Abstract
Introduction
EGFR mutation-positive lung adenocarcinoma (LUAD) displays impaired phosphorylation of ERK and Src-homology 2 domain-containing phosphatase 2 (SHP2) in comparison with EGFR wild-type LUADs. We hypothesize that SHP2 expression could be predictive in patients positive with resected EGFR mutation versus patients with EGFR wild-type LUAD.
Methods
We examined resected LUAD cases from Japan and Spain. mRNA expression levels of AXL, MET, CDCP1, STAT3, YAP1, and SHP2 were analyzed by quantitative reverse transcriptase polymerase chain reaction. The activity of SHP2 inhibitors plus erlotinib were tested in EGFR-mutant cell lines and analyzed by cell viability assay, Western blot, and immunofluorescence.
Results
A total of 50 of 100 EGFR mutation-positive LUADs relapsed, among them, patients with higher SHP2 mRNA expression revealed shorter progression-free survival, in comparison with those having low SHP2 mRNA (hazard ratio: 1.83; 95% confidence interval: 1.05–3.23; p = 0.0329). However, SHP2 was not associated with prognosis in the remaining 167 patients with wild-type EGFR. In EGFR-mutant cell lines, the combination of SHP099 or RMC-4550 (SHP2 inhibitors) with erlotinib revealed synergism via abrogation of phosphorylated AKT (S473) and ERK1/2 (T202/Y204). Although erlotinib translocates phosphorylated SHP2 (Y542) into the nucleus, either RMC-4550 alone, or in combination with erlotinib, relocates SHP2 into the cytoplasm membrane, limiting AKT and ERK1/2 activation.
Conclusions
Elevated SHP2 mRNA levels are associated with recurrence in resected EGFR mutation-positive LUADs, but not in EGFR wild-type. EGFR tyrosine kinase inhibitors can enhance SHP2 activation, hindering adjuvant therapy. SHP2 inhibitors could improve the benefit of adjuvant therapy in EGFR mutation-positive LUADs.
Keywords: Lung adenocarcinoma, EGFR, SHP2, Surgery, Recurrence
Introduction
Accurate prediction of recurrence in early-stage lung adenocarcinomas (LUADs) is attained by gene expression profiling,1 multiregion sequencing, and bespoke multiplex-polymerase chain reaction (PCR) assays.2,3 However, the benefit of adjuvant therapy in patients with high risk of recurrence has not been improved, neither with cisplatin-based chemotherapy (4% of survival benefit at 5 y), nor with bevacizumab.4 The 5-year survival rate in early, completely resected lung cancer varies from 67% in patients with T1-N0 (stage IA) to 23% for T1-3N2 (stage IIIA) disease.5 Moreover, adjuvant cisplatin-based chemotherapy in stage IA could have a detrimental effect on survival,6 which was not observed with adjuvant oral uracil-tegafur.7 In early resected EGFR mutation-positive LUADs, gefitinib and erlotinib were tested in the BR19 and RADIANT trials, respectively. The BR19 study included a small number of EGFR mutation-positive LUADs with better overall survival (OS) for the placebo-treated group (hazard ratio of death was 3.16, p = 0.15).8 Nor was any survival benefit seen with erlotinib versus placebo in stage IB to IIIA EGFR mutation-positive LUADs (RADIANT).9 The benefit of gefitinib versus vinorelbine plus cisplatin was seen in patients with stage II to IIIA EGFR mutation-positive LUAD.10 Several ongoing studies of adjuvant EGFR tyrosine kinase inhibitors (TKIs) are ongoing in resected lung cancer.11 Caution with adjuvant EGFR TKIs should be taken because EGFR-mutant lung cancer cells treated with erlotinib or afatinib activate Notch signaling, increasing aldehyde dehydrogenase stem-like cells.12,13 EGFR TKIs in combination with STAT3 inhibitors,13 AKT inhibitors,14 Src inhibitors,15 STAT3, and Src inhibitors16 or TPX-0005 (repotrectinib)17 can revert the resistance to EGFR TKIs in EGFR-mutant cells. Previously, we found that elevated mRNA expression of AXL and CDCP1 negatively influences progression-free survival and OS in advanced EGFR mutation-positive LUADs.17
Src-homology-2 domain containing phosphatase-2 (SHP2, encoded by the PTPN11 gene) is a promigratory signal from numerous growth-factors, cytokines and receptor tyrosine kinases (RTKs). Phosphorylated SHP2 (pSHP2)-Y542 is a readout of resistance to TKIs in melanoma.18 SHP2 activates Src family kinases, mainly Src, YES, and FYN, and downstream targets, inducing migration and invasion.19 In a previous study, we reported that high levels of SHP2 mRNA expression correlate with poor progression-free survival and OS in advanced EGFR mutation-positive LUADs treated with EGFR TKIs.20 Moreover, combinatory therapy with SHP2 inhibitors was noted to be effective in BRAF-mutant lung cancer cells.21 Early studies indicated that EGFR-mutant cells have limited ERK signaling and SHP2, which partly explains the sensitivity to EGFR TKIs.22 We assessed the mRNA levels of several RTKs and non-RTKs, including MET, AXL, CDCP1, STAT3, YAP1, and SHP2, in 267 resected LUADs, mostly stage IA. In this study, we investigated whether membranous SHP2 translocated to the nucleus as a mechanism of resistance to first-generation TKI (erlotinib) in EGFR-mutant cells and whether mRNA levels of SHP2 could be prognostic in EGFR mutation-positive surgically resected LUADs
