Abstract
Introduction
JNJ-64041757 (JNJ-757) is a live, attenuated, double-deleted Listeria monocytogenes–based immunotherapy expressing human mesothelin. JNJ-757 was evaluated in patients with advanced NSCLC as monotherapy (phase 1) and in combination with nivolumab (phase 1b/2).
Methods
Patients with stage IIIB/IV NSCLC who had received previous therapy were treated with JNJ-757 (1 × 108 or 1 × 109 colony-forming units [CFUs]) alone (NCT02592967) or JNJ-757 (1 × 109 CFU) plus intravenous nivolumab 240 mg (NCT03371381). Study objectives included the assessment of immunogenicity, safety, and efficacy.
Results
In the monotherapy study, 18 patients (median age 63.5 y; women 61%) were treated with JNJ-757 (1 × 108 or 1 × 109 CFU) with a median duration of 1.4 months (range: 0–29). The most common adverse events (AEs) were pyrexia (72%) and chills (61%), which were usually mild and resolved within 48 hours. Peripheral proinflammatory cytokines and lymphocyte activation were induced posttreatment with transient mesothelin-specific T-cell responses in 10 of 13 biomarker-evaluable patients. With monotherapy, four of 18 response-evaluable patients had stable disease of 16 or more weeks, including one patient with a reduction in target lesions. In the combination study, 12 patients were enrolled (median age 63.5 y; women 33%). The most common AEs with combination therapy were pyrexia (67%) and chills (58%); six patients had grade 3 AEs or greater, including two cases of treatment-related fatal pneumonitis. The best overall response for the combination was stable disease in four of nine response-evaluable patients.
Conclusions
As monotherapy, JNJ-757 was immunogenic and tolerable, with mild infusion-related fever and chills. The limited efficacy of JNJ-757, alone or with nivolumab, did not warrant further investigation of the combination.
Keywords: Non–small cell lung cancer, Mesothelin, LADD Lm, JNJ-757, Vaccine
Introduction
Lung cancer is the leading cause of cancer mortality worldwide, accounting for 18.4% of all cancer deaths in 2018, with most cases (85%) being NSCLCs.1, 2, 3 Despite recent advances in molecular testing and the introduction of targeted agents, the prognosis for patients with NSCLC remains poor, with a 5-year survival rate of 23% for all stages of NSCLC and 6% for metastatic disease.4 Therapies with immune checkpoint inhibitors (ICIs) have resulted in longer remissions and overall survival, with 5-year survival rates now approaching 15% in patients with advanced NSCLC.5,6 However, because of primary or acquired resistance, not all patients respond to ICIs, and those who do respond typically relapse. Improved biomarker-based selection of patients and possible combinations of ICIs with other therapeutic modalities may be needed to expand their clinical benefits in terms of the proportion of responders and duration of response.
Another immunotherapy approach for NSCLC is the use of therapeutic vaccinations to induce or increase innate and adaptive immune responses to target tumor cells. Several types of cancer vaccines have been developed for the treatment of NSCLC, including allogenic vaccines, peptide or protein vaccines, autologous dendritic cell vaccines, DNA vaccines, and vector-based vaccines.7 Although these vaccines are reported to stimulate cellular immunity and are well tolerated, most have not exhibited a substantial survival advantage in late-stage clinical trials,10, 11, 8, 9 possibly owing to an inability to overcome the immune evasion mechanisms used by NSCLC cells.12, 13, 14, 15
Live, attenuated, double-deleted (LADD) Listeria monocytogenes–based immunotherapy offers a novel approach to potentially tackle the issue of immune evasion by tumor cells. The LADD L. monocytogenes–based platform features a deletion of two virulence genes from the L. monocytogenes chromosome—actin assembly protein and internalin B—resulting in the prevention of cell-to-cell spread and infection of nonphagocytic cells, respectively, which leads to a 1000-fold attenuation in virulence without loss of its ability to induce innate and adaptive cellular immunity.16,17 On infection, virulence factors such as listeriolysin-O (LLO) and phospholipase C are secreted and dissolve the phagosome membrane, resulting in L. monocytogenes entering the host cell and any L. monocytogenes–secreted protein attaining direct access to the antigen processing pathway.18,19 In addition, L. monocytogenes–based immunotherapies inhibit the immunosuppression mediated by myeloid-derived suppressor cells and regulatory T-cells.20,21 Thus, LADD L. monocytogenes–based immunotherapies have the potential to enhance antigen processing and presentation, elicit strong antigen-specific tumor responses, and suppress the immune resistance of the tumor microenvironment.22
JNJ-64041757 (JNJ-757) is a LADD L. monocytogenes–based immunotherapy that encodes and expresses human mesothelin. Mesothelin, a glycoprotein expressed on the surface of cells lining the pleura, peritoneum, and pericardium, is a tumor-associated antigen that is overexpressed in 30% to 70% of NSCLCs.23, 24, 25, 26, 27 A study that evaluated mesothelin expression in patients with advanced lung adenocarcinoma (N = 93) reported that 24% of patients expressed high levels (≥25% of cells) of mesothelin; of these, 68% also had a KRAS mutation. In addition, high mesothelin expression was associated with poor prognosis, with a median overall survival of 18.2 months compared with 32.9 months in non–high expressers.28 In preclinical studies, JNJ-757 induced immune responses to mesothelin, as measured by intracellular cytokine staining of mouse splenocytes, and dose-dependently increased survival in a pulmonary metastatic mouse model (data on file). In murine models, the combination of programmed cell death protein 1 (PD-1) inhibition and JNJ-757 resulted in synergistic effects on adaptive responses to mesothelin, reduction of the mean tumor volume, and prolonged survival (data on file). These data suggest that JNJ-757 may have the potential to induce cellular immunity against mesothelin-expressing tumors and act synergistically with ICIs to improve efficacy compared with PD-1 inhibitor monotherapy.
Here, we report the findings from two clinical studies evaluating JNJ-757 in patients with advanced lung adenocarcinoma in a phase 1 monotherapy study and in a phase 1b/2 study in combination with the PD-1 inhibitor, nivolumab.
Materials and Methods
Study Conduct
All protocols were reviewed and approved by an institutional review board. The studies were conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practices and applicable regulatory requirements. Patients or their legally acceptable representatives provided their written consent to participate in the study after having been informed about the nature and purpose of the study.
