Abstract
Maternal immune activation (MIA) in response to infection during pregnancy has been linked through various epidemiological and preclinical studies to an increased risk of neurodevelopmental disorders such as autism spectrum disorder (ASD) and schizophrenia in exposed offspring. Sensory filtering disruptions occur in both of these disorders and are typically measured using the acoustic startle response in both humans and rodents. Our study focuses on characterizing the baseline reactivity, habituation and prepulse inhibition (PPI) of the acoustic startle response following exposure to MIA. We induced MIA using polyinosinic: polycytidylic acid (poly I:C) at gestational day (GD) 9.5 or 14.5, and we tested sensory filtering phenotypes in adolescent and adult offspring. Our results show that startle reactivity was robustly increased in adult GD9.5 but not GD14.5 poly I:C offspring. In contrast to some previous studies, we found no consistent changes in short-term habituation, long-term habituation or prepulse inhibition of startle. Our study highlights the importance of MIA exposure timing and discusses sensory filtering phenotypes as they relate to ASD, schizophrenia and the poly I:C MIA model. Moreover, we analyze and discuss the potential impact of between- and within-litter variability on behavioural findings in poly I:C studies.
Keywords: Poly I:C, Startle, Prepulse inhibition, Habituation, Sensorimotor gating, Schizophrenia, Autism Spectrum Disorder, Neurodevelopmental disorder, Litter variability
Highlights
-
•
Maternal immune activation (MIA) in rats by poly I:C is a model of neurodevelopmental disorder.
-
•
MIA at gestation day 9.5, but not 14.5, leads to robust startle hyperreactivity in response to sound.
-
•
No consistent changes in habituation or prepulse inhibition of startle are seen.
-
•
Analysis of within- and between-litter variability shows importance of experimental design to avoid random effects.
1. Introduction
Maternal infection during pregnancy is a known environmental risk factor for neurodevelopmental disorders such as schizophrenia (SCZ; Brown et al., 2004; Blomström et al., 2012), Autism Spectrum Disorder (ASD; Atladóttir et al., 2010; Zerbo et al., 2015), epilepsy (Sun et al., 2008; Whitehead et al., 2006) and intellectual disability (Lee et al., 2015). Since multiple types of infection are associated with a similar offspring risk (Nielsen et al., 2013), it is thought that the disruption of brain development is mediated by maternal cytokines, the inflammatory molecules of the maternal immune response (Canetta et al., 2014; Estes and McAllister, 2016; Jones et al., 2017; Koks et al., 2016).
Polyinosinic: polycytidylic acid (poly I:C) is a synthetic analog of double-stranded RNA which binds to toll-like-receptor 3 to initiate an acute antiviral-like immune reaction (Takeda and Akira, 2005). Administration of poly I:C at distinct time-points during pregnancy in rodents produces diverse phenotypes related to neurodevelopmental disorders (Careaga et al., 2017; Haddad et al., 2020; Solek et al., 2018). The poly I:C model has a high construct validity, as it closely relates to observations from human epidemiological studies (Li et al., 2009; Meyer et al., 2008, 2005; Mueller et al., 2018; Richetto et al., 2017; Shi et al., 2003). It also shows considerable face validity for neurodevelopmental disorders, as it induces behavioural offspring changes in sensorimotor gating, cognition, social behavior and stereotypy (Giovanoli et al., 2016; Haddad et al., 2020; Pacheco-López et al., 2013; Solek et al., 2018; Vuillermot et al., 2010), as well as molecular changes in dopaminergic, glutamatergic and GABAergic neurotransmission (Haddad et al., 2020; Rahman et al., 2017; Richetto et al., 2014, 2013).
The most commonly investigated phenotype across all poly I:C studies is the disruption of sensorimotor gating, which refers to pre-attentive filtering of redundant sensory information, measured as prepulse inhibition (PPI) of the acoustic startle response (ASR; Koch, 1999). Disruptions in PPI have been a consistent finding in patients with SCZ for decades and have also been reported in other psychiatric disorders such as ASD and Huntington’s Disease, although not as consistently (Kohl et al., 2013; Sinclair et al., 2017; Swerdlow et al., 2008). Given the direct translatability of ASR measures across species, PPI testing is a staple preclinical test in many relevant models and PPI deficits may reflect disruptions along with the cortico-striato-pallido-thalamic circuitry which modulates PPI (Swerdlow et al., 2016). Beyond PPI, the baseline startle amplitude can be used as a measure of implicit sensory reactivity. Furthermore, habituation of the startle response upon repeated stimulation is a form of non-associative memory which may also be considered a form of pre-attentive sensory filtering (Koch, 1999). Although startle reactivity and habituation of startle also represent valuable measures associated with sensory processing disruptions in ASD and SCZ, they have received far less attention in poly I:C literature in the past.
In this study, we characterize startle reactivity, startle short-term habituation (STH), long-term habituation (LTH) and PPI in the rat poly I:C model, and highlight the extent to which between- and within-litter variability influences these behavioural measures. Furthermore, we interpret our data specifically in the context of ASR methodology (e.g influence of habituation on startle reactivity measures, trial-by-trial vs. block-based analysis of habituation). Poly I:C was injected at GD9.5 and GD14.5, the most commonly studied timepoints across poly I:C studies. These time points are typically chosen because they represent rodent brain developmental equivalents of human first and second trimesters, which are the gestational periods when maternal infection imposes the greatest risk (Haddad et al., 2020).
2. Methods
2.1. Subjects
This study was conducted using wildtype Sprague Dawley (SD) adult male and female rats (Charles River Canada). Rats were housed in open cages with corn cob bedding, given ad libitum food and water, and kept on a 12 h light – 12 h dark cycle with lights turning on at 7:00 a.m. Cages were enriched with polycarbonate huts and wrinkled paper. Cage changes took place once a week except during behavioural testing procedures, during which cage changes were carried out at the end of the 5-day startle protocols to ensure LTH was not influenced by cage change stress. Same-sex animals were housed in groups of 2–4 per cage. Behavioural testing took place during the light phase (between 7:00 and 19:00 h). All animal procedures were approved by the Western Animal Care Committee and adhered to the guidelines of the Canadian Council on Animal Care.
2.2. Timed breeding
Adult male rats were paired with a maximum of two adult females at a time. After pairing overnight, a vaginal smear was collected from each female at 8 a.m. and inspected under a light microscope to track the estrus cycle and check for the presence of sperm. If sperm was detected in the smear, the female was considered pregnant and that day was considered as GD0.5. Pregnant females were then separated from the male and transferred into a single cage, where they were left undisturbed until injection day (GD9.5 or GD14.5). Each female was only bred once in this experiment.
2.3. Maternal immune activation
Pregnant females were randomly assigned to receive either poly I:C or saline injections. Maternal immune activation was induced using poly I:C (Sigma Lot#037M4011V), which had been previously aliquoted and stored at −20° C. Poly I:C aliquots were diluted in 0.9% saline to obtain a concentration of 4 mg/ml. At around 10 a.m. on GD9.5 or GD14.5, pregnant females were weighed and had their rectal temperature measured. Then, they underwent isoflurane anesthesia (5% induction, 2% maintenance), during which they were injected with either 0.9% saline or 4 mg/kg poly I:C into the tail vein. The entire injection from anesthesia induction to recovery procedure took an average of 15 min and rats were returned to their cages afterwards and were undisturbed besides temperature/weight measurement and weekly cage change. Temperature measurements were taken 3- and 24-h following injection, whereas maternal weight was recorded at 6 and 24-h following injection.
The day of parturition was designated as postnatal day (PND) 0. Offspring were weaned at PND21, and littermates were separated based on sex and housed in groups of 2–4 rats per cage. At the end of the experiment, the final number of litters per group was as follows: GD9.5 saline, 3 litters; GD9.5 poly I:C, 3 litters; GD14.5 saline, 4 litters; GD14.5 poly I:C, 4 litters. For the last 2 litters in each group, offspring were culled to 3–5 animals per sex per litter at 1 week of age, whereas in earlier cohorts all the offspring were tested. We did not record the full litter size for the last 2 litters from each group, but previous research suggests that prenatal poly I:C treatment similar to ours does not reduce litter size (Gray et al., 2019; Vernon et al., 2015; Vorhees et al., 2015, 2012). The final number of offspring included for analysis per group, litter, and sex is included in the statistical analysis section of the article.
2.4. qRT-PCR for quantification of the immune response
A total of six dams were used to confirm the efficacy of the batch of poly I:C used in this experiment. These dams were injected with either saline or poly I:C at GD9.5 (3 per group). They were sacrificed 6 h after maternal saline or poly I:C injection using mild carbon dioxide inhalation until respiratory failure, followed by cardiac puncture or decapitation. Two whole conceptuses (containing decidua, placenta, and embryo) were isolated per dam and either snap-frozen in liquid nitrogen or fixed in 10% neutral buffered formalin. RNA was extracted from the tissue by homogenizing in RiboZol (Amresco). The aqueous phase was then diluted with 70% ethanol, placed on RNeasy columns (Qiagen), treated with DNase I, and purified. cDNA was generated from purified RNA (50 ng/ml) using High Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific), diluted 1:10, and used for quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR). For qRT-PCR, cDNA was mixed with SensiFast SYBR green PCR Master Mix (FrogtaBio) and primers described in the supplemental information (Table S1). A CFX Connect Real-Time PCR system (Bio-Rad Laboratories) was used for amplification and fluorescence detection. Cycling conditions were as follows: an initial holding step (95 °C for 3 min), followed by 40 cycles of two-step PCR (95° for 10 s, 60 °C for 45 s), then a dissociation step (65 °C for 5 s and a sequential increase to 95 °C). Relative mRNA expression was calculated using the comparative cycle threshold (Ct; ΔΔCt) method. The geometric mean of Ct values obtained from the amplification of Ribosomal RNA 18s (Rn18s) was used as reference RNA. Ct values from each gene of interest (Interleukin-6, Interleukin-10, Tumor Necrosis Factor-α, and Interferon-γ) were stable among the conditions tested.