Methods
Patients
Surgical specimens of primary invasive resected LUADs between February 2007 and December 2015 at the Hiroshima University Hospital and between November 2017 and September 2018 at the University Hospital Mutua de Terrassa were collected. Patients, after preoperative chemotherapy, chemoradiotherapy or incomplete resection, or with unavailable specimens or clinicopathologic data, were excluded from the study. Patients with adenocarcinoma in situ and minimally invasive adenocarcinoma, were also excluded, owing to no recurrence after complete resection. All cases were diagnosed according to the 2015 WHO pathologic classification23 and the International Association for the Study of Lung Cancer eighth TNM staging system was utilized for staging.24 The Institutional Review Boards approved the design of this study for (IRB number: E-908 in Hiroshima University, EO/1745 in University Hospital Mutua de Terrassa), and all study participants provided informed written consent. The research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
Tumor Tissue Specimens Evaluation
Formalin-fixed paraffin-embedded (FFPE) surgically resected tissues were cut into four slices of 4 μm for RNA extraction. Sliced samples were deparaffinized using xylene. After the removal of xylene using ethanol, tissues were lysed in buffer containing tris-chloride, ethylenediaminetetraacetic acid, sodium dodecyl sulfate, and proteinase K. Next, RNA was extracted using a mixture of phenol and chloroform followed by precipitation using isopropanol, glycogen (10901393001, Darmstadt, Merck), and sodium acetate. The RNA was resolved in nuclease-free water and purified using a DNA removal kit (AM1906, Thermo Fisher Scientific, Waltham, MA). Complementary DNA was synthesized using reverse transcriptase-PCR with M-MLV (28025013, Thermo Fisher Scientific), dNTP Mix (110002, BIORON GmbH, Römerberg, Germany), Random primer (48190-011, Thermo Fisher Scientific), and RNase OUT (10777019, Thermo Fisher Scientific). The complementary DNA was mixed with Taqman Universal Master Mix (4318157, Thermo Fisher Scientific) containing specific primers and probes for each gene. The sequences of primers and probes are shown in Supplementary Table 1. Real-time PCR was performed using the ABI Prism 7900HT Sequence Detection System (Thermo Fisher Scientific), and mRNA expression was quantified using the following formula; 2−(ΔCt sample−ΔCt calibrator), in which ΔCt values of the calibrator and sample were determined by subtracting the cycle threshold (Ct) value of the house-keeping gene (β-actin) from the Ct value of the target gene. Commercial MVP Total RNA Human liver and lung controls were used as calibrators (540017 and 540019, Agilent Technologies, Santa Clara, CA). In the quantitative process, the SD of the Ct values was kept less than 0.30 in two or three independent analyses. The heatmap was made using SHINYHEATMAP.COM (http://shinyheatmap.com/) after the quantified mRNA was converted to logarithm and standardized using the following formula: [log(mRNA) – average value]/SD.
DNA was extracted from five slices of 5 μm FFPE tissues using the QIAamp DNA FFPE Tissue Kit (51304, Qiagen GmbH, Hilden, Germany) in accordance with the manufacturer’s protocol. The EGFR mutation status (G719X in exon 18, deletions in exon 19, and L858R/L861Q in exon 21) was detected by a peptide nucleic acid-locked nucleic acid PCR clamp-based detection test using a 7900HT Fast Real-Time PCR System (Thermo Fisher Scientific Inc.) as previously described.1,2
Cell Culture Experiments
Cell Lines and Reagents
Four EGFR-mutant lung cancer cell lines were used. The human LUAD PC9 cells, harboring EGFR exon 19 deletion (E746-A750) were provided by F. Hoffmann-La Roche Ltd. with the authorization of Dr. Mayumi Ono (Kyushu University, Fukuoka, Japan) H1975 cells, harboring both sensitizing L858R and resistant T790M mutation, were purchased by the American Type Culture Collection. HCC827 cells, harboring EGFR exon 19 deletion (E746-A750), and H1666 EGFR wild-type cells were purchased by American Type Culture Collection. Cells were cultured with Roswell Park Memorial Institute medium supplemented with 10% fetal bovine serum and 50 μg/mL penicillin-streptomycin. Cells were maintained in a humidified atmosphere with 5% carbon dioxide at 37°C. Erlotinib and the SHP2 inhibitor SHP099 were obtained from Selleckchem, Houston, Texas, whereas the SHP2 inhibitor RMC-4550 was obtained from CHEMIETEK, Indianapolis, Indiana. Erlotinib was used at concentrations from 0.5 μM to 2.5 μM, corresponding to the plasma concentrations of less than 50 mg/day and around 150 mg/day in clinical settings, respectively.5 Each reagent was used in a single-reagent treatment or in combination in the three cell lines. In the combination treatment, the synergistic effect of erlotinib plus SHP099 or RMC-4550 was evaluated. Details regarding the methods of the preclinical experiments, such as, cell viability assay, colony formation assay, Western blotting analysis, and immunofluorescence experiments are provided in the Methods section of the Supplementary Appendix.