Study Design
Phase 1 Monotherapy Study
This was a phase 1, open-label, multicenter, two-part study conducted at nine sites in the United States from December 8, 2015 to October 22, 2018 (NCT02592967). The primary objective of part 1 (dose escalation) was to confirm the recommended phase 2 dose (RP2D) of JNJ-757, and that of part 2 (dose expansion) was to further characterize the safety and to determine the preliminary immunologic activity at the RP2D in two expansion cohorts: 2A (patients with adenocarcinoma without selection on the basis of mesothelin expression) and 2B (patients with ≥50% mesothelin overexpression). The primary study end points were the incidence of dose-limiting toxicity (DLT) and adverse events (AEs) (part 1) and the incidence of AEs and antigen-specific T-cell response (part 2). For both parts, key secondary objectives included evaluating the preliminary clinical activity and assessing the blood culture and shedding profile of JNJ-757, and key exploratory objectives included evaluating the immunogenic activity of JNJ-757. Two doses of intravenous JNJ-757 were explored in up to 10 patients per cohort (1 × 108 [cohort 1A] and 1 × 109 colony-forming unit [CFU] [cohort 1B]). The doses were selected on the basis of previous clinical experience with another L. monocytogenes–based vaccine expressing human mesothelin, CRS-207, in which the 100 million (1 × 108) and 1 billion (1 × 109) CFU doses were tolerable, and 1 billion CFU was identified as the maximum tolerated dose after a DLT was reported at the 10 billion (1 × 1010) CFU dose.29 JNJ-757 was administered by means of a peripheral vein catheter over a 1-hour period. Treatment was administered once every 21 days. Dose escalation proceeded if none of the first three patients in cohort 1A experienced a DLT. Pretreatment and posttreatment biopsies of the metastatic lesion(s) for assessing mesothelin expression by immunohistochemistry were optional for patients in cohort 1 and cohort 2A but mandatory for cohort 2B. On the basis of evolving data, the decision was made to close the study early and proceed with a phase 1b/2 combination study.
Phase 1b/2 Combination Study
This was a multicenter, open-label, randomized phase 1b/2 study conducted at 10 sites in Spain, the United States, and Belgium from March 16, 2018 to October 9, 2018 (NCT03371381). The primary objective was to evaluate whether the efficacy of JNJ-757 combined with nivolumab was better than the efficacy of nivolumab monotherapy. The primary end point was the objective response rate. The key secondary objectives included evaluating the safety of JNJ-757 in combination with nivolumab and assessing the blood culture and shedding profile of JNJ-757. The key exploratory objectives included monitoring the markers of innate and adaptive immune responses. The phase 1b safety run-in phase was conducted with at least six patients to evaluate the incidence of DLTs and the tolerability of JNJ-757 in combination with nivolumab. Patients received intravenous nivolumab 240 mg over approximately 1 hour every 2 weeks in 28-day cycles. After completion of nivolumab infusion on day 1, JNJ-757, at a dose of 1 billion CFU, was administered intravenously over approximately 1 hour.
Patients
Eligible patients for the phase 1 monotherapy study were 18 years or older, had histologically or cytologically documented lung adenocarcinoma, stage IIIB or IV disease, at least one measurable site of disease (part 2 of the study only), and had Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1. Patients had received at least two previous lines of approved systemic therapies, of which one had to be platinum-based chemotherapy; previous treatment with ICIs was allowed but not required.
Eligible patients for the phase 1b/2 combination study were aged 18 years or older, had histologically documented NSCLC adenocarcinoma, stage IIIB or IV disease, ECOG PS of 0 or 1, mesothelin-positive tumor biopsy (>0% positive tumor cells by immunohistochemistry performed at a central laboratory) at screening (criterion added as a protocol amendment for phase 1b portion of the study), and progressive disease (PD) during or after platinum-based doublet chemotherapy.
Study Assessments
Safety was assessed by physical examinations, ECOG PS, laboratory tests, vital signs, electrocardiograms, monitoring of AEs, and concomitant medication usage. The DLT evaluation period was the first 21 or 28 days after the first infusion for part 1 of the monotherapy study and for the combination study, respectively. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Disease response was assessed using computed tomography scans of the chest, abdomen, and pelvis with intravenous contrast. Response to treatment was assessed by the investigator according to the Response Evaluation Criteria in Solid Tumors, version 1.1.
Blood cultures were performed during the treatment period, at the end-of-treatment visit, and during the follow-up phase. In the monotherapy study, surveillance blood samples were collected to investigate the clearance of JNJ-757 on C1D1 (before infusion and at 2 ± 0.5, 4 ± 0.5, 24 ± 4, and 48 ± 4 h after end-of-infusion); on C1D7 and C1D14; on C2D1 (before infusion); day 1 of subsequent cycles with a disease assessment (before infusion); and at the end-of-treatment visit. In the combination study, surveillance blood samples were collected on days 1, 2, and 15 of cycle 1; day 1 of cycles 2 and 3; day 1 of subsequent cycles with a disease assessment; and at the end-of-treatment visit. In both studies, patients were required to receive prophylactic antibiotics (intravenous amoxicillin 500 mg thrice daily [or oral trimethoprim 160 mg and sulfamethoxazole 800 mg twice daily for patients with penicillin allergy]) for 7 days after completion of treatment, starting on the day of the end-of-treatment visit. Patients with indwelling venous access devices had their first dose of antibiotics administered intravenously through the device as a prophylactic measure against device colonization. Surveillance blood samples were also collected during the follow-up phase after the required antibiotic treatment.
The potential shedding of JNJ-757 was studied in cultures of feces, urine, and saliva. Core-needle biopsies and blood samples were collected for biomarker analyses by interferon gamma enzyme-linked immunospot (ELISpot) assay, T-cell proliferation/intracellular cytokine staining, absolute lymphocyte counts, and cytokine release assays. ELISpot response was considered positive if the difference between background-subtracted response at any point after dosing and that at the screening was greater than or equal to 1.5 times the SD of response at baseline or at least 10 spot forming units greater.
Statistical Analyses
The all-treated population included patients who received at least one dose of study agent and was considered as primary for all efficacy and safety evaluations. The biomarker-evaluable population included patients who received at least one dose of study agent and had at least one pretreatment and posttreatment biomarker evaluation.
Data were summarized using descriptive statistics. Continuous variables were summarized using the number of observations, mean, SD, coefficient of variation, median, and range, as appropriate. Categorical values were summarized using the number of observations and percentages as appropriate.
