and persistent fatigue (Bower, 2007). The inflammation created by monocyte cells could exacerbate patient's quality of life as evidence showed these cells are associated with persistent fatigue, which negatively affects the quality of life in breast cancer survivors (Bower et al., 2000, 2002; Collado-Hidalgo et al., 2006). To improve outcomes in breast cancer survivors, strategies to reduce inflammation are needed. Exercise is a promising, non-pharmacological approach that reduces circulating pro-inflammatory cytokine levels in breast cancer survivors (Khosravi et al., 2019; Meneses-Echavez et al., 2016). Over the past 10 years, the exercise and immune-inflammation axis in cancer studies have grown rapidly (Khosravi et al., 2019). However, most have examined the effects of exercise training on circulating pro-inflammatory cytokine levels with little attention to the cells directly involved in cytokine production. Several mechanisms are suggested for the anti-inflammatory effect of exercise in healthy individuals, including reduced expression of toll-like receptors (TLRs) on monocytes (Gleeson et al., 2011), with limited data examining the specific cells that may contribute to the anti-inflammatory effects of exercise training in breast cancer survivors.
Monocytes are a heterogeneous group of cells, which can be divided into two subpopulations: CD14+CD16- cells are the major subgroup (80–95%) while CD14+CD16+ cells (5–15%) are a smaller subgroup but have higher pro-inflammatory characteristics (Belge et al., 2002; Grage-Griebenow et al., 2001). Exercise training reportedly decreased CD14+CD16+ proportions in the elderly (Bartlett et al., 2018; Markofski et al., 2014; Timmerman et al., 2008) and obese individuals (de Matos et al., 2019), although conflicting reports do exist (Child et al., 2013). Despite a role in breast cancer progression and the purported anti-inflammatory effects of exercise training, the acute and chronic effects of exercise on monocyte subgroup proportions and concentrations have not been evaluated in breast cancer patients.
Monocytes have several receptors involved in inflammatory responses. TLRs are transmembrane surface receptors that recognize antigens, leading to the activation of monocytes (Kawai and Akira, 2005). In response to lipopolysaccharide (LPS), the principal membrane component of Gram-negative bacteria, TLRs (mainly TLR4) activate monocytes (Guha and Mackman, 2001) and initiate intracellular signaling that increases pro-inflammatory gene expression, including tumor necrosis factor (TNF), IL-6, and IL-1ß (Guha and Mackman, 2001; Kawai and Akira, 2005). It has been hypothesized that exercise training may modulate monocytes' TLR2 and TLR4 expression, leading to a reduction in the inflammatory function of these cells (Flynn and McFarlin, 2006; Gleeson et al., 2006, 2011). However, some studies fail to show a reduction in TLR2 or TLR4 after exercise training (Child et al., 2013; Timmerman et al., 2008). Despite the fact that TLRs have been the focal point of many studies (Cavalcante et al., 2017; Flynn and McFarlin, 2006; Gleeson et al., 2006), monocyte inflammatory cytokine production, as the end result of TLRs activation, has rarely been studied in response to exercise training. We are aware of only one study that examined TLRs expression and intracellular cytokine production simultaneously with training. Indeed, 10 weeks of resistance training in elderly women was associated with lower monocyte mRNA expression of intracellular TNF and TLR4 but not IL-6 and IL-1ß (Flynn et al., 2003). While potentially promising, these findings need to be substantiated and also examined in clinical populations (e.g. breast cancer patients) with elevated inflammatory levels.
The monocyte response to an acute bout of exercise is not fully understood in breast cancer survivors. Generally, acute exercise affects monocyte trafficking and function, likely via activation of ß2-adrenergic receptors following epinephrine elevation (Dimitrov et al., 2013, 2017; Graff et al., 2018). However, epinephrine levels following acute exercise did not increase in breast cancer patients when compared to healthy age-matched women (Evans et al., 2016), with attenuated epinephrine responses to exercise also reported in prostate cancer patients (Hanson et al., 2018). The reduced catecholamine response could adversely affect monocyte mobilization but, to our knowledge, no studies have examined the regulation of these cells during acute exercise in breast cancer survivors. In non-cancer populations, acute exercise appears to have a differential response on TLRs. Resistance exercise reduced TLR4 levels whereas aerobic exercise resulted in conflicting findings (Cavalcante et al., 2017). Monocyte intracellular cytokine levels have mostly been studied in response to prolonged, exhaustive acute exercise with a greater proportion of monocytes producing intracellular TNF (Rhind et al., 2001; Starkie et al., 2000, 2001, 2005), IL-6 (Rhind et al., 2001; Starkie et al., 2000, 2001, 2005), IL-1ß (Rhind et al., 2001), and IL-1α (Starkie et al., 2000, 2001, 2005) while producing less TNF (Rhind et al., 2001; Starkie et al., 2000, 2001, 2005), IL-6 (Starkie et al., 2001, 2005), IL-1ß (Rhind et al., 2001), and IL-1α (Starkie et al., 2000, 2001) per cell. In contrast, moderate-intensity acute exercise in healthy (Dimitrov et al., 2017) and prehypertensive individuals (Dimitrov et al., 2013) demonstrated decreased monocyte TNF proportions and per-cell production. Thus, because of the important role of monocytes inflammatory function in breast cancer and the lack of information in this area, evaluating the dynamics of monocyte inflammatory function would shed light on the comprehensive understanding of the effects of exercise on monocyte regulation.
Acute exercise is a powerful stimulus for mobilizing the immune system (Pedersen and Hoffman-Goetz, 2000). Repetitive exposure to fluctuating immune cell levels following exercise training may create cross-tolerance leading to a reduced inflammatory response of the cells (Flynn and McFarlin, 2006). Despite the strong interaction between acute and chronic exercise, these two stimuli are often studied separately. Thus, to better understand how exercise affects monocyte regulation, we examined the acute exercise effect in sedentary breast cancer survivors before and after a 16 week combined exercise training program. For a comprehensive evaluation, we studied TLR2 and TLR4, production of TNF, IL-6, and IL-1ß in monocytes based on CD14 and CD16 expression. We hypothesized that exercise training would reduce the inflammatory CD14+CD16+ cells with minimal change in the CD14+CD16- monocytes proportion (Timmerman et al., 2008). Training would also reduce TLRs expression and pro-inflammatory cytokines (Flynn et al., 2003). Due to the use of moderate-intensity exercise used in this study, we hypothesized that CD14+CD16+ monocytes would be mobilized acutely with minimal changes in CD14+CD16- cells (Dimitrov et al., 2013). Acute exercise also would reduce pro-inflammatory cytokine production (Dimitrov et al., 2013, 2017) and TLR expression (Cavalcante et al., 2017) on monocytes. We hypothesized that these changes would be reduced following training because of the adaptation to exercise.
