Abstract
Introduction
The development of immune-related adverse events (irAEs) has been associated with improved efficacy of immune checkpoint inhibitors in patients with urothelial cancer, melanoma, and NSCLC. Whether this association exists in patients with SCLC is currently unknown.
Methods
We conducted a multicenter retrospective study to evaluate the relationship between irAEs and immunotherapy efficacy in SCLC. To account for the lead-time bias resulting from the time-dependent nature of irAEs, the development of irAEs was considered as a time-varying covariate in univariate and multivariate Cox proportional hazard models.
Results
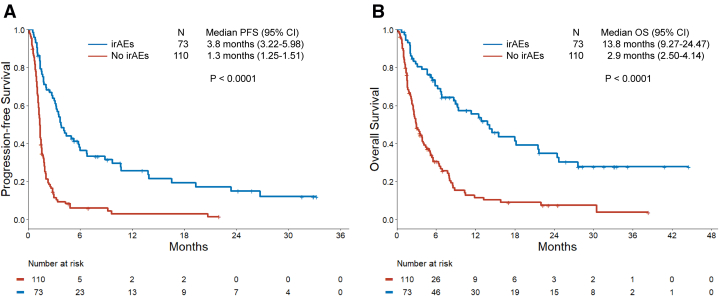
Of the 183 patients treated with immunotherapy, 73 (39.9%) experienced at least one irAE. A total of 42 patients (22.9%) had grade 1 to 2 irAEs, whereas 31 patients (16.9%) had grade 3 to 4 irAEs. The median time of onset to the first irAE was 24 days (interquartile range: 14–55). The baseline clinicopathologic features were well-balanced between patients with and without irAEs. At a median follow-up of 24 months (95% confidence interval [CI]: 17.0–31.6), the median progression-free survival was significantly longer in the irAE group than the non-irAE group (3.8 versus 1.3 mo, p < 0.0001). The median overall survival was also significantly longer among patients with irAEs than patients without irAEs (13.8 versus 2.9 mo, p < 0.0001). When analyzed as a time-varying covariate, the development of irAEs was associated with a significant improvement in progression-free survival (hazard ratio: 0.44 [95% CI: 0.29–0.66], p < 0.001) and overall survival (hazard ratio: 0.47 [95% CI: 0.32–0.71], p < 0.001) in multivariate models.
Conclusions
The development of irAEs is associated with improved clinical outcomes for immunotherapy in patients with advanced SCLC.
Keywords: SCLC, irAEs, PD-(L)1, CTLA-4
Introduction
Immunotherapies targeting programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) have changed the treatment paradigms for many cancer types. Despite their efficacy, these immune checkpoint inhibitors (ICIs) can trigger potentially serious immune-related adverse events (irAEs).1, 2, 3 Recently, the development of irAEs has been found to correlate with improved clinical outcomes in immunotherapy among patients with NSCLC, melanoma, and urothelial cancer.4, 5, 6, 7 Although the mechanisms underlying this association are still unknown, irAEs theoretically may be triggered by antigens that are common to both tumor and normal tissues.6,8 Under this hypothesis, activated T-cells would target both tissues, resulting in toxicity and response.8,9 PD-1 inhibitors have exhibited promising antitumor activity in a subset of patients with previously treated SCLC, with objective response rates (ORRs) of approximately 10% to 15%.10, 11, 12 In addition, the combination of chemotherapy with either of the PD-L1 agents, atezolizumab or durvalumab, has improved survival compared with chemotherapy plus placebo in the first-line setting for patients with extensive-stage SCLC.13,14 However, given that only a minority of patients with SCLC respond to ICIs, the risk of developing a severe irAE must be weighed against the potential clinical benefit in this population. We sought to determine the relationship between irAE development and clinical outcomes and immunotherapy in patients with extensive-stage SCLC, which has not been previously described.
