Abstract
SCLC is frequently associated with paraneoplastic syndromes, including dermatomyositis. Patients with malignancy-associated dermatomyositis express a specific autoantibody pattern usually positive for anti–transcription intermediary factor 1-γ (TIF1-γ), suggesting anti–TIF1-γ plays a role in development of malignancy-associated dermatomyositis. We present a case of a patient with SCLC, paraneoplastic dermatomyositis, positive anti–TIF1-γ, and a point mutation in TIF1-γ coding gene, with prominent clinical response to chemoradiation. We suggest that this point mutation is pathogenic, providing evidence for the development of paraneoplastic dermatomyositis through immune cross-reactivity.
Keywords: Small cell lung cancer, Dermatomyositis, Anti-TIF1-γ, Case report
Introduction
SCLC is a highly aggressive cancer, frequently associated with paraneoplastic syndromes.1 Dermatomyositis is an inflammatory disorder involving muscle weakness and typical skin rash and is often a manifestation of underlying malignancy, in particular SCLC.2,3 Patients with malignancy-associated dermatomyositis (MAD) have worse clinical symptoms, are unresponsive to corticosteroid treatment, and have worse prognosis.3 Patients with MAD express a distinct autoantibody pattern usually negative for dermatomyositis-specific autoantibodies yet positive for anti–transcription intermediary factor 1-γ (TIF1-γ), suggesting anti–TIF1-γ plays a role in MAD development.4 Nevertheless, the underlying mechanism remains unclear. We present a case of a patient with SCLC, paraneoplastic dermatomyositis, positive anti–TIF1-γ, and a mutation in the TIF1-γ coding gene.
Case Presentation
A 68-year-old male heavy smoker was evaluated on June 2020 for an itchy rash on sun-exposed areas, which started 3 weeks before his referral, accompanied by proximal muscle weakness with inability to walk. Skin biopsy result revealed hyperkeratosis, vacuolar degeneration, and perifascicular muscle inflammation with monoclonal infiltration. He was treated with high-dose corticosteroids for 12 days (methylprednisolone 1 mg/kg twice a day for 2 d followed by prednisone 1 mg/kg once a day for 10 days) with little to no improvement.
Chest radiograph result revealed an oval opacification in the left lung, and result from a positron emission tomography-computed tomography-fluorodeoxyglucose revealed a left upper lobe heterogeneous lung mass 4.9 cm in diameter with standard uptake volume maximum of 13 Hounsfield unit (Fig. 1A). In addition, transbronchial biopsy result revealed SCLC features with a mitotic index of 60%. Consequently, the patient was admitted to the oncology ward.
Figure 1.
PET-CT-FDG results of the patient. (A) During initial diagnosis on June 2020 revealing tumor size of 4.87 cm and uptake of 13.4 HU. (B) Follow-up PET-CT on September 2020 after chemoradiation treatment revealing reduction in tumor size to 2.4 cm and uptake to 3.4 HU. CT, computed tomography; FDG, fluorodeoxyglucose; HU, Hounsfield unit; PET, positron emission tomography; SUV, standard uptake volume.
On examination, the patient had scaly erythematous-confluent rash on his face, arms, and thighs together with hallmark shawl and V signs (Fig. 2A). There were proximal limb muscle weakness at two of five and laryngeal weakness with solid food dysphagia. Blood tests were prominent for creatinine kinase (CK) at 4842 U/liter (reference range; 22–198 U/liter), and a complete rheumatologic panel was positive for anti–TIF1-γ 64 U and negative for other dermatomyositis-specific autoantibodies.
Figure 2.
(A) Pictures taken during initial presentation on June 2020 revealing scaly erythematous-confluent rash, shawl, and V signs. (B) Pictures taken during follow-up on September 2020 after completion of chemoradiation revealing improvement in rash on hands, face, and chest.