2. Methodology
2.1. Participants
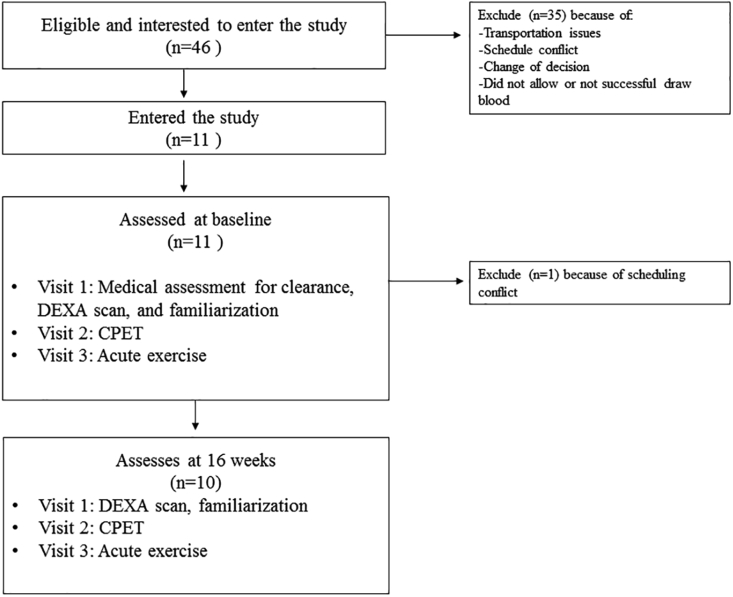
Women (n=11) aged between 45 and 67 years old with pathologically confirmed stage I, II, or III breast cancer who had completed their initial treatment (surgery, chemotherapy, and/or radiation therapy) within the previous year were evaluated. Patients were recruited at the North Carolina Cancer Hospital (Chapel Hill, NC, USA) and by the Get REAL and HEEL breast cancer rehabilitation program at the University of North Carolina at Chapel Hill (UNC). All participants provided written informed consent prior to participating in the study. All study procedures were approved by the Oncology Protocol Review Committee at the Lineberger Comprehensive Cancer Center and by the UNC Institutional Biomedical Review Board.
2.2. Baseline assessments
2.2.1. Preliminary assessments and familiarization (visit 1)
Participants visited the Exercise Oncology Research Laboratory on 3 different occasions. During the first visit, participants were screened to obtain a medical clearance prior to exercise testing, based on the American College of Sports Medicine (ACSM) guidelines (Riebe et al., 2018). These measures included a resting electrocardiogram, medical history, and the Physical Activity Readiness Questionnaire (PAR-Q), which were reviewed and approved by a study physician. To evaluate activity levels prior to enrolling in the study, participants completed an International Physical Activity Questionnaire (IPAQ). Body composition was determined using dual-energy X-ray absorptiometry (Hologic Discovery W; Bedford, MA). Participants were then familiarized with the cardiopulmonary exercise test (CPET) protocol by fitted with a mask and completing a submaximal exercise bout on an electronically braked cycle ergometer (Lode Corival, Groningen, The Netherlands). Familiarization was terminated when participants reached 75% of their calculated heart rate reserve (HRR).
2.2.2. Cardiopulmonary exercise testing (visit 2)
On day 2 of testing, participants returned to the laboratory to complete a maximal effort CPET to determine peak oxygen uptake (VO2peak). All patients were asked to refrain from eating or consuming caffeine for 2 h, and from exercising for 12 h prior to the CPET. The CPET was performed on a cycle ergometer using a 15W/min incremental ramp protocol after a 5-min light warm-up and followed standard ACSM exercise testing guidelines (Riebe et al., 2018). Expired gases were collected and analyzed using a TrueMax 2400 Metabolic System (Parvo Medics, Salt Lake City, UT) throughout the entire test. Vital signs were measured before and immediately after testing, with heart rate and rating of perceived exertion (RPE) were monitored continuously throughout the CPET. Testing was terminated when participants reached volitional exhaustion and/or researchers stopped the test due to a plateau in VO2 despite the increasing workload. VO2peak was calculated as the average of the three highest VO2 readings in the final minute of testing. The workload at termination was recorded as the peak power output completed in the CPET. Sixty percent of the peak power output from the CPET was applied as the cycling resistance for the acute exercise trial, which has been used previously (Hanson et al., 2018), and was completed on day 3 of testing.
2.2.3. Acute exercise trial (visit 3)
On the third visit, participants completed the acute exercise trial. The acute exercise protocol is based on previous work from our lab in breast (Evans et al., 2015) and prostate cancer survivors (Hanson et al., 2020) that initiated a pronounced leukocyte mobilization. Participants were asked to fast for at least 2 h and to avoid caffeine on the morning of the trial and to not have exercised in the previous 24 h. Previously, we have shown that immune and endocrine functions return to resting levels with 24 h following moderate-intensity exercise (Evans et al., 2015, 2016; Hanson et al., 2018). Prior to starting exercise, an intravenous catheter was inserted for repeat blood sampling. The first blood sample was drawn after 5 min of supine rest, and vital signs were obtained. The trial consisted of 10 intervals of 3 min of exercise at 60% of peak power output from the CPET followed by 1.5 min of rest, as used previously (Hanson et al., 2020). Participants warmed-up with 1 min of cycling with no resistance followed by 1 min at 50% of the calculated workload, with the entire exercise session being completed in 45 min. Immediately after exercise, a second blood sample was obtained (0 h). Participants began a seated 1-h recovery. During the recovery time, they were allowed to drink water ad libitum but no food or other beverages were consumed. At the end of the recovery, the final blood sample was drawn (1 h). All blood samples were kept on ice until they were processed.
2.2.4. Exercise training
All participants were asked to train 3 times per week, for 16 weeks at the Get REAL and HEEL breast cancer rehabilitation facility. The exercise was a combined aerobic and resistance training (RT) intervention and each session lasted approximately 1 h. Exercise duration (aerobic) and intensity (Borg RPE scale) was progressed across the 16 weeks (s Table 1). Rate of Perceived Exertion (RPE) was monitored by exercise physiologists to control the exercise intensity. Intensity started as low to moderate for weeks 1–5 and then increased to moderate to high for weeks 5–16. Resistance training duration was kept constant at 30 min whereas aerobic training duration began at 10–15 min and gradually increased to 30 min by week 8. Warm-ups and cool-downs were performed before and after the exercise. Following the completion of the 16-week exercise intervention, the same baseline assessments (visits 1, 2, and 3) were then repeated.
2.2.5. Hematology analysis
Complete blood counts with differential were determined using whole blood samples at each time points (Sysmex XP-300, Kobe, Japan). The differential breakdown included neutrophil counts, lymphocyte counts, and a mixed count population that was primarily monocytes but also included basophils and eosinophils. Sysmex samples were analyzed in duplicate and averaged, as performed previously (Hanson et al., 2017). If the total leukocyte count difference was >0.1 cells/μL, a third sample was run. Plasma volume shifts with exercise were determined as described previously (Dill and Costill, 1974).
2.2.6. Intracellular cytokines expression
Venous blood was collected in sodium heparin tubes and was used to assay intracellular cytokine production in monocytes. 100 μL of whole blood was incubated with 10 μg/mL Brefeldin-A (BD Bioscience, CA, USA) and 1 μg/mL LPS (Escherichia coli 026:B6, eBioscience, San Diego, CA, USA) or vehicle in RPMI with L-glutamine and 1% penicillin/streptomycin for 4 h at 37 °C and 5% CO2 (Starkie et al., 2005). After 4 h of incubation, samples were washed twice with 1% BSA in PBS (wash buffer) and were incubated with CD14 (APC, Biolegend, San Diego, CA, USA, clone HCD14) and CD16 (PE-Cy7, Biolegend, San Diego, CA, USA, clone 3G8) monoclonal antibodies in 100 ul of wash buffer for 20–30 min in the dark at 4 °C. Red blood cells were lysed using 1-step Fix Lyse buffer (Invitrogen/Thermo Fischer Scientific, Waltham, MA) in the dark at room temperature for 15 min. After being washed twice, samples were incubated in fixation medium (Medium A, Thermo Fisher Scientific, Waltham, MA) for 15 min in the dark at room temperature following the manufacturer's instructions. After washing, samples were permeabilized and stained for intracellular IL-6 (FITC, Biolegend, San Diego, CA, USA, clone MQ2-13A5), TNF (Alexa Fluor 700, Biolegend, San Diego, CA, USA, clone MAb11), and IL-1ß (PE, BD, Bioscience, CA, USA, clone AS10) using antibodies suspended in 100 μL of permeabilization medium (Medium B, Thermo Fisher Scientific, Waltham, MA) for 30 min in the dark at 4 °C. Excess antibody was washed and the cells were resuspended in 300 μL wash buffer and stored at 4 °C in the dark until they were analyzed. The majority of samples were analyzed the same day, with a limited number of samples run the next morning (i.e., ∼12 h later) due flow cytometer availability. Flow cytometry compensation beads (Invitrogen™, Thermo Fisher, USA) were used for single-color controls.