2.5. Acoustic startle response testing
Behavioural testing was conducted in adolescence (starting PND38-39) and adulthood (starting PND120-130). The same tests were conducted at each time point, and adolescent animals were handled at least twice before behavioural testing. Startle testing was performed as described before (Valsamis and Schmid, 2011). In brief, rats were initially acclimated to the experimental procedure, Plexiglas animal holders, and startle chambers (Med Associates, Vermont, USA) by undergoing three 5-min acclimation sessions, at least 6 h apart. During acclimation, the animals were exposed to a 65 dB sound pressure level (SPL) white noise background sound which is consistently present throughout all startle testing procedures. After acclimation sessions, startle reactivity in response to startle stimuli of varying intensities was measured. This startle reactivity test consisted of 12 white noise startle stimuli, each 20 ms in duration, at intensities ranging sequentially from 65 dB to 120 dB in 5 dB increments, with a fixed intertrial interval (ITI) of 15 s. Based on data from the startle reactivity session, platform sensitivity was adjusted for each rat to optimally detect the startle reflex and the adjusted platform gain for each rat was used throughout all the remaining testing sessions. Adjusting platform sensitivity prevents ‘maxing out’ of the startle response, which occurs with high startle responses where the peak of the response is beyond the limits of the recording software. It also allows for more accurate detection of low startle responses, where the response is close to background activity. Startle reactivity data shown below were all normalized by the platform sensitivity used for each animal, and therefore these data are directly comparable between animals and groups.
2.6. Habituation and PPI
Following startle reactivity testing, animals underwent 5 consecutive days of startle habituation and PPI testing to assess startle reactivity, PPI, STH, and LTH. Each day, animals were exposed to a 5-min acclimation period with background noise, followed by a habituation block of 20 trials and a PPI block of 50 trials. In the habituation block, each trial consisted of a 20 ms, 110 dB white noise stimulus. Trials were separated by a fixed ITI of 15 s. Besides analyzing habituation trials on a trial-by-trial basis, STH score was calculated from the 20 habituation trials on day 1 by dividing the average startle amplitude on the first 5 trials by the average startle amplitude on the last 5 trials (a value > 1 indicates reduced startle at the end of the habituation block and the presence of STH). It is important to note that initial STH measure across 20 consecutive trials measured at the start of the session is different from most of the human literature and the few poly I:C studies that measured this phenotype. In those studies, habituation was measured as the reduction of ASR amplitude on a final block of startle-only trials compared to the first block of startle-only trials, and these 2 blocks are usually separated by a PPI block of 30–40 startle and prepulse trials (Mena et al., 2016; Swerdlow et al., 2014; Takahashi et al., 2017).
In the PPI block, animals were exposed to either startle only trials as described above or prepulse trials. Prepulse trials included a 4-ms long white noise prepulse of 75 or 85 dB and preceded the startle stimulus by an interstimulus interval (ISI) of either 30 or 100 ms. ISI was determined as the time between the onset of the prepulse to the onset of the startle stimulus. Ten of each of the following prepulse-ISI combinations: 75–30, 75–100, 85–30, and 85–100 were presented, totaling 40 prepulse/pulse trials and 10 startle alone trials in a pseudorandomized order. The trials were separated by a 15s ITI. Peak to Peak maximum startle amplitude was measured. PPI was calculated as the amount of inhibition of startle in prepulse trials compared to startle-only trials in the PPI block: %PPI = [1 - (startle magnitude with prepulse/baseline startle without prepulse in PPI block)] × 100.
2.7. Statistical analysis
For weight and temperature analysis, separate univariate ANOVAs were conducted for temperature change at 3 h post-injection (GD9.5 Saline n = 10, GD9.5 Poly I:C n = 9, GD14.5 Saline n = 3, GD14.5 Poly I:C n = 3), temperature change 24 h post-injection (GD9.5 Saline n = 5, GD9.5 Poly I:C n = 7, GD14.5 Saline n = 3, GD14.5 Poly I:C n = 3) and weight change at 24 h post-injection (GD9.5 Saline n = 6, GD9.5 Poly I:C n = 6, GD14.5 Saline n = 4, GD14.5 Poly I:C n = 4). All the poly I:C injections in these rats were using the same batch of poly I:C. Sample size mismatch between analyses is evident because a) temperature was not collected in one saline and one poly I:C GD14.5 dam and b) 24-h weight/temperature could not be collected in dams that were sacrificed for qRT-PCR analysis, which was only performed at GD9.5. Some extra dams underwent the same injection procedure and weight/temperature measurement, but their offspring or tissue were used in other studies.
Data obtained from the behavioural tests described above were analyzed using IBM SPSS Statistics version 26 and graphed using GraphPad Prism version 7.00 for Windows. Data were scanned for extreme outliers (>3 interquartile ranges from the median) using the explore function in SPSS. The most extreme outlier across multiple behavioural tests was excluded in a litter-sex-specific manner (e.g the most extreme GD9.5 saline male in litter 1, the most extreme GD9.5 saline male in litter 2, etc.). After the exclusion of litter-sex-specific outliers, the same outlier analysis was performed but this time in a prenatal treatment-sex specific manner (e.g the most extreme GD9.5 saline male across all litters, the most extreme GD9.5 poly I:C male across all litters, etc.). After outlier analysis a total of 128 rats were included in the results: 24 GD9.5 saline offspring (11 males, 13 females), 37 GD9.5 poly I:C animals (20 males, 17 females), 32 GD14.5 saline animals (16 males, 16 females) and 35 GD14.5 poly I:C animals (19 males, 16 females). All data shown for all the different behavioural measures below represent the same animals per litter, prenatal treatment, and sex.
For each behavioural test, a three or four-way repeated-measures analysis of variance (ANOVA) was conducted separately for GD9.5 and GD14.5 offspring because GD9.5 and GD14.5 testing did not occur at the same time which may affect baseline values in our behavioural measures. Additionally, separate ANOVAs were conducted for adolescent and adult offspring as most of our measures are inherently influenced by weight. Although weight was not recorded in this experiment and therefore could not be used as a covariate in the startle reactivity analysis, unpublished data from our lab indicates that within sex/age groups (e.g adult males), weight fluctuations are not correlated with startle response amplitude. All ANOVAs included sex and prenatal treatment (saline vs poly I:C) as between-subject factors. Additional within-subject factors were included based on the measure of interest: Stimulus intensity for startle reactivity, trial number for STH of startle, day of testing for LTH of startle, and prepulse intensity and ISI for PPI.
ANOVAs were inspected for high-level interactions down to main effects. Analyses that showed high-level interactions were split up into multiple sub-analyses based on which factors were involved in the interaction (e.g after a high-level interaction of prenatal treatment, stimulus intensity and sex, males and females were analyzed separately for effects/interactions of prenatal treatment and stimulus intensity). Main effects were computed using t-tests with Bonferroni adjustment for multiple comparisons. Data in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9 are shown as mean ± standard error of the mean. In Fig. 10, individual data points are represented as dots, with each litter being shown as a different colour and the combined data from all litters are shown in black. Also in Fig. 10, the middle line in each data set indicates the median, and the error bars represent the 25th and 75th percentiles.
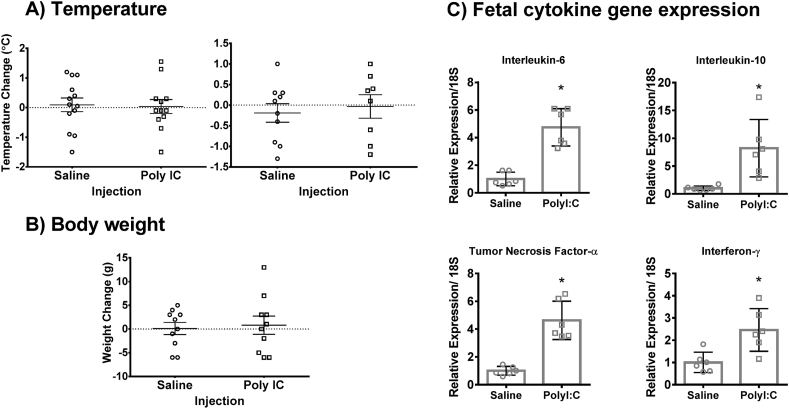
Fig. 1.
Maternal poly I:C injection induces a robust cytokine response in fetal tissue. Maternal temperature was measured at 3 (A, left) and 24 (A, right) hours following poly I:C or saline injection, whereas maternal body weight was measured ar 24 hours following injection (B). poly I:C did not influence maternal temperature at 3 hours (left; p=0.923), maternal temperature at 24 hours (right, p=0.723) or maternal weight at 24 hours (p=0.455) following injection. Fetal tissue was collected 6 h after GD9.5 poly I:C or saline injection, with a total of 6 fetuses per group and 2 fetuses per dam. Poly I:C fetuses exhibited an increase in gene expression of Interleukin-6 (p < 0.001), Interleukin-10 (p = 0.007), Tumor necrosis factor α (p < 0.001) and Interferon-γ (p = 0.007). N = 6 per group (C).
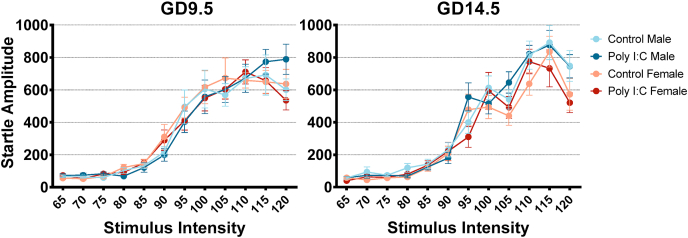
Fig. 2.
Neither GD9.5 nor GD14.5 poly I:C treatment change startle reactivity in adolescent offspring. Data is shown for both males and females from saline and poly I:C offspring for each time point. A 3-way repeated measures ANOVA conducted separately for GD9.5 and GD14.5 adolescent offspring showed a significant effect of stimulus intensity (p < 0.001) with no interactions or main effects associated with prenatal treatment.
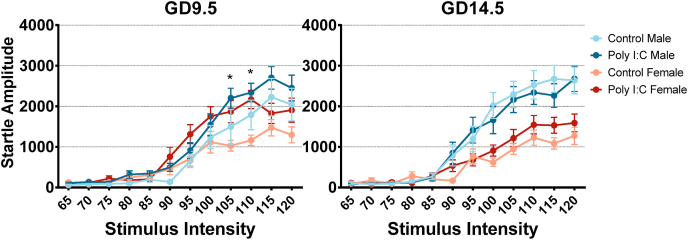
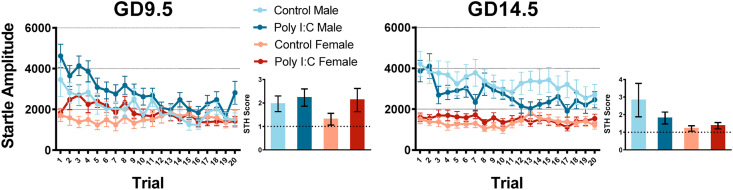
Fig. 3.
