Abstract
Objectives
Mutation analysis by massive parallel sequencing (MPS) is routinely performed in the clinical management of lung cancer in Sweden. We describe the clinical and mutational profiles of lung cancer patients subjected to the first 1.5 years of treatment predictive MPS testing in an autonomous regional health care region.
Methods
Tumors from all patients with lung cancer who had an MPS test from January 2015 to June 2016 in the Skåne health care region in Sweden (1.3 million citizens) were included. Six hundred eleven tumors from 599 patients were profiled using targeted sequencing with a 26-gene exon-focused panel. Data on disease patterns and characteristics of the patients subjected to testing were assembled, and correlations between mutational profiles and clinical features were analyzed.
Results
MPS with the 26-gene panel revealed alterations in 92% of the 611 lung tumors, with the most frequent mutations detected in the nontargetable genes TP53 (62%) and KRAS (37%). Neither KRAS nor TP53 mutations were associated with disease pattern, chemotherapy response, progression-free survival, or overall survival in advanced-stage disease treated with platinum-based doublet chemotherapy as a first-line treatment. Among targetable genes, EGFR driver mutations were detected in 10% of the tumors, and BRAF p.V600 variants in 2.3%. For the 71 never smokers (12%), targetable alterations (EGFR mutations, BRAF p.V600, MET exon 14 skipping, or ALK/ROS1 rearrangement) were detected in 59% of the tumors.
Conclusion
Although the increasing importance of MPS as a predictor of response to targeted therapies is indisputable, its role in prognostics or as a predictor of clinical course in nontargetable advanced stage lung cancer requires further investigation.
Keywords: Lung cancer, Massive parallel sequencing, Driver oncogenes, Chemotherapy, Never smoker
Introduction
Molecular diagnostics is a cornerstone in clinical management of NSCLC. Opportunities for personalized medicine in lung cancer evolve rapidly, and knowledge of how mutations and gene fusions influence the clinical course constantly increases.1 Lung cancers frequently harbor hotspot mutations that induce and sustain tumorigenesis. However, the spectra of genetic alterations differ between subtypes of NSCLC and between smokers and nonsmokers, emphasizing the need for broad molecular analysis to guide treatment. KRAS mutations are found in approximately 30% of lung adenocarcinomas but rarely in squamous cell carcinomas (SqCCs). In Western populations, targetable EGFR mutations occur in 10% to 15% and targetable BRAF in a smaller proportion of lung adenocarcinomas (ACs), whereas both genes are rarely mutated in SqCCs.2, 3, 4 In SqCC, mutations in the tumor suppressor gene TP53 have been reported in most tumor specimens and mutations in the PIK3CA oncogene in a smaller subset.2
Massive parallel sequencing (MPS) covering a wide range of genes is now the preferred method for mutational testing in lung cancer.5 The main focus of the testing is directed toward therapeutically actionable alterations in oncogenes such as EGFR, BRAF, and MET (splice site mutations leading to skipping of exon 14) and fusion gene analyses of ALK and ROS1 that are all predictive of targeted therapy responses.6, 7, 8, 9 However, the prognostic and predictive significance of co-occurring mutations is not fully understood. Furthermore, mutations in other genes might increase the understanding of the clinical course of lung tumors and be identified as new possible future targets for therapy. Since January 2015, MPS has been implemented as a routine method for lung cancer molecular diagnostics in the autonomous Skåne health care region in southern Sweden (region of Skåne; 1.3 million citizens in 201610). Here, we present the clinical aspects of the first 1.5 years with MPS mutational profiling using a 26-gene panel in consecutively tested patients with NSCLC in the Skåne region. We describe clinical features and mutation frequencies and investigate potential correlations of mutations and clinical outcomes.
Material and Methods
Patients and Tumors
All patients with lung cancer in the Skåne health care region of Sweden who were subjected to MPS testing from January 2015 to June 2016 were identified through referrals to the diagnostic MPS laboratory, using “lung cancer” or “unknown/cancer of unknown primary tumor” in the referral text as inclusion criteria (Fig. 1). Patients without lung cancer in the final diagnosis or without conclusive MPS results were excluded. All MPS tests had been requested by the patients’ treating pulmonologist or oncologist. Clinical features, including lung cancer treatment, treatment outcome, clinical course, metastatic pattern, smoking history, heredity of cancer, and occupational or environmental exposure, were obtained from patient files. History of smoking was classified into never smokers, former smokers, and current smokers. The definition of former smoker was cessation longer than 1 year before lung cancer diagnosis. Patients with a history of irregular light smoking during a very short period were included in the group of never smokers. Time of diagnosis was set as the date of the most lung cancer–specific histologic or cytologic proof of cancer. Date of recurrence was defined as the date of clinical, radiologic, or histologic findings recognized by a clinician as a recurrence. Clinical follow-up in patient files ended on May 8, 2018, and on the same date, information about other primary malignancies and eventual date of death was obtained from the Swedish Cancer Registry.
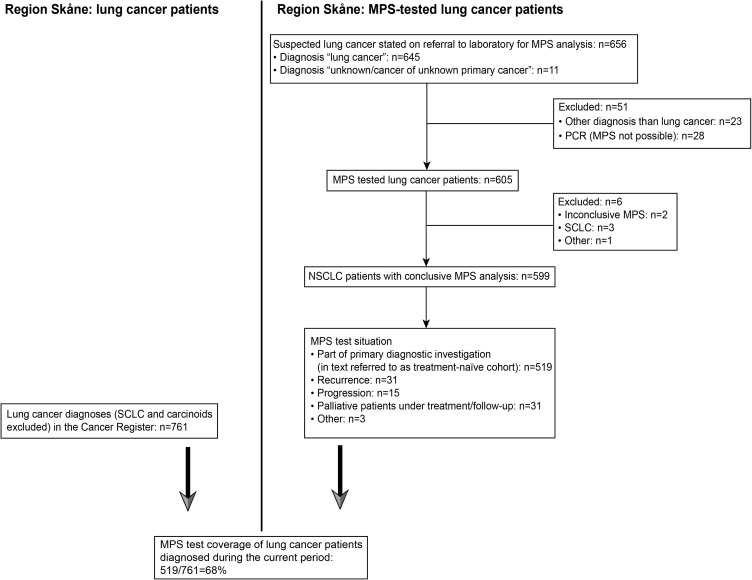
Figure 1.