2.2.7. TLR expression assay
50 μL of whole blood was aliquoted into FACS tubes and placed on ice immediately. Samples were incubated in the dark, on ice, for 1 h with pre-titrated concentrations of CD14 (Pacific Blue, BD Bioscience, USA, clone M5E2), CD16 (FITC, BD Bioscience, clone 3G8), and either TLR-2 (Alexa Fluor-647, BD Bioscience, clone 11G7), or TLR-4 (PE, BD Bioscience, clone TF901), or isotype-matched controls. Following incubation, cells were washed twice in PBS/1%BSA, before the addition of 2 mL of 1x Fix/Lyse solution (ThermoFisher, USA) and incubated at room temperature, in the dark for 15 min. Following incubation, cells were washed twice, as before, and resuspended in 300 μL of 1% BSA in PBS and analyzed immediately by flow cytometry. Fluorescence compensations and analyses were completed on 5,000 monocytes using FCS Express v6 (FCS Express, USA).
2.2.8. Flow cytometry
Monocyte phenotyping and intracellular cytokine production was obtained using a BD LSR Fortessa (BD, Bioscience, CA, USA) flow cytometer in the UNC Flow Cytometry Core Facility and TLR staining was quantified on a BD FACSCanto II (BD Bioscience, USA) flow cytometer in the Duke Cancer Institute Core Facility. Expression levels of surface receptors and intracellular cytokines were quantified by the percentage of cells positively expressing the relevant fluorescent staining, or the amount of receptor and cytokine expression by the MFI level detected in/on each cell. The gating strategy is presented in s Fig. 1. After collecting at least 50,000 cells, single cells were determined using forward scatter characteristics (FSC) for area (FSC-A) and height (FSC–H). CD14+ (albeit dim) neutrophils were excluded by gating first on CD14+ cells using CD14 vs. SSC-H, and then on distinct monocyte characteristics from FSC-A vs. SSC-H. From this, CD14+CD16- and CD14+ CD16+ were individually gated. T21he monocyte phenotypes were evaluated on the LPS negative samples. Intracellular cytokine levels were determined in both LPS positive and negative samples. Circulating cell number was determined by multiplying the percentage of monocytes expressing the markers of interest with the hematology total mixed cell count (see 2.6 Hematology Analysis). The percent of the two different monocyte subsets that expressing markers of intracellular cytokines and the median fluorescent intensity (MFI) of each cytokine were assessed and referred to as proportion and per-cell production of cytokines. The MFI of TLR2 and TLR4 were assessed by histogram analyses on each subtype. All analyses were performed using FlowJo v10 software (FlowJo, LLC Ashland, Oregon, USA) or FCS Express v6 (FCS Express, Pasadena, CA, USA).
2.2.9. Statistical analysis
To determine the effects of exercise training on resting outcomes, paired-samples t-tests were performed. Independent samples t-tests were used to determine the difference in baseline values between CD14+CD16- and CD14+CD16+ monocytes. Data normality was assessed using a Shapiro-Wilk test. Violations of normality were analyzed using a Wilcoxon signed rank test or Mann-Whitney U test. Changes with acute exercise before and after exercise training were examined using linear mixed modeling with time and training as fixed factors with a random intercept. All data were analyzed using Jamovi v1.0.2.0 (Sydney, Australia) with statistical significance set at P < 0.05. Effect sizes (Cohen's D) were calculated with 0.2, 0.5, and 0.8 representing small, medium, and large effects (Cohen, 2013). Percent change or mean difference was included for each value. Data presented as mean ± SE or (SD).
3. Results
3.1. Participant characteristics
Eleven breast cancer survivors participated in this study Fig. 1. Participant characteristics are reported in Table 1. Body mass index (BMI) indicated these women were overweight (∼27 kg/m2), with an average percent body fat of ∼41%.
Fig. 1.
Flow diagram of the study. Abbreviations: CPET, cardiopulmonary exercise test; DEXA, dual-energy X-ray absorptiometry.
Table 1.
Participant characteristics at baseline (n=11).
| Age (years) | 56.9 (7.9) |
|---|---|
| Race (%) | |
| Caucasian | 90 |
| African-American | 10 |
| Mass (kg) | 74.2 (14.8) |
| Height (cm) | 165.3 (4.6) |
| Body mass index (kg/m2) | 27.3 (6.5) |
| Body fat percentage (%) | 40.7 (5.6) |
| Some college degree (%) | 90 |
| Time since diagnosis (months) | 8.2 (3.5) |
| Time since completion of initial treatments (months) | 2.5 (2.2) |
| Disease stage (%) | |
| I | 30 |
| II | 60 |
| III | 10 |
| ER+ (%) | 90 |
| HER2+ (%) | 20 |
| Post menopause (%) | 60 |
| Radiation therapy (%) | 70 |
| Chemotherapy (%) | 60 |
| Hormone therapy (%) | 82 |
| Surgery (%) | |
| Mastectomy | 30 |
| Lumpectomy | 70 |
Data presented as mean (SD); ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
3.2. Effects of 16 weeks of exercise training
Ten women were assessed at 16 weeks (1 dropout). The physiological response to the CPET and the acute exercise session before and after the training are presented in Table 2. Body mass did not change after training (0.5%, 95% CI -1.5 to 0.79, ES=−0.223, p=0.499), however percentage of body fat showed a trend to decrease (−4.5%, 95% CI -0.03 to 3.71, ES=0.705, p=0.053). VO2peak also did not change after training (1.8%, 95% CI -2.73 to 2.01, ES=−0.108, p=0.741).
Table 2.
Physiological response to exercise.
| CPET Response | Pre-Training | Post-Training | P Value |
|---|---|---|---|
| Total time (min:sec) | 9:47 | 10:38 | <0.001 |
| VO2 peak (mL/kg/min) | 21.9 (5.2) | 22.2 (5.4) | 0.741 |
| HR maximum (beats/min) | 159 (14) | 159 (17) | 0.772 |
| Post exercise lactate levels (mmol/L) | 6.2 (1.5) | 7.3 (2.2) | 0.030 |
| RPE in the final stage | 17 (2) | 17 (2) | 0.209 |
| Peak power output (W) | 121 (14) | 136 (30) | <0.001 |
| Submaximal Exercise Response | |||
| HR final stage (beats/min) | 144 (17) | 142 (12) | 0.672 |
| RPE final stage | 14 (1.7) | 14 (0.8) | 0.654 |
| Workload (W) | 73 (15) | 81 (15) | <0.001 |
Data presented as mean (SD). CPET, cardiopulmonary exercise test; VO2peak, peak oxygen consumption; HR, heart rate; RPE, rating of perceived exertion.