Statistical Analysis
To estimate the prognostic impact of the biomarkers explored in this study, the mRNA expression levels between recurrent and nonrecurrent cases were compared considering the EGFR mutation status, and the recurrence-free survival (RFS) and OS. RFS was defined from the day of operation to the day when recurrence was detected radiologically or the death owing to any cause. OS was defined from the day of operation to the day of death from any cause. RFS and OS were calculated by the Kaplan-Meier method.
The significance of difference between RFS and OS curves was determined by the log-rank test unless survival curves cross the point of less than 80% of survival probability. The significance of frequencies for the data of the patients was compared using chi–square test or Yates-square test. Age was compared as continuous variables using Mann-Whitney U tests.
For the preclinical studies, the strength of interaction between reagents was determined by the combination index according to the method of the isobologram-combination index (Chou-Talalay method) as previously described.3 The expression levels of protein and mRNA were compared using the Wilcoxon t-test or Mann-Whitney U-test. The in vitro examinations were repeated at least three times. Two-sided statistics were employed and a p-value of less than 0.05 was regarded as significant. Statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY) and StatMate V (ATMS Co., Ltd., Tokyo, Japan).
Results
Effect of SHP2 Expression on the Outcome of Patients With Resected EGFR Mutation-Positive LUAD
Firstly, mRNA expression of the RTKs, AXL and MET, CDCP1, and the non-RTKs, YAP1, STAT3, and SHP2 were examined in EGFR mutation-positive and EGFR mutation-negative resected LUADs
The mRNA expression of the six biomarkers was evaluated in tumor samples from 267 surgically resected LUADs. The clinical characteristics of the patients are illustrated in Table 1. The median age of the patients was 69 years (range, 40–91) and 53.9% were male. Among them, 232 patients (86.9%) had pathologically confirmed N0-1 LUAD and 100 (37.7%) were EGFR mutation-positive LUADs. The median follow-up period was 45 months (range, 1.4–150 mo). Among the patients positive with EGFR mutation, 65 (65.0%) had pathologically verified stage I (IA1–IB), and half of them had a recurrence within a median period of 47 months (range, 5–150). As illustrated in Figure 1A and B, there was a trend for higher SHP2, MET, and CDCP1 mRNA expression for patients with tumor recurrence compared with those who did not have tumor recurrence. In contrast, the mRNA expression of YAP1, STAT3, and AXL was higher in patients without tumor recurrence.
Table 1.
Clinicopathologic Characteristics of Enrolled Patients With pN0-2 Adenocarcinoma (N = 267)
| Clinicopathologic Characteristic |
EGFR Status |
p Value | |
|---|---|---|---|
| Mutant (N = 100) | Wild Type (N = 167) | ||
| Positive EGFR mutation status, N (%) | |||
| G719X in exon 18 | 6 (6.0) | — | — |
| Deletion in exon19 | 38 (38.0) | — | — |
| Mutation in exon 21 | 52 (52.0) | — | — |
| L858R | 49 (49.0) | — | — |
| L861Q | 3 (3.0) | — | — |
| Double mutation | 4 (4.0) | — | — |
| Age, y | |||
| Median (interquartile range) | 70 (13.0) | 69 (14.0) | 0.635 |
| Range | 41–89 | 40–91 | — |
| Sex, N (%) | |||
| Male | 35 (35.0) | 109 (65.3) | <0.001 |
| Female | 65 (65.0) | 58 (34.7) | — |
| Smoking status, N (%) | |||
| Ex- or current | 37 (37.0) | 114 (68.3) | <0.001 |
| Never | 63 (63.0) | 52 (31.1) | <0.001 |
| Unknown | 0 (0.0) | 1 (0.6) | 0.795 |
| Surgical procedure, N (%) | |||
| Pneumonectomy | 0 (0.0) | 1 (0.6) | 0.795 |
| Lobectomy | 74 (74.0) | 117 (70.1) | 0.490 |
| Segmentectomy | 23 (23.0) | 35 (21.0) | 0.695 |
| Wedge resection | 3 (3.0) | 14 (8.4) | 0.138 |
| Predominant subtype, N (%) | |||
| Lepidic | 24 (24.0) | 34 (20.4) | 0.485 |
| Papillary | 58 (58.0) | 70 (41.9) | 0.0109 |
| Acinar | 8 (8.0) | 31 (18.6) | 0.0288 |
| Solid | 5 (5.0) | 20 (12.0) | 0.0936 |
| Micropapillary | 5 (5.0) | 7 (4.2) | 0.997 |
| Invasive mucinous | 0 (0.0) | 5 (3.0) | 0.200 |
| Pleural invasion, N (%) | |||
| Negative | 72 (72.0) | 125 (74.9) | 0.608 |
| Positive | 28 (28.0) | 42 (25.1) | — |
| Lymphatic invasion, N (%) | |||
| Negative | 71 (71.0) | 133 (79.6) | 0.108 |
| Positive | 29 (29.0) | 34 (20.4) | — |
| Vascular invasion, N (%) | |||
| Negative | 73 (73.0) | 122 (73.1) | 0.992 |
| Positive | 27 (27.0) | 45 (26.9) | — |
| Intrapulmonary metastasis, N (%) | |||
| Negative | 96 (96.0) | 161 (96.4) | 0.870 |
| Positive | 4 (4.0) | 6 (3.6) | — |
| Pathologic N descriptor, N (%) | |||
| N0 | 71 (71.0) | 140 (83.8) | 0.0127 |
| N1 | 8 (8.0) | 13 (7.8) | 0.864 |
| N2 | 21 (21.0) | 14 (8.4) | 0.00311 |
| Pathologic stage, N (%) | |||
| IA1–IA3 | 45 (45.0) | 86 (51.5) | 0.304 |
| IB | 20 (20.0) | 42 (25.1) | 0.335 |
| IIA–IIB | 11 (11.0) | 21 (12.6) | 0.701 |
| IIIA–IIIB | 24 (24.0) | 18 (10.8) | 0.00408 |
| Recurrence, N (%) | |||
| No | 53 (53.0) | 125 (74.9) | <0.001 |
| Yes | 47 (47.0) | 42 (25.1) | — |
| Locoregional | 35 (35.0) | 34 (20.4) | 0.633 |
| Distant | 8 (8.0) | 7 (4.2) | 0.811 |
| Locoregional + distant | 4 (4.0) | 1 (0.6) | 0.365 |
Figure 1.