One of the inherent challenges in determining the relationship between immunotherapy response and toxicity onset is that patients who are benefiting from ICI treatment have longer treatment exposure, making them more likely to develop adverse events over time. Comparing the clinical outcomes of patients who experience irAEs with those who do not, therefore, does not account for the time-dependent nature of irAE development. In particular, patients who experience disease progression or die within weeks after the start of immunotherapy will have a shorter follow-up period and shorter treatment exposure compared with those who stay on treatment, making them less likely to develop irAEs. Although statistical approaches with landmark analyses have been recently used to address this bias, this strategy is limited by somewhat arbitrary choices of the landmark interval. In addition, landmark analyses are not useful when the event of progression or death occurs very early, as in SCLC, in which the median progression-free survival (mPFS) with ICIs is only approximately 1.5 months.10, 11, 12
To account for the time-dependent nature of the relationship between toxicity and response in a tumor type with a very short mPFS on ICIs, we investigated the association between irAEs and clinical benefit from immunotherapy, estimating the association between irAEs and survival using a multivariable Cox model with irAEs included as a time-varying covariate.
Materials and Methods
Study Population
We retrospectively collected data from six participating academic medical centers: the Dana-Farber Cancer Institute (DFCI), the Massachusetts General Hospital, the Beth Israel Deaconess Medical Center, Columbia University, the Johns Hopkins University, and East Carolina University. Patients were included if they had consented to institutional review board–approved medical record review protocols at each institution and had advanced SCLC previously treated with at least one dose of a PD-(L)1 inhibitor alone or in combination with a CTLA-4 inhibitor. Patients who received a combination of chemotherapy and immunotherapy were excluded.
Tumor Mutational Burden Assessment
Tumor mutational burden (TMB), defined as the number of somatic, coding, base substitution, and indel mutations per megabase (mut/Mb) of genome evaluated, was available only for the DFCI cohort and was calculated from the DFCI OncoPanel next-generation sequencing platform, as previously described.15
Clinical Outcomes
To determine ORR and PFS, scans were reviewed by thoracic radiologists using Response Evaluation Criteria in Solid Tumors version 1.1. PFS was defined as the time from the start of immunotherapy to the date of disease progression or death, whichever occurred first. Patients who were alive without disease progression were censored on the date of their last adequate disease assessment. Overall survival (OS) was defined as the time from the start of immunotherapy to death. Patients who were still alive were censored at the date of the last contact. IrAEs were retrospectively assessed using the Common Terminology Criteria for Adverse Events (version 5.0).16
Statistical Analysis
Categorical and continuous variables were summarized descriptively using percentages and medians. The Wilcoxon ranked sum test and Kruskal-Wallis test were used to test for differences between continuous variables, and Fisher’s exact test was used to test for associations between categorical variables. Kaplan-Meier methodology was used to estimate event-time distributions, and the Greenwood formula was used to estimate the standard errors of the estimates. Log-rank tests were used to test for differences in event-time distributions, and Cox proportional hazards models were fitted to obtain estimates of hazard ratios (HRs) in univariate and multivariate models. To account for the lead-time bias resulting from the time-dependent nature of irAEs, the development of irAEs was considered as a time-varying covariate in univariate and multivariate Cox proportional hazard models. All p values were two-sided, and confidence intervals (CIs) were set at the 95% level, with statistical significance defined as p less than or equal to 0.05. All statistical analyses were performed using R version 3.6.1.
Results
Patients Characteristics
The clinicopathologic characteristics of the 183 patients with advanced SCLC who received immunotherapy between July 2014 and December 2018 are detailed in Supplementary Table 1. The median age of patients was 64 years (range: 34–84), 96.7% were current or former smokers, 76.1% had an Eastern Cooperative Oncology Group performance status of 0 to 1, and 73.7% received immunotherapy in the second-line setting. Immunotherapy consisted of PD-(L)1 inhibitor monotherapy in 59.6% of cases and combined PD-1 plus CTLA-4 inhibition in 40.4% of cases. Among the 69 cases (37.7%) that underwent successful targeted next-generation sequencing, the median TMB was 9.9 mut/Mb (range: 1.3–31.2).
