Abstract
Introduction
Pegargiminase (ADI-PEG 20; ADI) degrades arginine and potentiates pemetrexed (Pem) cytotoxicity in argininosuccinate synthetase 1 (ASS1)–deficient malignant pleural mesothelioma (MPM). We conducted a phase 1 dose-expansion study at the recommended phase 2 dose of ADI-PEG 20 with Pem and cisplatin (ADIPemCis), to further evaluate arginine-lowering therapy in ASS1–deficient MPM and explore the mechanisms of resistance.
Methods
A total of 32 patients with ASS1–deficient MPM (11 epithelioid; 10 biphasic;11 sarcomatoid) who were chemonaive received weekly intramuscular pegargiminase (36 mg/m2) with Pem (500 mg/m2) and cisplatin (75 mg/m2) intravenously, every 3 weeks (six cycles maximum). Maintenance pegargiminase was permitted until disease progression or withdrawal. Safety, pharmacodynamics, immunogenicity, and efficacy were determined. Biopsies were performed in progressing patients to explore the mechanisms of resistance to pegargiminase.
Results
The treatment was well tolerated. Most adverse events were of grade 1/2, whereas four nonhematologic grade 3/4 adverse events related to pegargiminase were reversible. Plasma arginine decreased whereas citrulline increased; this was maintained by 18 weeks of ADIPemCis therapy. The disease control rate in 31 assessed patients was 93.5% (n = 29 of 31; 95% confidence interval [CI]: 78.6%–99.2%), with a partial response rate of 35.5% (n = 11 of 31; 95% CI: 19.2%–54.6%). The median progression-free and overall survivals were 5.6 (95% CI: 4.0–6.0) and 10.1 (95% CI: 6.1–11.1) months, respectively. Progression biopsies on pegargiminase revealed a statistically significant influx of macrophages (n = 6; p = 0.0255) and patchy tumoral ASS1 reexpression (n = 2 of 6). In addition, we observed increased tumoral programmed death-ligand 1—an ADI-PEG 20 inducible gene—and the formation of CD3-positive T lymphocyte aggregates on disease progression (n = 2 of 5).
Conclusions
The dose expansion of ADIPemCis confirmed the high clinical activity and good tolerability in ASS1–deficient poor-prognosis mesothelioma, underpinning an ongoing phase 3 study (ClinicalTrials.govNCT02709512). Notably, resistance to pegargiminase correlated with marked macrophage recruitment and—along with the tumor immune microenvironment—warrants further study to optimize arginine deprivation for the treatment of mesothelioma.
Keywords: Arginine, ASS1, ADIPemCis, Mesothelioma, Macrophages
Introduction
Malignant pleural mesothelioma (MPM) is predominantly an asbestos-driven thoracic tumor notable for its chemoresistance and poor prognosis. Median survivals range from 3.5 to 6.6 months for the nonepithelioid, sarcomatoid, and biphasic variants and up to 18 months for the epithelioid subtype.1,2 No new frontline therapies for mesothelioma have been licensed since the antifolate pemetrexed with cisplatin in 2004.3
In preclinical studies, we identified arginine depletion as a rational antimetabolite strategy that targets mesothelioma cells displaying epigenetic inactivation of the urea cycle enzyme argininosuccinate synthetase 1 (ASS1).4 Arginine deprivation affects multiple biosynthetic pathways, including proteins, polyamines, nucleotides, and nitric oxide, emphasizing an essential role for the amino acid in the growth or auxotrophy of mesothelioma and other cancers.5, 6, 7 Consequently, bacteria-derived polyethylene glycol (PEG)ylated arginine deiminase (ADI-PEG 20, ADI, or pegargiminase) or bioengineered forms of human arginase are currently in development for patients with a range of advanced malignancies.10, 8, 9
Clinically, pegargiminase, which degrades arginine into citrulline and ammonia, improved progression-free survival (PFS) in patients with ASS1–deficient MPM in the ADAM (Arginine Deiminase And Mesothelioma) study, representing the first biomarker-driven randomized trial of arginine deprivation versus best supportive care in cancer.11 Moreover, ASS1 was prognostic, with the ASS1–deficient disease conferring a worse survival compared with ASS1–proficient disease, consistent with data linking dysregulation of urea cycle enzymes to accelerated tumorigenesis.5,11 In addition, when ADI-PEG 20 was combined with pemetrexed and cisplatin chemotherapy (ADIPemCis) in the phase I dose-escalation Tumors Requiring Arginine to Assess ADI-PEG 20, Pemetrexed and cisplatin (TRAP) study, a 100% disease control (78% partial response) rate was observed in nine patients with thoracic cancers (lung adenocarcinoma and MPM), including four of five patients with nonepithelioid MPM.12 Nevertheless, despite prolonged suppression of plasma arginine and a reciprocal increase in citrulline, patients progressed on ADIPemCis therapy, thereby implicating tumoral, rather than drug-innate, mechanisms of resistance to arginine deprivation.
First, reexpression of ASS1, and thus the recycling of citrulline to arginine, after long-term culture of tumor cell lines, including MPM cells in ADI-PEG 20, has been identified as a viable resistance mechanism with confirmatory studies in patients with melanoma.13, 14, 15 Second, autophagy (degradation and recycling of cellular components) is known to protect ASS1–negative MPM cells from arginine depletion.16 Third, the tumor microenvironment may also mediate cancer cell resistance to arginine withdrawal; however, this has not been addressed specifically in the context of pegargiminase. Tumor-associated macrophages (TAMs), in particular, constitute up to 30% of the total cell population of mesothelioma and play a key role in asbestos-mediated tumorigenesis.17, 18, 19, 20 As such, TAMs might also play a role in resistance to arginine deprivation therapy.