Comparison of mRNA expression of six RTKs and non-RTKs according to EGFR mutation, recurrent status, and prognostic impact of SHP2 expression. (A) Heatmap depicting mRNA expression of six RTKs and non-RTKs. The recurrent cases and nonrecurrent cases were gathered to upper and lower location, respectively. The expression patterns of SHP2, CDCP1, and MET are comparable. In contrast, YAP, STAT3, and AXL revealed similarity in expression patterns. (B) Comparison of mRNA level according to EGFR mutation and recurrent status. SHP2 expression is significantly higher in recurrent cases than recurrence-free cases with EGFR mutation. As the heatmap suggested, CDCP1 and MET reveal similarity with SHP2; CDCP1 and MET reveal a tendency for higher expression in recurrent cases than recurrence-free cases with EGFR mutation (the difference did not attain significance). YAP1, STAT3, and AXL are higher expressed in recurrence-free cases, regardless of EGFR mutation status. (C) Recurrence-free and OS curves according to EGFR mutation or SHP2 expression level. The cutoff level of mRNA expression was determined at the point where RFS was divided in the most efficient manner around the median expression level (from third to fifth octile of mRNA expression level). Wild-type cases revealed higher RFS compared with EGFR-mutant cases. In EGFR-mutant cases, higher expression of SHP2 reveals a significantly worse prognosis than cases with lower SHP2 expression. The 5-year RFS in EGFR mutation-positive LUADs was 31.5% with high SHP2 expression, versus 50.6% for those with low SHP2 (hazard ratio, 1.83; 95% CI: 1.05–3.23; p = 0.0329). The 5-year OS in wild-type LUADs was 79.9% with no influence of SHP2 mRNA. In EGFR mutation-positive LUADs with low SHP2 mRNA, the 5-year OS was 86.8% versus 60.7% for those with high SHP2 mRNA (hazard ratio, 2.28; 95% CI: 1.03–4.58; p = 0.0414). CI, confidence interval; LUAD, lung adenocarcinoma; OS, overall survival; RFS, recurrence-free survival; RTK, receptor tyrosine kinase; SHP2, Src-homology 2 domain-containing phosphatase 2.
The second part of the study focused on resected EGFR mutation-positive LUADs. The 5-year RFS in wild-type LUADs was 68.6%, without differences according to SHP2 mRNA expression. However, for EGFR mutation-positive LUADs with low SHP2 mRNA, the 5-year RFS was 50.6% versus 31.5% in those with high SHP2 mRNA (hazard ratio, 1.83; 95% CI: 1.05–3.23; p = 0.0329) (Fig. 1C). The 5-year OS in wild-type LUADs was 79.9% with no influence of SHP2 mRNA. In EGFR mutation-positive LUADs with low SHP2 mRNA, the 5-year OS was 86.8% versus 60.7% for those with high SHP2 mRNA (hazard ratio, 2.28; 95% CI: 1.03–4.58; p = 0.0414) (Fig. 1C). No significant differences in RFS or OS of patients with EGFR mutation-positive LUAD were observed according to CDCP1 and MET mRNA expression.