Patient inclusion. Study scheme outlining the cohort and describing the estimated coverage of conclusive MPS test among newly diagnosed lung cancers in the region. MPS, massive parallel sequencing; PCR, polymerase chain reaction; SCLC, small cell lung cancer.
Tumor samples were obtained from either primary tumor or metastatic sites and thus included both small biopsies or cytology and tumor resections, all of which were reviewed by a thoracic pathologist (KEL). The diagnoses were based on cellular structure and relevant immunohistochemical (IHC) stains in accordance with the fourth edition of the WHO classification of lung tumors.11 Tumors were staged according to TNM 7th edition,12 which was the valid staging edition at the time. Treatment response was evaluated on the basis of Response Evaluation Criteria in Solid Tumors (RECIST 1.1),13 with slight modifications due to the retrospective nature of the study with different types of performed radiology (x-ray, computed tomography, positron emission tomography, and magnetic resonance imaging). In one case, the response was evaluated by bronchoscopy result. Responses were described in terms of complete response, partial response (PR), stable disease, or progressive disease.
The study was approved by the Regional Ethical Review Board in Lund, Sweden (Registration no. 2014/32, 2015/575, and 2017/620).
Mutational Testing by MPS
Material for mutational profiling consisted of formalin-fixed paraffin-embedded tissues, cells from cytology slides, or cell blocks. Exon-focused targeted sequencing was performed using the Illumina TruSightTumor 26-gene panel on a MiSeq instrument. The methodology is described in detail in previous work by Lindquist Ericson et al.14 In addition, we filtered single nucleotide polymorphisms by identifying variants with a frequency of 1% or more in the general population, as reported in the Illumina Variant Studio software. Variants classified as single nucleotide polymorphisms are defined in Supplementary Table 1.
To separate variants of unknown significance from more tumorigenic relevant variants, we subsequently noted the following mutations as potentially prognostic or predictive driver oncogenes15, 16, 17, 18: (1) KRAS codon 12,13, and 6; (2) EGFR exon 19 deletions and insertions; (3) insertions in exon 20; and (4) substitutions in codon 719 (exon 18), codon 851 (exon 21), and 861 (exon 21). Furthermore, EGFR mutations in exon 18 deletion p.(E709_T710delinsD), p.(S768I) (exon 20), and p.(C797S) (exon 20) were included. BRAF codon 600; PIK3CA codon 542, 545, and 1047; NRAS codon 12,13, and 61; MAP2K1 codon 56 and 57; ERBB2 exon 20 insertions and in MET variants involving position c.3082 at the intron-exon junction leading to a MET exon 14 skipping variant were likewise included.
Fusion Gene Detection
ALK and ROS1 rearrangements were investigated through IHC, fluorescence in situ hybridization, or both, as part of clinical predictive testing during the study period. In parallel, and outside of clinically routine procedure, we performed multiplexed fusion gene detection at the RNA level by means of NanoString technology for a subset of the tumors, as previously described by Lindquist et al.14
Statistics
For description of clinical data, categorical variables were expressed as numbers and percentages, whereas continuous variables were expressed as median and range. Categorical variables were analyzed using the chi-square test. Overall survival (OS) was calculated from the date of lung cancer diagnosis to the date of death from any cause or was censored at the last follow-up date (May 8, 2018). Progression-free survival (PFS) was calculated from the date of start of treatment to the date of progression or death from any cause. Patients alive and progression-free were censored at the date of the latest appointment at the lung department. Differences in OS or PFS between groups were analyzed with the logrank test. Statistical tests were performed in R version 3.5.2.19
Data Availability
The data are available from the corresponding author on reasonable request.
Results
Patient Inclusion
Inclusion criteria of patients are summarized in Figure 1, which also describes the estimated coverage of conclusive MPS tests among newly diagnosed lung cancers in the region.
Most of the patients included (519 of 599, 87%) were tested in proximity to lung cancer diagnosis, with diagnostic material (from the primary tumor or metastases) obtained before the start of primary treatment. For the remaining 80 patients, an MPS test was requested in other treatment situations described in Supplementary Table 2.
Smoking Status, Occupation, and Environmental Exposure
We were able to group 98% of the patients into current smokers, former smokers, and never smokers and estimate tobacco smoking in pack years in 78% of cases. Seventy-one patients (12% of the study population) were never smokers, 66 (93%) of whom were patients with lung AC, and 45 (63%) were women. Eleven percent of the patients had smoked 50 pack years or more at the time of diagnosis.
Although the patients’ occupations were documented in 85% of the cases, information regarding exposure to carcinogens could be found in only 90 of 599 patient files (15%). Asbestos was the most frequently reported exposure. Forty patients reported asbestos exposure (including seven cases of “possible exposure”), and seven of these 40 patients reported exposure to asbestos in combination with other exposure (passive smoking, chemicals, airway irritants, dust, and isocyanates). Sixteen patients denied exposure to asbestos. Beyond asbestos and asbestos in combination with other exposures, eight patients had been exposed to passive smoking, and 22 patients reported other kinds of occupational or environmental exposure. Of the 71 never smokers, information regarding occupation was documented for 66 patients (93%). Information about exposure to occupational or environmental carcinogens was, however, present in only 16 (23%) of cases, of whom six denied exposure to asbestos and 10 reported some kind of exposure (asbestos, passive smoking, or other kind of exposure). Overall, 34 of the 71 never smokers (48%) were considered as having low-risk occupations (e.g., health care, education, or office work) and no other reported exposure, whereas the corresponding estimation in smokers and former smokers was 36%.