We treated a dose-expansion cohort of 32 patients with ASS1–deficient MPM at the recommended phase 2 dose (RP2D) of ADI-PEG 20 (36 mg/m2) in combination with standard doses of pemetrexed and cisplatin. The main aims of this phase 1 dose-expansion study were to define further the safety and preliminary activity of the ADIPemCis triplet in patients with MPM and to elucidate mechanisms of resistance to arginine deprivation by analyzing patients’ tumors at progression.
Materials and Methods
Patient Eligibility
The patients were 18 years or over and chemonaive with histologically proven ASS1–deficient advanced MPM (see Beddowes et al.12 for the methods). Additional eligibility included an Eastern Cooperative Oncology Group performance status of 0 or 1, no major comorbidities, a minimum expected survival of 3 months, and a measurable disease by modified Response Evaluation Criteria in Solid Tumors criteria for MPM. Exclusion criteria included recent major operation, history of another active primary cancer, and previous therapy with pegargiminase. All patients signed written informed consent.
Study Design and Treatment
This dose-expansion multicenter phase 1 study evaluated the RP2D of 36 mg/m2 weekly intramuscular ADI-PEG 20 plus 3-weekly 75 mg/m2 cisplatin and 500 mg/m2 pemetrexed derived from the previous dose-escalation TRAP study.11 Standard premedication was administered, including oral dexamethasone, daily folic acid, and 1000 μg intramuscular hydroxycobalamin every 9 weeks. The initial dose of intramuscular ADI-PEG 20 was administered 48 hours before the first dose of cytotoxic drugs. The patients received up to six cycles (18 wk) of ADIPemCis chemotherapy and could continue on maintenance pegargiminase until disease progression or withdrawal. Blood samples were taken at baseline, during ADIPemCis chemotherapy, and on disease progression or withdrawal from the study. Tumor biopsies were required at baseline and were optional at disease progression.
The primary objective of the dose-expansion study was to determine the safety and tolerability and to estimate the preliminary efficacy of ADIPemCis in patients with ASS1–deficient MPM. Secondary objectives included measuring pharmacodynamics, immunogenicity, and exploration of resistance mechanisms to pegargiminase.
Safety
Evaluation of safety was based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03, vital signs, physical examination, electrocardiogram, and laboratory blood analyses.
Pharmacodynamic and Efficacy Evaluations
Blood samples were analyzed by Polaris Pharmaceuticals, Inc. (San Diego, CA) for arginine and citrulline levels and anti–ADI-PEG 20 antibody titers, as described previously.11,12 Efficacy was assessed by computed tomography imaging using the modified Response Evaluation Criteria in Solid Tumors criteria every 6 weeks while on ADIPemCis and then every 2 months on maintenance pegargiminase.
Patient Tumor Immunohistochemistry
Tumor biopsy results were assessed for ASS1 expression using the monoclonal antibody 195-21-1 from Polaris Pharmaceuticals, Inc., San Diego, California. Infiltrating CD68pos macrophages were identified using a murine antihuman antibody (KP-1) and quantified as a percentage of the number of malignant cells, taking an average from five high-power fields at ×400 magnification. The expression of programmed death-ligand 1 (PD-L1) (Cell Signaling Technology E1L3N and Ventana-Roche SP-263 antibodies) and CD3 (Ventana-Roche 2GV6 antibody) was performed subsequent to the CD68 staining using residual tissue. PD-L1 was scored as a percentage of positive tumor cells and CD3 summarized descriptively.
Statistical Analyses
No formal sample size calculation was made for the dose-expansion TRAP study in patients with MPM, which aimed to recruit up to 30 patients as per protocol. Adverse events (AEs) were collated, and response rates, PFS, and overall survival (OS) were characterized according to the MPM subtype. The results of the patients’ tumor biopsies were analyzed using a paired t test in GraphPad Prism version 8.3.1 (GraphPad Software, San Diego, CA). A p value of less than 0.05 was considered to be statistically significant. This trial is registered with ClinicalTrials.gov, number NCT02029690.
Ethical Considerations
The clinical protocol (ClinicalTrials.gov identifier NCT02029690) was approved by the Leeds East Research Ethics Committee (14/YH/0090) and was sponsored by Polaris Pharmaceuticals, Inc.
Results
Patient Demographics
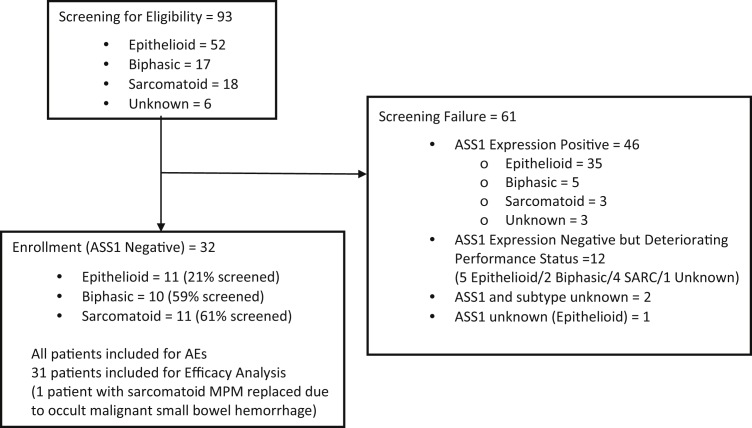
Patient enrollment in the dose-expansion study began in February 2015 and was completed in May 2016. A total of 93 patients were screened for recruiting 32 patients with ASS1–deficient MPM treated with ADIPemCis, including the following: 11 with epithelioid, 10 with biphasic, and 11 with the sarcomatoid subtype (Fig. 1). The protocol amendment for the dose-expansion cohort specified the enrollment of 30 patients at the RP2D; one patient was deemed ineligible and replaced owing to occult malignant melena, and an additional patient consented as the study recruitment was closing. All subjects were included for the safety analysis and 31 for the efficacy analysis (Table 1).
