Abstract
We previously published in the Journal of Thoracic Oncology the case of a patient with EGFR and MET-driven lung cancer and extracranial response to capmatinib and osimertinib. Here, we report on a second patient treated with the same combination, revealing complete and durable intracranial response. Adding capmatinib to osimertinib seems to be an effective salvage therapy for patients with EGFR-mutant lung cancer and acquired MET amplification.
Keywords: Lung cancer, Targeted therapy, Capmatinib, Osimertinib, Case report
Introduction
Osimertinib has superior activity against brain metastases over other tyrosine kinase inhibitors.1 Dose increase may delay progression in the central nervous system; however, long-term survival is limited, because tumors activate alternative signaling pathways.2 One of the most frequent molecular mechanisms of osimertinib resistance is MET amplification.3 Clinical trials are ongoing to test osimertinib in combination with MET inhibitors, including savolitinib or tepotinib. The MET inhibitor capmatinib as a single agent is approved for the treatment of patients with lung cancer and MET exon 14 skipping mutation.4 In the Journal of Thoracic Oncology, we previously published a case report of a patient with durable remission of pulmonary metastases on capmatinib plus osimertinib, on the basis of high-level MET amplification at the time of progression on osimertinib alone.5 The patient had previous irradiation of multiple brain metastases, and the disease is controlled for more than 22 months now, which encouraged us to treat a second patient with the same combination.
Case Presentation
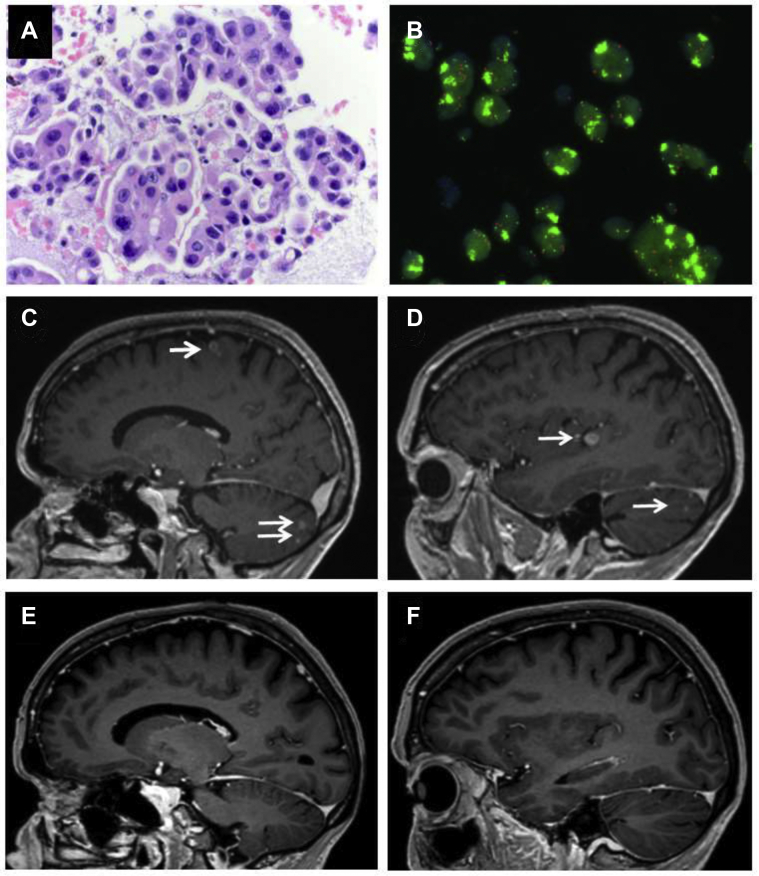
The second patient described here is a 69-year old female never smoker, diagnosed with having metastatic lung adenocarcinoma and EGFR deletion 19 in 2017. She had initial therapy with osimertinib plus stereotactic radiosurgery for a solitary brain metastasis, and developed disseminated liver metastases, and hilar lymph node metastases in August 2019. Bronchoscopic rebiopsy result revealed high-level MET amplification, with gene copy number of 20, MET immunohistochemistry of 3 plus, and MET-to-CEN7 ratio of greater than 5 (Fig. 1A and B). Osimertinib was stopped, the patient received chemotherapy with carboplatin and pemetrexed, but the disease progressed systemically and with disseminated brain metastases. In January 2020, the patient was enrolled in the early access program of Novartis, in which capmatinib 200 mg twice daily (50% of the recommended single-agent dose, on the basis of our previous patient) was started together with osimertinib 80 mg, leading to complete remission of all brain metastases (Fig. 1C–F). The patient remains on capmatinib (200 mg once daily) and osimertinib 80 mg for more than 12 months now (last imaging on January 2021). Tolerability is good, with grade 1 peripheral edema and grade 1 serum creatinine elevation.