Other Primary Malignancies and Family History of Cancer
Twenty-one percent (n = 126) of the study population had a history of other primary malignancies (nonmelanoma skin cancer excluded) than lung cancer. The most common malignancies among patients with a single other primary cancer were breast cancer (n = 28), prostate cancer (n = 25), bladder cancer (n = 13), and gastrointestinal cancers (n = 12). Fourteen patients (13 with a history of smoking and one with unknown smoking habits) had multiple other primary tumors, in which breast cancer (n = 6), gastrointestinal cancer (n = 6), and bladder cancer (n = 4) were the most common. The proportion of patients with another primary malignancy did not differ between never smokers and ever smokers, although some differences were noticed; for example, bladder cancer and multiple tumors were only found in the group of ever smokers. There was no correlation between another primary malignancy (of any kind) and the three most frequently mutated genes TP53, KRAS, and EGFR (chi-square test; p = 0.9, 0.4, and 0.9 respectively).
Information on family history of cancer was missing in most patient files (69%), but in the remaining cases, history of cancer in the family was negated by 67 patients (i.e., 11% of the total 599 patients), whereas 118 of 599 (20%) reported one or more family members with a malignancy, comprising 109 cases with a first-degree relative diagnosed with cancer. One or more relatives with lung cancer was reported by 39 patients, four with second-degree relatives with a lung cancer diagnosis, and the remaining 35 with a first-degree relative with a lung cancer diagnosis. There was no correlation between cancer (all tumor types) in a first-degree relative and the three most frequently mutated genes TP53, KRAS, and EGFR (chi-square test; p = 1.0, 0.7, and 0.8 respectively).
Histology
Most of the 611 MPS-tested tumors (70%) were AC, and 16% were SqCC, whereas according to the Swedish Cancer Registry, of all lung cancers diagnosed in the region during the period, AC and SqCC comprised 60% and 25%, respectively. This discrepancy is in accordance with SqCC not being part of the routine mutational testing during the entire study period. The other histologic types, constituting altogether 14%, were NSCLC marker null (36 tumors), NSCLC not further specified (34 tumors), large-cell neuroendocrine carcinoma (LCNEC; four tumors), NSCLC favor neuroendocrine (four tumors), NSCLC favor adenosquamous (three tumors), NSCLC with spindle cell (two tumors), large-cell carcinoma (LCC; two tumors), and sarcomatoid carcinoma (one tumor).
Mutation Spectra
The most frequently mutated genes (unselected regarding their driver potential, as defined previously) were TP53 (62%), KRAS (37%), and EGFR (10.5%). The frequency of mutations in each gene in the 26-gene panel is displayed in Figure 2A for all histologic subgroups and for AC in relation to stage in Figure 2B. Furthermore, the number of genes with variants detected per tumor are displayed in Figure 2C. More than one gene with at least one variant was more frequent in AC than SqCC (Fig. 2B), and at least one defined driver mutation was present in 52% of the tumors (62% in AC and 13% in SqCC). No tumor exhibited mutations in AKT1 or FGFR2.
Figure 2.
Mutation detection in the entire cohort. (A) Heatmap showing defined driver oncogene mutations and nondriver variants identified in all tumors (n = 611), AC (n = 429) and SqCC (n = 96). (B) heatmap with defined driver oncogene mutations and nondriver variants in each stage for AC (n = 429). (C) number of genes with at least one mutation identified in the entire cohort and in AC and SqCC. AC, adenocarcinoma; SqCC, squamous cell carcinoma.
Driver Oncogene Alterations
Driver oncogene mutations were defined in eight investigated genes, as previously described13: KRAS, EGFR, BRAF, PIK3CA, MET, MAP2K1, ERBB2, and NRAS. Cases with these driver variants are described in Table 1. In total, 15 tumors from 15 patients had more than one defined potential driver mutation in BRAF, KRAS, MAP2K1, NRAS, EGFR, ERBB2, or PIK3CA. In most cases, there was a clear difference in variant allele frequency between the driver variants, indicating that the drivers might be present in different subclones. The co-occurring mutations and corresponding variant allele frequencies are presented in Supplementary Table 3.
Table 1.
Tumors With Oncogene Driver Alterations
| Oncogene | KRAS | EGFR | BRAF | PIK3CA | MAP2K1 | MET | ERBB2 | NRAS | ALKa |
|---|---|---|---|---|---|---|---|---|---|
| Method | Massive parallel sequencing (Illumina TruSightTumor 26) | IHC / FISH /NanoString | |||||||
| Tumors (patients) | 223 (218) | 60 (59) | 14 (14) | 14 (14) | 9 (9) | 8 (8) | 7 (7) | 7 (7) | 18 |
| Most frequent alterations | c.34G>T: p.(gly12Cys) (43%) | Different exon 19 deletions (50%) c.2573T>G: p.(Leu858Arg) (33%) |
c.2573T>G: p.(V600E) (93%) | c.171G>T: p.(Lys545Asn) (64%) | c.1633G>A: p.(Lys57Asn) (67%) |
Splice site mutations, all including position c.3082 | c.2310_2311insGCATACGTGATG: p.(Glu770_AlainsAlaTyrValMet) (71%) |
c.35G>A: p.(Gly12Asp) (29%) c.182A>T: p.(Gln61Leu) (29%) |
ALK-EML4 |
| Age, y; median (range) | 69 (48–92) | 69 (32–88) | 68 (53–84) | 70 (65–84) | 73 (44–88) | 77 (66–86) | 70 (61–82) | 66 (59–83) | 69 (41–83) |
| Female Male |
57% 43% |
59% 41% |
64% 36% |
64% 36% |
56% 44% |
62% 38% |
57% 43% |
43% 57% |
72% 28% |
| Never smokers | 3% | 53% | 7% | 22% | 62% | 71% | 22% | ||
| Former smokers | 48% | 32% | 57% | 64% | 56% | 25% | 29% | 57% | 44% |
| Current smokers | 45% | 15% | 36% | 29% | 22% | 13% | 29% | 33% | |
| Former/current | 2% | ||||||||
| Unknown | 1% | 7% | 14% | ||||||
| 0 pack years | 3% | 53% | 7% | 22% | 62% | 71% | 22% | ||
| ≤10 pack years | 3% | 10% | 7% | 14% | 25% | 29% | 14% | 28% | |
| 11–20 pack years | 11% | 7% | 29% | 7% | 14% | ||||
| 21–30 pack years | 24% | 7% | 14% | 7% | 22% | 14% | 6% | ||
| 31–40 pack years | 11% | 3% | 29% | 11% | 29% | ||||
| 41–50 pack years | 13% | 3% | 7% | 7% | 11% | 11% | |||
| >50 pack years | 10% | 2% | 21% | 7% | 11% | 13% | 17% | ||
| Unknown | 25% | 15% | 14% | 29% | 22% | 29% | 17% | ||
| AC | 82% | 93% | 86% | 71% | 100% | 62% | 100% | 43% | 61% |
| SqCC | 3% | 2% | 7% | 14% | 13% | 14% | 6% | ||
| Other | 15% | 5% | 7% | 14% | 25% | 43% | 33% | ||
| Most frequently co-occurring mutated gene | TP53 (46%) | TP53 (60%) | TP53 (50%) |
TP53 (50%) KRAS (50%) |
TP53 (56%) | TP53 (38%) | TP53 (57%) | TP53 (57%) | TP53 (56%) |
| Genes with co-occurring driver alteration |
PIK3CA (3%) MAP2K1 (<1%) BRAF (<1%) NRAS (<1%) PIK3CA + NRAS (<1%) |
MAP2K1 (2%) PIK3CA (2%) |
KRAS (7%) |
KRAS (43%) KRAS + NRAS (7%) EGFR (7%) ERBB2 (7%) |
EGFR (22%) KRAS (22%) |
none | PIK3CA (14%) |
KRAS (14%) PIK3CA + KRAS (14%) |
KRAS (17%) BRAF (6%) |
ALK status available for 551/611 tumors.