3.2.1. Effects of 16 weeks exercise training on different monocyte subgroups
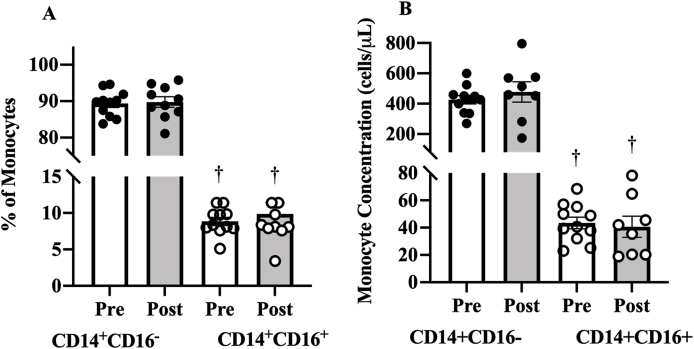
Exercise training did not change the resting levels of CD14+CD16- proportions (0.5%, 95% CI -4.73 to 3.69, ES=−0.08, p=0.78) or CD14+CD16+ proportion (−1.1%, 95% CI -4.51 to 2.30, ES=−0.232, p=0.481). Resting monocyte concentration also did not change after exercise training in CD14+CD16- (−48 cells/μl, 95% CI -238 to 141, ES=−0.21. p=0.565) or CD14+CD16+ monocytes (−4 cells/μl, 95% CI -20 to 27.8, ES=0.135, p=0.714).
Both resting CD14+CD16- proportion (80.5%, 95% CI 78 to 83, ES: 28.5, p < 0.001, Fig. 2A) and higher concentrations (382 cells/μl, 95% CI 324 to 440, ES: 5.89, p < 0.001, Fig. 2B) were higher than in CD14+CD16+ cells.
Fig. 2.
Effect of 16 weeks of exercise training on the resting monocytes (A) percentage and (B) concentration. Data presented as mean ± SE.
†P < 0.05 for comparison of resting values between CD14+CD16- and CD14+CD16+ monocytes.
3.2.2. Effects of 16 weeks exercise training on monocyte intracellular cytokines
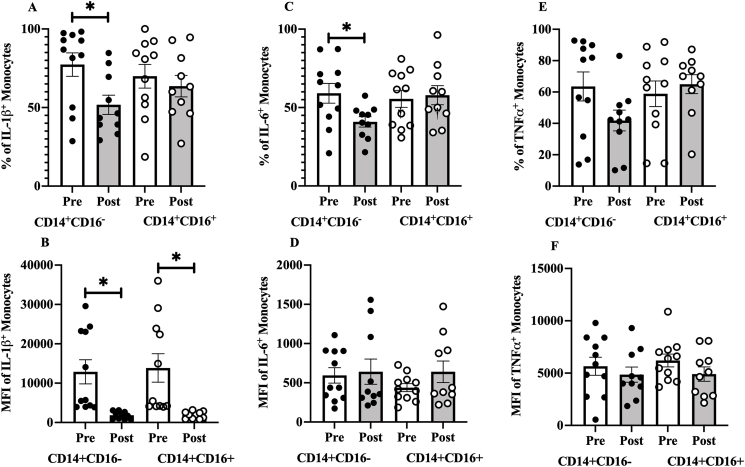
IL-1ß+CD14+CD16- proportions decreased after the exercise training (24.6%, 95% CI 5.79 to 43.5, ES=0.935, p=0.016, Fig. 3A), whereas IL-1ß+CD14+CD16+ proportion did not change (4.9%, 95% CI -22 to 31.9, ES=0.131, p=0.689). Exercise training also decreased IL-1ß MFI levels in both CD14+CD16- (−9989, 95% CI 2591 to 17387, ES=0.966, p=0.014) and CD14+CD16+ monocytes (−11101, 95% CI 2234 to 19968, ES=0.896, p=0.02, Fig. 3B). There were no differences in resting proportion (7.4%, 95% CI -14.7 to 29.5, ES=0.29, p=0.491) or MFI (−980, 95% CI -10892 to 8932, ES=−0.08, p=0.839) of IL-1ß+ cells between CD14+CD16- and CD14+CD16+ monocytes.
Fig. 3.
Effect of 16 weeks exercise training on the resting, LPS stimulated (A) monocytes expressing IL-1ß+ proportion along with (B) the median fluorescent intensity (MFI), (C) monocytes expressing IL-6+ proportion and (D) the MFI, and (E) monocytes expressing TNF+ proportion and (F) the MFI. Data presented as mean ± SE.
∗P < 0.05 for Pre-vs. Post-training.
Exercise training reduced IL-6+CD14+CD16- proportion (16.9%, 95% CI 0.5, 33.3, ES=0.74, P=0.04, Fig. 3C) whereas IL-6+CD14+CD16+ proportion remained unchanged (−4.2%, 95% CI -21.6 to 13.2, ES=−0.172, p=0.599). Exercise training did not change IL-6 MFI in CD14+CD16- (−79, 95% CI -329 to 171, ES=−0.227, p=0.49, Fig. 3D) or CD14+CD16+ monocytes (−224, 95% CI -489 to 40.7, ES=−0.606, p=0.088). There were no differences in resting proportion (3.7%, 95% CI -13.7 to 20.9, ES=0.183, p=0.672) or MFI (155, 95% CI -74.9 to 386, ES=0.06, p=0.175) of IL-6+ cells between CD14+CD16- and CD14+CD16+ monocytes.
Exercise training did not change TNF+CD14+CD16- (19.3%, 95% CI -4.30 to 42.9, ES=0.58, p=0.097, Fig. 3E) or TNF+CD14+CD16+ proportions (9%, 95% CI -35.5 to 17.3, ES=−0.246, p=0.457), nor did it alter TNF MFI for CD14+CD16- (−610, 95% CI -1960 to 3181, ES=0.17, p=0.604, Fig. 3F) or CD14+CD16+ cells (−1175, 95% CI -690 to 3040, ES=0.451, p=0.188). There were no differences in resting proportion (4.6%, 95% CI -21.1 to 30.4, ES=0.16, p=0.712) or MFI (−550, 95% CI -2757 to 1657, ES=−0.222, p=0.609) of TNF+ cells between CD14+CD16- and CD14+CD16+ monocytes.
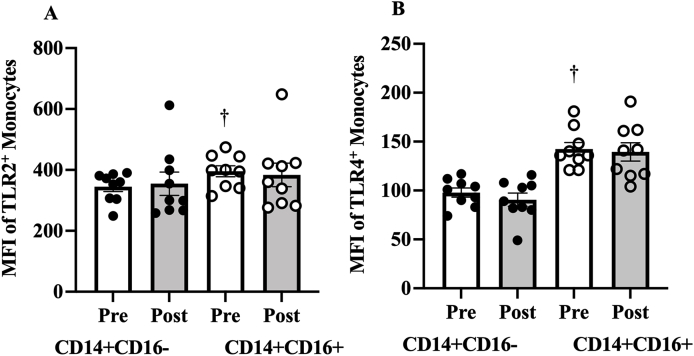
3.2.3. Effects of 16 weeks exercise training on TLR2 and TLR4 expression
TLR2 did not change after exercise training on CD14+CD16- (10.1, 95% CI -92.8 to 72.6, ES=0.09, p=0.785) or CD14+CD16+ monocytes (−12, 95% CI -92.3 to 116, ES=0.08, p=0.797, Fig. 4A). Exercise training had no effect on TLR4 expression in CD14+CD16- (−7.33, 95% CI -8.91 to 23.6, ES=0.347, p=0.328) or CD14+CD16+ monocytes (−3, 95% CI -26.3 to 32.3, ES=0.07, p=0.819, Fig. 4B).