GD9.5 but not GD14.5 poly I:C treatment increases startle reactivity in adult offspring regardless of sex. Data is shown for both males and females from saline and poly I:C offspring for each timepoint. For GD9.5 adult offspring, a 3-way repeated measures ANOVA showed a main effect of prenatal treatment (p = 0.007), as well as an interaction between stimulus intensity and treatment (p = 0.035). Post-hoc analysis with Bonferroni correction showed significant differences for stimulus intensities of 105 and 110 dB. There was no significant interaction between prenatal treatment and sex (p = 0.808). In contrast, a 3-way repeated measures ANOVA conducted for GD14.5 adult offspring showed no main effect (p = 0.709) of prenatal treatment or significant interactions associated with it.
Fig. 4.
Neither GD9.5 nor GD14.5 poly I:C treatment change short-term habituation of startle in adolescent offspring. Data is shown for both males and females from saline and poly I:C offspring for each timepoint. A 3-way repeated measures ANOVA conducted separately for GD9.5 and GD14.5 offspring showed a main effect of trial (p < 0.001 for both analyses), but no significant main effect of prenatal treatment or interactions between prenatal treatment and sex or prenatal treatment and trial, indicating short-term habituation across all groups and no effects of either prenatal poly I:C exposures. Insets show quantification of short term habituation using a ratio of startle amplitude on the first 5 trials divided by startle amplitude on the last 5 trials, with values > 1 indicating short term habituation of startle.
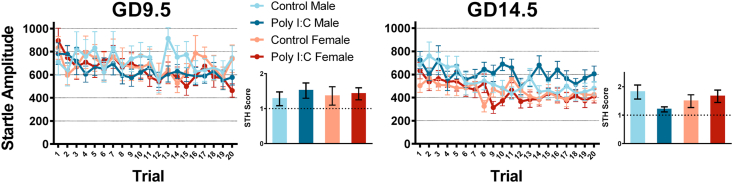
Fig. 5.
GD9.5 and GD14.5 adult offspring exhibit similar short-term habituation to controls, but GD9.5 offspring show a strong trend to increased startle reactivity in the first few trials of the habituation block. Data is shown for both malesand females from saline and poly I:C offspring for each timepoint. For GD9.5 offspring, a 3-way repeated measures ANOVA showed strong trends for a main effect of prenatal treatment (p = 0.074) and an interaction between trial and prenatal treatment (p = 0.064 with Greenhouse-Geisser correction). In contrast, there were no significant main effects or interactions associated with prenatal treatment for GD14.5 offspring. Insets show quantification of short term habituation using the ratio of startle amplitude of the 5 first trials divided by the last 5 trials.
Fig. 6.
Neither GD9.5 nor GD14.5 poly I:C treatment change adolescent startle reactivity or long-term habituation of startle as measured by the first 5 trials of the habituation block. Data is shown for both males and females from saline and poly I:C offspring for each timepoint. A 3-way repeated measures ANOVA conducted separately for GD9.5 and GD14.5 adolescent offspring showed no significant main effect of prenatal treatment or any interactions with day or sex. Interestingly, all adolescent offspring failed to show strong long-term habituation (p = 0.797 and p = 0.399 respectively for main effect of day).
Fig. 7.
GD9.5 and GD14.5 adult offspring exhibit similar long-term habituation to controls, but GD9.5 offspring show higher startle reactivity across days as measured by the first 5 trials of the habituation block. Data is shown for both males and females from saline and poly I:C offspring for each timepoint. For GD9.5 offspring, a 3-way repeated measures ANOVA revelead significant main effects of day, prenatal treatment and sex, but no interactions between them (p < 0.001, p = 0.036 and p = 0.01 respectively), providing evidence for changes in startle reactivity but not long-term habituation. For GD14.5 offspring, a similar analysis was conducted and a 3-way interaction followed by post-hoc testing revealed a significant decrease in startle reactivity only on day 3 for male poly I:C offspring, implying that these animals exhibit stronger long-term habituation across the first 3 days of testing but do not habituate further into days 4 and 5.
Fig. 8.
Neither GD9.5 nor GD14.5 poly I:C treatment change PPI in adolescent offspring across a variety of prepulse conditions. Data is shown for both males and females from saline and poly I:C offspring for each timepoint. A 4-way repeated measures ANOVA conducted separately for GD9.5 and GD14.5 adolescent offspring showed main effects of prepulse (PP) intensity and ISI for both groups but no main effect or interactions associated with prenatal treatment (see text for detailed statistics).
Fig. 9.
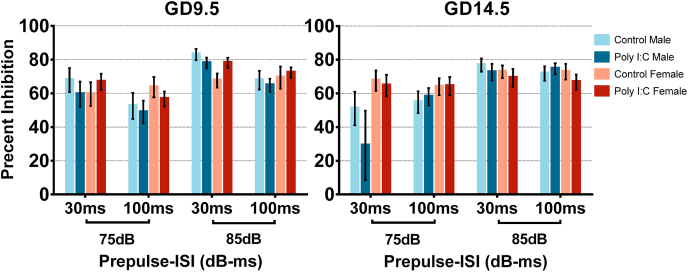
Neither GD9.5 nor GD14.5 poly I:C treatment change PPI in adult offspring across a variety of prepulse conditions. Data is shown for both males and females from saline and poly I:C offspring for each timepoint. A 4-way repeated measures ANOVA conducted separately for GD9.5 and GD14.5 adolescent offspring showed main effects of prepulse (PP) intensity and ISI for both groups, but no main effect or interactions associated with prenatal treatment, besides a significant three-way interaction between ISI, prenatal treatment and sex in GD9.5 offspring (see text for detailed statistics).
Fig. 10.
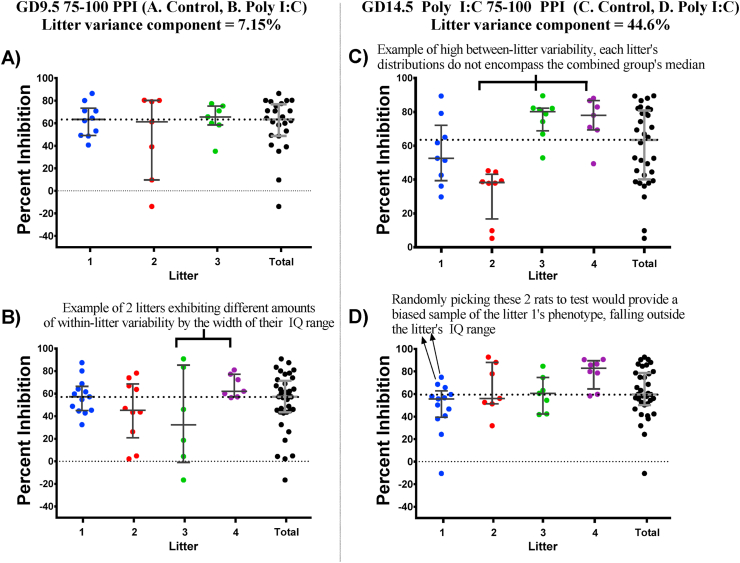
A representation of between and within litter variability in PPImeasures in our control and poly I:C offspring. Within each group, litters are depicted by different colours and each dot represents a single animal. The black group simply contains the entire group’s data, which was used to calculate effects in the results section. Error bars represent each group’s 25th and 75th quartiles are drawn in reference to the group’s median. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.8. Variability analysis
Between-litter variability: We quantified between-litter variability by analyzing litter as a random factor using a variance component analysis. In this case, random refers to the fact that our 3 or 4 litters are only a sample of infinitely many possible litters exposed to the same treatment. We measured the extent to which between-litter variability impacts the total variance in the whole group while taking into account fixed factors of sex and treatment. For example, Fig. 10C illustrates how much of the variance in the combined group shown in black is simply due to differences between the green, purple, and red subgroups. Specifically, we added the variance attributed to litter only and litter∗treatment interaction in the GD9.5 and GD14.5 groups and expressed that as a ratio of the total variance in the dataset. Litter only refers to between-litter variability that was similarly present in both poly I:C and saline offspring, whereas litter∗treatment refers to between-litter variability that was present to different extents in poly I:C offspring compared to saline offspring.
Within-litter variability: To quantify the extent of the sampling bias that can be present in instances of high within-litter variability, we analyzed data from the first litter of each experimental group, because those litters were not culled. Additionally, we only used PPI data, so that males and females could be combined to obtain a bigger sample. PPI data is normalized for each animal and there were no interactions between prenatal treatment and sex. Using this data set, we virtually recreated the process of culling, or randomly selecting offspring from the entire litter to include in the PPI experiment. We generated a thousand samples using RStudio (version 1.3.595) for every combination of sample size (2–7), age (adolescent or adult), treatments (GD9.5 control, GD9.5 poly I:C, GD14.5 control, GD14.5 poly I:C), and PPI parameters (prepulse-ISI of 75–30, 75–100, 85–30 or 85–100), and assessed how often the mean of those random samples fell outside the Interquartile (IQ) range of the full litter (in Fig. 10, the IQ range is outlined between the top and bottom error bars), i.e. the sample is not representative of the whole litter.
3. Results
3.1. Elevated cytokine gene expression in fetal tissue at 6 h following poly I:C injection, but no changes in body weight at 24 h or temperature at 3- or 24-h following poly I:C injection
Univariate ANOVAs conducted separately for temperature change at 3 or 24 h following poly I:C showed no significant main effect of poly I:C (Fig. 1A; 3 h: F(1,21) = 0.01, p = 0.923; 24 h: F(1,14) = 0.131, p = 0.723) and no interaction between poly I:C and GD (3 h: F(1,21) = 0.01, p = 0.923; 24 h: F(1,14) = 0.003, p = 0.956), indicating a similar lack of temperature response following poly I:C in both groups.
Similarly, a univariate ANOVA conducted for body weight change at 24 h post-injection revealed no significant effect of injection (Fig. 1B; F(1,16) = 0.586, p = 0.455) but a significant GD∗Injection interaction (F(1,19) = 4.931, p = 0.041). However, post-hoc testing with Bonferroni correction for multiple comparisons showed no significant effect of injection for either GD14.5 (p = 0.072 with poly I:C > saline) or GD9.5 (p = 0.267 with poly I:C < saline).
In a subset of dams from the animals described above in weight and temperature analysis, we confirmed the efficacy of the poly I:C batch used in this experiment using qRT-PCR. This was performed on fetal tissue collected 6 h after GD9.5 poly I:C or saline injection, and involved measuring gene expression of pro and anti-inflammatory cytokines commonly elevated following poly I:C. Unpaired t-tests conducted separately for each cytokine revealed a significant increase in the fetal gene expression of Interleukin-6 (t(10) = 6.40, p < 0.001), Interleukin-10 (t(10) = 3.40, p = 0.007), Tumor Necrosis Factor-α (t(10) = 6.26, p < 0.001) and Interferon-γ (t(10) = 3.37, p = 0.007) when compared to saline-injected control animals (Fig. 1C).