Results
Patients
A total of 18 patients were enrolled and treated in the monotherapy study. The median age was 64 years (range: 47–79), and 61% of patients were women (Table 1). During dose escalation, six patients were infused with JNJ-757 at 100 million CFU and three patients at 1 billion CFU. No DLTs were reported at either dose, and 1 billion CFU was selected as the RP2D to further assess the safety and explore antitumor activity in the dose-expansion phase. In total, nine patients were treated at 1 billion CFU in dose expansion: eight in cohort 2A and one in cohort 2B (patients with ≥50% mesothelin overexpression). All patients discontinued from study treatment owing to PD (83%), AE (6%), death (6%), or physician decision (6%) (Supplementary Fig. 1A and Supplementary Data 1). The monotherapy study was stopped after the combination study was initiated.
Table 1.
Demographic and Baseline Disease Characteristics
| Characteristic | Monotherapy |
Combination |
||
|---|---|---|---|---|
| 1 × 108 CFU n = 6 |
1 × 109 CFU n = 12 |
Total N = 18 |
Total N = 12 |
|
| Age, y, median (range) | 65 (56–73) | 64 (47–79) | 64 (47–79) | 64 (38–77) |
| Sex, n (%) | ||||
| Female | 4 (67) | 7 (58) | 11 (61) | 4 (33) |
| Male | 2 (33) | 5 (42) | 7 (39) | 8 (67) |
| Race, n (%) | ||||
| White | 2 (33) | 9 (75) | 11 (61) | 12 (100) |
| Black or African American | 3 (50) | 2 (17) | 5 (28) | 0 |
| Asian | 0 | 1 (8) | 1 (6) | 0 |
| Unknown | 1 (17) | 0 | 1 (6) | 0 |
| Cancer stage, n (%) | ||||
| IIIA | 0 | 0 | 0 | 1 (8)a |
| IIIB | 1 (17) | 0 | 1 (6) | 1 (8) |
| IV | 5 (83) | 12 (100) | 17 (94) | 10 (83) |
| ECOG PS, n (%) | ||||
| 0 | 2 (33) | 0 | 2 (11) | 5 (42) |
| 1 | 4 (67) | 12 (100) | 16 (89) | 7 (58) |
| Mutation status, n (%) | ||||
| EGFR mutation | 1 (17) | 3 (25) | 4 (22) | 0 |
| KRAS mutation | 1 (17) | 5 (42) | 6 (33) | 2 (17) |
| No mutationb | 2 (33) | 3 (25) | 5 (28) | 9 (75) |
| Unknown | 2 (33) | 1 (8) | 3 (17) | 1 (8) |
| Previous lines of therapy, n (%) | ||||
| 1 | 0 | 0 | 0 | 4 (33) |
| >1 | 6 (100) | 12 (100) | 18 (100) | 8 (67) |
| Previous systemic therapy, n (%) | 6 (100) | 12 (100) | 18 (100) | 12 (100) |
| Chemotherapy | 6 (100) | 12 (100) | 18 (100) | 12 (100) |
| PD-1/PD-L1 inhibitors | 3 (50) | 12 (100) | 15 (83) | 8 (67) |
| Tyrosine kinase inhibitor | 1 (17) | 4 (33) | 5 (28) | 1 (8) |
| Other | 4 (67) | 6 (50) | 10 (56) | 6 (50) |
CFU, colony-forming unit; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-1, programmed cell death protein-1; PD-L1, programmed death-ligand 1.
Initial diagnosis was stage IIIA but entered the study as stage IV.
Screened for EGFR, KRAS, ROS1, and ALK.
In the combination study, 12 patients were enrolled and treated in phase 1b. The median age was 64 years (range: 38–77), and 67% were men (Table 1). All patients received JNJ-757 1 billion CFU plus nivolumab 240 mg over approximately 1 hour every 2 weeks. The combination treatment was well tolerated, with no DLTs reported. All 12 patients discontinued study treatment owing to PD (50%), study closure (25%), AE (17%), and death (8%) (Supplementary Fig. 1B and Supplementary Data 1). The combination study was stopped before the initiation of the randomized phase 2 portion of the study owing to the evolving risk-benefit profile.
Safety
In the monotherapy study, AEs were reported for all patients who were administered JNJ-757 (Table 2). Pyrexia (13 of 18; 72%) and chills (11 of 18; 61%) were the most common AEs, which were usually mild and resolved within 48 hours and were consistent with transient activation of an innate immune response to JNJ-757. The most common grade 3 AEs or greater were hypertension (three of 18; 17%) and lymphopenia (two of 18; 11%). Four patients (22%) had grade 3 AEs or greater considered related to JNJ-757 by the investigator, which were as follows: (1) grade 3 hypertension (1 × 108 CFU); (2) grade 3 decreased lymphocyte count (1 × 109 CFU); (3) grade 3 hypotension (1 × 109 CFU); and (4) multiple grade 3 AEs (dyspnea, hypoxia, and pneumonitis) (1 × 109 CFU). Serious AEs were reported in eight patients (44%), including one patient treated with a dose of 1 billion CFU with treatment-related pyrexia and pneumonia. Treatment discontinuation owing to AEs was reported in four patients; none of these were considered related to JNJ-757 by the investigator. One death owing to an AE of malignant ascites was reported, which was considered not related to JNJ-757 by the investigator.
Table 2.