Immune-Related Adverse Events
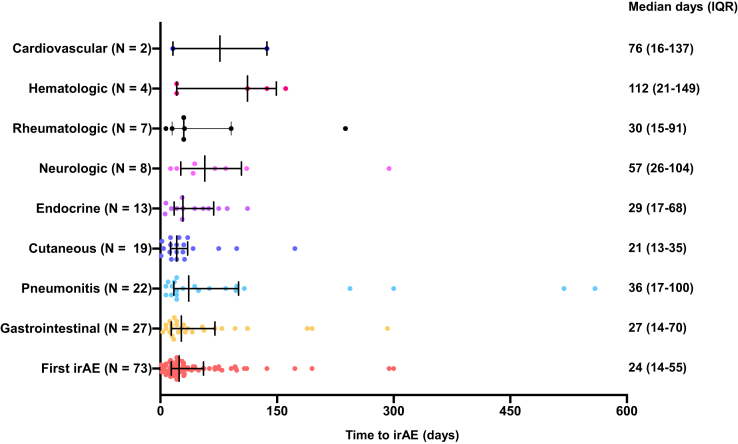
Among the 183 patients treated with immunotherapy, 73 (39.9%) experienced at least one irAE. A total of 42 patients (22.9%) had grade 1 to 2 irAEs, whereas 31 patients (16.9%) had grade 3 to 4 irAEs. Among the 73 patients with irAEs, 14.7% had gastrointestinal irAEs, 12.0% had pulmonary irAEs, 10.4% had cutaneous irAEs, 7.1% had endocrine iAEs, 3.8% had rheumatologic irAEs, 2.1% had hematologic irAEs, and 1.1% had cardiovascular irAEs. The full spectrum of irAEs observed in this cohort is detailed in Supplementary Table 2. Among the 73 patients with irAEs, the median time to onset to the first irAE was 24 days (interquartile range: 14–55), as illustrated in Figure 1.
Figure 1.
The median time to onset of the different irAE classes. irAE, immune-related adverse event; IQR, interquartile range.
Clinical Outcomes to Immunotherapy by irAE Development
In the entire cohort of 183 patients, the ORR to immunotherapy was 13.1% (95% CI: 8.6–18.8) (Supplementary Fig. 1A), the mPFS was 1.7 months (95% CI: 1.47–2.01) (Supplementary Fig. 1B), and the median OS (mOS) was 5.4 months (95% CI: 3.73–7.06) (Supplementary Fig. 1C). The ORR among patients treated with the combination of PD-1 plus CTLA-4 was 21.6% (95% CI: 12.89–32.72), which was significantly higher than the ORR of 7.3% (95% CI: 3.22–13.95) of patients treated with PD-(L)1 monotherapy (p = 0.007) (Supplementary Fig. 2A). No significant difference in terms of mPFS (2.1 versus 1.6 mo, HR: 81 [95% CI: 0.59–1.11], p = 0.19) and mOS (6.8 versus 4.6 mo, HR: 78 [95% CI: 0.55–1.10], p = 0.16) was observed between patients treated with the PD-1 plus CTLA-4 combination therapy and those treated with PD-(L)1 monotherapy (Supplementary Fig. 2B and C).