Effect of EGFR and SHP2 Inhibition in EGFR-Mutant Cell Lines
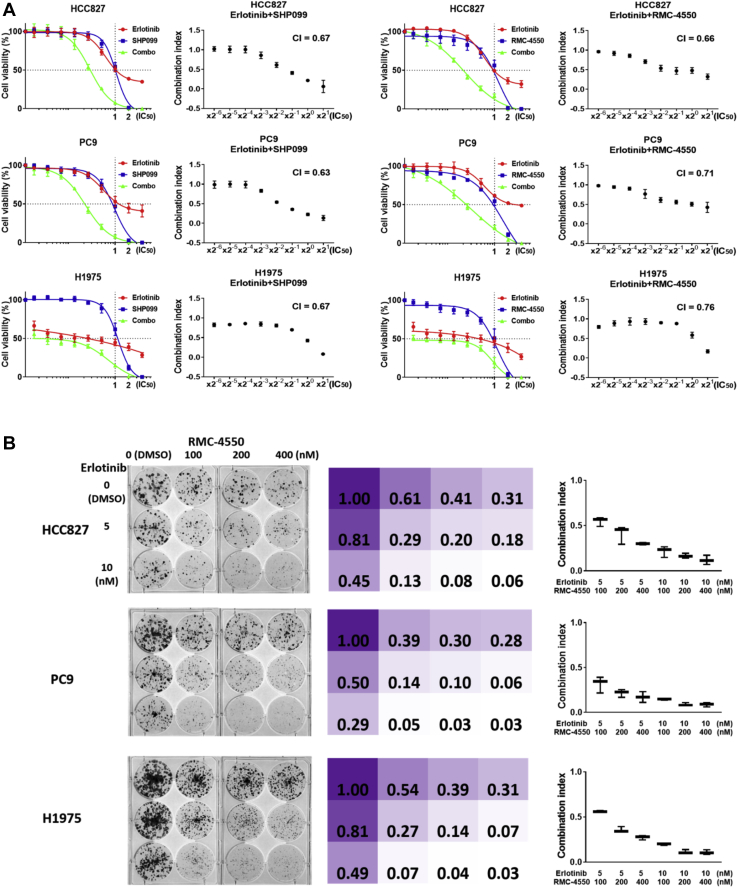
On the basis of our findings, we further explored the biological role of SHP2 in EGFR-mutant LUAD cell lines. In the PC9 EGFR-mutant cell line, SHP2 phosphorylation was increased upon erlotinib therapy, compared with the baseline levels, as illustrated by Western blotting in Figure 2A. The increased SHP2 phosphorylation upon erlotinib therapy in PC9 cells was confirmed with different doses of erlotinib (0.5–2.5 μM) for 24, 48, and 72 hours (Fig. 2B). When erlotinib was combined with the SHP2 inhibitor, RMC-4550, the phosphorylation of SHP2 (Y542), as well as, EGFR (Y1068) and downstream components, such as, AKT (S473) and ERK1/2 (T202/Y204), were significantly suppressed, and the suppression was more profound with the combination, compared with each of the inhibitors alone (Fig. 2A). In three EGFR-mutant cell lines (PC9, HCC827, and H1975) the combination of erlotinib with SHP2 inhibitors (RMC-4550 and SHP099) was synergistic, as revealed in a 3-day monolayer culture and in a long-term 10-day colony formation assay (Fig. 3A and B and Supplementary Table 1 in Supplementary Appendix).
Figure 2.
SHP2 inhibitor RMC-4550 decreases activation of protein involved in erlotinib resistance. (A) Erlotinib or RMC-4550 monotherapy or combination of these two compounds was tested in PC9 cell line for 18 hours and molecular effects were analyzed by Western blot technique. The expression level is shown as a fold change compared with control (= 1.0). Actin was used as a housekeeping protein. The experiment was performed at least three times. (B) Western blotting reveals the expression level of pSHP2 (Y542) after erlotinib treatment according to concentration (0.5 μM, 1.0 μM, and 2.5 μM) and treatment time (24, 48, and 72 hours) in PC9 cell lines. The expression level of pSHP2 (Y542) significantly increases through the reduced dose (0.5 μM, 1.0 μM) to clinically normal dose (2.5 μM), and assay period employed in this study (24–72 h). The quantification of phosphorylation level is reported as a fold change compared with control (= 1.0). Actin was used as a housekeeping protein. The experiment was performed at least three times. pAKT, phosphorylated AKT; pEGFR, phosphorylated EGFR; pERK, phosphorylated ERK; pSHP2, phosphorylated SHP2; SHP2, Src-homology 2 domain-containing phosphatase 2.
Figure 3.
Combination of erlotinib and SHP2 inhibitor potentiate cell viability and colony formation inhibition. (A) The combination treatment using erlotinib plus SHP2 inhibitor (SHP099 or RMC-4550) was assayed. Cells were exposed to increasing concentrations, on the basis of IC50 concentrations of erlotinib, SHP90 inhibitor (SHP099 or RMC-4550) or combination of erlotinib and SHP2 inhibitor, in the three EGFR-mutant cell lines, HCC827, PC9, and H1975 for 72 hours and cell viability was analyzed by MTT assay. The combination treatment indicates synergism in the three EGFR-mutant cell lines. The colored graph represents cell viability according to drug concentration (from 2−6 to 22 times of IC50 of each inhibitor). The dashed lines in x axis and y axis represent the IC50 concentration and the point of 50% cell viability, respectively. Black-white graphs reveal CIns at each drug concentration (from 2−6 to 21 times of IC50 of each inhibitor). Total CIn (calculated as the average of each CIn) ranged 0.63 to 0.76. The experiment was performed at least three times. (B) The three cell lines, HCC827, PC9, and H1975, were exposed to increasing doses of RMC-4550 in combination with increasing doses of erlotinib for x days. The colonies were then fixed and stained with crystal violet and a representative colony formation assay is showed. Colored chart representing the ratio of crystal violet concentration (control is fixed as 1.00). The graphs display CIns at each drug concentration. Erlotinib plus RMC-4550 combination revealed synergism in all three cell lines. Each CIn ranged synergism level: 0.559 to 0.088. The experiment was performed at least three times and a representative colony formation assay is reported. CI, confidence interval; CIn, combination index; IC50, concentration that inhibits 50%; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; SHP2, Src-homology 2 domain-containing phosphatase 2.