Figure 1.
CONSORT diagram. AE, adverse event; ASS1, argininosuccinate synthetase 1; MPM, malignant pleural mesothelioma; SARC, sarcomatoid.
Table 1.
Demographics
| Characteristic | Epithelioid (n = 11) | Nonepithelioid (n = 21) |
|
|---|---|---|---|
| Biphasic (n = 10) | Sarcomatoid (n = 11)∗ | ||
| Age (y), median (range) | 67 (61–77) | 66 (49–82) | 68 (58–79) |
| Sex | |||
| Male | 10 | 8 | 11 |
| Female | 1 | 2 | 0 |
| Performance status | |||
| 0 | 1 | 1 | 0 |
| 1 | 10 | 9 | 11 |
| Previous operation | |||
| Yes | 3 | 2 | 1 |
| No | 8 | 8 | 10 |
| Disease stagea | 1A (n = 1); 1B (n = 4) | 1A (n = 1) | 1B (n = 6) |
| II (n = 1) | 1B (n = 6) | II (n = 1) | |
| IIIA (n = 2) | IIIA (n = 1) | IIIA (n = 1) | |
| IV (n = 3) | IV (n = 2) | IV (n = 3) | |
| Time on study treatment (mo), median (range) | 4.6 (0.5–7.0) | 6.1 (1.9–18.0) | 4.1 (1.2–5.9) |
Eighth TNM classification for mesothelioma.
Safety
Consistent with the previous dose-escalation study, the ADIPemCis treatment was well tolerated (Supplementary Tables 1 and 2). AEs were reported in 24 of 32 patients (75%), most of which were related to cisplatin and pemetrexed (22 of 32; 68.8%), and to pegargiminase (12 of 32; 37.5%) patients. Most of the patients had grade 1 or 2 (116 of 137; 84.7%), particularly nausea and vomiting and decreased blood counts, with the remainder having grade 3 or 4 only (21 of 137; 15.3%). There were four nonhematologic grade 3 or 4 AEs related to pegargiminase, which include the following: increased alkaline phosphatase, hyperuricemia, skin rash, and posterior reversible encephalopathy syndrome. The last AE was unexpected and occurred in a patient with a sarcomatoid mesothelioma presenting with agitation and characteristic magnetic resonance imaging features during maintenance pegargiminase. He recovered completely after anxiolytics, steroids, and pegargiminase discontinuation (Supplementary Fig. 1).
Pharmacodynamics
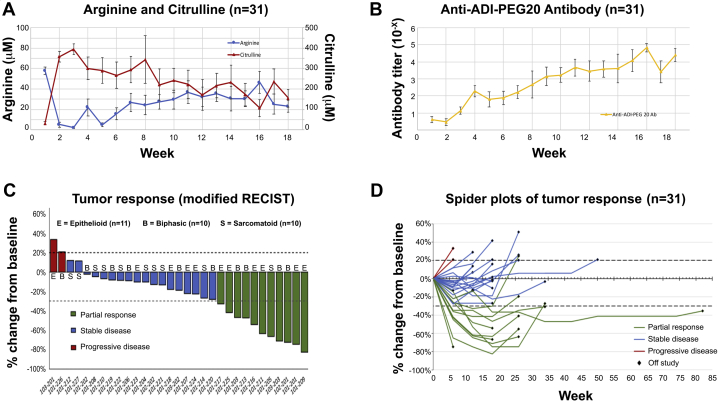
Pegargiminase decreased plasma arginine with a reciprocal increase in plasma citrulline levels in the patients (Fig. 2A). As reported in the dose-escalation cohort, plasma levels of the amino acids remained differentially altered compared with pretreatment levels by 18 weeks, despite a concomitant increase in anti–ADI-PEG 20 antibodies (Fig. 2B).
Figure 2.
Pharmacodynamics and response. (A) Pharmacodynamics of arginine and citrulline in patients treated with ADIPemCis. Serum arginine and citrulline are revealed by week of treatment (mean ± SEM). (B) Serum levels of anti–ADI-PEG 20 antibodies in all patients by week of ADIPemCis (mean ± SEM). (C) Waterfall plot of response by modified RECIST to ADIPemCis. (D) Spider plots revealing response duration to ADIPemCis. Ab, antibody; ADI, arginine deiminase; ADIPemCis, ADI-PEG 20 with pemetrexed and cisplatin; B, biphasic; E, epithelioid; PEG, PEGylated; RECIST, Response Evaluation Criteria in Solid Tumors; S, sarcomatoid.