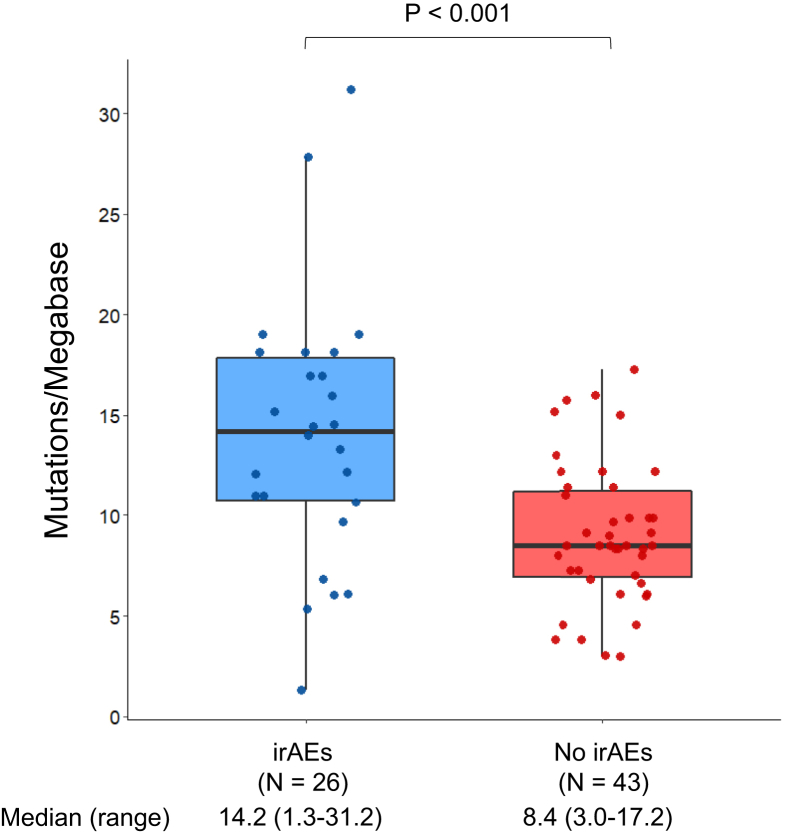
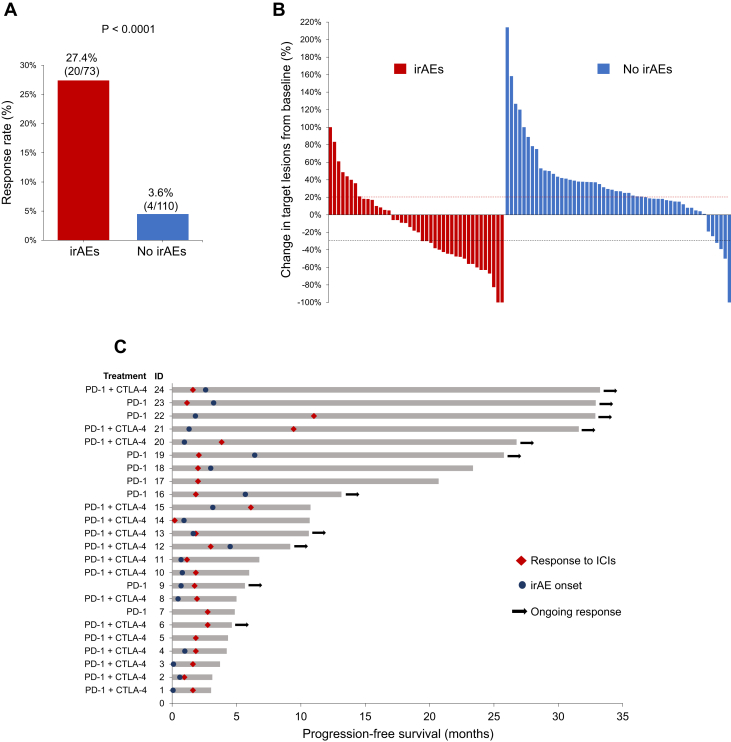
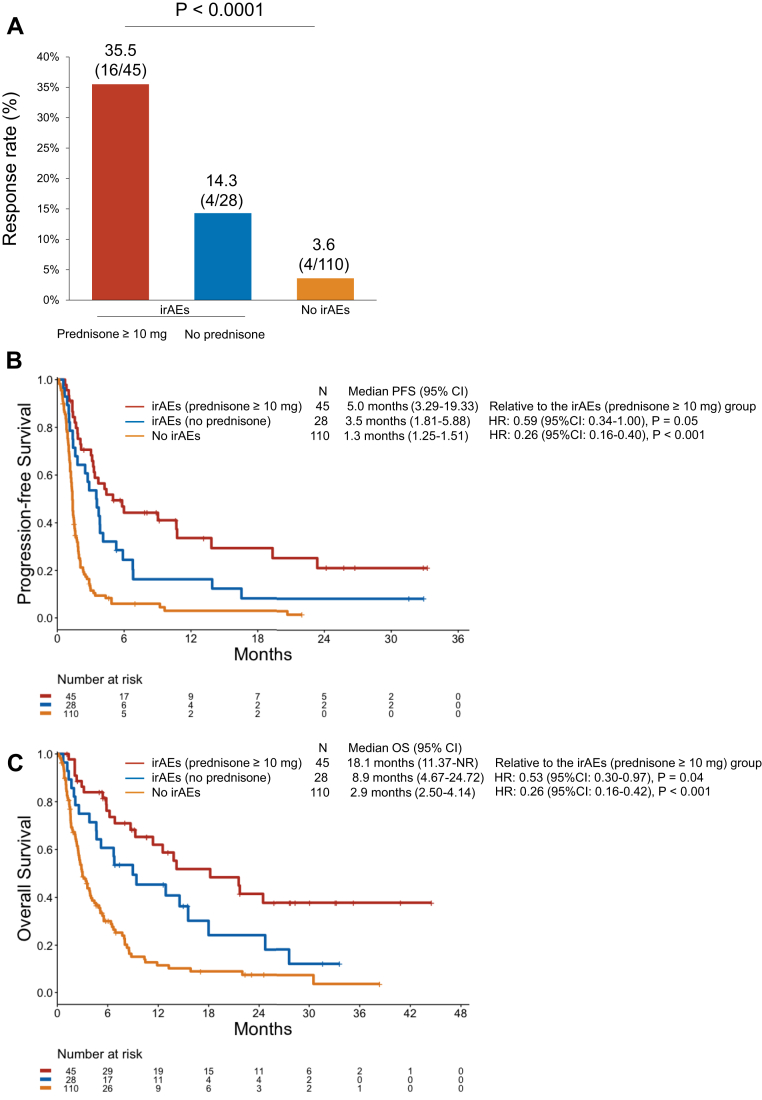
Next, we analyzed the clinical outcomes of immunotherapy by the development of irAEs. Baseline clinicopathologic features were generally well-balanced between patients with and without irAEs (Table 1). Patients who developed irAEs were younger (median age: 61 versus 66 years, p = 0.01) and more likely to have received CTLA-4 inhibition (p < 0.001). Among the 69 patients with TMB assessment, the median TMB was significantly higher among patients with irAEs compared with those without irAEs (14.2 versus 8.4 mut/Mb, p < 0.001) (Fig. 2). The ORR in patients with irAEs was 27.4% (95% CI: 17.6–39.1), which was significantly higher than the ORR of 3.6% (95% CI: 1.0–9.1) observed in patients without irAEs (p < 0.0001) (Fig. 3A and B). Among the 24 responders, 13 of the 20 patients (65%) with irAEs experienced the irAE before the radiological evidence of response to immunotherapy (Fig. 3C). The median time to response was 1.8 months (95% CI: 1.6–2.9) in patients with irAEs and 2.3 months (1.8–NR) in patients without irAEs. At a median follow-up of 24 months (95% CI: 17.0–31.6), the mPFS was significantly longer in the irAE group than in the no-irAE group (3.8 versus 1.3 mo, p < 0.0001) (Fig. 4A). The mOS was also significantly longer among patients with irAEs than those in the no-irAEs group (13.8 versus 2.9 mo, p < 0.0001) (Fig. 4B).