In the entire cohort, any targetable alteration (EGFR mutation, ALK or ROS1 rearrangement, BRAF p.[V600], or MET exon 14 skipping) was detected in 100 tumors (16%). However, the estimation is incomplete because ALK status was missing in 9.8%, and ROS1 status was missing in 67% of cases. Corresponding estimation in tumors from the 71 never smokers revealed targetable alterations in 42 tumors (59%). Here, ALK status was missing in 5.6% and ROS1 in 73% of cases. Considering histology, the 100 tumors with targetable alterations comprised 20% of AC tumors, 4% of SqCC tumors, and 14% of tumors of other histology. Corresponding numbers for driver mutations (targetable or nontargetable) were 63% of AC, 14% of SqCC, and 52% of tumors of histology other than SqCC or AC.
Tumors With EGFR Mutation
EGFR mutations were the dominant targetable alteration, detected in 64 tumors, of which 60 harbored EGFR mutations defined as driver alterations. Two of these tumors were synchronous tumors with different EGFR mutations (p.[L858R] and exon 19 deletion, respectively) in a surgically treated never smoker. Among the 60 tumors with driver EGFR mutations, exon 19 deletions and p.(L858R) mutations in exon 21 were the most frequent variants (Table 1), including four cases with concomitant p.(T790M) mutation. These four patients were all diagnosed with stage IV disease before MPS was routinely performed, and MPS was thus performed on new tumor material during treatment, that is, when progression on tyrosine kinase inhibitor (TKI) was confirmed. Other EGFR mutation variants in the 60 tumors included the following: (1) exon 20 insertions (n = 2); (2) codon 861 substitutions (n = 3); (3) exon 18 deletion (n = 1); (4) codon 719 substitution (n = 1); (5) compound mutations p.(G719S) and p.(L861Q) (n = 1); (6) compound mutations p.(G719C) and p.(S768I) (n = 1); and (7) one case with p.(T725M) mutation of unknown significance.
All in all, 56 (93%) of the tumors with driver variants of EGFR were AC, and the remaining four tumors constituted three NSCLC (two marker null and one not further specified) and one SqCC. Thirty-one of the 59 patients whose tumors harbored oncogene driver mutations in EGFR were never smokers (detailed smoking status is reported in Table 2).
Table 2.
Characteristics of the Treatment-Naive Cohort
| Clinicopathological Features | Stage I 103 Patients |
Stage II 40 Patients |
Stage III 111 Patientsc |
Stage IV 261 Patients |
Stage Unknownf 4 Patients |
All 519 Patients | |||
|---|---|---|---|---|---|---|---|---|---|
| IA 63 Patients | IB 40 Patients | IIA 17 Patients | IIB 23 Patients | IIIA 75 Patients | IIIB 36 Patients | ||||
| Median follow-up time, y | 2.6 | 2.5 | 2.7 | 2.5 | 3.1 | 2.3 | |||
| Age, y; median (range) | 72 (55–87) | 71 (54–85) | 70 (51–91) | 71 (39–86) | 71 (43–92) | 68 (47–84) | 70 (32–93) | 71 (69–84) | 70 (32–93) |
| Sex | |||||||||
| Female | 38 | 23 | 9 | 13 | 33 | 21 | 129 | 2 | 268 |
| Male | 25 | 17 | 8 | 10 | 42 | 15 | 132 | 2 | 251 |
| Performance status | |||||||||
| 0–1 | 57 | 38 | 14 | 20 | 66 | 28 | 171 | 4 | 398 |
| ≥2 | 6 | 2 | 3 | 3 | 9 | 8 | 90 | 0 | 121 |
| Smoking history | |||||||||
| Current | 25 | 21 | 6 | 6 | 31 | 18 | 106 | 0 | 213 |
| Former | 32 | 17 | 10 | 14 | 35 | 14 | 110 | 3 | 235 |
| Smoking cessation time unknown | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 7 |
| Never | 5 | 2 | 1 | 3 | 9 | 4 | 34 | 1 | 59 |
| Unknown | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 5 |
| Patients with synchronous tumors | 6a | 3 | 0 | 2 | 3c | 1 | 0 | - | 15 |
| Histologic diagnosis | |||||||||
| AC | 47 | 28 | 12 | 14 | 37 | 19 | 191 | 2 | 350 |
| SqCC | 7 | 8 | 5 | 6 | 23 | 11 | 23 | 1 | 84 |
| NSCLC not further specified | 1 | 0 | 0 | 0 | 6 | 1 | 18 | 0 | 26 |
| NSCLC marker null/LCC | 1 | 1 | 0 | 1 | 4 | 3 | 25 | 0 | 35 |
| Pleomorphic carcinoma/NSCLC with spindle cells | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Adenosquamous/NSCLC possibly adenosquamous | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 |
| LCNEC/possibly LCNEC | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 5 |
| Patients with synchronous tumors | 6a | 3 | 0 | 2 | 3c | 1 | 0 | - | 15 |
| Metastases at baseline (stage IV) | |||||||||
| Pericardium, pleura, contralateral lung | - | - | - | - | - | - | 154 | - | 154 |
| Skeletal | - | - | - | - | - | - | 112 | - | 112 |
| Liver | - | - | - | - | - | - | 44 | - | 44 |
| CNS | - | - | - | - | - | - | 54 | - | 54 |
| Adrenal glands | - | - | - | - | - | - | 47 | - | 47 |
| Other | - | - | - | - | - | - | 53d | - | 53d |
| Single metastases | - | - | - | - | - | - | 29e | - | 29e |
| Initial treatment | |||||||||
| No oncological treatment ± local palliative treatment | 1 | 0 | 1 | 2 | 9 | 6 | 78 | 2 | 99 |
| Radiotherapy lung tumor ± lgll | 18 | 2 | 2 | 2 | 4 | 4 | 4 | 0 | 36 |
| Operation | 37 | 35 | 11 | 12 | 32 | 3 | 0 | 0 | 130 |
| Neoadjuvant | 0 | 0 | 0 | 2 | 13 | 1 | 0 | 0 | 16 |