Efficacy
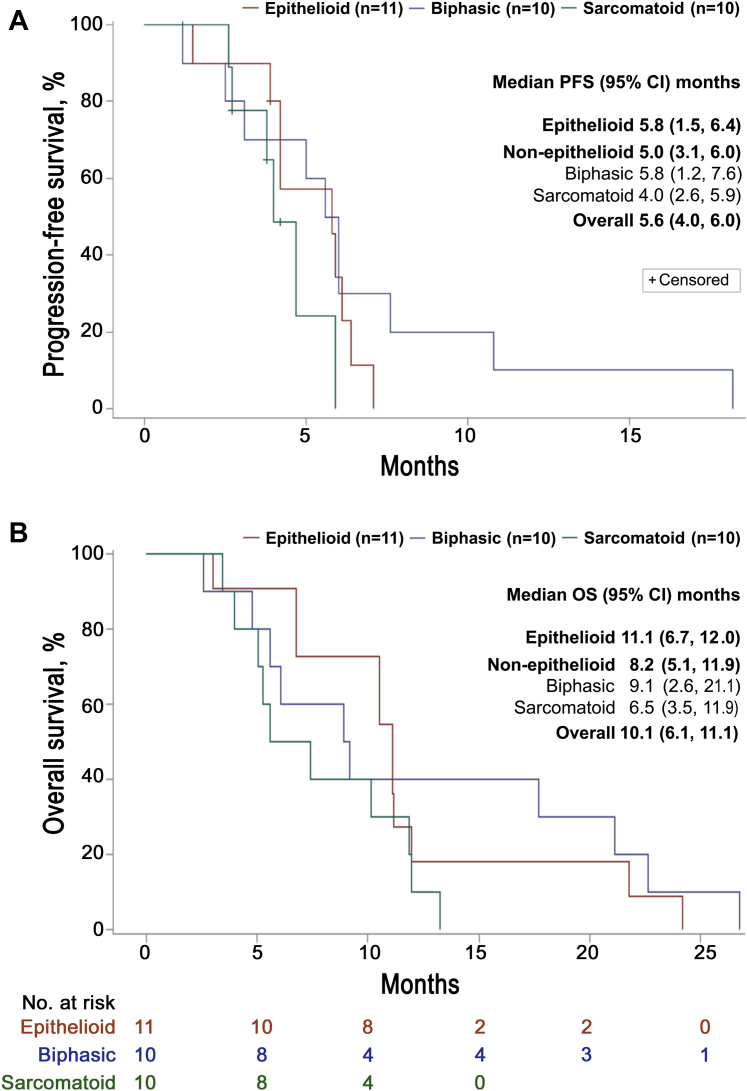
The ADIPemCis treatment induced a high disease control rate of 93.5% (n = 29 of 31; 95% confidence interval [CI]: 78.6%–99.2%), with a partial response rate of 35.5% (n = 11 of 31; 95% CI: 19.2%–54.6%) in a cohort of patients enriched by ASS1 loss for nonepithelioid MPM (Fig. 2C and D). The median PFS and OS were 5.6 (95% CI: 4.0–6.0) and 10.1 (95% CI: 6.1–11.1) months, respectively (Fig. 3A and B). Subsequently, 11 of 31 patients (35.5%) received antiprogrammed cell death protein 1 (PD-1) therapy with pembrolizumab, achieving stable disease in one patient (9.1%), whereas nine patients had progressive disease (81.8%), and one patient was nonassessable (9.1%). PD-L1 expression before treatment was available in nine of 11 patients ranging from 0% (n = 4) to 15% to 30% (n = 5). Owing to rapidly progressive disease, a few patients received second-line and subsequent therapies (vinorelbine and gemcitabine).
Figure 3.
Survival outcomes. (A) PFS by MPM histologic subtype. (B) Kaplan-Meier survival estimates by MPM histologic subtype. CI, confidence interval; MPM, malignant pleural mesothelioma; OS, overall survival; PFS, progression-free survival.
Exploratory Tumor Biopsies
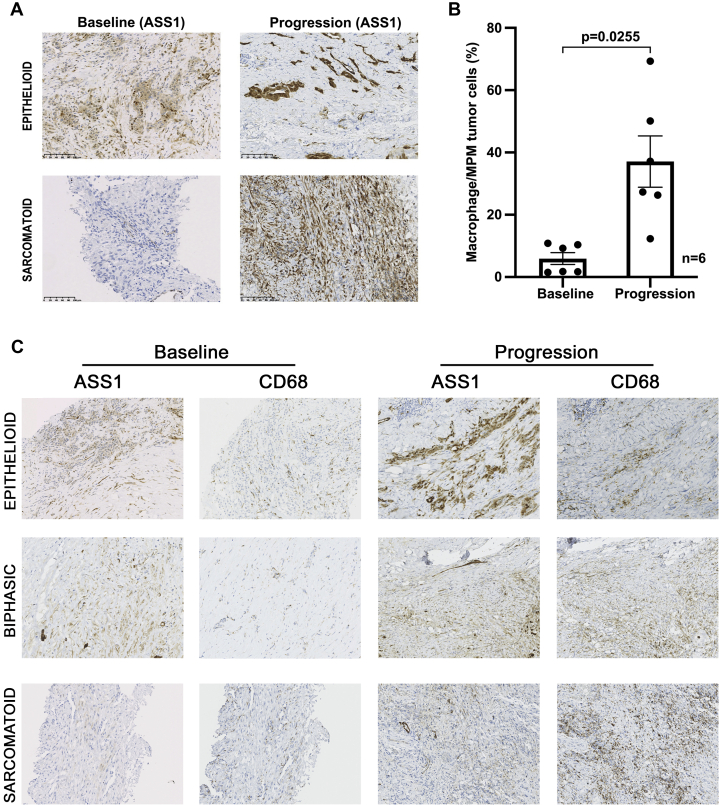
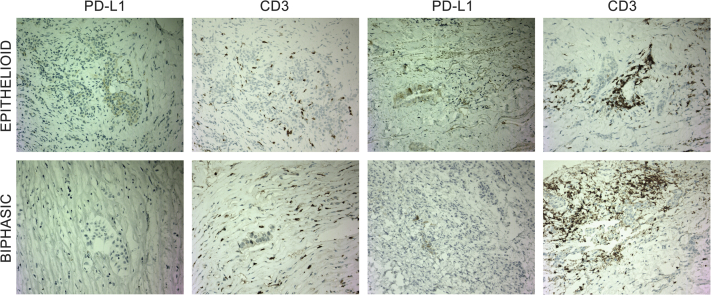
To understand drug resistance, six patients on pegargiminase therapy consented to a tumor rebiopsy at progression allowing a comparison with the pretreatment biopsies. The ASS1 levels increased in a subpopulation of MPM cells in two of six patients with epithelioid and sarcomatoid disease during cycles 5 and 6 of ADIPemCis (Fig. 4A). There was a significant increase of CD68pos ASS1pos macrophages at disease progression in ASS1neg tumor areas, which included four patients receiving maintenance pegargiminase for up to 18 months (p = 0.0255; n = 6; Fig. 4B and C). Owing to patchy ASS1 tumoral reexpression found in two patients only, we were unable to quantify the amount of macrophage infiltration specifically in ASS1pos tumor areas. We also noted an increase in tumoral PD-L1 expression and clustering of CD3pos T lymphocytes within MPM tumor cell islands in two of five patients with available tissue for immunohistochemistry (Fig. 5). In the remaining three patients, we detected variable effects on PD-L1 expression and T cell localization at disease progression (Supplementary Table 3).