Fig. 4.
Effect of 16 weeks of exercise training o resting (A) TLR2 and (B) TLR4 expression. Data presented as mean ± SE.
†P < 0.05 for comparison of resting values in CD14+CD16- and CD14+CD16+ monocytes.
Resting expression of TLR4 and TLR2 were higher in CD14+CD16+ compare to CD14+CD16- monocytes (TLR4: 44.6, 95% CI -61.9 TO -27.2, ES=−2.57, P < 0.001, Fig. 4B; TLR2: 50.9, 95% CI -102 to 0.08, ES=−0.99, p=0.05, Fig. 4A).
3.3. Acute exercise responses
As time x training interactions were not significant for all measures, the pooled effects of acute exercise are reported in Fig. 5, Fig. 6 and Table 2.
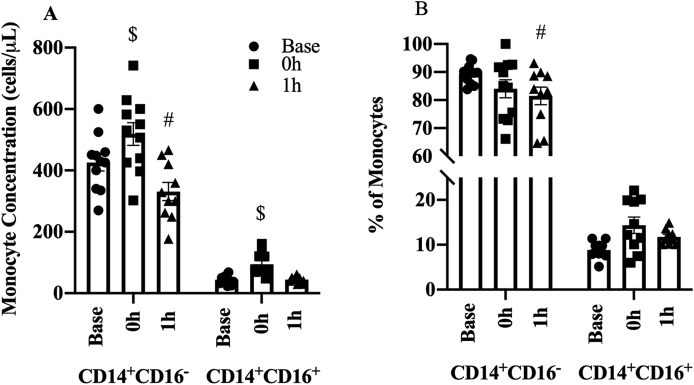
Fig. 5.
Changes in monocyte subpopulation (A) concentrations and (B) proportions with acute exercise. Data presented as mean ± SE.
$ P < 0.05 between baseline and 0 h; #P < 0.05 between baseline and 1 h.
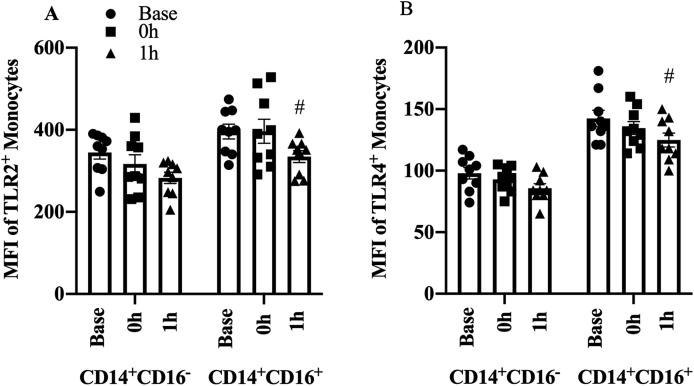
Fig. 6.
Expression of (A) TLR2 and (B) TLR4 in different monocytes in response to the acute exercise. Data presented as mean ± SE.
# indicated P < 0.05 between baseline and 1 h.
3.3.1. Physiological response to CPET and submaximal exercise
The physiological response to the CPET and the acute exercise session before and after the exercise intervention are presented in Table 2. The highest oxygen uptake obtained during the CPET was considered to be VO2peak. Based on VO2peak values, most participants (60%) had poor cardiorespiratory fitness (Riebe et al., 2018). All participants completed the 45-min submaximal test except one participant who completed 7 intervals (30 min) and requested to stop.
3.3.2. Leukocytes response to the acute exercise
Exercise significantly mobilized leukocytes, mixed cells, lymphocytes, and neutrophils (sTable 2). All these cells increased immediately after exercise and then decreased to baseline levels at 1 h post-exercise.
3.3.3. Different monocytes subgroup's response to the acute exercise
Acute exercise mobilized both CD14+CD16- and CD14+CD16+ monocyte populations (p < 0.01). CD14+CD16- monocyte concentration increased by 24% at 0 h (109 cells/μl, 95% CI 32.79 to 180.1, p=0.007, Fig. 5A), then dropped by −23% at 1 h relative to baseline (−103 cells/μl, 95% CI -178.21 to −25.1 p=0.013). CD14+CD16+ monocyte concentration increased by 121% at 0 h (53 cells/μl CI 28.4 to 75, p < 0.001) before returning to baseline levels at 1 h (−15 cells/μl, 95% CI -9.22 to 384, p=0.23).
Overall, CD14+CD16- proportion was decreased with acute exercise (p=0.006). There was a 5%, albeit marginally significant, decrease at 0 h (4.6%, 95 CI -9.39 to 0.069, p=0.059, Fig. 5B) that continued to decrease at 1 h relative to baseline (8.4%, 95% CI -13.19 to −3.59, p=0.001). CD14+CD16+% did not change at 0 h (2.1%, 95% CI -0.87 to 50.03, p=0.174) or 1 h (2.3%, 95% CI -0.73 to 5.25, p=0.146).
3.3.4. Monocyte intracellular cytokines response to acute exercise
The acute exercise did not alter TNF, IL-6, or IL-1ß production by monocytes (Table 3). Although time effects were not statistically significant, all three cytokines proportion and MFI showed a decreasing trend in response to exercise except for IL-6+CD14+CD16- proportion.
Table 3.
Monocytes intracellular cytokines changes following the acute exercise.
| 95% CI |
|||||
|---|---|---|---|---|---|
| Time | B | Lower | Upper | P | |
| IL-1ß+CD14+CD16- % | 0 h-Baseline | −6.4 | −22.4 | 9.6 | 0.439 |
| 1 h–0h | −5.4 | −21.4 | 10.6 | 0.510 | |
| IL-1ß+CD14+CD16- MFI | 0 h-Baseline | −1486 | −4623 | 1651 | 0.358 |
| 1 h–0h | −1870 | −5006 | 1267 | 0.249 | |
| IL-1ß+CD14+CD16+ % | 0 h-Baseline | −4.8 | −20.7 | 11.2 | 0.560 |
| 1 h–0h | −3.9 | −19.8 | 12.1 | 0.640 | |
| IL-1ß+CD14+CD16+ MFI | 0 h-Baseline | −2038 | −5567 | 1491 | 0.264 |
| 1 h–0h | −2131 | −5659 | 1398 | 0.243 | |
| IL-6+CD14+CD16- % | 0 h-Baseline | 1.6 | −9.0 | 12.2 | 0.772 |
| 1 h–0h | 2.3 | −8.1 | 13.1 | 0.649 | |
| IL-6+CD14+CD16- MFI | 0 h-Baseline | −23 | −200 | 153 | 0.795 |
| 1 h–0h | −65 | −242 | 112 | 0.475 | |
| IL-6+CD14+CD16+ % | 0 h-Baseline | −0.2 | −10.7 | 9.5 | 0.959 |
| 1 h–0h | −5.4 | −15.2 | 4.4 | 0.287 | |
| IL-6+CD14+CD16+ MFI | 0 h-Baseline | −60 | −212 | 93 | 0.447 |
| 1 h–0h | −44 | −199 | 111 | 0.582 | |
| TNF+CD14+CD16- % | 0 h-Baseline | −5.9 | −18.5 | 6.8 | 0.369 |
| 1 h–0h | −6.2 | −18.8 | 6.5 | 0.342 | |
| TNF+CD14+CD16- MFI | 0 h-Baseline | −537 | −1637 | 563 | 0.344 |
| 1 h–0h | −6618 | −1780 | 456 | 0.252 | |
| TNF+CD14+CD16+ % | 0 h-Baseline | −10.5 | −23.8 | 3.7 | 0.158 |
| 1 h–0h | −1.9 | −15.9 | 12.0 | 0.786 | |
| TNF+CD14+CD16- MFI | 0 h-Baseline | −621 | −1786 | 544 | 0.302 |
| 1 h–0h | −142 | −1345 | 1061 | 0.818 | |
Data presented as Mean ± SE. IL, interleukin; TNF, tumor necrosis factor; MFI, mean fluorescence intensity; CD, cluster of differentiation.