Because of the clinical features, elevated CK, positive anti–TIF1-γ, and findings of muscle and lung pathologies, the patient was diagnosed as having limited SCLC with paraneoplastic dermatomyositis. The patient was treated with cisplatin (75 mg/m2 once a day) and etoposide (100 mg/m2 once a day for three days). One week after admission, the patient began to have improvement in his symptoms, including improvement of the rash, renewed ability to walk with a walker, and sallow solid food. CK level was down to 1300 U/liter.
In July to September 2020, the patient completed three more cycles of cisplatin-etoposide with concurrent radiotherapy, for a total dose of 66 Gy. On follow-up examination done on September 2020, there was complete resolution of the rash (Fig. 2B), improvement of proximal strength, and the patient was now able to walk unaided. CK levels were normalized, and anti–TIF1-γ levels were reduced from 64 U measured during the initial diagnosis 3 months earlier to 19 U measured on follow-up. Result from repeat positron emission tomography-computed tomography-fluorodeoxyglucose revealed reduction of tumor size and uptake to 2.4 cm with standard uptake volume of 3.4 Hounsfield unit (Fig. 1B).
Whole-gene next-generation hybrid-capture DNA and RNA sequencing (next-generation sequencing [NGS]) analysis was performed on the tumor sample obtained from transbronchial biopsy. There were RB1 and P53 loss-of-function mutations and MYC copy number gain with a corresponding MYC and TOP2A overexpression. Furthermore, there was a missense point mutation c.2519T>C in the TIF1-γ coding gene, TRIM33 with matching RNA mutation with median gene coverage of 462 (25–75 percentile in 124–575 coverage, accordingly).
Discussion
We presented a case of a patient with newly diagnosed limited SCLC with a prodrome of paraneoplastic dermatomyositis, positive anti–TIF1-γ, and a mutation in the TIF1-γ coding gene. Impressive response to chemoradiation was evident in clinical features of dermatomyositis, imaging of primary tumor, and reduction of anti–TIF1-γ.
TIF1-γ is a regulator of cellular proliferation and is considered a tumor suppressor, through its regulation of the TGF-β and Smad pathway, by its ability to ubiquitinate or compete with Smad3 or 4. Inactivation, mutation, or down-regulation of TIF1-γ results in tumorigenesis and metastasis development, as evident in several malignancies.5
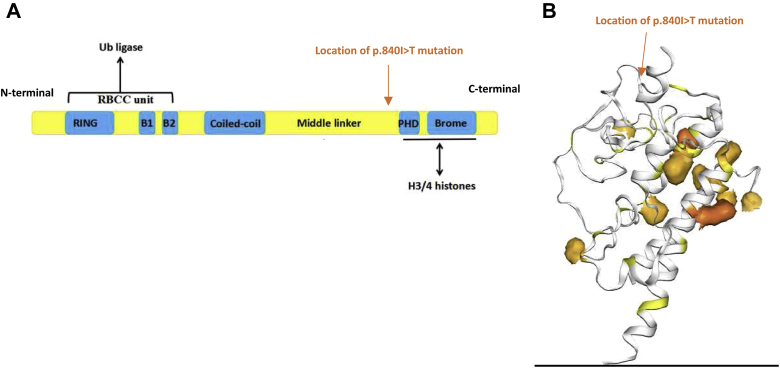
The mutation p.I840T found in this case is located near the plant homeodomain of TIF1-γ (Fig. 3). Using SWISS-MODEL, we predicted that this mutation would modify protein structure and binding. In vitro studies have revealed that ubiquitination of Smad4 by TIF1-γ is dependent on integral binding of plant homeodomain to Smad4. In addition, loss of TIF1-γ attenuates down-regulation of MYC,5 which was found to be overexpressed in our case.
Figure 3.
(A) Schematic representation of TIF1-γ protein containing a ubiquitin ligase with a ring-box–coiled coil region on the n-terminal and a PHD and bromodomain with high H3 chromatin affinity on the c-terminal. Location of point mutation found in our case p.840I>T noted in orange. (B) 3D representation of TIF1-γ. PHD/bromodomain-histone complexes represented in dark orange. Taken from COSMIC. Location of point mutation found in our case p.840I>T noted in orange. 3D, three dimensional; COSMIC, Catalogue Of Somatic Mutations In Cancer; PHD, plant homeodomain; TIF1-γ, transcription intermediary factor 1-γ.