3.2. Increased startle reactivity in the adult
Startle reactivity was measured to increasing startle stimulus intensities between 70 and 110 dB in 5 dB increments. Neither GD9.5 nor GD14.5 poly I:C offspring group showed changes in startle reactivity in adolescence (Fig. 2). Three-way repeated-measures ANOVAs with stimulus intensity as a within-subject factor revealed no significant interactions between prenatal treatment, sex, and stimulus intensity. Main effects of prenatal treatment and sex were also not significant, and the only significant effect was that of stimulus intensity (GD9.5: Greenhouse-Geisser correction applied; F(5.475,627) = 79.897, p < 0.001, partial η2 = 0.584, ε = 0.498; GD14.5: Greenhouse-Geisser correction applied; F(5.285,682) = 117.262, p < 0.001, partial η2 = 0.654, ε = 0.480).
In contrast, adult offspring of the GD9.5, but not of the GD14.5 poly I:C group, exhibited increased startle reactivity independent from sex, particularly at 105- and 110-dB stimulus intensities (Fig. 3). Three-way repeated-measures ANOVAs with stimulus intensity as a within-subject factor revealed no significant three-way interactions between prenatal treatment, sex, and stimulus intensity. However, there was a significant interaction between stimulus intensity and GD9.5 prenatal treatment (Greenhouse-Geisser correction applied; F(4.112, 627) = 2.607, p = 0.035, partial η2 = 0.044, ε = 0.374). Post-hoc comparison of startle amplitude between GD9.5 poly I:C and saline offspring at each stimulus intensity with Bonferroni correction showed a significant increase in startle reactivity at 105 and 110 dB stimulus intensity for GD9.5 poly I:C offspring (p = 0.008 and 0.003 respectively). For GD14.5 offspring, there was no interaction between stimulus intensity and prenatal treatment (Greenhouse-Geisser correction applied; F(5.074, 693) = 0.250, p = 0.941, partial η2 = 0.0004, ε = 0.461) and no main effect of prenatal treatment (F(1,63) = 0.141, p = 0.709, partial η2 = 0.002).
3.3. No changes in short-term habituation of startle
Short-term startle habituation was measured through the animals’ startle amplitude across the first 20 habituation trials on day 1 of startle testing. All adolescent offspring showed STH, and neither GD9.5 nor GD14.5 poly I:C treatment had an impact on STH (Fig. 4). Three-way repeated-measures ANOVAs with trial number as a within-subject factor revealed no significant three-way or two-way interactions when considering the factors of trial number, prenatal treatment, and sex. However, both GD9.5 and GD14.5 offspring exhibited a main effect of trial number, indicating STH across trials (GD9.5: Greenhouse-Geisser correction applied; F(8.774,1083) = 2.466, p = 0.010, partial η2 = 0.041, ε = 0.452; GD14.5: Greenhouse-Geisser correction applied; F(11.417,1197) = 4.467, p < 0.001, partial η2 = 0.066, ε = 0.601). All offspring groups showed STH as measured by STH score values larger than 1. Univariate ANOVAs were conducted separately for GD9.5 and GD14.5 offspring on the STH score with sex and prenatal treatment as between-subject factors. Neither analysis showed significant interactions with or main effects of prenatal treatment, indicating that neither GD9.5 nor GD14.5 impact STH score in adolescent offspring.
In adulthood, all offspring also showed STH across trials (Fig. 5). Three-way repeated-measures ANOVAs with trial number as a within-subject factor revealed a non-significant trend for an interaction between trial and prenatal treatment in GD9.5 offspring, potentially pointing towards an impact of prenatal poly I:C on STH (GD9.5: Greenhouse-Geisser correction applied; F(8.852,1083) = 1.815, p = 0.064, partial η2 = 0.031, ε = 0.466; GD14.5: Greenhouse-Geisser correction applied; F(9.697,1197) = 1.480, p = 0.145, partial η2 = 0.023, ε = 0.510). STH scores were calculated as described in the previous paragraph, and univariate ANOVAs were conducted separately for GD9.5 and GD14.5 offspring on the STH score with sex and prenatal treatment as between-subject factors. Once again, neither analysis showed significant interactions with or main effects of prenatal poly I:C treatment, indicating that the trend towards a prenatal treatment and trial interaction in GD9.5 offspring is likely due to the increased startle reactivity in adult poly I:C offspring rather than changes in STH.
3.4. Changes of long-term habituation of startle in the adult
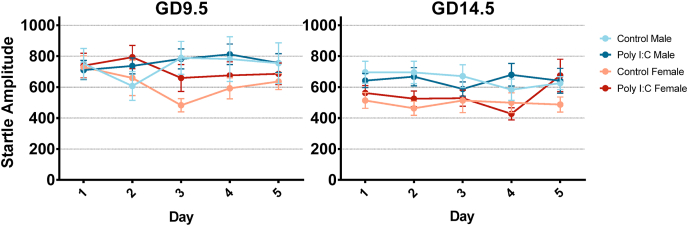
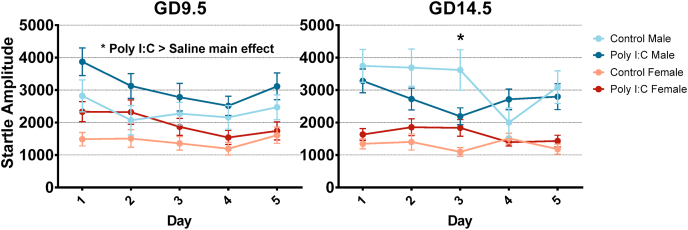
LTH was measured through the animals’ startle amplitude on the first 5 trials of startle testing across 5 days of testing. LTH was not impacted in adolescent offspring (Fig. 6). Three-way repeated-measures ANOVAs with day as a within-subject factor revealed no significant interactions or main effects associated with prenatal treatments for either timepoint. Interestingly, neither adolescent groups showed a main effect of day (GD9.5: Greenhouse-Geisser correction applied; F(3.408,228) = 0.416, p = 0.766, partial η2 = 0.007, ε = 0.852; GD14.5: Greenhouse-Geisser correction applied; F(3.438,252) = 1.018, p = 0.392, partial η2 = 0.016, ε = 0.859), indicating a lack of long-term habituation in all groups, which may be attributed to the offspring’s age or the nature of protocol used.
In adulthood, startle reactivity was increased in the GD9.5 poly I:C group (see above), but there was no change in LTH (Fig. 7). In the GD14.5 poly I:C group, the rate but not extent of LTH was changed in males. Three-way repeated-measures ANOVAs conducted for GD9.5 offspring with day as a within-subject factor revealed significant main effects of prenatal treatment (F(1,57) = 4.616 p = 0.036), sex (F(1,57) = 12.237, p = 0.001) and day (F(4,228) = 5.690, p < 0.001) with no interactions between the 3 factors. These results indicate that while startle amplitude is generally increased across all 5 days in GD9.5 poly I:C offspring, LTH is intact, although generally weak LTH in females may be a confound (Fig. 7). A similar analysis conducted for GD14.5 offspring revealed a significant three-way interaction between prenatal treatment, sex, and day (F(4,232) = 5.627, p < 0.001). Two-way repeated-measures ANOVAs were then conducted separately for GD14.5 males and females and those revealed a significant interaction between prenatal treatment and day in male offspring (F(4,112) = 3.321, p = 0.013). Post-hoc comparison with Bonferroni correction showed a significant decrease in startle amplitude for GD14.5 poly I:C males on day 3 (p = 0.019), potentially indicating faster LTH, although the total habituation after 5 days of testing was unchanged as evidenced by a lack of difference in startle amplitude on days 1 and 5.
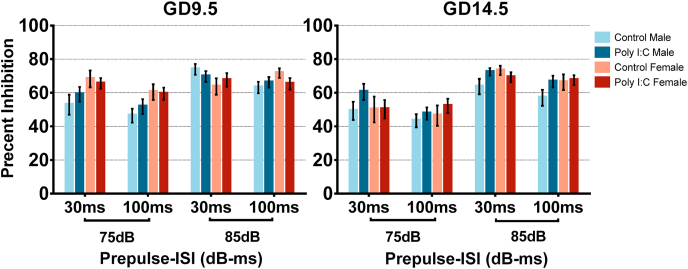
3.5. No changes in prepulse inhibition of startle
PPI was measured on day 1 of startle testing with a variety of prepulse intensities and ISI. Four-way repeated-measures ANOVAs with prepulse intensity and ISI as within-subject factors revealed no significant interactions with or main effects of prenatal treatment in adolescent offspring of the GD9.5 or GD14.5 poly I:C group (Fig. 8). Higher prepulses and shorter ISIs were associated with increased PPI across all groups (GD9.5 prepulse main effect F(1,57) = 22.239, p < 0.001; GD9.5 ISI main effect F(1,57) = 6.110, p = 0.016; GD14.5 prepulse main effect F(1,63) = 72.062, p < 0.001; GD14.5 ISI main effect F(1,63) = 12.482, p < 0.001).
Four-way repeated measures ANOVAs conducted for adult GD9.5 offspring with prepulse intensity and ISI as a within-subject factors revealed a significant three-way interaction between ISI, prenatal treatment, and sex (F(1,57) = 4.483, p = 0.039). Three-way repeated-measures ANOVAs were then conducted separately for GD9.5 males and females and those revealed a significant interaction between ISI and prenatal treatment in female offspring (F(1,28) = 6.824, p = 0.014) with a trend for increased PPI in poly I:C offspring at 30 ms ISI, but the result did not reach statistical significance after Bonferroni correction for multiple comparisons (F(1,28) = 3.740, p = 0.063). A similar analysis for adult GD14.5 offspring revealed no significant interactions or main effects associated with prenatal treatment (Fig. 9).
3.6. Variability analysis
In the process of data analysis, we noticed instances of high variability of some measures between litters of the same experimental group, and also within specific litters (Fig. 10). In the next section, we attempt to describe the extent to which between- and within-litter variability impacted our results.
3.6.1. Between-litter variability
To have an estimate on the extent to which between-litter variability impacts the total variance in the whole group, we performed a variance component analysis. The results are shown in Table 1. When considering all 7 measures and ages, between-litter variability accounted for 18% of the variance in GD14.5 offspring data (saline and poly I:C offspring combined), but only 9.6% of the variance in GD9.5 offspring data. Between-litter variability was also more prominent in adolescence in GD9.5 offspring data (11.5% compared to 7.7% in adulthood) and in adulthood in GD14.5 offspring data (20.4% compared to 15.7% in adolescence). Splitting up by phenotype, the between-litter effects do not impact all behavioural measurements to the same extent. For example, between-litter variability accounted for approximately 18.6% of all PPI variance (averaged across ages, treatments, parameter subtypes), but only about 3% of all habituation score data variance. Even within the same paradigm (PPI), between-litter effects were different across different testing parameters, most prominently seen in GD14.5 adult offspring, where almost half of the data’s variance (44%) was attributed to between-litter effects in conditions with an ISI of 100 ms.