3.3.5. TLR2 and TLR4 response to the acute exercise
Acute exercise altered TLR2 expression in CD14+CD16- monocytes (p=0.008). The initial decrease in MFI at 0 h did not reach significance (−22, 95% CI -60.1 to 15.8, p=0.259, Fig. 6A) but was reduced at 1 h relative to baseline (−63, 95 CI -100.9 to −25, p=0.002). TLR2 expression in CD14+CD16+ monocytes did not change at 0 h (1, 95% CI -53.7 to 55.5, p=0.975) or 1 h (−32, 95% CI -87 to 22.3, p=0.253).
TLR4 expression in CD14+CD16- monocytes did not change at 0 h (−3, 95% CI -11.48 to 5.59, p=0.503, Fig. 6B) while there was a trend to decrease MFI at 1 h (−8, 95% CI -16.92 to 0.14, p=0.06) relative to baseline. TLR4 expression in CD14+CD16+ monocytes changed with acute exercise (p= 0.019), with no change initially at 0 h (−6, 95% CI -18 to 6.34, p=0.35) that was decreased at 1 h relative to baseline (−18, 95% CI -30.2 to −5.83, p=0.006).
4. Discussion
4.1. Findings
Exercise training may reduce inflammation in breast cancer survivors (Khosravi et al., 2019; Meneses-Echavez et al., 2016), however, the mechanism that underpins these effects remain unclear. One key postulated mechanism is through the regulation of monocytes (Gleeson et al., 2011), yet limited data exist in breast cancer survivors. in the current study, we examined the effects of acute and chronic exercise on monocytes, a major source of cytokine production (Hsi and Remick, 1995) in breast cancer survivors. Following training, IL-1ß and IL-6 proportions in CD14+CD16- and per-cell IL-1ß in both monocyte subgroups were reduced. Acute exercise mobilized both monocytes subgroups, with increased cell concentration immediately after exercise, and then returned to baseline levels at 1 h. Acute exercise also reduced TLR2 and TLR4 expression. Collectively, this indicated an anti-inflammatory effect of the combined exercise training and the intermittent aerobic exercise on monocytes through reduced cytokine production and TLR2/4 expression respectively, in the breast cancer survivor.
4.2. Training effects
We found the proportion of cells producing IL-1ß and IL-6 in CD14+CD16- and per cell production of IL-1ß in both monocyte subgroups decreased after 16 weeks of combined exercise training. Several pro-inflammatory cytokines are linked to impaired central nervous system activity leading to “sickness behavior” (Cleeland et al., 2003). For instance, inflammatory cytokines produced by monocytes are directly linked to fatigue, with high levels of intracellular IL-ß, IL-6, and TNF were reported in fatigued breast cancer patients (Collado-Hidalgo et al., 2006; Saligan and Kim, 2012). Our findings in breast cancer survivors are consistent with previous work showing monocytes pro-inflammatory cytokines production was lower in trained compared to untrained apparently healthy individuals (Selkirk et al., 2009). Also, monocyte pro-inflammatory cytokine mRNA expression was reported to decrease after resistance training (RT) (Flynn et al., 2003). It has been hypothesized that endogenous ligands, such as heat shock proteins, increase with a bout of exercise, and with the frequent occurrence, it creates a cross-tolerance, which may decrease monocyte TLRs expression while reducing the inflammatory response (Flynn and McFarlin, 2006). In a large randomized controlled trial, exercise training was associated with decreased levels of stress hormones such as cortisol and corticosterone (Friedenreich et al., 2019). Reduced stress hormone levels might play a role in the reduced inflammatory response of immune cells to exercise. Despite a reduction in pro-inflammatory cytokines production, we found no changes in TLR2 or TLR4 expression. The lack of change in TLR4 in the current study is consistent with other work that used combined training (Timmerman et al., 2008). Contrary to our results, Flynn et al. (2003) found a reduction of TLR4 mRNA expression on CD14+ monocytes after RT in elderly women, and Coen et al. (2010) observed reduced TLR4 expression on monocytes after RT in hypercholesterolemic individuals. RT has previously been shown to reduce TLR2 and TLR4 expression while aerobic training (AT) produced less consistent results, with some studies indicating increases in TLR2 and TLR4 expression (Cavalcante et al., 2017). These conflicting results may be partially explained by differences in modes of exercise but most importantly, the fact that none of the previous studies have used cancer population. Thus, we suggest that a combined exercise training reduces the IL-1ß and IL-6 production in monocytes in breast cancer survivors. However, reductions occur independently of TLR4 and TLR2 reduction.
We found no effects of 16 weeks of combined exercise training on CD14+CD16- or CD14+CD16+ proportion in breast cancer survivors. However, a reduced CD14+CD16+ monocyte proportion was reported following high-intensity interval training (Bartlett et al., 2018; de Matos et al., 2019), RT (Markofski et al., 2014), and combined training (Timmerman et al., 2008) in healthy individuals. As there is no similar study in breast cancer patients, it is unclear if this finding is specific to breast cancer-related characteristics or treatment, or differences in study designs or intensities. Additional studies with increased sample sizes are needed to confirm this finding.
4.3. Acute exercise effects
TLR2 and TLR4 expression were reduced following the acute exercise. The reasons for this change are unclear, with the shedding of these receptors from the cell's surface, downregulation of TLR gene expression, or different expression of TLRs on cells all being possible (Berger and Dannenberg, 2013). Despite the decrease in TLR expression, we did not find any changes in monocyte intracellular cytokine production. However, there were trends for reduced expression levels, with a non-negligible effect, in all cytokines except for the IL-6+CD14+CD16- proportion. This suggests that the study may have been underpowered to detect these effects, although considerable variation between subjects was also likely a factor (Dimitrov et al., 2017). Decreased monocyte intracellular TNF production in response to moderate acute exercise has been reported in healthy populations, with ß2-adrenergic activation potentially involved in this effect (Dimitrov et al., 2017). Total monocyte cell number and the proportion increased immediately after exercise and returned to baseline at 1 h. Acute exercise, irrespective of intensity and duration, leads to monocytosis (Shinkai et al., 1992; Woods et al., 1999). However, in one study that examined breast cancer patients' response to half-marathon running, monocytes decreased immediately after exercise (Zimmer et al., 2016), which was likely due to the exercise intensity and the delayed timing of the post-exercise blood draw (Rooney et al., 2018). There is no similar study in cancer survivors to compare our findings with and as noted above, breast cancer or its treatments might affect endocrine response to the exercise (Evans et al., 2016; Hanson et al., 2018). More studies in the breast cancer population need to confirm these results.