Figure 4.
Baseline and progression biopsies analyzed for ASS1 and CD68. (A) Tumoral ASS1 reexpression at progression noted in two patients (×200 magnification; epithelioid and sarcomatoid). (B) Increase in CD68pos macrophages at disease progression in ASS1–deficient tumoral regions (n = 6; p = 0.0255; paired t test); two epithelioid, one sarcomatoid, and three biphasic tumors (N.B. one epithelioid tumor was reclassified as biphasic on operative rebiopsy). (C) Representative serial sections of epithelioid, biphasic, and sarcomatoid MPM at baseline and progression stained for ASS1 and CD68, revealing the increase in ASS1posCD68pos macrophages at progression (×200 magnification). ASS1, argininosuccinate synthetase 1; MPM, malignant pleural mesothelioma; N.B., nota bene; pos, positive.
Figure 5.
Baseline and progression biopsies analyzed for PD-L1 and CD3 modulation of PD-L1 expression and CD3pos lymphocytes in two patients at progression (×200 magnification; epithelioid and biphasic). PD-L1 increased from 10% to 30% (in epithelioid disease) and 0% to 5% (in biphasic disease) with clustering of CD3pos T cells in both patients at progression. PD-L1, programmed death-ligand 1; pos, positive.
Discussion
In this ASS1 biomarker-led study, we observed good tolerability and a high rate of disease control in patients with poor-prognosis MPM treated with the RP2D of ADIPemCis, expanding on the preliminary signal in the dose-escalation trial. Nonetheless, tumor progression on pegargiminase was universal, and instead of widespread ASS1 tumoral reexpression, correlated with robust macrophage infiltration on rebiopsy, pointing to a stromal-mediated resistance pathway that may be leveraged to optimize arginine-depleting cancer therapeutics. We also describe induction of tumoral PD-L1 expression and modulation of T lymphocytes, which segues into the developing area of mesothelioma immunotherapy.
The toxicities were mostly grade 1 or 2 nausea and vomiting and hematologic and injection skin reactions, whereas grade 3 or 4 events were manageable and reversible. There was only one serious grade 3 toxicity attributed to pegargiminase maintenance therapy, namely posterior reversible encephalopathy syndrome, a known complication of several biochemotherapies, including bevacizumab and the enzyme therapeutic, asparaginase, but described here for the first time with arginine deprivation.21
The median OS and PFS of 10.1 and 5.6 months, respectively, are encouraging in a patient cohort enriched for poor-prognosis ASS1–deficient disease. Biomarker screening selected two to three times as many patients with nonepithelioid compared with epithelioid disease, consistent with previous data sets for ASS1 loss in MPM, and accounting for the unusually high rate of patients enrolled with the nonepithelioid disease (65.6%).4,12,22 The median OS for epithelioid disease was 11.1 months, lower than that reported in recent phase 3 studies with a median OS of 16.1 months for patients with predominantly epithelioid disease (81%–97%) in the standard chemotherapy groups (LUME-meso [Nintedanib in combination with pemetrexed and cisplatin in mesothelioma] and MAPS [Mesothelioma Avastin Cisplatin Pemetrexed Study]).23,24 Moreover, twice as many patients were alive at 15 months with biphasic compared with the epithelioid disease (40% versus 20%), indicating that the latter subgroup is at the aggressive end of the spectrum and concurring with the poor-prognosis epithelioid disease defined by nuclear grading and p16 loss on multivariate analysis.25,26
Notably, the 8.2 month median OS for nonepithelioid disease compares favorably with the recent SWOG S0905 trial reporting a median OS of 6.3 months for PemCis plus placebo or 6.5 months for PemCis plus the vascular endothelial growth factor receptor antagonist, cediranib (n = 23; nonepithelioid).27 In addition, we observed a doubling of the median survival (6.5 versus 3.5 mo) and a three-fold increase in survival at 12 months (30% versus 10%), compared with historical controls for sarcomatoid mesothelioma.1,26 Although response assessment in mesothelioma is challenging, and reported infrequently in trials for the nonepithelioid disease, the 93.5% disease control rate is encouraging and consistent with the earlier dose-escalation study findings.12 Collectively, these data benchmarked the design of the ATOMIC-meso (ADI-PEG 20 Targeting Of Malignancy Induces Cytotoxicity-Mesothelioma) study, which transitioned from phase 2 to phase 3 earlier this year after successful recruitment of 176 patients with nonepithelioid mesothelioma; a further 210 patients are being enrolled to report on the primary end point of OS (ClinicalTrials.gov identifier NCT02709512).
A key exploratory aim of the dose-expansion study was to understand resistance to the ADI-PEG 20-based therapy by sampling patients’ tumors on progression. Six patients consented to repeat biopsies, which were incorporated into patient management, most often for control of a recurrent pleural effusion. Owing to limited baseline tissue, we analyzed the ASS1 status followed by CD68 expression on macrophages, and lastly, PD-L1 expression and CD3 expression on lymphocytes. Patchy induction of tumoral ASS1 was identified in two patients, supporting a limited role for ASS1 reexpression as a mechanism of resistance to pegargiminase as identified in the long-term MPM cell line culture under ADI-PEG 20.13 This contrasted with a robust and statistically significant influx of CD68pos ASS1pos macrophages in ASS1–deficient tumoral regions, which is of particular interest because myeloid cells are known to express abundant ASS1 under proinflammatory cytokine control.28 Moreover, arginine metabolism is a critical component of macrophage function, including nitric oxide synthesis for pathogen recognition and polyamine synthesis for wound healing.29 Interestingly, we also observed an influx of CD68pos ASS1pos macrophages in ASS1–deficient tumoral regions in the rebiopsy of two patients in a separate expansion study of ADIPemCis in patients with NSCLC, in which p equaleds 0.0079 for the entire thoracic patient cohort of ADIPemCis (Supplementary Fig. 2).30 Separately, we have identified a novel mechanism whereby ADI-PEG 20 induces several chemoattractant proinflammatory cytokines from MPM cells, triggering resistance to arginine deprivation by macrophage-derived argininosuccinate, the immediate precursor for arginine synthesis (manuscript under review).