Table 1.
Baseline Clinicopathologic Characteristics of Patients With Versus Without irAEs
| Characteristic | irAEs 73 (%) | No irAEs 110 (%) | p Value |
|---|---|---|---|
| Age, y, median (range) | 61 (47–84) | 66 (34–84) | 0.01 |
| Sex | 0.76 | ||
| Male | 33 (45.2) | 47 (42.7) | |
| Female | 40 (54.8) | 63 (57.3) | |
| Smoking status | 0.68 | ||
| Ever | 70 (95.9) | 107 (97.3) | |
| Never | 3 (4.1) | 3 (2.7) | |
| Stage at diagnosis | 0.62 | ||
| Limited | 23 (31.5) | 30 (27.3) | |
| Extensive | 50 (68.5) | 80 (72.7) | |
| ECOG PS | 0.23 | ||
| 0–1 | 52 (76.5) | 73 (67.0) | |
| ≥2 | 16 (23.5) | 36 (33.0) | |
| Not available | 5 | 1 | |
| Line of therapy for ICIs | 0.86 | ||
| 2nd | 53 (72.6) | 82 (72.6) | |
| ≥3rd | 20 (27.4) | 28 (25.5) | |
| Treatment received | < 0.001 | ||
| PD-(L)1 monotherapy | 32 (43.8) | 77 (70.0) | |
| PD-1 + CTLA-4 | 41 (56.2) | 33 (30.0) | |
| Cycles of ICI received, median (range) | 3.5 (1–71) | 2 (1–18) | < 0.001 |
| Any history of brain metastases before ICI | 0.87 | ||
| No | 49 (67.1) | 72 (65.5) | |
| Yes | 24 (32.9) | 38 (34.5) |
Note: CTLA-4, cytotoxic T-lymphocyte–associated antigen 4; ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; PD-L1, programmed death-ligand 1; PD-1, programmed cell death protein 1.
