Abstract
Background
Immune checkpoint inhibitors (ICIs) have become an increasingly important tool in cancer treatment, revealing durable responses in several different types of tumors, including NSCLCs. Nevertheless, ICIs carry a risk of immune-mediated toxicities. There is a paucity of data for concurrent use of these agents in patients with autoimmune disorders, such as multiple sclerosis (MS).
Case Presentation
We report a case of a man with a history of MS and metastatic NSCLC with brain metastases who had cancer progression after receiving chemotherapy, whole-brain radiation therapy, and stereotactic radiosurgery to brain lesions and was treated with the programmed death-ligand 1 inhibitor, atezolizumab. He had dramatic clinical and radiographic benefit but developed a severe MS flare and neurologic decline precluding further treatment. Considerable growth of a previously radiated brain lesion prompted resection, with pathologic findings consistent with radiation necrosis and demyelination without viable tumor cells.
Conclusions
Although patients with preexisting autoimmune diseases, including MS, might be at an increased risk of developing immune-related adverse events with ICIs, they may also experience anticancer benefit. Intracranial disease can be challenging to accurately diagnose in a patient with MS who previously underwent radiation, as progressing lesions can be tumor growth, MS flare, or radiation necrosis.
Keywords: Brain metastases, Case report, Immune checkpoint inhibitor, Non–small cell lung cancer, Multiple sclerosis
Introduction
Immune checkpoint inhibitor (ICI) use in patients with NSCLC and preexisting autoimmune diseases, such as multiple sclerosis (MS), remains an area of clinical uncertainty owing to the risk of immune-related adverse events. Here, we describe the potential benefits and challenges in managing a patient with a history of relapsing-remitting MS and NSCLC metastatic to the brain. We provide a framework for determining the cause of various intracranial lesions.
Case Presentation
A 45-year-old man with a history of optic neuritis (diagnosed in 2001) and well-controlled relapsing-remitting MS on fingolimod since 2005 presented with several months of back and hip pain in 2016. Pelvic magnetic resonance imaging (MRI) revealed a destructive lesion in the right iliac bone with extension into the surrounding soft tissue. Positron emission tomography-computed tomography revealed hypermetabolism in a 1.9 cm mass in the minor fissure of the right lung, right cervical lymph node, and the right iliac lesion. Brain MRI revealed five new, enhancing parenchymal lesions with surrounding edema in the right temporal, bilateral occipital, and bilateral cerebellar lobes consistent with metastases. Extensive abnormal fluid-attenuated inversion recovery white matter hyperintensities were redemonstrated, consistent with his history of MS. Biopsy of the iliac lesion was consistent with metastatic, high-grade large cell neuroendocrine carcinoma of the lung. Molecular testing result was negative for targetable mutations.
The patient underwent whole-brain radiation therapy (WBRT) followed by three cycles of cisplatin and etoposide. Restaging imaging 3 months after initiating treatment revealed disease progression in the right hip, for which he was treated with palliative radiation therapy. A repeat brain MRI revealed multiple new cerebral and cerebellar enhancing lesions. He was transferred to our institution in early 2017 and underwent stereotactic radiosurgery (SRS) to 16 intracranial lesions.
In the approximately 6 months since his diagnosis, the patient experienced worsening fatigue, anorexia resulting in a 50-pound weight loss, and severe hip pain. He was seen in our office for consultation 7 months after the initial diagnosis. After a detailed discussion regarding the risks of ICIs and in consultation with the patient's neurologist, he received atezolizumab 1200 mg.
In the subsequent 3 weeks, the patient developed blurred vision, generalized weakness, and confusion. Brain MRI results revealed numerous new enhancing lesions within the cerebrum, cerebellum, and brainstem. Bilateral enhancements of the optic sheath complexes were also present. Cerebrospinal fluid analysis was notable for oligoclonal bands without malignant cells. Cerebrospinal fluid antibodies for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, amphiphysin, antiglial nuclear, CRMP-5 immunoglobulin G, gamma aminobutyric acid–B-receptor, GAD65, neuronal nuclear antibodies (type 1, 2, and 3), N-methyl-D-aspartate-receptor, Purkinje cell cytoplasmic antibodies (type 1, 2, and Tr), and VGKC complex were negative. He was treated for an MS flare with high-dose glucocorticoids tapered in 3 weeks with symptom resolution. Further atezolizumab was withheld owing to the patient’s neurologic deterioration.