Table 1.
Between litter variability in 7 startle phenotypes. All values in the table represent the percent variance component of litter + litter∗treatment within each prenatal exposure group (GD9.5 or GD14.5).
| Average across all animals for each measure | ||
|---|---|---|
| Startle Reactivity | 11.3% | |
| Habituation Score | 3.1% | |
| Startle Baseline on Day 1 | 8.1% | |
| PPI | 18.6% | |
| Average across all measures for each exposure timing | ||
| GD9.5 | 9.6% | |
| GD14.5 | 18.1% | |
| Measure and age-specific | ||
| GD9.5 | GD14.5 | |
| Adolescence: Average across all 7 measures | 11.5% | 15.7% |
| Startle Reactivity to 110 dB | 0.0% | 22.8% |
| Habituation Score | 5.1% | 7.3% |
| Startle baseline (average of first 5 trials) on day 1 | 19.2% | 0.0% |
| PPI | ||
| 75–30 | 3.9% | 19.5% |
| 75–100 | 14.1% | 27.7% |
| 85–30 | 0.7% | 6.6% |
| 85–100 | 37.7% | 26.3% |
| Adulthood: Average across all 7 measures | 7.7% | 20.4% |
| Startle Reactivity to 110 dB | 22.5% | 0.0% |
| Habituation Score | 0.0% | 0.0% |
| Startle baseline (average of first 5 trials) on day 1 | 1.6% | 11.6% |
| PPI | ||
| 75–30 | 7.8% | 14.1% |
| 75–100 | 7.2% | 44.6% |
| 85–30 | 15.0% | 29.0% |
| 85–100 | 0.0% | 43.4% |
3.6.2. Within-litter variability
When looking closely at individual litters, we observed that even within the same experimental group, one litter’s data could be much more variable compared to another litter (Fig. 10B). For example, as depicted in Fig. 10D, choosing the two animals tagged by arrows would provide a biased overestimate of litter 1’s PPI phenotype at 75 dB prepulse and 100 ms ISI, which we refer to as sampling bias.
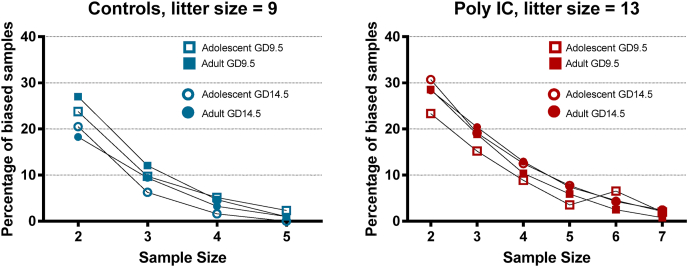
Fig. 11 shows the percentage of samples, drawn from litter 1 of each group, that had a mean falling outside the IQ range of the full litter (Y-axis, probability of obtaining a biased sample). It clearly shows that testing more animals per litter reduces the probability of obtaining biased estimates of the litter’s phenotype. This relationship holds regardless of age or prenatal treatment. Despite all lines in Fig. 11 following a similar downward trajectory with an increased sample size, there is still some variation between different groups (differences between symbols within the same graph), which could be attributed to each group’s data distribution. We wanted to see precisely which aspects of a full litter’s data could explain this variation. For example, perhaps it is more likely to pick biased samples from a litter whose data has a higher standard deviation, or whose data is not normally distributed. For this purpose, we split up all the PPI conditions to obtain more datasets, each with their unique distribution. We found a positive correlation between the probability of obtaining a biased sample and the kurtosis or absolute skewness of the litter’s data, regardless of the sample size (Supplementary Table 2 and supplementary figure 2 for a graphical representation for sample size of 2). Skewness and kurtosis are both measures of normality and give an idea of the shape of the distribution. For example, in Fig. 10D, the litter 3 (green) is relatively non-skewed, with the top and bottom quartile bars being a similar distance from the median, whereas litters 2 and 4 (red and purple) are skewed in different directions. To interpret this correlation using our results, the odds of a sample chosen from litter 2 misrepresenting litter 2’s full data are higher than the odds of a sample chosen from litter 3 misrepresenting litter 3’s full data.
Fig. 11.
The probability of a random sample of 2–7 animals picked out from litter 1 of each group to produce a biased estimate of the litter’s PPI phenotype Data was averaged across all 4 PPI conditions (prepulse-ISI combinations) used in our experiment. A biased estimate was considered to be a sample mean falling outside of the full litter’s IQ range. A thousand random samples of size 2–7 were generated from each group’s first litter, where animals were not culled, for each PPI condition. Each sample’s average was compared to the full litter’s 25th and 75th quartiles for that PPI condition. The number of samples out of 1000 that fell above the 75th or below the 25th quartiles averaged across all 4 PP-ISI conditions is represented on the Y-axis.
4. Discussion
In this study, we thoroughly investigated phenotypes associated with the baseline startle reflex and its modulations in animals exposed to prenatal immune activation using poly I:C. Acoustic startle reactivity, habituation, and PPI are all common phenotypes measured in ASD and schizophrenia clinical studies, two disorders that have been repeatedly linked with an increased risk after maternal infection. We report a robustly increased startle response amplitude in offspring after an early (GD9.5) maternal immune activation, but not after GD14.5. We did not find any consistent effects on STH or LTH, nor PPI of startle.
4.1. Immune response following poly I:C
In our study, neither GD9.5 nor GD14.5 influenced maternal body weight at 24 h post-injection, which conflicts with previous studies using similar MIA rat models (Chou et al., 2015; Howland et al., 2012; Murray et al., 2017; Sangha et al., 2014; Zhang et al., 2012). Similarly, we did not detect changes in maternal temperature at 3 h following poly I:C, which has previously been shown following 5 mg/kg of poly I:C (Murray et al., 2019). However, others have reported no significant changes in body weight gain (Vernon et al., 2015) or temperature (Murray et al., 2017; Sangha et al., 2014), following poly I:C administration, although the latter 2 studies measured maternal temperature at 8 h.
Overall, we conclude that maternal temperature and body weight gain following poly I:C are unreliable measures of the maternal immune response, especially given our confirmation of a robust gene expression response in fetal tissue 6 h following administration of the same batch of poly I:C in GD9.5 dams, which suggests that the poly I:C used in this experiment contained sufficient amounts of high molecular weight strands known to induce a cytokine response at 6 h post-injection (Careaga et al., 2018). Although we did not conduct a similar gene expression test in GD14.5 dams, there is ample evidence to support the efficacy of poly I:C when injected at GD14.5 (Haddad et al., 2020) and little to suggest that poly I:C leads to substantially different immune responses when injected at different gestational time points in rats.
4.2. Startle reactivity
Our data show that GD9.5 but not GD14.5 poly I:C administration increases startle reactivity in the offspring in adulthood. Previous studies investigating startle reactivity in adults with ASD have reported an increase in startle reactivity to 110 dB startle stimuli, as well as an increase in the probability of startle to 80 dB prepulse stimuli (Kohl et al., 2014). In our study, GD9.5 poly I:C offspring also exhibited an increase in startle reactivity to 110 dB stimulation (Fig. 3). However, startle amplitude was unchanged at lower stimulus intensities and due to the methods of data acquisition, we were unable to accurately determine the probability of startle for these stimuli. One limitation of the GD9.5 poly I:C model in replicating startle reactivity changes seen in ASD is the relatively late phenotype manifestation. Startle reactivity is increased in children and adolescents with ASD to sounds as quiet as 65 dB (Takahashi et al., 2016, 2014) whereas our adolescent GD9.5 poly I:C offspring did not exhibit changes in startle reactivity to any sounds in the range of 65–120 dB.
The majority of poly I:C studies that investigated startle reactivity in GD9.5 poly I:C offspring report no change in this phenotype (Meyer et al., 2010; Vuillermot et al., 2011; Meehan et al., 2017), although some conflicting reports of increased reactivity exist (Kim et al., 2018). We believe our startle reactivity measurements are more sensitive to detecting changes in ASR amplitude than previous poly I:C studies, since we measure the ASR amplitude in a separate session with a minimum number of trials to avoid any effects of STH on our readout. Our STH results show that habituation is intact in GD9.5 poly I:C offspring and could therefore mask the startle reactivity changes if reactivity is measured using an average that includes habituated trials. Our STH curves indicate by the 10th trial of our habituation block, startle reactivity is indistinguishable between GD9.5 poly I:C and control offspring (Fig. 5). In further support of this notion, ASR amplitude to startle only trials during the PPI block of our experiment was not significantly different between GD9.5 poly I:C and control offspring (data not shown). We also did not detect significant changes in ASR amplitude at 120 dB, suggesting that a ceiling effect may prevent the detection of reactivity changes in poly I:C rodent studies. Furthermore, previous poly I:C studies almost exclusively utilize 120 dB startle stimuli that are 40 ms in duration (Kim et al., 2018; Meyer et al., 2008; Song et al., 2011; Van den Eynde et al., 2014; Wolff and Bilkey, 2010; Zhang and van Praag, 2015). Our choice of a less intense 20 ms, 110 dB stimulus may therefore be more suitable to detect changes in startle reactivity.
4.3. Startle habituation
Neither GD9.5 nor GD14.5 poly I:C influenced STH in the offspring, as measured by the decrease in startle reactivity over 20 consecutive startle-only trials (Figs. 4 and 5), and this is in line with the handful of poly I:C studies that investigated STH (Meyer et al., 2005; O’Leary et al., 2014; Wolff and Bilkey, 2008; Zhang and van Praag, 2015). These findings are more akin to normal habituation in ASD (Ebishima et al., 2019; Kohl et al., 2014; Takahashi et al., 2017) as compared to habituation deficits sometimes observed in patients with schizophrenia (Meincke et al., 2004; Mena et al., 2016). However, some conflicting evidence reporting normal habituation in schizophrenia does exist (Oranje and Glenthøj, 2013; Swerdlow et al., 2014). As described in the methods section, we took a different approach to measure STH compared to previous studies. We believe our consideration of all trials in the habituation block, which occurs at the beginning of the testing session, gives a better representation of trial-by-trial STH and also more clearly identifies the presence or absence of startle sensitization (e.g Fig. 4 GD9.5 Females trials 15–17), which often occurs in later trials and may confound startle habituation scores.