Although previous studies reported higher levels of pro-inflammatory cytokines in CD14+CD16+ monocytes (Belge et al., 2002; Grage-Griebenow et al., 2001), we observed no significant difference in resting pro-inflammatory cytokines between CD14+CD16+ and CD14+CD16- monocytes. Breast cancer disease or treatment might be an explanation for that as above mentioned studies were conducted on a healthy population. Intracellular production of IL-6 and TNF increased in fatigued breast cancer in the general monocyte population (Collado-Hidalgo et al., 2006). Also, cytokine gene polymorphisms were reported in breast cancer survivors (Collado-Hidalgo et al., 2008). These results indicated a possible different response of breast cancer patients compared to healthy individuals.
4.4. Limitations and recommendation for further research
There are several limitations to this study. Since all assays were run on freshly isolated cells, we were only able to perform these experiments on a subset of the cohort (11 out of 31 breast cancer survivors), thus the sample size was small. Moreover, there was a relatively high degree of variability in the data obtained in this study. Collectively, these factors reduced the power of the study and make it a preliminary analysis. The lack of a non-exercise control group makes it unclear how much of the response was due to exercise training or a greater time since the end of treatment, which could also alter the inflammatory response. In the current study, we only evaluated monocyte intracellular cytokine production in whole blood. Evaluation of supernatant cytokine production of monocyte culture after LPS stimulation and/or circulating cytokines may provide more information about monocyte cytokine regulation, however, that needs additional assays including sort the cells and/or culture them, which was beyond the scope of this initial study. Also, obesity may alter the immune system and inflammatory responses (Gil et al., 2007). in the future, analyzing the results based on the obesity levels will shed light on the possible confounding effects of that status on response to exercise.
4.5. Conclusions
Searching for the mechanisms of anti-inflammatory effects of exercise in breast cancer patients, we examined how acute and chronic exercise altered the phenotype and function of monocytes. Sixteen-week combined aerobic and resistance training reduced monocyte production of intracellular pro-inflammatory cytokines, especially IL-1ß, although these markers did not change acutely. While acute exercise downregulated the expression of TLR2 and TLR4 on monocytes, this was not sustained over the course of training. Therefore, combined exercise training appears to be associated with the reduced inflammatory function of monocytes, which in turn may contribute to a better quality of life and improved survival outcomes for breast cancer survivors. Also, while preliminary in nature, our reports of reduced IL-1ß provide support for targeting this pathway as a promising strategy to prevent or control tumor growth and spread.
Funding
This work was supported by the Breast Cancer Research Foundation of New York (New York, NY). The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. Research reported in this publication was supported by the Center for AIDS Research award number 5P30AI050410.
Declaration of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100216.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bartlett D.B., Willis L.H., Slentz C.A., Hoselton A., Kelly L., Huebner J.L., Kraus V.B., Moss J., Muehlbauer M.J., Spielmann G., Kraus W.E., Lord J.M., Huffman K.M. Ten weeks of high-intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: a pilot study. Arthritis Res. Ther. 2018;20:127. doi: 10.1186/s13075-018-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belge K.-U., Dayyani F., Horelt A., Siedlar M., Frankenberger M., Frankenberger B., Espevik T., Ziegler-Heitbrock L. The proinflammatory CD14+ CD16+ DR++ monocytes are a major source of TNF. J. Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- Berger N.A., Dannenberg A.J. Springer; 2013. Obesity, Inflammation and Cancer. [Google Scholar]
- Bower J.E. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav. Immun. 2007;21:863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J.E., Ganz P.A., Aziz N., Fahey J.L. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom. Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bower J.E., Ganz P.A., Desmond K.A., Rowland J.H., Meyerowitz B.E., Belin T.R. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J. Clin. Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- Cavalcante P.A.M., Gregnani M.F., Henrique J.S., Ornellas F.H., Araújo R.C. Aerobic but not resistance exercise can induce inflammatory pathways via toll-like 2 and 4: a systematic review. Sports medicine-open. 2017;3:42. doi: 10.1186/s40798-017-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child M., Leggate M., Gleeson M. Effects of two weeks of high-intensity interval training (HIIT) on monocyte TLR2 and TLR4 expression in high BMI sedentary men. Int. J. Exerc. Sci. 2013;6:10. [Google Scholar]
- Cleeland C.S., Bennett G.J., Dantzer R., Dougherty P.M., Dunn A.J., Meyers C.A., Miller A.H., Payne R., Reuben J.M., Wang X.S. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer: Interdiscipl. Int. J. Am. Canc. Soc. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- Coen P.M., Flynn M.G., Markofski M.M., Pence B.D., Hannemann R.E. Adding exercise to rosuvastatin treatment: influence on C-reactive protein, monocyte toll-like receptor 4 expression, and inflammatory monocyte (CD14+CD16+) population. Metab. Clin. Exp. 2010;59:1775–1783. doi: 10.1016/j.metabol.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Cohen J. Academic press; 2013. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Collado-Hidalgo A., Bower J.E., Ganz P.A., Cole S.W., Irwin M.R. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Canc. Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A., Bower J.E., Ganz P.A., Irwin M.R., Cole S.W. Cytokine gene polymorphisms and fatigue in breast cancer survivors: early findings. Brain Behav. Immun. 2008;22:1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Matos M.A., Garcia B.C.C., Vieira D.V., de Oliveira M.F.A., Costa K.B., Aguiar P.F., Magalhaes F.C., Brito-Melo G.A., Amorim F.T., Rocha-Vieira E. High-intensity interval training reduces monocyte activation in obese adults. Brain Behav. Immun. 2019;80:818–824. doi: 10.1016/j.bbi.2019.05.030. [DOI] [PubMed] [Google Scholar]
- Dill D.B., Costill D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Dimitrov S., Hulteng E., Hong S. Inflammation and exercise: inhibition of monocytic intracellular TNF production by acute exercise via β 2-adrenergic activation. Brain Behav. Immun. 2017;61:60–68. doi: 10.1016/j.bbi.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S., Shaikh F., Pruitt C., Green M., Wilson K., Beg N., Hong S. Differential TNF production by monocyte subsets under physical stress: blunted mobilization of proinflammatory monocytes in prehypertensive individuals. Brain Behav. Immun. 2013;27:101–108. doi: 10.1016/j.bbi.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E.S., Hackney A.C., McMurray R.G., Randell S.H., Muss H.B., Deal A.M., Battaglini C.L. Impact of acute intermittent exercise on natural killer cells in breast cancer survivors. Integr. Canc. Ther. 2015;14:436–445. doi: 10.1177/1534735415580681. [DOI] [PubMed] [Google Scholar]