Safety Summary
| AE, n (%) | Monotherapy |
Combination |
||
|---|---|---|---|---|
| 1 × 108 CFU n = 6 | 1 × 109 CFU n = 12 | Total N =18 | Total N = 12 | |
| Any AE | 6 (100) | 12 (100) | 18 (100) | 12 (100) |
| Related AEsa | 6 (100) | 11 (92) | 17 (94) | 12 (100) |
| Grade ≥3 AEs | 4 (67) | 8 (67) | 12 (67) | 6 (50) |
| Related grade ≥3 AEsa | 1 (17) | 3 (25) | 4 (22) | 3 (25) |
| Serious AEs | 1 (17) | 7 (58) | 8 (44) | 5 (42) |
| Related serious AEsa | 0 | 1 (8) | 1 (6) | 3 (25) |
| Grade ≥3 serious AEs | 1 (17) | 7 (58) | 8 (44) | 5 (42) |
| AEs leading to treatment discontinuation | 1 (17) | 3 (25) | 4 (22) | 3 (25) |
| AEs leading to death | 1 (17) | 0 | 1 (6) | 4 (33) |
| Most Common AEs,b n (%) | ||||
| Pyrexia | 4 (67) | 9 (75) | 13 (72) | 8 (67) |
| Chills | 3 (50) | 8 (67) | 11 (61) | 7 (58) |
| Fatigue | 3 (50) | 5 (42) | 8 (44) | 2 (17) |
| Nausea | 4 (67) | 4 (33) | 8 (44) | 5 (42) |
| Hypotension | 2 (33) | 6 (50) | 8 (44) | 2 (17) |
| Decreased appetite | 2 (33) | 6 (50) | 8 (44) | 4 (33) |
| Influenza-like illness | 3 (50) | 4 (33) | 7 (39) | 0 |
| Vomiting | 4 (67) | 3 (25) | 7 (39) | 3 (25) |
| Headache | 3 (50) | 3 (25) | 6 (33) | 1 (8) |
| Diarrhea | 2 (33) | 3 (25) | 5 (28) | 0 |
| Tachycardia | 2 (33) | 3 (25) | 5 (28) | 1 (8) |
| Asthenia | 0 | 0 | 0 | 6 (50) |
| Dyspnea | 0 | 3 (25) | 3 (17) | 5 (42) |
| Anemia | 1 (17) | 0 | 1 (6) | 5 (42) |
| Most common grade ≥3 AEs,c n (%) | ||||
| Hypertension | 1 (17)d | 2 (17)d | 3 (17)d | 0 |
| Treatment-related | 1 (17)d | 0 | 1 (6)d | 0 |
| Lymphopenia | 0 | 2 (17)e | 2 (11)e | 0 |
| Treatment-related | 0 | 1 (8)d | 1 (6)d | 0 |
| Pneumonitis | 0 | 1 (8)d | 1 (6)d | 2 (17)f |
| Treatment-related | 0 | 1 (8)d | 1 (6)d | 2 (17)f |
| Hyponatremia | 0 | 0 | 0 | 2 (17)d |
| Treatment-related | 0 | 0 | 0 | 0 |
AE, adverse event; CFU, colony-forming unit.
Assessed by the investigator as possibly, probably, or likely related to the study agent.
Any grade AEs occurring in at least five patients in either study.
Grade greater than or equal to 3 AEs occurring in at least two patients in either study.
Grade 3.
Grade 3 (n = 1) and grade 4 (n = 1).
Grade 3 (n = 1) and grade 5 (n = 1).
In the combination study, AEs were also reported in all patients (Table 2). The most frequently reported all-grade AEs were pyrexia (eight of 12; 67%), chills (seven of 12; 58%), and asthenia (six of 12; 50%). Hyponatremia (two of 12; 17%) and pneumonitis (two of 12; 17%) were the most common grade greater than or equal to 3 AEs, with both cases of pneumonitis resulting in patient death. Of the two patients with pneumonitis, one had concurrent sputum culture of Klebsiella pneumoniae, and the other had significant emphysema on imaging. Five patients (42%) reported serious AEs in the combination study (Table 2), of which pneumonitis (two of 12; 17%) and erythema (one of 12; 8%) were considered related to study treatment. AEs with the outcome of grade 5 were reported in four patients: two patients admitted to the hospital with arthralgia and intestinal obstruction owing to PD (neither AE was considered by the investigator to be related to study treatment) and two patients with pneumonitis (considered by the investigator to be related to study treatment).
Blood Cultures and Bacterial Shedding
In the monotherapy study, blood cultures were predominantly negative, with three of 18 patients reporting positive Listeria cultures at either 2 or 4 hours postdose on cycle 1 day 1; all subsequent cultures for these patients were negative for Listeria. In the combination study, one patient had a positive LADD L. monocytogenes blood culture, but the sample was erroneously drawn immediately after the infusion of JNJ-757, and the subsequent scheduled culture was negative.
The shedding profile of JNJ-757 was assessed through scheduled fecal, urine, and saliva cultures, with samples collected at 4, 24, and 48 hours after infusion in the monotherapy study and on days 1 (4 hours after JNJ-757 infusion), 2, and 15 of cycle 1 and on day 1 of cycles 2 and 3. All samples from both studies were negative for Listeria growth, with the exception of one patient in the combination study who had a positive fecal sample, which was later identified to be wild-type L. monocytogenes (non–LADD L. monocytogenes) by sequencing.
Clinical Activity
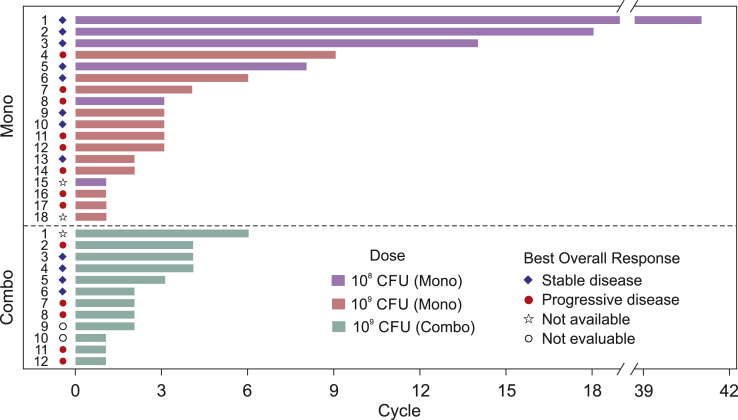
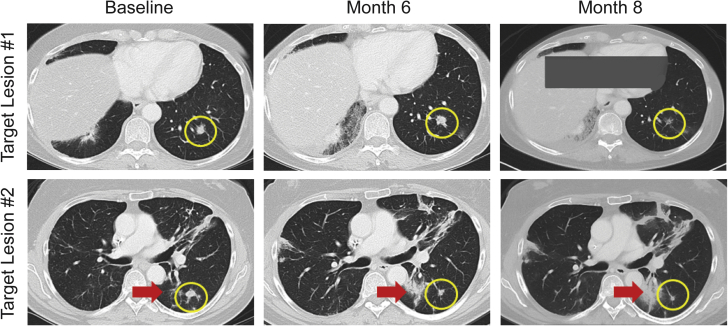
In the monotherapy study, the median total dose of JNJ-757 was 2.0 billion (range: 0.1–10.0 billion) CFU, and the median duration of exposure was 1.4 (range: 0–29) months. The best overall response was stable disease in eight of 18 patients, including one patient with stable disease through 41 cycles (Fig. 1). Four patients maintained a clinically significant response of stable disease for at least 16 weeks (4.6, 9.9, 10.2, and 29.7 mo), including three who had received previous anti–programmed death-ligand 1 (anti–PD-L1) therapy. A reduction of target lesions was observed in one patient (53% reduction at the third disease evaluation) who also had a concurrent increase in nontarget lesions (Fig. 2). Eight patients had PD as the best overall response, and two patients did not have investigator-assessed best responses available (Fig. 1). Although there were signals of clinical activity in terms of disease stabilization, the lack of objective responses led to a decision to close the monotherapy study and proceed with the combination study.