| Adjuvant | 0 | 9 | 6 | 7 | 15 | 1 | 0 | 0 | 38 |
| Chemotherapy ± local palliative treatment | 0 | 0 | 2 | 1 | 7 | 7 | 151 | 1 | 169 |
| Concomittant CRT | 0 | 0 | 1 | 1 | 5 | 3 | 0 | 0 | 10 |
| Sequential CRT | 0 | 0 | 0 | 3 | 14 | 11 | 0 | 0 | 28 |
| TKI ± local palliative treatment | 1 | 0 | 0 | 0 | 1 | 1 | 28 | 1 | 32 |
| Treatment synchronous tumors | |||||||||
| Operation ± adjuvant | 3 | 3 | 0 | 2b | 2 | 1 | 0 | 0 | 11 |
| Operation and radiotherapy | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Radiotherapy | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
Clinicopathological features of the 519 patients with MPS testing as part of primary diagnostic procedure. Most frequent alterations, clinicopathologic data, and co-occurring alterations are summarized for tumors with driver mutations as detected by the TST 26. ALK status (available for 90% of the tumors) is shown for comparison.
AC, adenocarcinoma; ALK, anaplastic lymphoma kinase; CNS, central nervous system; CRT, conformal radiation therapy; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; LCC, large-cell carcinoma; LNEC, large-cell neuroendocrine carcinoma; MPS, massive parallel sequencing; SqCC, squamous cell carcinoma; TKI, tyrosine kinas inhibitors; TST, TruSight Tumor.
One patient with a synchronous tumor and a previous metachronous tumor, all three tumors were tested by MPS.
Operation of one of the two tumors. Radiotherapy was planned against the other tumor, but fatal postoperative complications occurred.
One patient with multiple tumors in one lung. Three tumors (AC) were sequenced by MPS, displaying different mutational profiles. One of the other tumors had a different histologic diagnosis (LCNEC) and was considered a synchronous tumor. In addition, there were two more AC, either metastases or synchronous tumors. No known lymph node dissemination. If all tumors are considered , highest stage will be IB, and if some of the lesions are considered metastases, the most advanced stage will be IIIA.
Two patients with clinically considered disseminated lung cancer (pleural and skeletal metastasis and skeletal metastasis respectively) had a suspicious lesion in a kidney. Because of disseminated lung cancer, no further diagnostics on the kidney lesions were performed. These two patients are not included in metastasis category “other.”
Two patients were excluded because of uncertainties regarding metastases: one patient with a kidney lesion (explained above) and one skeletal metastasis and one patient with a presumed thyroid metastasis (examination revealed malignancy, not suspected to be a primary thyroid cancer) and radiologically a lesion in the breast without further diagnosis that could be a metastasis or a primary breast cancer.
In one of these patients, it cannot be excluded that dissemination in lungs represented metastatic spread from another primary tumor. Another patient previously described had either two different tumor components or two synchronous tumors. In addition, a third patient had either two synchronous tumors or a stage IV disease with one lung metastasis in the contralateral lung, deceased in infection shortly after diagnostic bronchoscopy. The fourth patient was diagnosed with a second primary lung tumor and metastases that could have originated from any of the two tumors.
Mutations in other genes co-occurring with oncogenic EGFR mutations were most frequent in TP53 (in 36 of the 60 EGFR-mutated tumors) and CTNNB1 (n = 5). Other co-occurring mutations were in PIK3CA (n = 4, one tumor with a tumorigenic variant), NRAS (n = 2, none with tumorigenic variants), MAP2K1 (n = 2, both with driver variants), and MET (n = 1, no tumorigenic variant).
Treatment-Naive Cohort
Of the 599 patients, 519 were subject to MPS testing as part of primary diagnostic procedure. Baseline characteristics of these 519 patients, from here on named treatment-naive cohort, are further described in Table 2. The most common disease pattern among patients diagnosed with stage IV was intrathoracic spread (without any other dissemination), appearing in 25%, followed by (1) skeletal metastases alone (11%), (2) intrathoracic and skeletal spread combined (10%), (3) central nervous system metastasis alone (6.5%), and (4) intrathoracic spread in combination with adrenal metastasis (6.5%). Oncogene driver mutation frequency of the two largest groups of drivers, KRAS and EGFR, in each metastatic site at baseline is presented in Supplementary Table 4. There was only a moderate difference in mutation frequency between metastatic sites. It should be noted that other diagnostics, for example, radiology of the central nervous system, were only performed at baseline if symptoms indicated metastases. Proportions of patients alive at the end of the study and median follow-up times are described per disease stage in Table 2, whereas overall survival is displayed in Supplementary Figure 1.
