A 66-year-old man presented to the pulmonology clinic with cough and sputum that had been present for 3 weeks and for evaluation of a mass in the right lower lobe of the lung that was revealed on computed tomography (CT) of the chest. He was an ex-smoker with a smoking history of 40 pack-years. The physical examination was unremarkable, and laboratory evaluation only revealed an elevated erythrocyte sedimentation rate of 50 mm/hour and a C-reactive protein of 26.87 mg/L, suggesting the presence of an underlying inflammatory reaction.
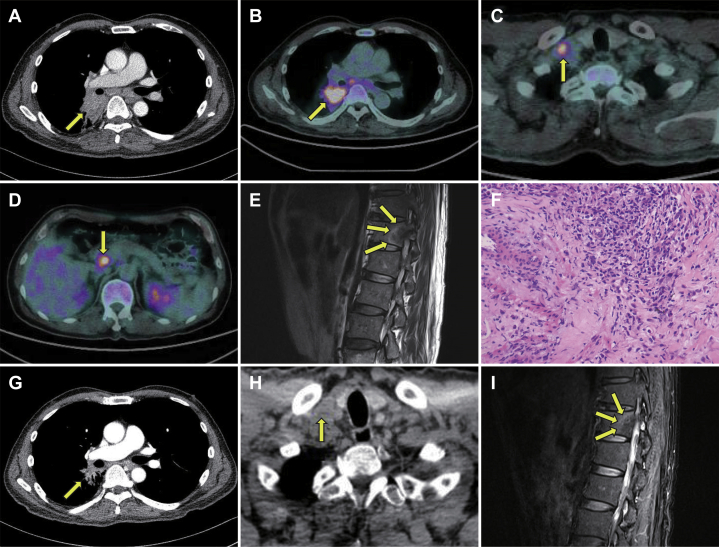
CT scan revealed a locally invasive mass (4.0 cm × 5.3 cm) in the right lower lobe (Fig. 1A) and multiple lymph node metastases in both the supraclavicular regions, the mediastinal region, and the portocaval region. 18F-fluorodeoxyglucose-positron emission tomography–CT revealed substantial 18F-fluorodeoxyglucose-positron emission tomography avidity in all of these lesions (Fig. 1B, C, and D). T2-weighted magnetic resonance imaging of the thoracic spine revealed a peripherally enhanced mass-like lesion involving the T12 vertebra (Fig. 1E). On the basis of these findings, advanced lung cancer with distant metastases was suspected.
Figure 1.
(A) Computed tomography (CT) of the chest revealed mass-like opacity (arrow); (B, C, and D)18F-fluorodeoxyglucose positron emission tomography–CT scan revealed high metabolic activity in the pulmonary lesion, the supraclavicular region, and the portocaval region (arrows); (E) T2-weighted magnetic resonance imaging (MRI) revealed a peripherally enhanced mass-like lesion involving T12 vertebra (arrows); (F) pathologic examination of pulmonary lesion revealed proliferation of myofibroblastic spindle cells with lymphoplasmacytic infiltration, a finding consistent with inflammatory myofibroblastic tumor (IMT) (hematoxylin and eosin stains). After systemic corticosteroid therapy (G, H, and I), the CT scan revealed a shrinkage of IMT lesions (G and H, arrows), and T2-weighted MRI revealed a decreased enhancement of the T12 vertebra lesion (I, arrows).
Pathologic examination of biopsy specimens obtained by percutaneous transthoracic needle biopsy reported the proliferation of fibroblasts with lymphoplasmacytic infiltration (Fig. 1F). Immunohistochemical staining was positive for smooth muscle markers such as actin and desmin and negative for the thyroid transcription factor-1, Immunoglobulin G4, and anaplastic lymphoma kinase-1. These findings were consistent with the diagnosis of inflammatory myofibroblastic tumor (IMT). Excisional biopsy of the right supraclavicular lymph node and the thoracic vertebra revealed no evidence of malignant cells. On the basis of these findings, a pathologist confirmed that these lesions were metastases from the pulmonary IMT.
On the basis of a careful review of previous reports on IMT, the patient was administered clarithromycin at a dosage of 500 mg twice a day.1 However, there was no obvious improvement in the follow-up chest CT scan after the initiation of the therapy. Subsequently, the patient was administered methylprednisolone at the dosage of 0.5 mg/kg once a day.2 Interestingly, a dramatic improvement in these IMT lesions was reported in the follow-up images 5 weeks after the initiation of the corticosteroid therapy (Fig. 1G, H, and I).
IMTs are rare tumors that account for 0.04% to 0.1% of all pulmonary neoplasms.3 IMTs are composed of myofibroblastic spindle cells with an inflammatory infiltrate of plasma cells and lymphocytes. Although IMTs are benign histopathologically, they may invade surrounding structures, recur, or even metastasize clinically.4
Complete surgical resection is the mainstay treatment of IMT of the lung. In the case of inoperable IMT, there is currently no established treatment.4 However, recent studies reveal the importance of molecular biological assessment of the disease and the therapeutic potential of crizotinib in the treatment of unresectable IMT harboring anaplastic lymphoma kinase gene rearrangement.5
In this case, given the extensive involvement of the disease, surgical resection or targeted therapy was not appropriate for the patient. Instead, clarithromycin, followed by a systemic corticosteroid, was administered on the basis of sporadic case reports supporting its anti-inflammatory role.1,2 Although there was no discernible improvement with clarithromycin, a dramatic improvement was seen after systemic corticosteroid therapy in the follow-up images.
Based on our experience, a systemic corticosteroid may be one of the therapeutic options for unresectable IMT with extensive metastases, which has limited treatment options.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, Information and Communications Technologies, and Future Planning (NRF-2017R1A2A1A05000747; YCL), and by the fund of Biomedical Research Institute, Jeonbuk National University Hospital.
Footnotes
Drs. Lee and Jeong equally contributed to this work.
Disclosure: Dr. Yong C. Lee reports grants from Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, Information and Communications Technologies, and Future Planning, Biomedical Research Institute, Jeonbuk National University Hospital, during the conduct of the study. The remaining authors declare no conflict of interest.
References
- 1.Watanabe H., Uruma T., Tazaki G. Remission of ALK-negative primary pulmonary inflammatory myofibroblastic tumor on treatment with clarithromycin: a case report and review of the literature. Oncol Lett. 2016;11:1757–1761. doi: 10.3892/ol.2016.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee M.H., Lee H.B., Lee Y.C. Bilateral multiple inflammatory myofibroblastic tumors of the lung successfully treated with corticosteroids. Lung. 2011;189:433–435. doi: 10.1007/s00408-011-9314-3. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher C.D.M., Unni K.K., Mertens F. 3rd ed. IARC Press; France: 2002. World Health Organization classification of tumors: pathology and genetics of tumours or soft tissue and bone. [Google Scholar]
- 4.Khatri A., Agrawal A., Sikachi R.R., Mehta D., Sahni S., Meena N. Inflammatory myofibroblastic tumor of the lung. Adv Respir Med. 2018;86:27–35. doi: 10.5603/ARM.2018.0007. [DOI] [PubMed] [Google Scholar]
- 5.Schoffski P., Sufliarsky J., Gelderblom H. Crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumours with and without anaplastic lymphoma kinase gene alterations (European organization for research and treatment of cancer. 2018 90101 CREATE): a multicenter, single-drug, prospective, non-randomised phase 2 trial. Lancet Respir Med. 2018;6:431–441. doi: 10.1016/S2213-2600(18)30116-4. [DOI] [PubMed] [Google Scholar]