It was further examined whether the synergistic effect of erlotinib with SHP2 inhibitors is related to the increased SHP2 phosphorylation observed with erlotinib monotherapy. Intriguingly, in the PC9 cell line, the sensitivity to RMC-4550 decreased after exposure to erlotinib (Supplementary Fig. 1), suggesting that the erlotinib-induced increment of SHP2 phosphorylation does not augment the effectiveness of the SHP2 inhibitor in the EGFR-mutant PC9 cell line. The protein tyrosine phosphatase SHP2 is required for complete ERK activation downstream of EGFR and other RTKs. SHP2 is distributed to both the cytoplasm and nucleus. The localization of SHP2 in the nucleus potentially promotes tumor invasion, migration and metastases.25 The cellular localization of pSHP2 in the PC9 cell line, and upon treatment with erlotinib, RMC-4550 (SHP2 inhibitor), and the combination, were, then, analyzed by immunofluorescence analyses. As illustrated in Figure 4 and Supplementary Figure 2, pSHP2 was in the nucleus and cytoplasm of the PC9 cell line. Upon erlotinib treatment, pSHP2 translocated partially to the nucleus, whereas upon RMC-4550 treatment, it was assembled on the cell membrane. Erlotinib combined with RMC-4550 prevented the nuclear import of pSHP2 (Fig. 4). Intensive staining in the nucleus and membrane was confirmed as a spike of pixel intensity after erlotinib or RMC-4550 treatment, respectively (Supplementary Fig. 2). The SHP2 phosphorylation in the wild-type cell line, H1666, was widely diffused in the nuclei (Supplementary Fig. 3A). In patients with EGFR wild type, no differences in RFS and OS were seen according to SHP2 levels (Supplementary Fig. 3B).
Figure 4.
Immunostaining experiments estimating pSHP2 (Y542) expression after monotherapy or combination therapy. Representative immunofluorescence images of location of pSHP2 in PC-9 cells after erlotinib or RMC-4550 treatment. Fixed cells were stained using DAPI (blue) for nuclear counterstaining, phalloidin (red) for actin, and pSHP2 (Y542) (green) antibody. The scale bar corresponds to 50 μm. The shape of PC9 cells changes from spindle to round after erlotinib or RMC-45500 treatment. DAPI, 4′,6-diamidino-2-phenylindole; pSHP2, phosphorylated SHP2; SHP2, Src-homology 2 domain-containing phosphatase 2.
Phosphorylated EGFR (pEGFR) on the tyrosine residue 1068 was expressed in the nucleus and cytoplasm in the PC9 cell line. Upon RMC-4550 treatment, pEGFR remained in the perinuclear region, whereas upon erlotinib monotherapy and combination with RMC-4550, the pEGFR expression was reduced (Supplementary Fig. 4).
Discussion
Patients from Hiroshima, Japan and Terrassa, Spain, mostly early-stage LUADs, were examined, including 100 EGFR mutation-positive LUADs. The frequency of EGFR mutations is geographically distinct by regions, with a high frequency in Asians, in comparison with Caucasians.26 The recent Nederlands-Leuvens Longkanker Screenings Onderzoek study has revealed a reduction in lung cancer mortality with volume-based low-dose computed tomography screening among former and current male smokers. Screening-detected lung cancers are more often stage IA or IB (58.6%).27 Most patients included in our study, with or without harboring EGFR mutations, were stage IA (IA1, IA2, and IA3) or IB (T2a > 3–4 cm).28 SHP2 surfaced as the only significant marker of RFS and OS, but exclusively in EGFR mutation-positive NSCLC. This finding was partly expected. Activation of SHP2 by phosphorylation at tyrosine 542 is required for ERK activation in response to growth factors. It was previously reported that SHP2 Y542 phosphorylation was induced in H1666 cells in response to EGF by 5 minutes, but its phosphorylation was not induced in H3255 cells with EGFR mutations at any time point. The results shed light on the seminal finding that SHP2 function is impaired in NSCLC cells expressing mutant versus wild-type EGFR.25 In EGFR-mutant cells, SHP2 is sequestered at the plasma membrane, impeding the ability of SHP2 to promote ERK activity. Weak ERK activity can favor initial response to EGFR TKIs. In this study, fluorescently labeled SHP2 was translocated to the nucleus and only faintly visible in the cell membrane after erlotinib treatment (Fig. 4). However, after RMC-4550 treatment (SHP2 inhibitor), SHP2 was repositioned in the cell membrane (Fig. 4). Furthermore, the combination of erlotinib and RMC-4550 prevented nuclear accumulation of pSHP2 (Fig. 4). It was observed that the combination of erlotinib and RMC-4550 reduced the nuclear expression of pEGFR in PC9 cells (Supplementary Fig. 3). In this study, we were unable to reveal that YAP1 mRNA levels predicted RFS and OS. In a previous study, we reported that either gefitinib or osimertinib (first- and third-generation EGFR TKIs, respectively) induced YAP phosphorylation and elevated YAP mRNA levels were associated with shorter progression-free survival and OS in patients with EGFR-mutant NSCLC treated with EGFR TKIs.16 In this study, the YAP mRNA levels were not related to prediction of RFS or OS, neither in EGFR mutant LUADS nor wild-type LUADs. This apparent discrepancy can be explained by the fact that resected EGFR mutation-positive LUADs did not receive neoadjuvant therapy and, therefore, neither SHP2, YAP, nor