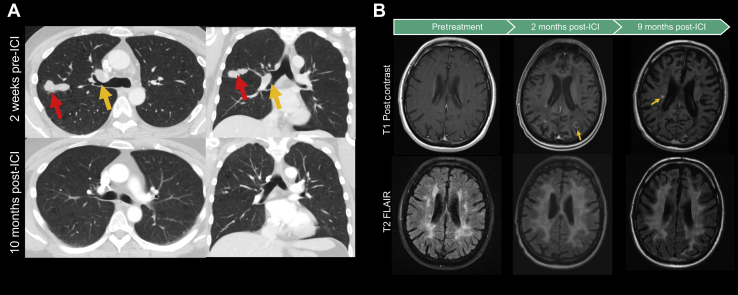
In the next 6 months, the patient had a dramatic clinical recovery from his cancer, including a 40-pound weight gain and substantial improvement in his performance status. Repeat imaging result revealed improved disease burden, including regression or resolution of multiple lung nodules (Fig. 1A) and stable pelvic metastases. Brain MRI result revealed new enhancing lesions in the right centrum semiovale and right temporal lobes; however, given the flame-shaped appearance, periventricular location (Fig. 1B), and his overall clinical improvement, the lesions were interpreted as demyelinating lesions. Imaging also revealed accelerated diffuse brain atrophy and parenchymal loss (Fig. 1B).
Figure 1.
(A) Representative axial and coronal CT images of the primary lung tumor 2 weeks before (top) and 10 months after (bottom) ICI therapy. The red arrow in the left image indicates a 4.0 × 2.3 × 1.5 cm lobulated and spiculated mass within the right upper lobe of the lung, which completely resolved after ICI therapy. The yellow arrow indicates a perihilar mass that similarly improved. (B) Representative MR images of the brain before any therapy (left), 2 months after atezolizumab (middle), and 9 months after atezolizumab (right). T1 postgadolinium contrast images reveal the development of enhancing lesions after 2 months on ICI therapy. An incomplete, ring-enhancing lesion (yellow arrow in middle panel) and periventricular lesion (yellow arrow in right panel) are suggestive of actively demyelinating lesions. T2-FLAIR sequence images reveal progressive white matter changes throughout the patient’s course. Accelerated brain atrophy is also noted. CT, computed tomography; FLAIR, fluid-attenuated inversion recovery; ICI, immune checkpoint inhibitor; MR, magnetic resonance.
After 6 months of ICI, the patient developed progressive left lower extremity weakness. Imaging result revealed multiple new enhancing lesions in the bilateral cerebral and cerebellar hemispheres without extracranial disease progression. The patient was treated with high-dose corticosteroids and rituximab 1000 mg with symptom improvement.
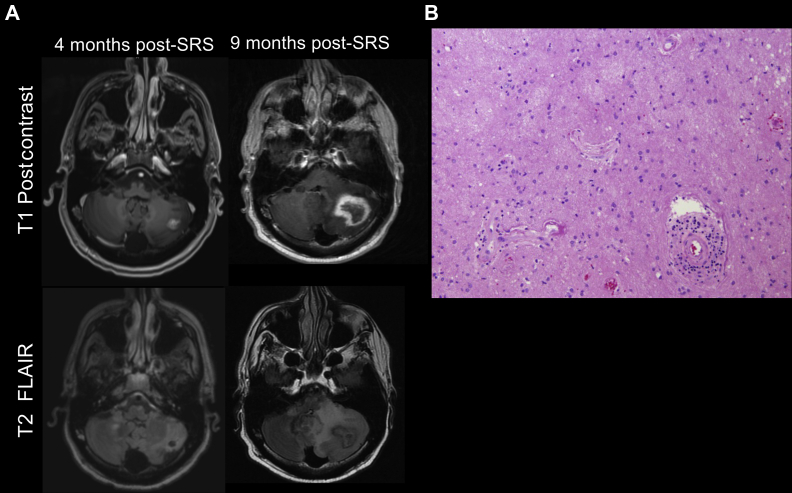
The patient presented on the following month with worsening ataxia and confusion. MRI result of the brain revealed several enhancing lesions, including an enlarging left cerebellar 3.5 cm mass at the site of a lesion previously treated with SRS (Fig. 2A). The patient received dexamethasone and the cerebellar mass was resected given the risk of herniation. Pathologic findings were consistent with radiation necrosis and demyelination, with no viable tumor cells (Fig. 2B).
Figure 2.