Figure 1.
Best overall response. The best overall response for patients treated with JNJ-757 monotherapy and with JNJ-757 in combination with nivolumab is presented. Purple bars represent patients treated with 100 million (1 × 108) CFU JNJ-757, green bars represent patients treated with 1 billion (1 × 109) CFU JNJ-757, and red bars represent patients treated with 1 billion CFU JNJ-757 in combination with nivolumab 240 mg. The best response is noted for each patient: stable disease (blue diamond), progressive disease (red circle), not available (open star), and not evaluable (open black circle). CFU, colony-forming unit; combo, combination study; mono, monotherapy study.
Figure 2.
Radiographic imaging of target and nontarget lesions in the monotherapy study. Yellow circles indicate reductions in target lesions. Red arrows mark an increase in a nontarget lesion.
Because the combination study was also stopped early, limited efficacy results were available. The median total dose of JNJ-757 was 2.0 billion (range: 1–6 billion) CFU, and the median duration of exposure was 1.3 (range: 0.5–5) months. Among 12 patients, eight had a previous exposure to anti–PD-L1 agent, with three patients each exposed to pembrolizumab, atezolizumab, and nivolumab. The best overall response was stable disease in four patients and PD in five patients; two patients were not evaluable, and one did not have a response available (Fig. 1).
Biomarker Analysis in the Monotherapy Study
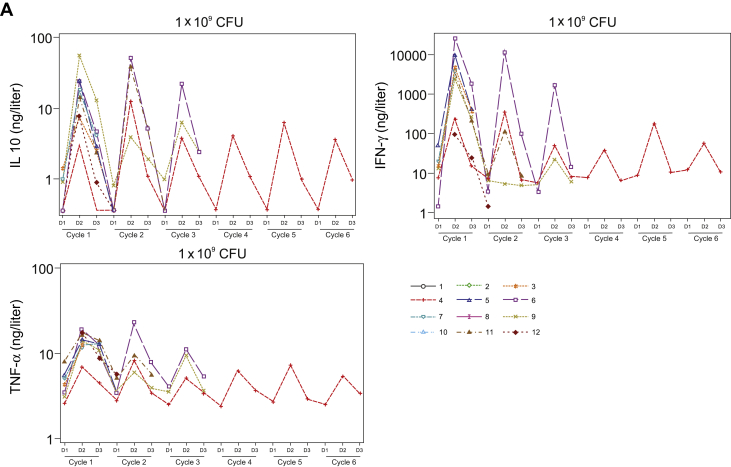
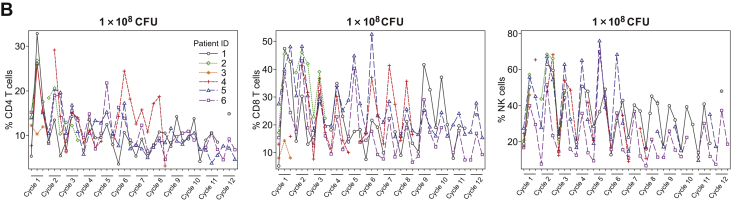
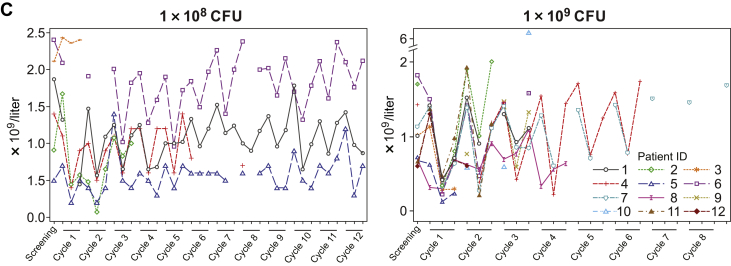
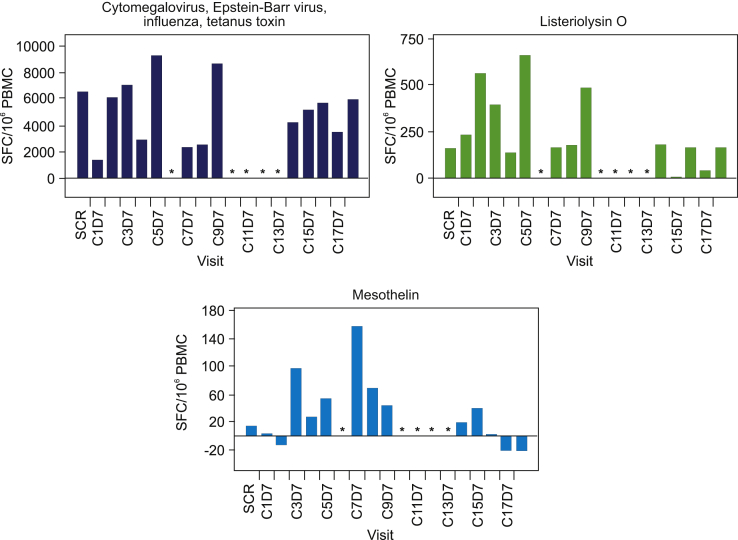
Activation of the innate immune response, as measured by transient increases in serum cytokine levels after JNJ-757 administration, was observed in all patients enrolled in the monotherapy study (N = 18). In general, levels of serum proinflammatory cytokines including, but not limited to, interferon gamma, tumor necrosis factor-α, and interleukin-10 were highest at 24 hours and returned to baseline levels by 48 hours after infusion (Fig. 3A). Consistently, transient activation of CD4, CD8 T-cells, and natural killer cells were observed in most patients at 24 hours after infusion (Fig. 3B). Transient lymphopenia, observed at approximately 24 to 48 hours after infusion, coincided with peak lymphocyte activation and cytokine elevation and was thought to represent lymphocyte margination (Fig. 3C). Adaptive immune responses were assessed by ELISpot analysis in 13 patients who had peripheral blood mononuclear cell samples that met the predefined quality control criteria on the basis of viability, recovery, and the number of cells available for screening and at least one dosing cycle sample. All biomarker-evaluable patients exhibited T-cell responses to the cytomegalovirus, Epstein-Barr virus, influenza, and tetanus toxoid control epitopes, and 12 patients exhibited reactivity to the Listeria antigen LLO (representative sample illustrated in Fig. 4). Antigen-specific T-cell responses to mesothelin were variable in magnitude and persistence and were consistently lower than the responses to Listeria antigen LLO or cytomegalovirus, Epstein-Barr virus, influenza, and tetanus (Fig. 4). Biomarker analysis was not performed in the combination study because of the small number of patients available.