Our analysis of resistance was limited by the availability of patient tissue, especially the polarization of the infiltrating CD68pos ASS1pos macrophages (i.e., M1 and M2 macrophage subtypes) and the potential role of autophagy which will require further study.31,32 Indeed, autophagy was inferred in a separate expansion cohort study of ADIPemCis in glioblastoma multiforme with a patient exhibiting prolonged remission on maintenance ADI-PEG 20 with quinine sulfate, an antimalarial autophagy inhibitor, and on a background of durable arginine depletion (20.8 mo).33 Indeed, autophagy as a contributory resistance mechanism has been described preclinically in various cancer cell lines, including MPM cells and is similarly abrogated with chloroquine.16,34,35 Finally, pharmacologic resistance owing to neutralizing antibodies to ADI-PEG 20 cannot be excluded entirely, as arginine levels, although persistently low compared with pretreatment levels, increased at the end of the 18-week sampling period (with reciprocal changes in citrulline). Nonetheless, the pharmacodynamic changes were durable in the dose-escalation ADIPemCis study, which reported a higher median OS of 13.9 months in patients with thoracic cancers; these interstudy differences may be explained in part by a variation in the amount of blood sampling performed at each time point owing to earlier subject withdrawal in this study (Supplementary Table 4).12 It is also relevant that blood draws were performed weekly and just before ADI-PEG 20 dosing, reflecting static rather than dynamic changes in amino acid levels.
The limited analysis of tumoral PD-L1 expression and CD3 lymphocytes at progression in the remaining five biopsies was insufficient to draw firm conclusions. However, the up-regulation of PD-L1 and CD3 lymphocytes in two of the five patients on rebiopsy is consistent with earlier preclinical work on ADI-PEG 20 inducing PD-L1 in tumor cell lines and T cell infiltration in syngeneic tumor mouse models.36 Recently, a phase 1 study of pegargiminase and pembrolizumab in solid tumors completed accrual with on-treatment biopsies that evaluate the effects of pegargiminase specifically on T cell markers in the tumor microenvironment before PD-1 blockade (ClinicalTrials.gov identifier NCT03254732).37 Although a third of the patients received pembrolizumab on progression (6 of 11 epithelioid and 5 of 11 nonepithelioid), the disease control rate of 11.1% (n = 1 of 11; biphasic disease) was disappointing and lower than that reported in larger patient studies of PD-1/PD-L1 blockade in mesothelioma (47%–72%).38, 39, 40, 41 However, four of the patients treated with pembrolizumab expressed PD-L1 less than 1% (n = 4 of 9 or 44.4), which is known to correlate with lower responses to PD-1 blockade compared with greater than 1% PD-L1 MPM expression (Supplementary Table 5).41 Furthermore, the influx of TAMs reported previously may have contributed potentially to a more immunosuppressive environment constraining PD1–based immune checkpoint therapy.42
Clearly, further dissection of the complex effects of arginine deprivation on the mesothelioma microenvironment will be needed to understand the role of ADI-PEG 20 in the context of mesothelioma immunotherapy.43, 44, 45 Moreover, studies in urea cycle–dysregulated cancers suggest that biomarker analysis will be of increasing importance in guiding prognosis and therapeutic response to arginine-based therapeutics.46 We propose that the macrophage influx may be exploited pharmacologically to optimize arginine deprivation as a novel antimetabolite therapy for mesothelioma and other treatment-resistant cancers. Indeed, several approaches are under clinical evaluation, including CSF-1R, CXCR2, CD47 (don’t eat me), and PD-1 antagonists, to retarget TAMs for tumor cell killing.47, 48, 49, 50, 51, 52
In summary, ADIPemCis is safe and active in an expansion cohort of patients with aggressive ASS1–deficient MPM, and a phase 3 trial is underway. Our data also provide novel insights into resistance pathways to arginine deprivation, going beyond tumoral ASS1 reexpression, namely macrophage trafficking. Validation of this innate-immunometabolic relationship by targeting macrophages alongside tumor cells with pegargiminase therapy has the potential to improve patient outcomes with mesothelioma and other arginine-auxotrophic cancers.
Acknowledgments
This work was funded and sponsored by Polaris Pharmaceuticals, Inc., and supported by Barts (Queen Mary University of London), King’s College (University of London), and Cambridge Experimental Cancer Medicine Centers and the National Institute for Health Research Cambridge Clinical Research Facility. The Cambridge Human Research Tissue Bank is supported by the National Institute for Health Research Cambridge Biomedical Research Centre. The authors are indebted to all the patients and their families who took part in the dose-expansion Tumors Requiring Arginine to Assess ADI-PEG 20, Pemetrexed and cisplatin (Thoracic cohort) study. The authors also thank Suzanne Jordan for her clinical pathology services in Barts.
Footnotes
Disclosure: Dr. Szlosarek has received support from the Higher Education Funding Council for England and research support from the Polaris Group. Drs. Bomalaski, Johnson, and Feng are paid employees of Polaris Pharmaceuticals. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100093.