Radiographic and histologic images revealing radiation necrosis. (A) Representative axial MR images 4 months (left) and 9 months (right) after SRS to a left cerebellar lesion in the T1 postgadolinium contrast (top row) and T2-FLAIR (bottom row) sequences. On the left, a 1.5 cm irregularly enhancing lesion is noted in the left cerebellar hemisphere. On the right, approximately 5 months after, the same lesion has increased to 3.5 cm and is peripherally enhancing, centrally necrotic with irregular margins, as may be found with radiation necrosis. (B) H&E staining of the resected left cerebellar lesion revealing reactive gliosis, the presence of macrophages, and hyalinized vessel walls with perivascular inflammation. Findings represent radiation necrosis without identifiable tumor. FLAIR, fluid-attenuated inversion recovery; H&E, hematoxylin and eosin; MR, magnetic resonance; SRS, stereotactic radiosurgery.
In the subsequent months, the patient’s motor coordination improved, though he developed progressive cognitive decline. He ultimately expired 10 months after receiving the ICI.
Discussion
ICIs have not been extensively studied in patients with a history of MS. A previous review included 2 patients with NSCLC and preexisting MS treated with anti-programmed cell death protein-1/programmed death-ligand 1 inhibitors, neither of whom developed MS flares while on therapy.1 Four patients with melanoma and MS described in the literature received anti–CTLA-4 monotherapy for advanced melanoma.2 Only 1 patient developed an MS flare while on ICI therapy, which was treated with corticosteroids and the resumption of glatiramer acetate. The patient ultimately achieved a complete response. Our patient’s MS flare after ICI therapy was more severe and rapid than previously reported, occurring soon after a single dose of therapy.
Our patient had several risk factors for developing neurologic adverse events, including an underlying demyelinating disorder, brain metastases, and previous intracranial radiation therapy. It is important to note that several of these characteristics are shared by many patients with metastatic NSCLC. Although the combination of ICIs and intracranial radiation therapy does not increase the overall risk of adverse events in patients with NSCLC with brain metastases, an increased risk of radiation necrosis has been reported with SRS and ICI therapy.3 Furthermore, patients with MS are thought to be more sensitive to radiation-induced neurologic injury.4 Though radiation-induced demyelination is more often described in patients who have received higher doses of radiation or have more generalized radiation fields, such as WBRT, cases associated with SRS have also been described.4 Our patient had received both WBRT and SRS, and the severity of his neurologic symptoms was likely due to the previous multimodal therapy in addition to his underlying autoimmune process.
An important element complicating our patient’s course was differentiating metastatic disease progression, ICI-associated pseudoprogression, demyelinating lesions, and radiation necrosis. These lesions may seem radiographically identical, and identifying the correct underlying disease process has important treatment implications. Clinical features that may help narrow the diagnosis include the timing of presentation and radiographic subtleties (Table 1). Demyelinating lesions are typically linear or ovoid in shape and have incomplete ring enhancement, unlike metastases which are more likely to be round in shape with diffuse or complete ring enhancement.5 In our clinical experience, lesions with incomplete peripheral enhancement favor demyelinating lesions (Fig. 1B). In addition, we have observed variable rates of growth among radiation necrosis lesions, as was found in our patient. The mechanisms regulating differential growth remain unclear at this time.
Table 1.
Clinical and Radiographic Characteristics of Enhancing CNS Lesions
| Lesion Type | Common Clinical Features | Common Radiographic Features |
|---|---|---|
| Tumor progression | Nodular growth on sequential imaging ≥3 mo apart Progression in systemic disease |
Rounded with either homogeneous or ring enhancement and clear delineation of lesion edge Perilesional edema Higher rCBV on perfusion imaging Higher Cho/Cr and Cho/NAA on MRS FDG PET of limited value Potential role for amino acid PET |
| ICI-related pseudoprogression | Growth within weeks of ICI initiation Regression on sequential imaging separated by ≤3 mo Clinical stability |
Can be indistinguishable from tumor progression |
| Radiation necrosis | More often found ≥6 mo after radiation therapy Increased incidence after ICI therapy May spontaneously regress on longitudinal imaging even if presenting with symptoms |
Rim-enhancing pseudopodic lesion with central necrosis on T1-postcontrast Perilesional edema of previous SRS target on FLAIR Lower rCBV on PWI Lower Cho/Cr and Cho/NAA on MRS |
| Demyelinating lesion | History of autoimmune disease History of high-dose or high-brain volume radiation therapy Little to no growth on longitudinal imaging |
T2 hyperintense, T1 hypointense Little to no surrounding edema Juxtacortical, periventricular Homogenous enhancements; however, heterogeneous, nodular, ring-like (typically open ring), or tumefactive patterns may be found |
Cho, choline; CNS, central nervous system; Cr, creatine; FDG, 18F-2-fluoro-2-deoxy-D-glucose; FLAIR, fluid-attenuated inversion recovery; ICI, immune checkpoint inhibitor; MR, magnetic resonance; MRS, magnetic resonance spectroscopy; NAA, N-acetyl aspartate; PET, positron emission tomography; PWI, perfusion-weighted imaging; rCBV, relative cerebral blood volume; SRS, stereotactic radiosurgery.