other transcription factors, could be activated. The combination of erlotinib and RMC-4550 was highly synergistic in three EGFR-mutant cell lines (Fig. 2). In other studies, SHP2 depletion also leads to substantial inhibition of colony formation in RTK-dependent cancer cells.29, 30, 31 Moreover, SHP2 inhibition prevents resistance to MEK inhibitors in several tumors.32 This study reveals that SHP2 plays a crucial role in EGFR-mutant cells and erlotinib causes mislocalization of SHP2 in the nucleus, potentially promoting tumor invasion, migration, and metastases. Therefore, studies in EGFR mutation-positive LUADs with the combination of EGFR TKI and SHP2 inhibitors should be promoted. Moreover, this study was able to include patients with stage IA, which reinforces the need to determine SHP2 expression in this patient subgroup for further selection of optimal adjuvant combinatory therapy where SHP2 inhibitors could be an essential part of the treatment.33 Nevertheless, our study has some limitations. Firstly, although most patients were stage I, a few patients with stage III were also included, and no homogenous adjuvant therapy was administered. The Japanese patients received adjuvant oral uracil-tegafur, but there is no reliable data on the adherence to adjuvant treatment. Notwithstanding, the study highlights the function of SHP2 in EGFR mutation-positive LUAD patients, and the data in EGFR-mutant cells advocates adjuvant treatment with TKIs plus SHP2 inhibitors. In addition, we recently reviewed 1155 stage IAI to IIIA resected LUADs and confirmed that RFS was significantly lower in the subgroup of EGFR mutation-positive cases (excluding minimally invasive carcinomas, adenocarcinoma in situ, and lepidic variants), in comparison with EGFR wild-type LUADs.34 “We validated the synergism in MTT by colony forming assay. However, the range of reagent concentrations was not wide and high synergism might be partly due to repeated treatments every 3 days in colony forming assay. Further validation including the toxicity should be performed in other cell lines, with a wider range of concentration, or in vivo.” In conclusion, SHP2 is an important mechanism of recurrence in EGFR mutation-positive NSCLC and the combination with SHP2 inhibitors can improve adjuvant therapy with EGFR TKIs. Further studies can also define whether SHP2 can prevent metastasis by abrogating myeloid-derived suppressor cells.35 Lewis lung cancer mice treated with adjuvant epigenetic therapy have longer survival than those treated with chemotherapy.36
Acknowledgments
This study was conducted in part at the Analysis Center of Life Science, Natural Science Center for Basic Research and Development, Hiroshima University, Hiroshima, Japan, and supported by a La Caixa Foundation grant and the Spanish Association Against Cancer (PROYE18012ROSE).
Footnotes
Disclosure: The authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100084.
Supplementary Data
References
- 1.Kratz J.R., He J., Van Den Eeden S.K. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet. 2012;379:823–832. doi: 10.1016/S0140-6736(11)61941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamal-Hanjani M., Wilson G.A., McGranahan N. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 3.Abbosh C., Birkbak N.J., Wilson G.A. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution [published correction appears in Nature. 2017;:] Nature. 2017;545:446–451. doi: 10.1038/nature22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakelee H.A., Dahlberg S.E., Keller S.M. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2017;18:1610–1623. doi: 10.1016/S1470-2045(17)30691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kris M.G., Gaspar L.E., Chaft J.E. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol. 2017;35:2960–2974. doi: 10.1200/JCO.2017.72.4401. [DOI] [PubMed] [Google Scholar]
- 6.NSCLC Meta-analyses Collaborative Group. Arriagada R., Auperin A. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato H., Ichinose Y., Ohta M. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350:1713–1721. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 8.Goss G.D., O’Callaghan C., Lorimer I. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31:3320–3326. doi: 10.1200/JCO.2013.51.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly K., Altorki N.K., Eberhardt W.E. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol. 2015;33:4007–4014. doi: 10.1200/JCO.2015.61.8918. [DOI] [PubMed] [Google Scholar]
- 10.Zhong W.Z., Wang Q., Mao W.M. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19:139–148. doi: 10.1016/S1470-2045(17)30729-5. [DOI] [PubMed] [Google Scholar]