Figure 1.
Tumor rebiopsy result revealing (A) lung adenocarcinoma, (B) MET-FISH result with clusters of green signals indicating high-level MET amplification. Brain MRI at the time of (C, D) tumor progression after osimertinib and chemotherapy, revealing multiple brain metastases (arrows), and (E, F) after 4 months of therapy with capmatinib plus osimertinib, revealing complete intracranial response. FISH, fluorescence in situ hybridization; MRI, magnetic resonance imaging.
Discussion
Addition of low-dose capmatinib to full-dose osimertinib is feasible and can induce durable remission of intracerebral and extracerebral metastases from tumors with EGFR mutation and acquired resistance to osimertinib associated with MET amplification. Rechallenge with osimertinib alone may also induce tumor remission to some extent, but brain metastatic tissue or serial blood samples were not available for the testing of MET and drug plasma levels. Rebiopsy under chemotherapy was not feasible, because the condition of the patient required immediate therapy. However, the extent and durability of intracranial and extracranial response on capmatinib plus osimertinib, and the absence of skin and mucosal toxicity suggest that intracranial response is indeed mediated by dual target inhibition, rather than by osimertinib alone, or by elevated osimertinib plasma level by capmatinib.
Conclusion
Combination therapy with capmatinib and osimertinib seems to be an effective salvage therapy for patients with osimertinib-resistant lung cancer and acquired MET amplification. Clinical trials combining MET inhibitors with osimertinib are ongoing.
Acknowledgments
The patient provided consent to this publication. The authors thank Dr. Mannhard (Cham, Switzerland) for patient referral, Dr. Roos and A. Hirschmann from our institution for the images, and Novartis (Basel, Switzerland) for capmatinib and approval of this report.
Footnotes
Disclosure: Prof. Dr. Gautschi is a consultant for Amgen and Eli Lilly and attended advisory boards for Eli Lilly and Bayer. Dr. Diebold declares no conflict of interest.
Gautschi O, Diebold J. Intracranial Activity of osimertinib plus capmatinib in a patient with EGFR and MET-driven lung cancer: case report. JTO Clin Res Rep. 2021;2:100162.
References
- 1.Reungwetwattana T., Nakagawa K., Cho B.C. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:3290–3297. doi: 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 2.Ahn M.J., Chiu C.H., Cheng Y. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol. 2020;15:637–648. doi: 10.1016/j.jtho.2019.12.113. [DOI] [PubMed] [Google Scholar]
- 3.Ramalingam S.S., Cheng Y., Zhou C. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29(suppl 8):VIII740. [Google Scholar]
- 4.Wolf J., Seto T., Han J.Y. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 5.Gautschi O., Menon R., Bertrand M., Murer C., Diebold J. Capmatinib and osimertinib combination therapy for EGFR-mutant lung adenocarcinoma. J Thorac Oncol. 2020;15:e13–e15. doi: 10.1016/j.jtho.2019.07.027. [DOI] [PubMed] [Google Scholar]