Supplementary Data
References
- 1.Klebe S., Brownlee N.A., Mahar A. Sarcomatoid mesothelioma: a clinical-pathologic correlation of 326 cases. Mod Pathol. 2010;23:470–479. doi: 10.1038/modpathol.2009.180. [DOI] [PubMed] [Google Scholar]
- 2.Vigneswaran W.T., Kircheva D.Y., Ananthanarayanan V. Amount of epithelioid differentiation is a predictor of survival in malignant pleural mesothelioma. Ann Thorac Surg. 2017;103:962–966. doi: 10.1016/j.athoracsur.2016.08.063. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzang N.J., Rusthoven J.J., Symanowski J. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 4.Szlosarek P.W., Klabatsa A., Pallaska A. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res. 2006;12:7126–7131. doi: 10.1158/1078-0432.CCR-06-1101. [DOI] [PubMed] [Google Scholar]
- 5.Allen M.D., Luong P., Hudson C. Prognostic and therapeutic impact of argininosuccinate synthetase 1 control in bladder cancer as monitored longitudinally by PET imaging. Cancer Res. 2014;74:896–907. doi: 10.1158/0008-5472.CAN-13-1702. [DOI] [PubMed] [Google Scholar]
- 6.Rabinovich S., Adler L., Yizhak K. Diversion of aspartate in ASS1-deficient tumors fosters de novo pyrimidine synthesis. Nature. 2015;527:379–383. doi: 10.1038/nature15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keshet R., Szlosarek P., Carracedo A., Erez A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat Rev Cancer. 2018;18:634–645. doi: 10.1038/s41568-018-0054-z. [DOI] [PubMed] [Google Scholar]
- 8.Ensor C.M., Holtsberg F.W., Bomalaski J.S., Clark M.A. PEGylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002;62:5443–5450. [PubMed] [Google Scholar]
- 9.Cheng P.N., Lam T.L., Lam W.M. PEGylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007;67:309–317. doi: 10.1158/0008-5472.CAN-06-1945. [DOI] [PubMed] [Google Scholar]
- 10.Phillips M.M., Sheaff M.T., Szlosarek P.W. Targeting arginine-dependent cancers with arginine-degrading enzymes: opportunities and challenges. Cancer Res Treat. 2013;45:251–262. doi: 10.4143/crt.2013.45.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szlosarek P.W., Steele J.P., Nolan L. Arginine deprivation with PEGylated arginine deiminase in patients with argininosuccinate synthetase 1-deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol. 2017;3:58–66. doi: 10.1001/jamaoncol.2016.3049. [DOI] [PubMed] [Google Scholar]
- 12.Beddowes E., Spicer J., Chan P.Y. Phase 1 dose-escalation study of PEGylated arginine deiminase, cisplatin, and pemetrexed in patients with argininosuccinate synthetase 1-deficient thoracic cancers. J Clin Oncol. 2017;35:1778–1785. doi: 10.1200/JCO.2016.71.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locke M., Ghazaly E., Freitas M.O. Inhibition of the polyamine synthesis pathway is synthetically lethal with loss of argininosuccinate synthase 1. Cell Rep. 2016;16:1604–1613. doi: 10.1016/j.celrep.2016.06.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai W.B., Aiba I., Lee S.Y., Feun L., Savaraj N., Kuo M.T. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1alpha/Sp4. Mol Cancer Ther. 2009;8:3223–3233. doi: 10.1158/1535-7163.MCT-09-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feun L.G., Marini A., Walker G. Negative argininosuccinate synthetase expression in melanoma tumors may predict clinical benefit from arginine-depleting therapy with PEGylated arginine deiminase. Br J Cancer. 2012;106:1481–1485. doi: 10.1038/bjc.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battisti S., Valente D., Albonici L., Bei R., Modesti A., Palumbo C. Nutritional stress and arginine auxotrophy confer high sensitivity to chloroquine toxicity in mesothelioma cells. Am J Respir Cell Mol Biol. 2012;46:498–506. doi: 10.1165/rcmb.2011-0195OC. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Balkwill F.R., Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Burt B.M., Rodig S.J., Tilleman T.R., Elbardissi A.W., Bueno R., Sugarbaker D.J. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer. 2011;117:5234–5244. doi: 10.1002/cncr.26143. [DOI] [PubMed] [Google Scholar]
- 20.Miselis N.R., Wu Z.J., Van Rooijen N., Kane A.B. Targeting tumor-associated macrophages in an orthotopic murine model of diffuse malignant mesothelioma. Mol Cancer Ther. 2008;7:788–799. doi: 10.1158/1535-7163.MCT-07-0579. [DOI] [PubMed] [Google Scholar]
- 21.Peddi P.F., Peddi S., Santos E.S., Morgensztern D. Central nervous system toxicities of chemotherapeutic agents. Expert Rev Anticancer Ther. 2014;14:857–863. doi: 10.1586/14737140.2014.911089. [DOI] [PubMed] [Google Scholar]
- 22.Bueno R., Stawiski E.W., Goldstein L.D. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–416. doi: 10.1038/ng.3520. [DOI] [PubMed] [Google Scholar]
- 23.Scagliotti G.V., Gaafar R., Nowak A.K. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2019;7:569–580. doi: 10.1016/S2213-2600(19)30139-0. [DOI] [PubMed] [Google Scholar]
- 24.Zalcman G., Mazieres J., Margery J. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial [published correction appears in Lancet. 2016;387:e24] Lancet. 2016;387:1405–1414. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 25.Forest F., Patoir A., Dal Col P. Nuclear grading, BAP1, mesothelin and PD-L1 expression in malignant pleural mesothelioma: prognostic implications. Pathology. 2018;50:635–641. doi: 10.1016/j.pathol.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Chou A., Toon C.W., Clarkson A., Sheen A., Sioson L., Gill A.J. The epithelioid BAP1-negative and p16-positive phenotype predicts prolonged survival in pleural mesothelioma. Histopathology. 2018;72:509–515. doi: 10.1111/his.13392. [DOI] [PubMed] [Google Scholar]