Figure 2.
TMB in SCLCs from patients treated with immunotherapy who did or did not develop irAEs. irAE, immune-related adverse event; TMB, tumor mutational burden.
Figure 3.
(A) The proportion of patients who achieved a complete or partial response to immunotherapy in the irAEs group and no-irAEs group. (B) Waterfall plot illustrating the percentage change of tumor target lesions compared with baseline in patients with assessable target lesions. (C) Swimmer plot illustrating the duration of PFS among the 24 patients who responded to immunotherapy. The time to response and the time to irAE onset are also illustrated. irAE, immune-related adverse event; PFS, progression-free survival.
Figure 4.
(A) PFS and (B) OS in patients with SCLC treated with immunotherapy in the irAEs and no-irAEs cohorts. irAE, immune-related adverse event; OS, overall survival; PFS, progression-free survival.
In the light of the lead-in bias of the time-dependent nature of irAEs, we analyzed the survival outcomes to immunotherapy including irAEs as a time-varying covariate in the Cox proportional hazard model and found that the development of irAEs was significantly associated with improved PFS (unadjusted HR: 0.45 [95% CI: 0.30–0.67], p < 0.001) and OS (unadjusted HR: 0.46 [95% CI: 0.31–0.68], p < 0.001). The cumulative hazard of death in patients with and without irAEs is illustrated in Supplementary Figure 3. After adjusting for age and Eastern Cooperative Oncology Group performance status, the development of irAEs retained a significant association with improved PFS (adjusted HR: 0.44 [95% CI: 0.29–0.66], p < 0.001) and OS (adjusted HR: 0.47 [95% CI: 0.32–0.71], p < 0.001) in a multivariate model (Supplementary Table 3). When analyzing the relationship between irAEs and clinical outcomes to immunotherapy by treatment received, this association seemed to be driven primarily by the group of patients who received the combination of PD-1 and CTLA-4 inhibition (Supplementary Table 4).
Overall, 45 patients (61.6%) required systemic corticosteroids for the treatment of irAEs (Supplementary Table 2). Five patients (6.8%) with immune-related pneumonitis were treated with the interleukin-6 receptor inhibitor tocilizumab in addition to systemic corticosteroids. A total of 32 patients (17.5%) discontinued treatment because of irAE development. Among patients with irAEs, the administration of systemic corticosteroids did not negatively affect clinical outcomes to immunotherapy (Fig. 5A–C).
Figure 5.
(A) Response rate, (B) PFS, and (C) OS to immunotherapy in patients with irAEs according to corticosteroid administration and in those without irAEs. irAE, immune-related adverse event; OS, overall survival; PFS, progression-free survival.
When analyzing clinical outcomes according to the grade of irAEs, there was no difference in terms of PFS (HR: 0.97 [95% CI: 0.55–1.72], p = 0.93) and OS (HR: 0.72 [95% CI: 0.39–1.34], p = 0.31) between patients with grade 1/2 irAEs, and those with grade 3/4 irAEs. The cumulative hazard of death in patients with grade 1/2, grade 3/4, and no irAEs is illustrated in Supplementary Figure 4.
Discussion
To our knowledge, this is the first study to report that the development of irAEs is associated with substantial improvements in clinical outcomes to immune checkpoint blockade among patients with SCLC. Immunotherapy toxicity represents a major concern in oncology when treating patients with ICIs, and the risk of developing severe adverse effects must be weighed against the potential for durable responses. We found that with SCLC, as with other tumor types such as melanoma, NSCLC, and urothelial cancer, there may be a trade-off between therapeutic benefit and treatment-related toxicities that will need to be managed by medical teams and their patients.