Some reports suggest that advanced imaging techniques, such as MR spectroscopy and amino acid-labeled positron emission tomography, may be helpful in determining whether lesions are metastatic, demyelinating, or inflammatory in nature, though such modalities have limitations and are neither often used nor widely available. Numerous challenges remain, particularly given the limited body of evidence for advanced imaging techniques.
Our patient’s cognitive decline ultimately led to de-escalation of his medical care. Brain atrophy was noted (Fig. 1B), which may be found with both radiation therapy and advanced MS in the course of several years. The accelerated course may have resulted from a combination of radiation and demyelinating disease.
Conclusion
Although our patient experienced neurologic complications, the remarkable cancer response to a single dose of ICI is noteworthy. We estimate that without treatment, his survival might have been on the order of several weeks to a few months given his rapid decline before initiating therapy. During this time, he had several months of functional and nutritional recovery. Given the clinical benefits observed, we believe that ICIs should be considered a viable treatment option for many patients with metastatic NSCLC and preexisting autoimmune diseases, including MS. The difficulty in radiographically discerning metastatic from demyelinating lesions or radiation necrosis imposes considerable challenges for disease surveillance, and we recommend multidisciplinary discussions and careful clinical correlation to help guide therapy.
CRediT Authorship Contribution Statement
Benjamin Y. Lu and Sarah B. Goldberg: analyzed and interpreted the patient case, created figures, wrote and edited the manuscript.
Cigdem Isitan, Veronica Chiang, Sarah F. Wesley, and Sarah B. Goldberg: coordinated the patient’s care.
Amit Mahajan: performed radiologic interpretation and provided the imaging figures.
Anita Huttner: provided the pathologic interpretation, provided the histology images, and edited the manuscript.
Jackson Robinson Mitzner: interpreted the case and edited the manuscript.
Benjamin Y. Lu, Cigdem Isitan, Amit Mahajan, Veronica Chiang, Anita Huttner, Jackson Robinson Mitzner, Sarah F. Wesley, and Sarah B. Goldberg: read and approved the final manuscript.
Acknowledgments
Patient information was collected from medical records in accordance with research protocols approved by the Yale University Institutional Review Board (HIC #1603017333). Written informed consent was obtained with the research protocol for publication. Data sharing is not applicable to this article as no data sets were generated or analyzed during this study.
Footnotes
Disclosure: Dr. Chiang reports receiving personal fees from Monteris Medical, Inc., MRI Interventions, Inc., and Brainlab outside of the submitted work. Dr. Goldberg reports receiving grants and personal fees from AstraZeneca and Boehringer Ingelheim and personal fees from Bristol-Myers Squibb, Genentech, Eli Lilly, Amgen, Spectrum, Blueprint Medicines, Sanofi Genzyme, Daiichi-Sankyo, Regeneron, Janssen, and Takeda outside of the submitted work. The remaining authors declare no conflict of interest.
Cite this article as: Lu BY, Isitan C, Mahajan A, et al. Intracranial complications from immune checkpoint therapy in a patient with NSCLC and multiple sclerosis: case report. JTO Clin Res Rep. 2021;2:100183.
References
- 1.Leonardi G.C., Gainor J.F., Altan M. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol. 2018;36:1905–1912. doi: 10.1200/JCO.2017.77.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdel-Wahab N., Shah M., Lopez-Olivo M.A., Suarez-Almazor M.E. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med. 2018;168:121–130. doi: 10.7326/M17-2073. [DOI] [PubMed] [Google Scholar]
- 3.Colaco R.J., Martin P., Kluger H.M., Yu J.B., Chiang V.L. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurol Surg. 2016;125:17–23. doi: 10.3171/2015.6.JNS142763. [DOI] [PubMed] [Google Scholar]
- 4.Lowell D., Tatter S.B., Bourland J.D. Toxicity of gamma knife radiosurgery in the treatment of intracranial tumors in patients with collagen vascular diseases or multiple sclerosis. Int J Radiat Oncol Biol Phys. 2011;81:e519–e524. doi: 10.1016/j.ijrobp.2011.02.056. [DOI] [PubMed] [Google Scholar]
- 5.Hemond C.C., Bakshi R. Magnetic resonance imaging in multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8:a028969. doi: 10.1101/cshperspect.a028969. [DOI] [PMC free article] [PubMed] [Google Scholar]