Figure 3.
Transient increase in (A) Serum proinflammatory cytokines, (B) lymphocyte activation, and (C) lymphocyte margination after JNJ-757 infusion. Levels of the serum proinflammatory cytokines IL-10, interferon gamma, and TNF-α were highest at 24 hours after treatment with JNJ-757. (A) By 48 hours after treatment, serum cytokine levels returned to basal levels. (B) Transient lymphocyte margination was observed at both doses and coincided with peak cytokine elevation and lymphocyte activation. Activation of CD4, CD8, and NK cells was observed in most patients 24 hours after treatment with JNJ-757. (C) Lymphocyte activation status returned to baseline levels 48 to 72 hours after JNJ-757 administration. CFU, colony-forming unit; D, day; ID, identification document; IFN, interferon; IL-10, interleukin-10; NK, natural killer; TNF-α, tumor necrosis factor-α.
Figure 4.
Sample ELISpot analysis of a patient treated with JNJ-757 100 million CFU. Adaptive immune responses were assessed using interferon gamma ELISpot analysis. The ELISpot analysis for the patient with the longest treatment duration is illustrated here. Responses to the positive control HLA class II-restricted T-cell epitopes and to the Listeria antigen LLO were observed. T-cell responses to mesothelin were also observed in this patient; ∗ not analyzed. C, Cycle; CFU, colony-forming unit; D, day; ELISpot, enzyme-linked immunospot; HLA, human leukocyte antigen; IFN, interferon; LLO, listeriolysin-O; PBMC, peripheral blood mononuclear cell; SCR, screening; SFU, spot forming unit.
Discussion
In two phase 1 studies, the safety, and immunogenicity of JNJ-757 were evaluated as monotherapy and in combination with nivolumab in patients with advanced NSCLC adenocarcinoma. In both studies, JNJ-757 was well tolerated, mostly with mild AEs that were consistent with an innate immune response to JNJ-757. No DLTs were reported in either study; treatment-related grade 3 AEs or greater were reported in 22% to 25% of patients. None of the AEs resulting in treatment discontinuations in the monotherapy study were considered related to JNJ-757. On the basis of the overall safety profile in the dose escalation part of the monotherapy study, 1 billion CFU was selected as the RP2D, which was consistent with the safety profile of another L. monocytogenes–based vaccine expressing mesothelin.29 One patient died because of malignant ascites (not considered related to JNJ-757) in the monotherapy study (100 million CFU). In the combination study, two treatment-related deaths occurred because of pneumonitis, suggesting that the combination could potentially increase the risk of this toxicity known to be associated with anti–PD-1 or anti–PD-L1 therapies.30
In the monotherapy study, the best overall disease response was stable disease in eight of 18 patients, including four patients with stable disease of more than or equal to 16 weeks and one with stable disease of more than 1 year (41 cycles). Administration of JNJ-757 monotherapy elicited an innate immune response, and mesothelin-specific T-cell responses on the basis of protocol-specified criteria were observed in 10 of 13 biomarker-evaluable patients. Among eight patients with the best response of stable disease, four had mesothelin-specific T-cell responses. However, the magnitude and persistence of these T-cell responses were variable, and the significance of the adaptive immune response is unclear, given the lack of an antitumor response. On the basis of the lack of objective responses, the monotherapy study was stopped to focus on the potential safety and efficacy of the combination with nivolumab, which represents the current standard of care and could act synergistically with the observed activity of JNJ-757. Because JNJ-757 monotherapy induced innate and limited adaptive immune response, with some evidence of clinical benefit in terms of disease stabilization, it was expected that the combination with nivolumab might induce some tumor response. However, the best overall disease response with the combination was stable disease in four of 12 patients. Because of the lack of objective disease response to the combination and potential increased risk of pneumonitis, the decision was made to not proceed to phase 2. As a result, a major limitation of both studies was the low number of patients and limited data collected for analysis.
Blood culture assessments for both studies confirmed the rapid clearance of JNJ-757 and a low risk for bacterial colonization. The shedding profile of JNJ-757 in both studies suggested a low potential for transmission of LADD L. monocytogenes, as no fecal, saliva, or urine samples were positive for LADD L. monocytogenes.
Many cancer vaccine approaches for NSCLC have revealed promise in preclinical studies that have not translated into clinically meaningful responses in late-stage clinical trials, especially in lung cancer. Several factors could be contributing to the lack of clinical response observed with the cancer vaccine studies. Patients enrolled in these trials had an advanced-stage disease and may not represent the best candidates for vaccine-based approaches, which may need to be introduced at an earlier stage of the disease. In addition, the time involved in initiating an adaptive response against the tumor and the potential need for repeated challenges to obtain clinically significant T- cell responses may be prohibitive in patients with advanced and progressing disease. As our study enrolled patients with stage III or IV NSCLC, JNJ-757 treatment in less advanced disease might possibly have resulted in a more robust clinical response. Another challenge is that use of a single target antigen may be suboptimal, and multiple antigens or combination with other therapeutic agents may be required to elicit an effective immunologic response and to overcome the immune evasion mechanisms in the tumor microenvironment. As with ICIs, the selection of patients on the basis of predictive biomarkers may be needed for a successful cancer vaccine in NSCLC. Evaluation of JNJ-757 in mesothelin-positive NSCLC was planned for phase 2 of the combination study; however, the study did not proceed to the randomized phase 2 portion because of the lack of observed responses with either monotherapy or combination therapy, the lower magnitude of adaptive responses suggested by the ELISpot assays, and the potential increased risk of pneumonitis. To date, two cancer vaccines are approved for advanced NSCLC: (1) the CIMAvax-EGF in Cuba, Peru, and Venezuela for patients who progressed after first-line chemotherapy,31, 32, 33 and (2) racotumomab in Cuba and Argentina as maintenance therapy.34,35 Both vaccines have exhibited improved overall survival compared with the placebo arm in the randomized phase 3 studies, and both are being further evaluated in other patient populations and as combination therapy.
Despite evidence of activation of the innate and adaptive responses elicited by JNJ-757 as monotherapy, the evolving clinical profile of the combination therapy did not warrant further evaluation in the planned phase 2 randomized study, as the combination seemed unlikely to provide the additional clinical benefit (higher response rates and/or longer response duration) needed in patients with NSCLC.