Targeted Therapy in the Treatment-Naive Cohort
Figure 3A summarizes patients with EGFR TKI as first-line therapy regarding treatment situation, duration of treatment, subsequent treatments, and mutation findings. Twenty-seven patients in the treatment-naive cohort (i.e., the patients with MPS testing as part of the primary diagnostic procedure) were treated with EGFR TKI as first-line treatment either immediately after diagnosis or at recurrence or progression after operation or curatively intended chemotherapy and radiotherapy. A tendency of better response on TKI for patients with exon 19 deletions compared with patients with rare EGFR mutations was observed. However, as shown in Figure 3A, some patients had a very short treatment duration not possible to evaluate, and the patients displayed differences in stage and type of TKI. Because of the limited number of TKI-treated patients, it is difficult to draw conclusions from co-occurring mutations among EGFR-positive patients. TP53 mutations were the most frequently mutated gene in the entire cohort and also the most often co-occurring mutation among the TKI-treated patients.
Figure 3.
First-line TKI treatment in treatment-naive cohort. (A) EGFR TKI and (B) Crizotinib. Chemo, chemotherapy; IT, immunotherapy; L, treatment line; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
Few patients in this study received ALK inhibitors as first-line treatment during the current period because of guidelines at the time. Seventeen tumors in the treatment-naive cohort had positive ALK rearrangement according to IHC or fluorescence in situ hybridization in clinical testing, of which 10 were in stage IV at the time of diagnosis. Altogether, nine patients received ALK inhibition as first-line and crizotinib was given in each case. Patients on ALK inhibitor as first-line are illustrated in Figure 3B.
Chemotherapy in Stage IV in Treatment-Naive Cohort
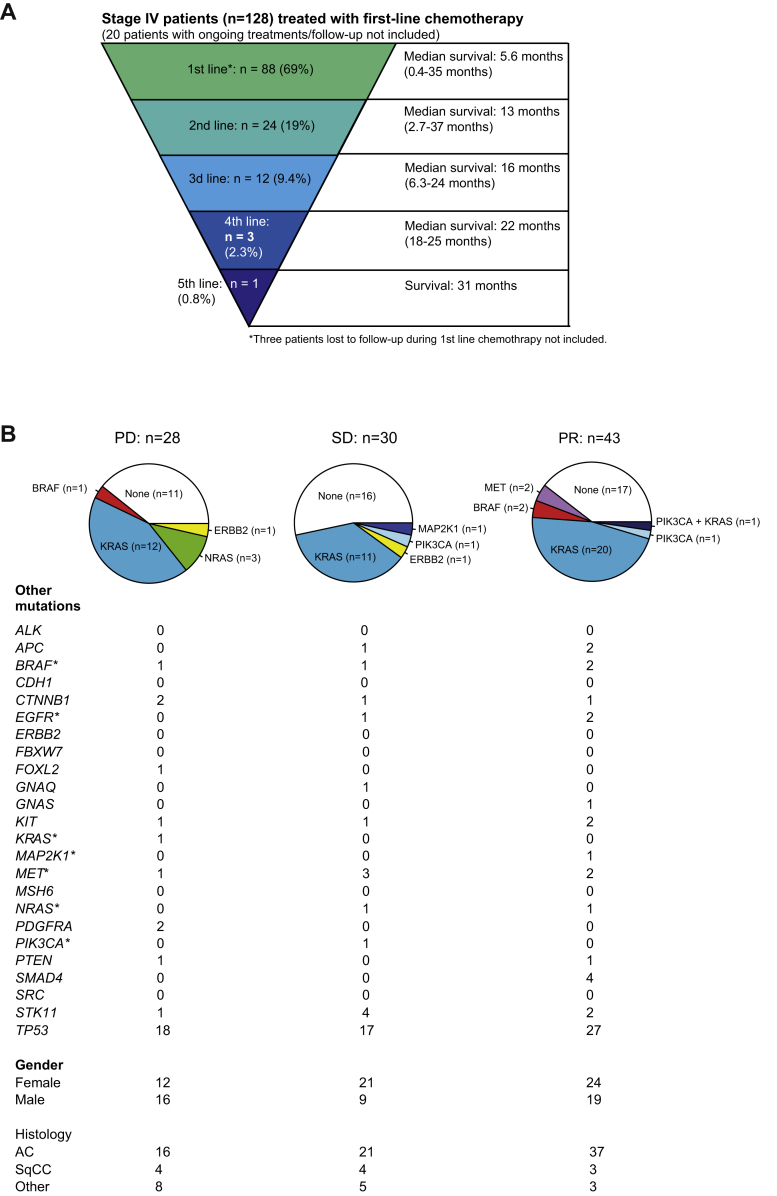
As presented in Table 2, 151 patients with stage IV disease had chemotherapy (single or combinations) as first-line treatment. At the end of the study, 20 of 151 patients receiving chemotherapy as first-line treatment still had ongoing treatments in different lines or were on treatment break. In addition, three patients were lost to follow-up during first-line chemotherapy (moved outside the health care region). Numbers of systemic oncologic treatment lines for the remaining 128 patients starting with chemotherapy as first-line treatment are presented in Figure 4A. The majority of the patients received no further treatment after first-line chemotherapy. However, as seen by the range of survival times, this large group with only one line of chemotherapy also comprises patients with long response duration as do the other groups with a larger number of total systemic treatment lines. Furthermore, those with treatment beyond first-line chemotherapy have received treatments from different main groups of systemic anticancer therapies, but in most cases chemotherapy.
Figure 4.
Chemotherapy in patients with stage IV cancer. (A) Number of systemic treatments for patients with chemotherapy as first-line therapy. (B) Oncogene drivers in relation to therapy response for patients receiving platinum doublet chemotherapy as first-line. Other sequencing results, sex, and histologic diagnosis are presented below each chart. AC, adenocarcinoma; PD, progressive disease; PR, partial response; SqCC, squamous cell carcinoma; SD, stable disease.