- 11.Sandler J.E., D’Aiello A., Halmos B. Changes in store for early-stage non-small cell lung cancer. J Thorac Dis. 2019;11:2117–2125. doi: 10.21037/jtd.2019.05.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arasada R.R., Amann J.M., Rahman M.A., Huppert S.S., Carbone D.P. EGFR blockade enriches for lung cancer stem-like cells through Notch3-dependent signaling. Cancer Res. 2014;74:5572–5584. doi: 10.1158/0008-5472.CAN-13-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Codony-Servat C., Codony-Servat J., Karachaliou N. Activation of signal transducer and activator of transcription 3 (STAT3) signaling in EGFR mutant non-small-cell lung cancer (NSCLC) Oncotarget. 2017;8:47305–47316. doi: 10.18632/oncotarget.17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsen K., Bertran-Alamillo J., Molina M.A. Convergent Akt activation drives acquired EGFR inhibitor resistance in lung cancer. Nat Commun. 2017;8:410. doi: 10.1038/s41467-017-00450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichihara E., Westover D., Meador C.B. SFK/FAK signaling attenuates osimertinib efficacy in both drug-sensitive and drug-resistant models of EGFR-mutant lung cancer. Cancer Res. 2017;77:2990–3000. doi: 10.1158/0008-5472.CAN-16-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaib I., Karachaliou N., Pilotto S. Co-activation of STAT3 and YES-associated protein 1 (YAP1) pathway in EGFR-mutant NSCLC. J Natl Cancer Inst. 2017;109:djx014. doi: 10.1093/jnci/djx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karachaliou N., Chaib I., Cardona A.F. Common co-activation of AXL and CDCP1 in EGFR-mutation-positive non-smallcell lung cancer associated with poor prognosis. EBioMedicine. 2018;29:112–127. doi: 10.1016/j.ebiom.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prahallad A., Heynen G.J., Germano G. PTPN11 is a central node in intrinsic and acquired resistance to targeted cancer drugs. Cell Rep. 2015;12:1978–1985. doi: 10.1016/j.celrep.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Sausgruber N., Coissieux M.M., Britschgi A. Tyrosine phosphatase SHP2 increases cell motility in triple-negative breast cancer through the activation of SRC-family kinases. Oncogene. 2015;34:2272–2278. doi: 10.1038/onc.2014.170. [DOI] [PubMed] [Google Scholar]
- 20.Karachaliou N., Cardona A.F., Bracht J.W.P. Integrin-linked kinase (ILK) and src homology 2 domain-containing phosphatase 2 (SHP2): novel targets in EGFR-mutation positive non-small cell lung cancer (NSCLC) EBioMedicine. 2019;39:207–214. doi: 10.1016/j.ebiom.2018.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bracht J.W.P., Karachaliou N., Bivona T. BRAF mutations classes I, II, and III in NSCLC patients included in the SLLIP trial: the need for a new pre-clinical treatment rationale. Cancers (Basel) 2019;11:1381. doi: 10.3390/cancers11091381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazzara M.J., Lane K., Chan R. Impaired SHP2-mediated extracellular signal-regulated kinase activation contributes to gefitinib sensitivity of lung cancer cells with epidermal growth factor receptor-activating mutations. Cancer Res. 2010;70:3843–3850. doi: 10.1158/0008-5472.CAN-09-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Travis W.D., Brambilla E., Noguchi M. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstraw P., Chansky K., Crowley J. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Furcht C.M., Muñoz Rojas A.R., Nihalani D., Lazzara M.J. Diminished functional role and altered localization of SHP2 in non-small cell lung cancer cells with EGFR-activating mutations. Oncogene. 2013;32:2346–2355. doi: 10.1038/onc.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitsudomi T. Molecular epidemiology of lung cancer and geographic variations with special reference to EGFR mutations. Transl Lung Cancer Res. 2014;3:205–211. doi: 10.3978/j.issn.2218-6751.2014.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Koning H.J., van der Aalst C.M., de Jong P.A. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 28.Rami-Porta R., Asamura H., Travis W.D., Rusch V.W. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:138–155. doi: 10.3322/caac.21390. [DOI] [PubMed] [Google Scholar]
- 29.Dardaei L., Wang H.Q., Singh M. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med. 2018;24:512–517. doi: 10.1038/nm.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mainardi S., Mulero-Sánchez A., Prahallad A. SHP2 is required for growth of KRAS-mutant non-small-cell lung cancer in vivo. Nat Med. 2018;24:961–967. doi: 10.1038/s41591-018-0023-9. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y.N., LaMarche M.J., Chan H.M. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535:148–152. doi: 10.1038/nature18621. [DOI] [PubMed] [Google Scholar]
- 32.Fedele C., Ran H., Diskin B. SHP2 inhibition prevents adaptive resistance to MEK inhibitors in multiple cancer models. Cancer Discov. 2018;8:1237–1249. doi: 10.1158/2159-8290.CD-18-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q., Qu J., Zhao M., Xu Q., Sun Y. Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharmacol Res. 2019;152:104595. doi: 10.1016/j.phrs.2019.104595. [DOI] [PubMed] [Google Scholar]
- 34.Ito M., Miyata Y., Tsutani Y. Positive EGFR mutation status is a risk of recurrence in pN0-1 lung adenocarcinoma when combined with pathological stage and histological subtype: a retrospective multi-center analysis. Lung Cancer. 2020;141:107–113. doi: 10.1016/j.lungcan.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Marasco M., Berteotti A., Weyershaeuser J. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci Adv. 2020;6 doi: 10.1126/sciadv.aay4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Z., Zou J., Li S. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature. 2020;579:284–290. doi: 10.1038/s41586-020-2054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.