- Evans E.S., Hackney A.C., Pebole M.M., McMurray R.G., Muss H.B., Deal A.M., Battaglini C.L. Adrenal hormone and metabolic biomarker responses to 30 min of intermittent cycling exercise in breast cancer survivors. Int. J. Sports Med. 2016;37:921–929. doi: 10.1055/s-0042-110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn M.G., McFarlin B.K. Toll-like receptor 4: link to the anti-inflammatory effects of exercise? Exerc. Sport Sci. Rev. 2006;34:176–181. doi: 10.1249/01.jes.0000240027.22749.14. [DOI] [PubMed] [Google Scholar]
- Flynn M.G., McFarlin B.K., Phillips M.D., Stewart L.K., Timmerman K.L. Toll-like receptor 4 and CD14 mRNA expression are lower in resistive exercise-trained elderly women. J. Appl. Physiol. 2003;95:1833–1842. doi: 10.1152/japplphysiol.00359.2003. [DOI] [PubMed] [Google Scholar]
- Friedenreich C.M., Wang Q., Shaw E., Heer E.V., Zhou R., Brenner D.R., Courneya K.S., Wynne-Edwards K.E. The effect of prescribed exercise volume on biomarkers of chronic stress in postmenopausal women: results from the Breast Cancer and Exercise Trial in Alberta (BETA) Prev. Med. Rep. 2019;15:100960. doi: 10.1016/j.pmedr.2019.100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil A., Aguilera C.M., Gil-Campos M., Canete R. Altered signalling and gene expression associated with the immune system and the inflammatory response in obesity. Br. J. Nutr. 2007;98:S121–S126. doi: 10.1017/S0007114507838050. [DOI] [PubMed] [Google Scholar]
- Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11:607. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Gleeson M., McFarlin B., Flynn M. Exercise and toll-like receptors. Exerc. Immunol. Rev. 2006;12:34–53. [PubMed] [Google Scholar]
- Graff R.M., Kunz H.E., Agha N.H., Baker F.L., Laughlin M., Bigley A.B., Markofski M.M., LaVoy E.C., Katsanis E., Bond R.A., Bollard C.M., Simpson R.J. beta2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. 2018;74:143–153. doi: 10.1016/j.bbi.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Grage-Griebenow E., Flad H.D., Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Hanson E.D., Danson E., Nguyen-Robertson C.V., Fyfe J.J., Stepto N.K., Bartlett D.B., Sakkal S. Maximal exercise increases mucosal associated invariant T cell frequency and number in healthy young men. Eur. J. Appl. Physiol. 2017;117:2159–2169. doi: 10.1007/s00421-017-3704-z. [DOI] [PubMed] [Google Scholar]
- Hanson E.D., Sakkal S., Evans W.S., Violet J.A., Battaglini C.L., McConell G.K., Hayes A. Altered stress hormone response following acute exercise during prostate cancer treatment. Scand. J. Med. Sci. Sports. 2018;28:1925–1933. doi: 10.1111/sms.13199. [DOI] [PubMed] [Google Scholar]
- Hanson E.D., Sakkal S., Que S., Cho E., Spielmann G., Kadife E., Violet J.A., Battaglini C.L., Stoner L., Bartlett D.B. Natural killer cell mobilization and egress following acute exercise in men with prostate cancer. Exp. Physiol. 2020;105:1524–1539. doi: 10.1113/EP088627. [DOI] [PubMed] [Google Scholar]
- Hooning M.J., Botma A., Aleman B.M., Baaijens M.H., Bartelink H., Klijn J.G., Taylor C.W., Van Leeuwen F.E. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J. Natl. Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- Hsi E.D., Remick D.G. Monocytes are the major producers of interleukin-1 beta in an ex vivo model of local cytokine production. J. Interferon Cytokine Res. : Off. J. Int. Soc. Interferon Cytokine Res. 1995;15:89–94. doi: 10.1089/jir.1995.15.89. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 2005;17:338–344. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Khosravi N., Stoner L., Farajivafa V., Hanson E.D. Exercise training, circulating cytokine levels and immune function in cancer survivors: a meta-analysis. Brain Behav. Immun. 2019;81:92–104. doi: 10.1016/j.bbi.2019.08.187. [DOI] [PubMed] [Google Scholar]
- Markofski M.M., Flynn M.G., Carrillo A.E., Armstrong C.L., Campbell W.W., Sedlock D.A. Resistance exercise training-induced decrease in circulating inflammatory CD14+ CD16+ monocyte percentage without weight loss in older adults. Eur. J. Appl. Physiol. 2014;114:1737–1748. doi: 10.1007/s00421-014-2902-1. [DOI] [PubMed] [Google Scholar]
- Meneses-Echavez J.F., Correa-Bautista J.E., Gonzalez-Jimenez E., Schmidt Rio-Valle J., Elkins M.R., Lobelo F., Ramirez-Velez R. The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Canc. Epidemiol. Biomarkers Prev. : Publ. Am. Assoc. Canc. Res. Cosponsored Am. Soc. Prev. Oncol. 2016;25:1009–1017. doi: 10.1158/1055-9965.EPI-15-1061. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol. Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Rhind S.G., Castellani J.W., Brenner I.K., Shephard R.J., Zamecnik J., Montain S.J., Young A.J., Shek P.N. Intracellular monocyte and serum cytokine expression is modulated by exhausting exercise and cold exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R66–R75. doi: 10.1152/ajpregu.2001.281.1.R66. [DOI] [PubMed] [Google Scholar]
- Riebe D., Ehrman J., Liguori G., Magal M. Lippincott Williams & Wilkins; 2018. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]
- Rooney B.V., Bigley A.B., LaVoy E.C., Laughlin M., Pedlar C., Simpson R.J. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: a detailed temporal analysis of leukocyte extravasation. Physiol. Behav. 2018;194:260–267. doi: 10.1016/j.physbeh.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Saligan L., Kim H. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav. Immun. 2012;26:830–848. doi: 10.1016/j.bbi.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkirk G.A., McLellan T.M., Wright H.E., Rhind S.G. Expression of intracellular cytokines, HSP72, and apoptosis in monocyte subsets during exertional heat stress in trained and untrained individuals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R575–R586. doi: 10.1152/ajpregu.90683.2008. [DOI] [PubMed] [Google Scholar]
- Shinkai S., Shore S., Shek P., Shephard R. Acute exercise and immune function. Int. J. Sports Med. 1992;13:452–461. doi: 10.1055/s-2007-1021297. [DOI] [PubMed] [Google Scholar]
- Starkie R., Angus D., Rolland J., Hargreaves M., Febbraio M. Effect of prolonged, submaximal exercise and carbohydrate ingestion on monocyte intracellular cytokine production in humans. J. Physiol. 2000;528:647–655. doi: 10.1111/j.1469-7793.2000.t01-1-00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkie R., Hargreaves M., Rolland J., Febbraio M. Heat stress, cytokines, and the immune response to exercise. Brain Behav. Immun. 2005;19:404–412. doi: 10.1016/j.bbi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Starkie R., Rolland J., Angus D., Anderson M., Febbraio M. Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-α levels after prolonged running. Am. J. Physiol. Cell Physiol. 2001;280:C769–C774. doi: 10.1152/ajpcell.2001.280.4.C769. [DOI] [PubMed] [Google Scholar]
- Timmerman K.L., Flynn M.G., Coen P.M., Markofski M.M., Pence B.D. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J. Leukoc. Biol. 2008;84:1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- Woods J.A., Davis J.M., Smith J.A., Nieman D.C. Exercise and cellular innate immune function. Med. Sci. Sports Exerc. 1999;31:57–66. doi: 10.1097/00005768-199901000-00011. [DOI] [PubMed] [Google Scholar]
- Zimmer P., Baumann F.T., Bloch W., Zopf E.M., Schulz S., Latsch J., Schollmayer F., Shimabukuro-Vornhagen A., von Bergwelt-Baildon M., Schenk A. Impact of a half marathon on cellular immune system, pro-inflammatory cytokine levels, and recovery behavior of breast cancer patients in the aftercare compared to healthy controls. Eur. J. Haematol. 2016;96:152–159. doi: 10.1111/ejh.12561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.