To further analyze chemotherapy response in advanced stages, we studied patients with stage IIIB/IV, all treated with platinum doublet chemotherapy as first-line treatment, without radiotherapy against the lung tumor before or concomitant with chemotherapy. Of the 297 patients in stage IIIB and IV, 101 (34%) were treated with at least two cycles of platinum-based chemotherapy combination as first-line treatment, without previous or concomitant radiotherapy. Patients with platinum doublet chemotherapy that discontinued after less than four cycles for other reasons than progression were not included in the 101 patients. None of the 101 patients had EGFR-mutated tumors or confirmed ALK rearrangement (not tested or inconclusive in eight patients and negative in the remaining 93 cases). In total, 11 of these patients received two to three cycles of platinum doublet chemotherapy, 88 received four cycles, and two received five to six cycles. Response to platinum doublet chemotherapy was PR in 43 patients, stable disease in 30, and progressive disease in 28. Distribution of oncogene drivers in each group of response is displayed in Figure 4B.
The most frequently mutated gene in the MPS panel in this group was TP53 (61 tumors, 61%). Driver oncogenes were almost exclusively found in KRAS (44 tumors, 44%) but also in BRAF (3 tumors), ERBB2 (2 tumors), PIK3CA (3 tumors), NRAS (3 tumors), and MAP2K1 (1 tumor). In addition, two patients had MET exon 14 splice site mutations. No correlation between mutational status in KRAS, TP53, or any combination of KRAS and TP53 (KRAS mutation and TP53 mutation, no KRAS/TP53 mutation, KRAS mutation without TP53 mutation, or TP53 mutation without KRAS mutation) and chemotherapy response according to RECIST 1.1 criteria was found. Neither OS nor PFS were significantly different in these patients when grouped according to KRAS and TP53 mutation status (Supplementary Fig. 2). Notably, two of three patients with driver oncogene BRAF mutations (two former smokers with approximately 15 and 29 pack years, respectively) had PR after four to five cycles of platinum-based doublet chemotherapy and continued with maintenance therapy. The third patient, a never smoker, had progression after four cycles of platinum-based combination, progression after second-line paclitaxel, and third-line gemcitabine was discontinued after one cycle. Similar to that with the BRAF mutations, targeted therapy in cases with MET exon 14 skipping was not implemented at the time. These two patients with MET splice site mutations were both never smokers and had PR on chemotherapy.
Discussion
The mutation spectra (Fig. 2) detected within our first 1.5 years of experience of mandatory clinical MPS testing largely reflects the European lung cancer population.20,21 As expected, the panel was more informative in AC than in SqCC, and female patients were slightly overrepresented among cases with driver alterations. Among the 15% of females and 9% of males in this study who were never smokers, targetable alterations were detected in 59% of cases. This observation clearly stresses the importance of complete molecular diagnostics in the clinical management of these patients, not the least considering that a panel detecting additional targetable variants in MET and, likewise, a higher inclusion grade for fusion gene testing could potentially have identified additional targetable cases in our study. However, 47.5% of patients with EGFR mutations were former or current smokers, and patients with BRAF p.(V600) mutations were not associated with never-smoking status, thus highlighting the need for broader mutational profiling in smokers as well. In contrast with the most often mutually exclusive alterations detected within KRAS, EGFR, BRAF, and MET in our cohort, mutations in PIK3CA were seldom single drivers but instead frequently co-occurred with other oncogene driver alterations (Table 1, Supplementary Table 3). This may suggest that PIK3CA may be more challenging to target in therapy for NSCLC.
To elucidate further clinical implications of the mutational profiles (including driver variants and co-occurring alterations), we performed extensive clinical characterization of the treatment-naive cohort (519 patients), exemplified by first-line treatment duration in EGFR- and ALK- positive cases (Fig. 3) and by a well-defined subcohort of 101 patients with advanced NSCLC with unambiguous response evaluations of first-line platinum-based standard chemotherapy (further discussed below).
Whereas the common mutations in exon 19 and 21 generally respond to EGFR TKI and exon 20 insertions generally do not, less is known about some rare EGFR mutations. Responses in these cases have been suggested to depend on the type of mutation, whether there is a sole mutation or complex variants, and on the choice of TKI, where second-generation EGFR inhibitors tend to be superior to first-generation ones.22 One patient in the treatment-naive cohort with such a nonclassical mutation, the exon 18 deletion p.(E709_T710delinsD), was diagnosed in stage IV with tumor dissemination in both lungs but no extrathoracic spread. Erlotinib was given as first-line treatment, with a treatment duration of 8 months; the treatment was discontinued because of progression of the cancer. The p.(E709_T710delinsD) has indeed been reported to respond to second-generation EGFR TKI in a previous case report.23 Furthermore, an in vitro study by Kobayashi et al. displayed increased responses to second-generation EGFR TKI compared with those to first- and third-generation EGFR TKI for lung tumors harboring exon 18 mutations.17
Despite decades of chemotherapy use in lung cancer, no successful molecular markers predictive of response have been established. Previous studies have shown conflicting results regarding KRAS mutation as a prognostic or predictive variable, potentially owing to the influence of different comutations and tumor stage.24 In our study, we did not detect any correlation between mutation status and chemotherapy response in patients with advanced cancer treated with platinum-based chemotherapy as first-line treatment when stratified for TP53 and KRAS mutation status (Fig. 4). Neither did we detect any correlation between these mutations and PFS or OS (Supplementary Fig. 2).
Our study has several limitations. By covering only the first 1.5 years of testing within our health care region, the cohort is small, despite being consecutive and population based. Larger patient groups could potentially have enabled the identification of correlations between clinical and mutational profiles, for example, regarding metastatic patterns or the predictive potential of co-occurring alterations. Furthermore, information in patient files about heredity and exposure was limited and worth improving, considering different causes and possible stratification of tumors and mutational patterns. More important, although heredity was rarely noted in patient files, 35 patients (5.8%) in our cohort reported that they had a first-degree relative with lung cancer. This information on unconfirmed lung cancer diagnoses should be carefully interpreted. Nevertheless, this still indicates that the patterns of lung cancer heredity are important to identify and could potentially serve as future indicators needed for lung cancer screening, which is now impending for high-risk groups (primarily smokers or former smokers).