Pan-cancer search of cBioPortal.org and Catalogue Of Somatic Mutations In Cancer found that although TRIM 33 mutations occur in less than 1% of cancers, p.I840T appears with the most frequency, including in 84 cases of non-SCLC and 37 cases of endometrial cancer harboring improved prognosis. These results suggest that the mutation found in our case is likely pathogenic. A point mutation c.3299T>C in TRIM33 has been previously described in one other patient diagnosed with having MAD with positive anti–TIF1-γ yet, with unknown clinical context or recurrence of this mutation in other malignancies.
Anti–TIF1-γ is highly specific to MAD and reacts with TIF1-γ antigens found mostly in skin and muscle tissues. Hence, it is suggested that the immune response to altered TIF1-γ in the tumor cross-reacts with native TIF1-γ antigens in muscle and skin tissues, causing MAD.4 The likely pathogenic mutation found in our case and the anti–TIF1-γ reduction in response to treatment provide important evidence for this hypothesis. Furthermore, cross-reactivity is considered to be the mechanism causing neurologic paraneoplastic syndromes.1 To the best of our knowledge, none of the exciting NGS panels contain TRIM33. We suggest that this gene be added to NGS panels owing to its importance as a tumor suppressor and a marker for paraneoplastic syndromes.
Conclusion
The mutation c.2519T>C in TRIM33 is likely pathogenic and provides evidence of the development of MAD through immune cross-reactivity. Future analysis of TRIM33 in NGS panels might provide further proof for this finding.
CRediT Authorship Contribution Statement
JohnathanArnon: Conceptualization, Investigation, Writing—original draft.
Anna Elia: Resources.
Yuval Nevo: Investigation, Formal analysis.
Alexander Lossos: Resources, Investigation, Writing—review and editing.
Hovav Nechushtan: Conceptualization, Supervision, Writing—review and editing.
Acknowledgments
No outside funding support was used in this case report. The patient involved in this case report gave his informed consent authorizing use, disclosure, and publication of his health information and personal photographs. Special acknowledgment to the Monsa Foundation for assitance with genetic analysis funding.
Footnotes
Disclosure: The authors declare no conflict of interest.
Cite this article as: Arnon J, Elia A, Nevo Y, Lossos A, Nechushtan H. SCLC, paraneoplastic dermatomyositis, positive transcription intermediary factor 1-γ, and point mutation in the transcription intermediary factor 1-γ coding gene: a case report. JTO Clin Res Rep. 2021;2:100217.
References
- 1.Gandhi L., Johnson B.E. Paraneoplastic syndromes associated with small cell lung cancer. JNCCN J Natl Compr Cancer Netw. 2006;4:631–638. doi: 10.6004/jnccn.2006.0052. [DOI] [PubMed] [Google Scholar]
- 2.Takashima R., Takamatsu K., Shinkawa Y., Yagita M., Fukui M., Fujita M. Dermatomyositis associated with lung neuroendocrine carcinoma. Intern Med. 2017;56:719–724. doi: 10.2169/internalmedicine.56.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.András C., Ponyi A., Constantin T. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol. 2008;35::438–444. [PubMed] [Google Scholar]
- 4.De Vooght J., Vulsteke J.B., De Haes P., Bossuyt X., Lories R., De Langhe E. Anti-TIF1-γautoantibodies: warning lights of a tumour autoantigen. Rheumatology (Oxford) 2020;59:469–477. doi: 10.1093/rheumatology/kez572. [DOI] [PubMed] [Google Scholar]
- 5.Yu C., Ding Z., Liang H., Zhang B., Chen X. The roles of TIF1γ in cancer. Front Oncol. 2019;9:979. doi: 10.3389/fonc.2019.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]