Data Sharing Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access Project site at http://yoda.yale.edu.
Acknowledgments
Medical writing was provided by Tracy T. Cao, PhD (Janssen Global Services, LLC) and funded by Janssen Global Services, LLC. The Sponsor and its employees were involved in the study design, collection, analysis, and interpretation of data, writing of the report, and in the decision to submit the manuscript for publication. The authors thank the study participants, without whom this study would never have been accomplished, and the investigators for their participation in the studies that provided these data. Drs. Brahmer, Johnson, Viteri, Sukari, Awad, Papadimitrakopoulou, and Rajan contributed to study execution, review, and analysis of data of the manuscript. Dr. Cobo contributed to study execution, data support, review, and analysis of data of the manuscript. Coves contributed to the study execution of the manuscript. Drs. Salgia, Allred, Mason, Zudaire, and Knoblauch contributed to study conception and design, study execution, review, and analysis of data of the manuscript. Dr. Bandyopadhyay contributed to study conception and design, study execution, review and analysis of data, and formal analyses of the manuscript. Mr. Wade and Dr. Stone contributed to the review and analysis of data of the manuscript. Dr. Lorenzi and Hasan contributed to the study conception and design of the manuscript. All authors contributed to writing the manuscript, approved the submitted version of the manuscript, and agreed to be accountable for all aspects of the work.
Footnotes
Disclosure: Dr. Brahmer reports being in a consulting or advisory role for Bristol-Myers Squibb, Eli Lilly, Celgene, Syndax, Janssen Oncology, Merck, Amgen, and Genentech; receiving travel and accommodation expense assistance from Bristol-Myers Squibb and Merck; receiving other forms of assistance from Bristol-Myers Squibb and Merck; and receiving research funding for her institution from Bristol-Myers Squibb, Merck, AstraZeneca, Incyte, Janssen Oncology, and Spectrum Pharmaceuticals. Dr. Johnson reports being in a consulting or advisory role for Otsuka and Astellas Pharma for an immediate family member; reports being in a consulting or advisory role for Achilles Therapeutics, Arcus Biosciences, AstraZeneca, Atreca, Boehringer Ingelheim, Calithera Biosciences, EMD Serono, Genentech, GlaxoSmithKline, Gritstone Oncology, Guardant Health, Incyte, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Ribon Therapeutics, and Sanofi-Aventis; and receiving research funding for her institution from AbbVie, Acerta Pharma, Adaptimmune, Amgen, AstraZeneca, Apexigen, Array BioPharma, Atreca, BeiGene, BerGenBio, Boehringer Ingelheim, Calithera Biosciences, Checkpoint Therapeutics, Corvus Pharmaceuticals, CytomX, Daiichi Sankyo, Dynavax Technologies, EMD Serono, Foundation Medicine, Genentech, Genmab, Genocea Biosciences, GlaxoSmithKline, Gritstone Oncology, Guardant Health, Hengrui Therapeutics, Immunocore, Incyte, Janssen, Jounce Therapeutics, Kadmon Pharmaceuticals, Eli Lilly, Loxo Oncology, Lycera, Neovia Oncology, Merck, Novartis, OncoMed Pharmaceuticals, Pfizer, Regeneron Pharmaceuticals, Ribon Therapeutics, Sanofi-Aventis, Seven and Eight Biopharmaceuticals, Shattuck Labs, Stem CentRx, Syndax Pharmaceuticals, Takeda Pharmaceuticals, Tarveda, TCR2 Therapeutics, University of Michigan, and WindMIL Therapeutics. Dr. Viteri reports being in a consulting or advisory role for Roche and Bristol-Myers Squibb; receiving travel accommodation and expenses from Merck Serono, Roche, and OSE Pharma; was a member of speakers bureau for Roche and Bristol-Myers Squibb; and receiving research funding from OSE Pharma, Boehringer Ingelheim, Novocure, Merck Serono, Roche, Exelixis, Takeda, and IO Biotech. Dr. Sukari reports being in a consulting or advisory role for Eisai and Bayer Pharmaceuticals; participating in speakers bureau for Merck and Eisai; and receiving research funding from Eisai. Dr. Awad reports being in a consulting or advisory role for Genentech, Merck, Pfizer, Boehringer Ingelheim, AbbVie, AstraZeneca/MedImmune, Clovis Oncology, Nektar, Bristol-Myers Squibb, ARIAD Pharmaceuticals, Foundation Medicine, Syndax, Novartis, Blueprint Medicines, and Maverick Therapeutics; receiving research funding from Bristol-Myers Squibb; and receiving research funding for his institution from Genentech/Roche, Eli Lilly, AstraZeneca, and Bristol-Myers Squibb. Dr. Papadimitrakopoulou is currently an employee of Pfizer, Inc. and reports being in a consulting or advisory role for Clovis Oncology, Genentech, Merck, Biothera, Eli Lilly, Janssen, AstraZeneca, ARIAD Pharmaceuticals, Nektar, Loxo Oncology, Araxes Pharma, AbbVie, Bristol-Myers Squibb, Exelixis, Pfizer, Novartis, Takeda Pharmaceuticals, TRM Oncology, Tesaro, Arrys Therapeutics, Gritstone Oncology, Leeds Biolabs, Bolt Biotherapeutics, and G2 innovation; receiving funding for travel and accommodation expenses from AstraZeneca and Roche; receiving honoraria and other assistance from Roche; and receiving research funding for her institution from Merck, Novartis, Celgene, Clovis Oncology, Bayer, Bristol-Myers Squibb, AstraZeneca, Pfizer, Janssen Oncology, ACEA Biosciences, Nektar, Roche, Eli Lilly, Checkmate Pharmaceuticals, Incyte, and Guardant Health. Dr. Rajan reports receiving research funding for his institution from Aduro Biotech, Morphotek, TCR2, and Bayer Pharmaceuticals. Dr. Bandyopadhyay, Dr. Allred, Mr. Wade, Dr. Mason, Dr. Zudaire, Dr. Knoblauch, and Dr. Stone report employment, stock, or other ownership in Johnson & Johnson. Dr. Lorenzi reports employment, stock, or other ownership in and also receiving funding for travel and accommodation expenses from Johnson & Johnson. Dr. Hassan reports receiving research funding for his institution from Aduro Biotech, Morphotek, TCR2, and Bayer Pharmaceuticals. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100103.