To summarize, in this population-based lung cancer cohort with well-characterized follow-up, MPS by a 26-gene exon-focused panel of common oncogenes and tumor suppressors in cancer revealed alterations in 92% of the tumors but could not predict metastatic patterns, chemotherapy responses, or patient survival. For most mutations covered by this panel, we confirmed low frequencies, whereas EGFR, KRAS, and TP53 alterations were also frequently detected. The impact of individual as well as co-occurring mutations on clinical features deserves further study. In conclusion, the increasing importance of MPS as predictor of response to targeted therapies is indisputable, but, at least for the gene panel size used in our study, its role in prognostics or as predictor of clinical course in nontargetable lung cancer may be limited and requires further extended investigations.
Acknowledgments
The authors thank all patients and families who participated in the study. The authors also thank Carolina Andersson Rios for administrative help and thank the funders of this work—the Swedish Cancer Society (grant numbers CAN2016/786, CAN2017/1096 and CAN2015/555), Sjöberg Foundation (years 2019-2021), the Mrs. Berta Kamprad Foundation (grant number FBKS-2018-17-170), the Gustav V’s Jubilee Foundation (grant number 164151), and governmental funding (grant number ALF40604).
Footnotes
Disclosures: Dr. Staaf reports receiving grants from Sjöberg Foundation and Swedish Cancer Society during the conduct of the study. Dr. Planck reports receiving grants from Sjöberg Foundation, Swedish Cancer Society, the Mrs. Berta Kamprad Foundation, and the Gustav V’s Jubilee Foundation; he also reports receiving government funding during the conduct of the study. Dr. Öhman reports receiving personal fees from Boehringer-Ingelheim, Astra-Zeneca, MSD, Roche, Takeda, and Novartis outside of the submitted work. The remaining authors declare no conflicts of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100013.
Supplementary Data
Supplementary Figure 1.
Supplementary Figure 2.
References
- 1.Lindeman N.I., Cagle P.T., Aisner D.L. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol. 2018;13:323–358. doi: 10.1016/j.jtho.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paik P.K., Arcila M.E., Fara M. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29:2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sholl L. Molecular diagnostics of lung cancer in the clinic. Transl Lung Cancer Res. 2017;6:560–569. doi: 10.21037/tlcr.2017.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch T.J., Bell D.W., Sordella R. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 7.Odogwu L., Mathieu L., Blumenthal G. FDA approval summary: dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring BRAF V600E mutations. Oncologist. 2018;23:740–745. doi: 10.1634/theoncologist.2017-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon B.J., Mok T., Kim D.W. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 9.Shaw A.T., Ou S.H., Bang Y.J. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Statistics Sweden. https://www.scb.se/hitta-statistik/statistik-efter-amne/befolkning/befolkningens-sammansattning/befolkningsstatistik/pong/tabell-och-diagram/helarsstatistik--kommun-lan-och-riket/folkmangd-i-riket-lan-och-kommuner-31-december-och-befolkningsforandringar/ Accessed February 10, 2019.
- 11.Travis W.D., Brambilla E., Burke A.P., Marx A., Nicholson A.G., editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. IARC Press; Lyon, France: 2015. [DOI] [PubMed] [Google Scholar]
- 12.Goldstraw P. eds., editor. TNM Classification of Malignant Tumours. 7th ed. Wiley-Blackwell; Chichester, UK: 2009. [Google Scholar]
- 13.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Lindquist K.E., Karlsson A., Leveen P. Clinical framework for next generation sequencing based analysis of treatment predictive mutations and multiplexed gene fusion detection in non-small cell lung cancer. Oncotarget. 2017;8:34796–34810. doi: 10.18632/oncotarget.16276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.My cancer genome. https://www.mycancergenome.org/ Accessed February 10, 2019.
- 16.Hagemann I.S., Devarakonda S., Lockwood C.M. Clinical next-generation sequencing in patients with non-small cell lung cancer. Cancer. 2015;121:631–639. doi: 10.1002/cncr.29089. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi Y., Togashi Y., Yatabe Y. EGFR Exon 18 mutations in lung cancer: molecular predictors of augmented sensitivity to afatinib or neratinib as compared with first- or third-generation TKIs. Clin Cancer Res. 2015;21:5305–5313. doi: 10.1158/1078-0432.CCR-15-1046. [DOI] [PubMed] [Google Scholar]
- 18.Frampton G.M., Ali S.M., Rosenzweig M. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850–859. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 19.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2019. https://www.R-project.org/. Accessed January 15, 2019.
- 20.Planchard D., Remon J., Nowak F., Soria J.C. Future genetic/genomic biomarker testing in non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2017;37:12–17. doi: 10.1200/EDBK_100007. [DOI] [PubMed] [Google Scholar]
- 21.Konig K., Peifer M., Fassunke J. Implementation of amplicon parallel sequencing leads to improvement of diagnosis and therapy of lung cancer patients. J Thorac Oncol. 2015;10:1049–1057. doi: 10.1097/JTO.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 22.Shen Y.C., Tseng G.C., Tu C.Y. Comparing the effects of afatinib with gefitinib or erlotinib in patients with advanced-stage lung adenocarcinoma harboring non-classical epidermal growth factor receptor mutations. Lung Cancer. 2017;110:56–62. doi: 10.1016/j.lungcan.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim U., Saqib A., Atallah J.P. EGFR exon 18 delE709_T710insD mutated stage IV lung adenocarcinoma with response to afatinib. Lung Cancer. 2017;108:45–47. doi: 10.1016/j.lungcan.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Arbour K.C., Jordan E., Kim H.R. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res. 2018;24(2):334–340. doi: 10.1158/1078-0432.CCR-17-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from the corresponding author on reasonable request.