- 27.Tsao A.S., Miao J., Wistuba I.I. Phase II trial of cediranib in combination with cisplatin and pemetrexed in chemotherapy-naïve patients with unresectable malignant pleural mesothelioma (SWOG S0905) J Clin Oncol. 2019;37:2537–2547. doi: 10.1200/JCO.19.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nussler A.K., Billiar T.R., Liu Z.Z., Morris S.M., Jr. Coinduction of nitric oxide synthase and argininosuccinate synthetase in a murine macrophage cell line. Implications for regulation of nitric oxide production. J Biol Chem. 1994;269:1257–1261. [PubMed] [Google Scholar]
- 29.Phillips M., Szyszko T., Hall P. Abstract B33: expansion study of PEGylated arginine deiminase (ADI-PEG 20), pemetrexed, and cisplatin in patients with ASS1-deficient non-squamous non-small cell lung cancer (TRAP) Clin Cancer Res. 2018;24(suppl) [Google Scholar]
- 30.Phillips M., Szlosarek P.W. In: Tumour-Associated Macrophages. Lawrence T., Hagemann T., editors. Springer; New York, NY: 2012. Arginine metabolism and tumour associated macrophages; pp. 77–90. [Google Scholar]
- 31.Jayasingam S.D., Citartan M., Thang T.H., Mat Zin A.A., Ang K.C., Ch’ng E.S. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Front Oncol. 2020;9:1512. doi: 10.3389/fonc.2019.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klionsky D.J., Abdelmohsen K., Abe A. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) [published correction appears in Autophagy. 2016;12:443. Selliez, Iban [corrected to Seiliez, Iban]] Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall P.E., Lewis R., Syed N. A phase I study of PEGylated arginine deiminase (pegargiminase), cisplatin, and pemetrexed in argininosuccinate synthetase 1-deficient recurrent high-grade glioma. Clin Cancer Res. 2019;25:2708–2716. doi: 10.1158/1078-0432.CCR-18-3729. [DOI] [PubMed] [Google Scholar]
- 34.Kim R.H., Coates J.M., Bowles T.L. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009;69:700–708. doi: 10.1158/0008-5472.CAN-08-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delage B., Luong P., Maharaj L. Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis. 2012;3:e342. doi: 10.1038/cddis.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brin E., Wu K., Lu H.T., He Y., Dai Z., He W. PEGylated arginine deiminase can modulate tumor immune microenvironment by affecting immune checkpoint expression, decreasing regulatory T cell accumulation and inducing tumor T cell infiltration. Oncotarget. 2017;8:58948–58963. doi: 10.18632/oncotarget.19564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang K.Y., Chiang N.J., Yen C.J. A phase Ib study of ADI-PEG 20 plus pembrolizumab in advanced solid cancers. J Clin Oncol. 2018;36(supple15) doi: 10.1080/2162402X.2021.1943253. 2556–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alley E.W., Lopez J., Santoro A. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18:623–630. doi: 10.1016/S1470-2045(17)30169-9. [DOI] [PubMed] [Google Scholar]
- 39.Quispel-Janssen J., van der Noort V., de Vries J.F. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol. 2018;13:1569–1576. doi: 10.1016/j.jtho.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 40.Hassan R., Thomas A., Nemunaitis J.J. Efficacy and safety of avelumab treatment in patients with advanced unresectable mesothelioma: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5:351–357. doi: 10.1001/jamaoncol.2018.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada M., Kijima T., Aoe K. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, Japanese phase II study in malignant pleural mesothelioma (MERIT) Clin Cancer Res. 2019;25:5485–5492. doi: 10.1158/1078-0432.CCR-19-0103. [DOI] [PubMed] [Google Scholar]
- 42.Awad M.M., Jones R.E., Liu H. Cytotoxic T cells in PD-L1-positive malignant pleural mesotheliomas are counterbalanced by distinct immunosuppressive factors. Cancer Immunol Res. 2016;4:1038–1048. doi: 10.1158/2326-6066.CIR-16-0171. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez P.C., Ochoa A.C., Al-Khami A.A. Arginine metabolism in myeloid cells shapes innate and adaptive immunity. Front Immunol. 2017;8:93. doi: 10.3389/fimmu.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray P.J. Amino acid auxotrophy as a system of immunological control nodes. Nat Immunol. 2016;17:132–139. doi: 10.1038/ni.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemos H., Huang L., Prendergast G.C., Mellor A.L. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat Rev Cancer. 2019;19:162–175. doi: 10.1038/s41568-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 46.Lee J.S., Adler L., Karathia H. Urea cycle dysregulation generates clinically relevant genomic and biochemical signatures. Cell. 2018;174:1559–1570. doi: 10.1016/j.cell.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ries C.H., Cannarile M.A., Hoves S. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 48.Sharma B., Nawandar D.M., Nannuru K.C., Varney M.L., Singh R.K. Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Mol Cancer Ther. 2013;12:799–808. doi: 10.1158/1535-7163.MCT-12-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matlung H.L., Szilagyi K., Barclay N.A., van den Berg T.K. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276:145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 50.Schürch C.M., Forster S., Brühl F., Yang S.H., Felley-Bosco E., Hewer E. The “don’t eat me” signal CD47 is a novel diagnostic biomarker and potential therapeutic target for diffuse malignant mesothelioma. Oncoimmunology. 2017;7 doi: 10.1080/2162402X.2017.1373235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon S.R., Maute R.L., Dulken B.W. PD-1 expression by tumor-associated macrophages inhibits phagocytosis and tumor immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vitale I., Manic G., Coussens L.M., Kroemer G., Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30:36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.