The time-dependent nature of irAEs constitutes a challenge to the interpretation of studies investigating the association between irAEs and clinical outcomes to immunotherapy. Are patients who receive longer courses of immunotherapy (because their cancer is responding to treatment) simply more likely to develop irAEs over time owing to greater ICI exposure? Although other retrospective studies have addressed this bias by using landmark analyses,4 because of the very short PFS of patients with SCLC treated with ICIs in our cohort, this was not the optimal approach to properly address this question. In addition, one limitation of landmark analyses is that the choice of a time point cutoff is somewhat arbitrary. Therefore, in our study, we analyzed the development of irAEs as a time-varying covariate in a Cox regression model and found a significant association between irAEs and improved PFS and OS to immunotherapy. This approach minimized the lead-time bias associated with the time-dependent nature of irAEs. Furthermore, we found that the onset of irAEs among patients with SCLC was often very rapid (median time: 24 days), suggesting that the development of irAEs is not typically a late event that occurs after long periods of immunotherapy exposure. One concern with irAE management is whether the use of immunosuppressive agents will impart a deleterious effect on the benefits of immunotherapy. As with other studies,17,18 we found that corticosteroid administration had no effect on the management of irAEs in response to immunotherapy.
The mechanisms underlying the association between irAEs and improved clinical outcomes are poorly understood. Immune checkpoint blockade may activate exhausted T-cells that can cross-react both with tumor antigens and self-antigens, resulting in autoimmune tissue damage. In addition, epitope spreading can occur when tumor cell death releases antigens, including neoantigens, that may prime lymphocytes against the wild-type antigens in normal tissue.8,9,18 Cancers with high TMB, such as NSCLC and SCLC, have recently been found to have a higher risk of developing irAEs during anti–PD-1 therapy, possibly because of the underlying neoantigenic potential that may increase the chance of molecular mimicry between cancer antigens and normal tissues.19 Consistent with this hypothesis, we also found that the median TMB was significantly higher among patients with irAEs compared with those without irAEs. However, this finding should be interpreted with caution in the light of the small sample size of TMB-assessable cases and the known correlation between higher TMB and immunotherapy benefit in SCLC.20,21
Limitations of this study include the retrospective design and retrospective assessment of iAEs, which may have introduced bias. In addition, the relatively small sample size did not allow us to investigate the association between specific irAE subtypes and clinical outcomes to immunotherapy. Furthermore, the new standard of care for extensive-stage SCLC is to combine immunotherapy with chemotherapy in the first-line setting rather than use immunotherapy alone. It is hoped that our findings in patients previously treated with chemotherapy will lay the basis for additional studies exploring the relationship between irAEs and clinical outcomes among patients treated with chemotherapy plus immunotherapy.
In conclusion, in this study, we found that irAEs occur early and are associated with improved clinical outcomes to immunotherapy in patients with SCLC. Additional studies are necessary to identify the mechanistic basis for improved immunotherapy efficacy in patients who develop irAEs.