In contrast to STH, LTH of startle has been rarely studied in poly I:C studies. Our results show that similar to STH, LTH is not influenced by prenatal exposure to GD9.5 or GD14.5 poly I:C (Fig. 6, Fig. 7). Typically, LTH is not part of human startle testing protocols, likely due to the difficulty of bringing in patients for recurrent testing across days. However, one previous report of normal LTH in individuals with ASD (Ornitz et al., 1993) is in line with our results. We also found that adolescent animals, regardless of prenatal treatment, did not exhibit substantial LTH across testing days (Fig. 6), which may be related to the developmental time course of habituation mechanisms. For example, Pletnicov et al. (1995) showed that pre-weanling rats exhibit STH but not LTH of acoustic startle. In contrast, adult offspring demonstrated robust LTH across 5 days of testing, as shown by a decrease in startle baseline amplitude measured as an average of the first 5 trials across test days (Fig. 7). Interestingly, GD14.5 poly I:C males showed faster LTH than controls, although the significance of this result is diminished by the observation that controls only required one more day to reach a similar degree of LTH.
4.4. Prepulse inhibition of startle
To our surprise, neither GD9.5 nor GD14.5 poly I:C treatment showed a consistent impact on PPI in adolescent or adult offspring. The closest trend to a significant effect was an increase in PPI at 30-ms ISI in adult GD9.5 poly I:C females, although the effects were not significant after adjustment for multiple comparisons. Since startle reactivity was indistinguishable between groups at the end of the habituation block (Fig. 4, Fig. 5), we do not assume that changes in baseline startle reactivity impacted PPI. In the context of ASD and schizophrenia, the two disorders most commonly linked with MIA, these findings are more similar to reports of no change in PPI in ASD (Ebishima et al., 2019; Madsen et al., 2014; Oranje et al., 2013; Takahashi et al., 2016) as opposed to the overwhelming evidence of PPI disruptions in schizophrenia (Csomor et al., 2009; Hammer et al., 2011; Swerdlow et al., 2018, 2014).
The lack of PPI disruption in our study is in contrast to previous reports of decreased PPI in GD9.5 (Meehan et al., 2017; Meyer et al., 2008; Vuillermot et al., 2011) and GD14.5 (Luchicchi et al., 2016; Wolff and Bilkey, 2010; Zhang and van Praag, 2015) poly I:C studies. However, there are also numerous reports of no changes in PPI for either GD (Abazyan et al., 2010; Ballendine et al., 2015; Chou et al., 2015; Gray et al., 2019; Lipina et al., 2013; Missault et al., 2014; Vorhees et al., 2012). It is important to note that early timepoints of poly I:C exposure such as GD9.5 are not well studied in rats, as discussed in a recent review that classified studies based on poly I:C exposure categories of dose, timing, and route of administration (Haddad et al., 2020). Moreover, contrasting rat data to mouse data is not straightforward, given the slight but substantial difference in developmental timeline across the two species (http://translatingtime.org; Haddad et al., 2020).
We confirmed the efficacy of the poly I:C we used in eliciting an innate immune response in a subset of dams injected with poly I:C at GD9.5 (Fig. 1). Despite not performing this analysis in GD14.5 dams, previous studies support the efficacy of poly I:C administered using similar procedures at GD14.5 in rats (Clark, 2019; Gray 192). Similar dosing and administration methods are followed in virtually all GD14.5 poly I:C rat literature (see Haddad, 2020 for review), and we are not aware of literature that suggests different poly I:C responses based on gestational timing in rats. Therefore, we can rule out that the poly I:C we used was ineffective.
Our PPI protocol contained shorter stimulus durations for both the startle pulse (20 ms compared to the most commonly used 40 ms) and the prepulse (4 ms compared to the most commonly used 20 ms). Shorter prepulses produce less intense PPI (Reijmers and Peeters, 1994). As a result, our shorter prepulses may have lowered the baseline PPI values across all groups, making it more difficult to detect subtle differences in PPI between groups. We intentionally chose shorter prepulse durations to reduce PPI and avoid ceiling effects when using shorter ISIs of 30 ms, which typically produce greater PPI compared to longer (and more commonly used) ISIs of 100 ms (Azzopardi et al., 2018; Schmid et al., 2011; Valsamis and Schmid, 2011; Yang et al., 2016). Additionally, we did not include an intermediate prepulse between 75 and 85 dB to avoid a large number of trials, which often leads to extensive habituation that makes it difficult to detect the startle response. Some studies also indicate that PPI phenotypes may differ if the startle stimulus intensity is changed, which could explain the variability in PPI findings across studies (Weber-Stadlbauer et al., 2017; Mueller et al., 2018; Luan et al., 2018; Vuillermot et al., 2010).
4.5. Variability analysis
Litter variability has been a topic of discussion in toxicology and neurodevelopmental models for several decades, specifically in relation to models that investigate prenatal exposures such as poly I:C (Golub and Sobin, 2020)). Within the poly I:C literature, there is a trend towards increasing the number of litters and reducing the number of pups used per litter, typically 1–2 animals are used per litter (e.g see recent poly I:C literature: Clark et al., 2019, De Felice et al., 2018, Di Biase et al., 2020, Haida et al., 2019, Kleinmans and Bilkey, 2018, Purves-Tyson et al., 2019; Zhang et al., 2019). In this case, within-litter variability has implications for whether the chosen rats are representative of the litter’s phenotype. Alternatively, between-litter variability has implications for whether the full group’s phenotype is attributable to the treatment or is simply an artifact of differences between litters. We therefore attempted to analyze between- and within-litter variability in our results to highlight important considerations for the poly I:C MIA field.
Overall, between-litter variability was more evident in GD14.5 data, particularly for PPI (Table 1). This may have masked our ability to fully decipher the effects of poly I:C on PPI and may be the reason why our findings of no change in PPI conflict with some previous reports of PPI disruption. In support of this notion, the vast majority of poly I:C literature measures PPI with 100 ms ISI, which is where we found the biggest between-litter variability in GD14.5 offspring (approximately 44%, Fig. 10C and D). The high between-litter variability seems problematic in that it increases the variation of the final outcome. It makes PPI a relatively unreliable measure, especially when a limited number of litters are tested. It has been shown that there is a strong genetic component to PPI (Schwabe et al., 2007), which might cause the large between-litter effects in these outbred rats. Moreover, there might be a differential susceptibility to poly I:C, so differences in impact of poly I:C MIA on PPI may help uncover factors that exacerbate or protect against MIA. Future studies can account for between-litter variability by conducting a similar variance component analysis to ours or by analyzing data using mixed models that consider litter as a random factor.
In addition to between-litter variability, within-litter variability has many implications for experimental designs where litters are culled, or only 1–2 animals are used per litter for the experiment. The most crucial observation from our within-litter analysis (Fig. 11) is that picking only two or three pups out of a litter for testing leads to a high probability of a biased sample (up to 30% for large litters) that are not representative of the full litter’s data, whereas measuring just over half of the litter (5/9 or 7/13) almost fully eliminates the probability of the sampled group of animals over or underestimating the full litter’s phenotype. We also show that litter data with skewness and kurtosis values that indicate deviation from normality are more likely to produce biased samples (see supplemental table and figure S2). Therefore, at least half of an average litter should be tested for an accurate representation of the effects of poly I:C on the offspring. Additionally, if a behavioural test is known to produce skewed data in littermates, more animals per litter should be tested to minimize sampling bias.
In summary, we here show that baseline startle reactivity is the only startle-related phenotype that is robustly altered in poly I:C offspring, whereas STH, LTH, and PPI were unaffected in our study. Moreover, our study supports previous evidence highlighting the importance of MIA timing (Li et al., 2009; Meyer et al., 2008; Richetto et al., 2017), as startle reactivity changes were present in offspring exposed to GD9.5 but not GD14.5 poly I:C. Finally, our study quantifies the potential impact of between- and within-litter variability on MIA results, suggesting that at least half the average litter size should be tested and that litter should be included rather than avoided in statistical analysis to fully understand the effects of MIA on offspring behavior.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
We want to thank Salonee Patel and Nikita Thakkar for help with postmortem tissue processing. This study was supported by the Natural Science and Engineering Council (NSERC, Canada, Discovery grant, to SS), the Simons Foundation for Autism Research (SFARI, US, to SS), and the Joshua & Jonathan Memorial Fund Canada (to FLH). We thank Cleusa DeOliveira for her help with this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100156.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abazyan B., Nomura J., Kannan G., Ishizuka K., Tamashiro K.L., Nucifora F., Pogorelov V., Ladenheim B., Yang C., Krasnova I.N., Cadet J.L., Pardo C., Mori S., Kamiya A., Vogel M.W., Sawa A., Ross C.A., Pletnikov M.V. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol. Psychiatr. 2010;68:1172–1181. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladóttir H.Ó., Thorsen P., Østergaard L., Schendel D.E., Lemcke S., Abdallah M., Parner E.T. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Azzopardi E., Louttit A.G., DeOliveira C., Laviolette S.R., Schmid S. The role of cholinergic midbrain neurons in startle and prepulse inhibition. J. Neurosci. 2018;38:8798–8808. doi: 10.1523/JNEUROSCI.0984-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballendine S.A., Greba Q., Dawicki W., Zhang X., Gordon J.R., Howland J.G. Behavioral alterations in rat offspring following maternal immune activation and ELR-CXC chemokine receptor antagonism during pregnancy: implications for neurodevelopmental psychiatric disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2015;57:155–165. doi: 10.1016/j.pnpbp.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomström Å., Karlsson H., Wicks S., Yang S., Yolken R.H., Dalman C. Maternal antibodies to infectious agents and risk for non-affective psychoses in the offspring—a matched case–control study. Schizophr. Res. 2012;140:25–30. doi: 10.1016/j.schres.2012.06.035. [DOI] [PubMed] [Google Scholar]