Supplementary Data
References
- 1.Ferlay J., Colombet M., Soerjomataram I. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [published correction appears in CA Cancer J Clin. 2020;70:313] CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Molina J.R., Yang P., Cassivi S.D., Schild S.E., Adjei A.A. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlander N., Noone A.M., Krapcho M. SEER cancer statistics review, 1975–2016. National Cancer Institute. https://seer.cancer.gov/csr/1975_2016/
- 5.Topalian S.L., Hodi F.S., Brahmer J.R. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5:1411–1420. doi: 10.1001/jamaoncol.2019.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garon E.B., Hellmann M.D., Rizvi N.A. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37:2518–2527. doi: 10.1200/JCO.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveres H., Caglevic C., Passiglia F., Taverna S., Smits E., Rolfo C. Vaccine and immune cell therapy in non-small cell lung cancer. J Thorac Dis. 2018;10(suppl 13):S1602–S1614. doi: 10.21037/jtd.2018.05.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vansteenkiste J.F., Cho B.C., Vanakesa T. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:822–835. doi: 10.1016/S1470-2045(16)00099-1. [DOI] [PubMed] [Google Scholar]
- 9.Giaccone G., Bazhenova L.A., Nemunaitis J. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer. 2015;51:2321–2329. doi: 10.1016/j.ejca.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Creelan B.C., Antonia S., Noyes D. Phase II trial of a GM-CSF-producing and CD40L-expressing bystander cell line combined with an allogeneic tumor cell-based vaccine for refractory lung adenocarcinoma. J Immunother. 2013;36:442–450. doi: 10.1097/CJI.0b013e3182a80237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butts C., Socinski M.A., Mitchell P.L. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 12.Guo C., Manjili M.H., Subjeck J.R., Sarkar D., Fisher P.B., Wang X.Y. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake C.G., Jaffee E., Pardoll D.M. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 14.De Pas T., Giovannini M., Rescigno M. Vaccines in non-small cell lung cancer: rationale, combination strategies and update on clinical trials. Crit Rev Oncol Hematol. 2012;83:432–443. doi: 10.1016/j.critrevonc.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Campoli M., Ferrone S., Zea A.H., Rodriguez P.C., Ochoa A.C. Mechanisms of tumor evasion. Cancer Treat Res. 2005;123:61–88. doi: 10.1007/0-387-27545-2_3. [DOI] [PubMed] [Google Scholar]
- 16.Brockstedt D.G., Giedlin M.A., Leong M.L. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahabi V., Reyes-Reyes M., Wallecha A., Rivera S., Paterson Y., Maciag P. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother. 2008;57:1301–1313. doi: 10.1007/s00262-008-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf B.J., Princiotta M.F. Processing of recombinant Listeria monocytogenes proteins for MHC class I presentation follows a dedicated, high-efficiency pathway. J Immunol. 2013;190:2501–2509. doi: 10.4049/jimmunol.1201660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pamer E.G., Sijts A.J., Villanueva M.S., Busch D.H., Vijh S. MHC class I antigen processing of Listeria monocytogenes proteins: implications for dominant and subdominant CTL responses. Immunol Rev. 1997;158:129–136. doi: 10.1111/j.1600-065x.1997.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 20.Wallecha A., Singh R., Malinina I. Listeria monocytogenes (Lm)-LLO immunotherapies reduce the immunosuppressive activity of myeloid-derived suppressor cells and regulatory T cells in the tumor microenvironment. J Immunother. 2013;36:468–476. doi: 10.1097/CJI.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 21.Mkrtichyan M., Chong N., Abu Eid R. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer. 2013;1:15. doi: 10.1186/2051-1426-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood L.M., Paterson Y. Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol. 2014;4:51. doi: 10.3389/fcimb.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ordóñez N.G. The diagnostic utility of immunohistochemistry in distinguishing between epithelioid mesotheliomas and squamous carcinomas of the lung: a comparative study. Mod Pathol. 2006;19:417–428. doi: 10.1038/modpathol.3800544. [DOI] [PubMed] [Google Scholar]
- 24.Ordóñez N.G. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol. 2003;27:1031–1051. doi: 10.1097/00000478-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen M., Sarlomo-Rikala M. Expression of calretinin, thrombomodulin, keratin 5, and mesothelin in lung carcinomas of different types: an immunohistochemical analysis of 596 tumors in comparison with epithelioid mesotheliomas of the pleura. Am J Surg Pathol. 2003;27:150–158. doi: 10.1097/00000478-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Kushitani K., Takeshima Y., Amatya V.J., Furonaka O., Sakatani A., Inai K. Immunohistochemical marker panels for distinguishing between epithelioid mesothelioma and lung adenocarcinoma. Pathol Int. 2007;57:190–199. doi: 10.1111/j.1440-1827.2007.02080.x. [DOI] [PubMed] [Google Scholar]
- 27.Frierson H.F., Jr., Moskaluk C.A., Powell S.M. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum Pathol. 2003;34:605–609. doi: 10.1016/s0046-8177(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 28.Thomas A., Chen Y., Steinberg S.M. High mesothelin expression in advanced lung adenocarcinoma is associated with KRAS mutations and a poor prognosis. Oncotarget. 2015;6:11694–11703. doi: 10.18632/oncotarget.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le D.T., Brockstedt D.G., Nir-Paz R. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correale P., Saladino R.E., Giannarelli D. HLA expression correlates to the risk of immune checkpoint inhibitor-induced pneumonitis. Cells. 2020;9:E1964. doi: 10.3390/cells9091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saavedra D., Crombet T. CIMAvax-EGF: a new therapeutic vaccine for advanced non-small cell lung cancer patients. Front Immunol. 2017;8:269. doi: 10.3389/fimmu.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez P.C., Popa X., Martínez O. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2016;22:3782–3790. doi: 10.1158/1078-0432.CCR-15-0855. [DOI] [PubMed] [Google Scholar]
- 33.Neninger Vinageras E., de la Torre A., Osorio Rodríguez M. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:1452–1458. doi: 10.1200/JCO.2007.11.5980. [DOI] [PubMed] [Google Scholar]
- 34.Alfonso S., Valdes-Zayas A., Santiesteban E.R. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2014;20:3660–3671. doi: 10.1158/1078-0432.CCR-13-1674. [DOI] [PubMed] [Google Scholar]
- 35.Alfonso S., Diaz R.M., de la Torre A. 1E10 anti-idiotype vaccine in non-small cell lung cancer: experience in stage IIIb/IV patients. Cancer Biol Ther. 2007;6:1847–1852. doi: 10.4161/cbt.6.12.5000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.