Footnotes
Disclosure: Dr. Walker reports receiving personal fees from and owns interest in Circulogene outside of the submitted work. Dr. Farago reports serving as a consultant for AbbVie, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Genentech, Loxo, Merck, Pfizer, Pharmamar, and Roche; and has received research support from AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Genentech, Loxo, Merck, PharmaMar, and Roche. Dr. Gainor reports serving as a consultant for or has received honoraria from Bristol-Myers Squibb, Genentech/Roche, Ariad/Takeda, Loxo, Gilead, Blueprint, Pfizer, Incyte, Novartis, Merck, Agios, Amgen, Regeneron, Oncorus, Array, Jounce, and Clovis Oncology; has received research support from Novartis, Genentech/Roche, and Ariad/Takeda; received institutional research support from Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array Biopharma, Merck, Adaptimmune, Novartis, and Alexo; and has an immediate family member who is an employee of Ironwood Pharmaceuticals. Dr. Sholl reports serving as a consultant for Foghorn Therapeutics. Dr. Nishino reports serving as a consultant for Daiichi Sankyo and AstraZeneca; has received a research grant from Merck, Canon Medical Systems, and AstraZeneca; and has received an honorarium from Roche. Dr. Henick reports having previous stock ownership in AbbVie and participated in Boehringer Ingelheim Clinical Congress at the American Society of Clinical Oncology 2017. Dr. Sands reports serving as a consultant for AbbVie, AstraZeneca, Celgene, Foundation Medicine, Genentech, Guardant, Incyte, Loxo, Merk, and Troovagene; and has received an honorarium from Pharma Mar. Dr. Naidoo reports having received research funding from AstraZeneca and Merck; has served as consultant/advisory board member for Bristol-Myers Squibb, AstraZeneca, and Roche/Genentech; and has received honoraria from Bristol-Myers Squibb. Dr. Rizvi reports serving as a consultant for AstraZeneca, MedImmune, Genentech, Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb, Pfizer, Eli Lilly, AbbVie, Regeneron, Janssen Pharmaceuticals, EMD Serono, GlaxoSmithKline, and NeoGenomics Laboratories. Dr. Awad reports serving as a consultant/advisory board member for Bristol-Myers Squibb, AstraZeneca, Achilles, AbbVie, Neon, Maverick, Nektar, Hegrui, Syndax, and Gritstone; and has received research funding from BMS, AstraZeneca, Eli Lilly, and Genentech. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100074.
Supplementary Data
References
- 1.Boutros C., Tarhini A., Routier E. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann L., Forschner A., Loquai C. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Zimmer L., Goldinger S.M., Hofmann L. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–225. doi: 10.1016/j.ejca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Haratani K., Hayashi H., Chiba Y. Association of immune-related adverse events with nivolumab efficacy in non-small cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman-Keller M., Kim Y., Cronin H., Richards A., Gibney G., Weber J.S. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua C., Boussemart L., Mateus C. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 7.Maher V.E., Fernandes L.L., Weinstock C. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37:2730–2737. doi: 10.1200/JCO.19.00318. [DOI] [PubMed] [Google Scholar]
- 8.Pauken K.E., Dougan M., Rose N.R., Lichtman A.H., Sharpe A.H. Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol. 2019;40:511–523. doi: 10.1016/j.it.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koon H., Atkins M. Autoimmunity and immunotherapy for cancer. N Engl J Med. 2006;354:758–760. doi: 10.1056/NEJMe058307. [DOI] [PubMed] [Google Scholar]
- 10.Antonia S.J., López-Martin J.A., Bendell J. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 11.Ott P.A., Elez E., Hiret S. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35:3823–3829. doi: 10.1200/JCO.2017.72.5069. [DOI] [PubMed] [Google Scholar]
- 12.Goldman J.W., Dowlati A., Antonia S.J. Safety and antitumor activity of durvalumab monotherapy in patients with pretreated extensive disease small-cell lung cancer (ED-SCLC) J Clin Oncol. 2018;36(suppl 15) 8518–8518. [Google Scholar]
- 13.Horn L., Mansfield A.S., Szczsna A. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 14.Paz-Ares L., Dvorkin M., Chen Y. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 15.Garcia E.P., Minkovsky A., Jia Y. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–758. doi: 10.5858/arpa.2016-0527-OA. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services Common terminology criteria for adverse events (CTCAE), version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 17.Wakuda K., Miyawaki T., Miyawaki E. The impact of steroid use on efficacy of immunotherapy among patients with lung cancer who have developed immune-related adverse events. J Clin Oncol. 2019;37(suppl 15) e20583–e20583. [Google Scholar]
- 18.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 19.Bomze D., Hasan Ali O., Bate A., Flatz L. Association between immune-related adverse events during anti-PD-1 therapy and tumor mutational burden. JAMA Oncol. 2019;5:1633–1635. doi: 10.1001/jamaoncol.2019.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricciuti B., Kravets S., Dahlberg S.E. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer. 2019;7:87. doi: 10.1186/s40425-019-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellmann M.D., Callahan M.K., Awad M.M. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33:853–861.e4. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.