- Brown A.S., Begg M.D., Gravenstein S., Schaefer C.A., Wyatt R.J., Bresnahan M., Babulas V.P., Susser E.S. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatr. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Canetta S., Sourander A., Surcel H.-M., Hinkka-Yli-Salomäki S., Leiviskä J., Kellendonk C., McKeague I.W., Brown A.S. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Aust. J. Pharm. 2014;171:960–968. doi: 10.1176/appi.ajp.2014.13121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M., Murai T., Bauman M.D. Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biol. Psychiatr. 2017;81:391–401. doi: 10.1016/j.biopsych.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M., Taylor S.L., Chang C., Chiang A., Ku K.M., Berman R.F., Van de Water J.A., Bauman M.D. Variability in PolyIC induced immune response: implications for preclinical maternal immune activation models. J. Neuroimmunol. 2018;323:87–93. doi: 10.1016/j.jneuroim.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S., Jones S., Li M. Adolescent olanzapine sensitization is correlated with hippocampal stem cell proliferation in a maternal immune activation rat model of schizophrenia. Brain Res. 2015;1618:122–135. doi: 10.1016/j.brainres.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark Sarah M., Notarangelo Francesca M., Li Xin, Chen Shuo, Schwarcz Robert, Tonelli Leonardo H. Maternal immune activation in rats blunts brain cytokine and kynurenine pathway responses to a second immune challenge in early adulthood. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2019;89:286–294. doi: 10.1016/j.pnpbp.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csomor P.A., Yee B.K., Feldon J., Theodoridou A., Studerus E., Vollenweider F.X. Impaired prepulse inhibition and prepulse-elicited reactivity but intact reflex circuit excitability in unmedicated schizophrenia patients: a comparison with healthy subjects and medicated schizophrenia patients. Schizophr. Bull. 2009;35:244–255. doi: 10.1093/schbul/sbm146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice Marta, Melis Miriam, Aroni Sonia, Muntoni Anna Lisa, Fanni Silvia, Frau Roberto, Devoto Paola, Pistis Marco. The PPARα agonist fenofibrate attenuates disruption of dopamine function in a maternal immune activation rat model of schizophrenia. CNS Neurosci. Ther. 2018 doi: 10.1111/cns.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase Maria A., Katabi Gili, Piontkewitz Yael, Cetin-Karayumak Suheyla, Weiner Ina, Pasternak Ofer. Increased extracellular free-water in adult male rats following in utero exposure to maternal immune activation. Brain Behav. Immun. 2020;83:283–287. doi: 10.1016/j.bbi.2019.09.010. [DOI] [PubMed] [Google Scholar]
- Ebishima K., Takahashi H., Stickley A., Nakahachi T., Sumiyoshi T., Kamio Y. Relationship of the acoustic startle response and its modulation to adaptive and maladaptive behaviors in typically developing children and those with autism spectrum disorders: a pilot study. Front. Hum. Neurosci. 2019;13 doi: 10.3389/fnhum.2019.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M.L., McAllister A.K. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353:772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S., Weber-Stadlbauer U., Schedlowski M., Meyer U., Engler H. Prenatal immune activation causes hippocampal synaptic deficits in the absence of overt microglia anomalies. Brain Behav. Immun. 2016;55:25–38. doi: 10.1016/j.bbi.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Golub Mari S., Sobin Christina A. Statistical modeling with litter as a random effect in mixed models to manage “intralitter likeness”. Neurotoxicol. Teratol. 2020;77 doi: 10.1016/j.ntt.2019.106841. [DOI] [PubMed] [Google Scholar]
- Gray A., Tattoli R., Dunn A., Hodgson D.M., Michie P.T., Harms L. Maternal immune activation in mid-late gestation alters amphetamine sensitivity and object recognition, but not other schizophrenia-related behaviours in adult rats. Behav. Brain Res. 2019;356:358–364. doi: 10.1016/j.bbr.2018.08.016. [DOI] [PubMed] [Google Scholar]
- Haddad F.L., Patel S.V., Schmid S. Maternal immune activation by poly I:C as a preclinical model for neurodevelopmental disorders: a focus on autism and schizophrenia. Neurosci. Biobehav. Rev. 2020;113:546–567. doi: 10.1016/j.neubiorev.2020.04.012. [DOI] [PubMed] [Google Scholar]
- Haida Obelia, Sagheer Tareq Al, Balbous Anais, Francheteau Maureen, Matas Emmanuel, Soria Federico, Fernagut Pierre Oliver, Jaber Mohamed. Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Transl. Psychiatry. 2019;9(1):1–12. doi: 10.1038/s41398-019-0457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer T.B., Oranje B., Fagerlund B., Bro H., Glenthøj B.Y. Stability of prepulse inhibition and habituation of the startle reflex in schizophrenia: a 6-year follow-up study of initially antipsychotic-naive, first-episode schizophrenia patients. Int. J. Neuropsychopharmacol. 2011;14:913–925. doi: 10.1017/S1461145711000034. [DOI] [PubMed] [Google Scholar]
- Howland J.G., Cazakoff B.N., Zhang Y. Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience. 2012;201:184–198. doi: 10.1016/j.neuroscience.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.L., Croen L.A., Yoshida C.K., Heuer L., Hansen R., Zerbo O., DeLorenze G.N., Kharrazi M., Yolken R., Ashwood P., Water J.V. de. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol. Psychiatr. 2017;22:273–279. doi: 10.1038/mp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-J., Won H., Im J., Lee H., Park J., Lee S., Kim Y.-O., Kim H.-K., Kwon J.-T. Effects of Panax ginseng C.A. Meyer extract on the offspring of adult mice with maternal immune activation. Mol. Med. Rep. 2018;18:3834–3842. doi: 10.3892/mmr.2018.9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinmans Maren, Bilkey David K. Reversal learning impairments in the maternal immune activation rat model of schizophrenia. Behav. Neurosci. 2018;132(6):520–525. doi: 10.1037/bne0000275. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog. Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Kohl S., Heekeren K., Klosterkötter J., Kuhn J. Prepulse inhibition in psychiatric disorders – apart from schizophrenia. J. Psychiatr. Res. 2013;47:445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Kohl S., Wolters C., Gruendler T.O.J., Vogeley K., Klosterkötter J., Kuhn J. Prepulse inhibition of the acoustic startle reflex in high functioning autism. PloS One. 2014;9 doi: 10.1371/journal.pone.0092372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koks N., Ghassabian A., Greaves-Lord K., Hofman A., Jaddoe V.W.V., Verhulst F.C., Tiemeier H. Maternal C-reactive protein concentration in early pregnancy and child autistic traits in the general population. Paediatr. Perinat. Epidemiol. 2016;30:181–189. doi: 10.1111/ppe.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.K., Magnusson C., Gardner R.M., Blomström Å., Newschaffer C.J., Burstyn I., Karlsson H., Dalman C. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav. Immun. 2015;44:100–105. doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Cheung C., Wei R., Hui E.S., Feldon J., Meyer U., Chung S., Chua S.E., Sham P.C., Wu E.X., McAlonan G.M. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PloS One. 2009;4 doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina T.V., Zai C., Hlousek D., Roder J.C., Wong A.H.C. Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice. J. Neurosci. 2013;33:7654–7666. doi: 10.1523/JNEUROSCI.0091-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan W., Hammond L.A., Vuillermot S., Meyer U., Eyles D.W. Maternal vitamin D prevents abnormal dopaminergic development and function in a mouse model of prenatal immune activation. Sci. Rep. 2018;8:9741. doi: 10.1038/s41598-018-28090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchicchi A., Lecca S., Melis M., De Felice M., Cadeddu F., Frau R., Muntoni A.L., Fadda P., Devoto P., Pistis M. Maternal immune activation disrupts dopamine system in the offspring. Int. J. Neuropsychopharmacol. 2016;19 doi: 10.1093/ijnp/pyw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen G.F., Bilenberg N., Cantio C., Oranje B. Increased prepulse inhibition and sensitization of the startle reflex in autistic children. Autism Res. 2014;7:94–103. doi: 10.1002/aur.1337. [DOI] [PubMed] [Google Scholar]
- Meehan C., Harms L., Frost J.D., Barreto R., Todd J., Schall U., Shannon Weickert C., Zavitsanou K., Michie P.T., Hodgson D.M. Effects of immune activation during early or late gestation on schizophrenia-related behaviour in adult rat offspring. Brain Behav. Immun. 2017;63:8–20. doi: 10.1016/j.bbi.2016.07.144. [DOI] [PubMed] [Google Scholar]
- Meincke U., Light G.A., Geyer M.A., Braff D.L., Gouzoulis-Mayfrank E. Sensitization and habituation of the acoustic startle reflex in patients with schizophrenia. Psychiatr. Res. 2004;126:51–61. doi: 10.1016/j.psychres.2004.01.003. [pii] [DOI] [PubMed] [Google Scholar]
- Mena A., Ruiz-Salas J.C., Puentes A., Dorado I., Ruiz-Veguilla M., De la Casa L.G. Reduced prepulse inhibition as a biomarker of schizophrenia. Front. Behav. Neurosci. 2016;10 doi: 10.3389/fnbeh.2016.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U., Feldon J., Schedlowski M., Yee B.K. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci. Biobehav. Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U., Nyffeler M., Yee B.K., Knuesel I., Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav. Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Meyer U., Spoerri E., Yee B.K., Schwarz M.J., Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr. Bull. 2010;36:607–623. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missault S., Van den Eynde K., Vanden Berghe W., Fransen E., Weeren A., Timmermans J.P., Kumar-Singh S., Dedeurwaerdere S. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav. Immun. 2014;42:138–146. doi: 10.1016/j.bbi.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Mueller F.S., Polesel M., Richetto J., Meyer U., Weber-Stadlbauer U. Mouse models of maternal immune activation: mind your caging system! Brain Behav. Immun. 2018;73:643–660. doi: 10.1016/j.bbi.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Murray B.G., Davies D.A., Molder J.J., Howland J.G. Maternal immune activation during pregnancy in rats impairs working memory capacity of the offspring. Neurobiol. Learn. Mem. 2017;141:150–156. doi: 10.1016/j.nlm.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Murray K.N., Edye M.E., Manca M., Vernon A.C., Oladipo J.M., Fasolino V., Harte M.K., Mason V., Grayson B., McHugh P.C., Knuesel I., Prinssen E.P., Hager R., Neill J.C. Evolution of a maternal immune activation (mIA) model in rats: early developmental effects. Brain Behav. Immun. 2019;75:48–59. doi: 10.1016/j.bbi.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Nielsen P.R., Laursen T.M., Mortensen P.B. Association between parental hospital-treated infection and the risk of schizophrenia in adolescence and early adulthood. Schizophr. Bull. 2013;39:230–237. doi: 10.1093/schbul/sbr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary C., Desbonnet L., Clarke N., Petit E., Tighe O., Lai D., Harvey R., Waddington J.L., O’Tuathaigh C. Phenotypic effects of maternal immune activation and early postnatal milieu in mice mutant for the schizophrenia risk gene neuregulin-1. Neuroscience. 2014;277:294–305. doi: 10.1016/j.neuroscience.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Oranje B., Glenthøj B.Y. Clonidine normalizes sensorimotor gating deficits in patients with schizophrenia on stable medication. Schizophr. Bull. 2013;39:684–691. doi: 10.1093/schbul/sbs071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranje B., Lahuis B., van Engeland H., Jan van der Gaag R., Kemner C. Sensory and sensorimotor gating in children with multiple complex developmental disorders (MCDD) and autism. Psychiatr. Res. 2013;206:287–292. doi: 10.1016/j.psychres.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Ornitz E.M., Lane S.J., Sugiyama T., de Traversay J. Startle modulation studies in autism. J. Autism Dev. Disord. 1993;23:619–637. doi: 10.1007/BF01046105. [DOI] [PubMed] [Google Scholar]
- Pacheco-López G., Giovanoli S., Langhans W., Meyer U. Priming of metabolic dysfunctions by prenatal immune activation in mice: relevance to schizophrenia. Schizophr. Bull. 2013;39:319–329. doi: 10.1093/schbul/sbr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnicov M.V., Storozheva Z.I., Sherstnev V.V. Developmental analysis of habituation of the acoustic startle response in the preweanling and adult rats. Behav. Process. 1995;34:269–277. doi: 10.1016/0376-6357(95)00004-E. [DOI] [PubMed] [Google Scholar]
- Purves-Tyson Tertia D., Weber-Stadlbauer Ulrike, Richetto Juliet, Rothmond Debora A., Labouesse Marie A., Polesel Marcello, Robinson Kate, Weickert Cynthia Shannon, Meyer Urs. Increased levels of midbrain immune-related transcripts in schizophrenia and in murine offspring after maternal immune activation. Mol. Psychiatr. 2019:1–15. doi: 10.1038/s41380-019-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman T., Zavitsanou K., Purves-Tyson T., Harms L.R., Meehan C., Schall U., Todd J., Hodgson D.M., Michie P.T., Weickert C.S. Effects of immune activation during early or late gestation on N-Methyl-d-Aspartate receptor measures in adult rat offspring. Front. Psychiatr. 2017;8 doi: 10.3389/fpsyt.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers L.G., Peeters B.W. Effects of acoustic prepulses on the startle reflex in rats: a parametric analysis. Brain Res. 1994;661:174–180. doi: 10.1016/0006-8993(94)91204-1. [DOI] [PubMed] [Google Scholar]
- Richetto J., Calabrese F., Meyer U., Riva M.A. Prenatal versus postnatal maternal factors in the development of infection-induced working memory impairments in mice. Brain Behav. Immun. 2013;33:190–200. doi: 10.1016/j.bbi.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Richetto J., Calabrese F., Riva M.A., Meyer U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr. Bull. 2014;40:351–361. doi: 10.1093/schbul/sbs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetto J., Massart R., Weber-Stadlbauer U., Szyf M., Riva M.A., Meyer U. Genome-wide DNA methylation changes in a mouse model of infection-mediated neurodevelopmental disorders. Biol. Psychiatr. 2017;81:265–276. doi: 10.1016/j.biopsych.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Sangha S., Greba Q., Robinson P.D., Ballendine S.A., Howland J.G. Heightened fear in response to a safety cue and extinguished fear cue in a rat model of maternal immune activation. Front. Behav. Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S., Azzopardi E., De Jaeger X., Prado M.A., Prado V.F. VAChT knock-down mice show normal prepulse inhibition but disrupted long-term habituation. Gene Brain Behav. 2011;10:457–464. doi: 10.1111/j.1601-183X.2011.00686.x. [DOI] [PubMed] [Google Scholar]
- Schwabe K., Freudenberg F., Koch M. Selective breeding of reduced sensorimotor gating in Wistar rats. Behav. Genet. 2007;37:706–712. doi: 10.1007/s10519-007-9166-z. [DOI] [PubMed] [Google Scholar]
- Shi L., Fatemi S.H., Sidwell R.W., Patterson P.H. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D., Oranje B., Razak K.A., Siegel S.J., Schmid S. Sensory processing in autism spectrum disorders and Fragile X syndrome—from the clinic to animal models. Neuroscience & Biobehavioral Reviews, SI:IBNS-2015. 2017;76:235–253. doi: 10.1016/j.neubiorev.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solek C.M., Farooqi N., Verly M., Lim T.K., Ruthazer E.S. Maternal immune activation in neurodevelopmental disorders. Dev. Dynam. 2018;247:588–619. doi: 10.1002/dvdy.24612. [DOI] [PubMed] [Google Scholar]
- Song X., Li Wenqiang, Yang Y., Zhao J., Jiang C., Li Wei, Lv L. The nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate reduces polyinosinic-polycytidilic acid-induced immune response in pregnant rats and the behavioral defects of their adult offspring. Behav. Brain Funct. 2011;7:50. doi: 10.1186/1744-9081-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Vestergaard M., Christensen J., Nahmias A.J., Olsen J. Prenatal exposure to maternal infections and epilepsy in childhood: a population-based cohort study. Pediatrics. 2008;121:e1100–e1107. doi: 10.1542/peds.2007-2316. [DOI] [PubMed] [Google Scholar]
- Swerdlow N.R., Braff D.L., Geyer M.A. Sensorimotor gating of the startle reflex: what we said 25 years ago, what has happened since then, and what comes next. J. Psychopharmacol. 2016;30:1072–1081. doi: 10.1177/0269881116661075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N.R., Light G.A., Sprock J., Calkins M.E., Green M.F., Greenwood T.A., Gur R.E., Gur R.C., Lazzeroni L.C., Nuechterlein K.H., Radant A.D., Ray A., Seidman L.J., Siever L.J., Silverman J.M., Stone W.S., Sugar C.A., Tsuang D.W., Tsuang M.T., Turetsky B.I., Braff D.L. Deficient prepulse inhibition in schizophrenia detected by the multi-site COGS. Schizophr. Res. 2014;152:503–512. doi: 10.1016/j.schres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N.R., Light G.A., Thomas M.L., Sprock J., Calkins M.E., Green M.F., Greenwood T.A., Gur R.E., Gur R.C., Lazzeroni L.C., Nuechterlein K.H., Radant A.D., Seidman L.J., Siever L.J., Silverman J.M., Stone W.S., Sugar C.A., Tsuang D.W., Tsuang M.T., Turetsky B.I., Braff D.L. Deficient prepulse inhibition in schizophrenia in a multi-site cohort: internal replication and extension. Schizophrenia Research, Impaired sensorimotor Gating in Schizophrenia. 2018;198:6–15. doi: 10.1016/j.schres.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N.R., Weber M., Qu Y., Light G.A., Braff D.L. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Komatsu S., Nakahachi T., Ogino K., Kamio Y. Relationship of the acoustic startle response and its modulation to emotional and behavioral problems in typical development children and those with autism spectrum disorders. J. Autism Dev. Disord. 2016;46:534–543. doi: 10.1007/s10803-015-2593-4. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Nakahachi T., Komatsu S., Ogino K., Iida Y., Kamio Y. Hyperreactivity to weak acoustic stimuli and prolonged acoustic startle latency in children with autism spectrum disorders. Mol. Autism. 2014;5:1–8. doi: 10.1186/2040-2392-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Nakahachi T., Stickley A., Ishitobi M., Kamio Y. Stability of the acoustic startle response and its modulation in children with typical development and those with autism spectrum disorders: a one-year follow-up. Autism Res. 2017;10:673–679. doi: 10.1002/aur.1710. [DOI] [PubMed] [Google Scholar]
- Takeda K., Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Valsamis B., Schmid S. Habituation and prepulse inhibition of acoustic startle in rodents. JoVE. 2011;e3446 doi: 10.3791/3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eynde K., Missault S., Fransen E., Raeymaekers L., Willems R., Drinkenburg W., Timmermans J.-P., Kumar-Singh S., Dedeurwaerdere S. Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav. Brain Res. 2014;258:179–186. doi: 10.1016/j.bbr.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Vernon A.C., So P.-W., Lythgoe D.J., Chege W., Cooper J.D., Williams S.C.R., Kapur S. Longitudinal in vivo maturational changes of metabolites in the prefrontal cortex of rats exposed to polyinosinic–polycytidylic acid in utero. Eur. Neuropsychopharmacol. 2015;25:2210–2220. doi: 10.1016/j.euroneuro.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V., Graham D.L., Braun A.A., Schaefer T.L., Skelton M.R., Richtand N.M., Williams M.T. Prenatal immune challenge in rats: effects of polyinosinic–polycytidylic acid on spatial learning, prepulse inhibition, conditioned fear, and responses to MK-801 and amphetamine. Neurotoxicol. Teratol. 2015;47:54–65. doi: 10.1016/j.ntt.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Graham D.L., Braun A.A., Schaefer T.L., Skelton M.R., Richtand N.M., Williams M.T. Prenatal immune challenge in rats: altered responses to dopaminergic and glutamatergic agents, prepulse inhibition of acoustic startle, and reduced route-based learning as a function of maternal body weight gain after prenatal exposure to poly IC. Synapse. 2012;66:725–737. doi: 10.1002/syn.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillermot S., Feldon J., Meyer U. Nurr1 is not essential for the development of prepulse inhibition deficits induced by prenatal immune activation. Brain Behav. Immun. 2011;25:1316–1321. doi: 10.1016/j.bbi.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Vuillermot S., Weber L., Feldon J., Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J. Neurosci. 2010;30:1270–1287. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Stadlbauer U., Richetto J., Labouesse M.A., Bohacek J., Mansuy I.M., Meyer U. Transgenerational transmission and modification of pathological traits induced by prenatal immune activation. Mol. Psychiatr. 2017;22:102–112. doi: 10.1038/mp.2016.41. [DOI] [PubMed] [Google Scholar]
- Whitehead E., Dodds L., Joseph K.S., Gordon K.E., Wood E., Allen A.C., Camfield P., Dooley J.M. Relation of pregnancy and neonatal factors to subsequent development of childhood epilepsy: a population-based cohort study. Pediatrics. 2006;117:1298–1306. doi: 10.1542/peds.2005-1660. [DOI] [PubMed] [Google Scholar]
- Wolff AmyR., Bilkey DavidK. The maternal immune activation (MIA) model of schizophrenia produces pre-pulse inhibition (PPI) deficits in both juvenile and adult rats but these effects are not associated with maternal weight loss. Behav. Brain Res. 2010;213:323–327. doi: 10.1016/j.bbr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Wolff A.R., Bilkey D.K. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav. Brain Res. 2008;190:156–159. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Yang A., Daya T., Carlton K., Yan J.H., Schmid S. Differential effect of clomipramine on habituation and prepulse inhibition in dominant versus subordinate rats. Eur. Neuropsychopharmacol. 2016;26:591–601. doi: 10.1016/j.euroneuro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- Zerbo O., Qian Y., Yoshida C., Grether J.K., Van de Water J., Croen L.A. Maternal infection during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2015;45:4015–4025. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cazakoff B.N., Thai C.A., Howland J.G. Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology. 2012;62:1299–1307. doi: 10.1016/j.neuropharm.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., van Praag H. Maternal immune activation differentially impacts mature and adult-born hippocampal neurons in male mice. Brain Behav. Immun. 2015;45:60–70. doi: 10.1016/j.bbi.2014.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.