Abstract
Introduction
The clinical successes seen with anti–programmed cell death protein 1/programmed death-ligand 1 (anti–PD-[L]1) agents have galvanized the field of immuno-oncology. We evaluated the landscape and trends in immunotherapy trials involving the PD-(L)1 axis in intrathoracic tumors.
Methods
We identified clinical trials involving anti–PD-(L)1 agents on the ClinicalTrials.gov registry through November 13, 2020 for NSCLC, SCLC, mesothelioma, and thymic epithelial tumor. Clinical trials were indexed according to monotherapy versus combination approaches, PD-(L)1 agents under investigation, clinical settings, trial start date, and partner drug(s). We assessed redundancy among the clinical trials.
Results
We found 686 clinical trials investigating anti–PD-(L)1 agents for intrathoracic tumors (540 trials in NSCLC, 96 in SCLC, 38 in mesothelioma, and 12 in thymic epithelial tumor). A total of 23 PD-(L)1 inhibitors are undergoing clinical development. A total of 81% of trials assess combination treatment. The number of clinical trials has been growing exponentially in the past decade. PD-(L)1 blockade was frequently combined with chemotherapy or immunomodulatory therapy. Various strategies are in development to overcome resistance to PD-(L)1 blockade in metastatic NSCLC. PD-(L)1 blockade is also increasingly evaluated in neoadjuvant and adjuvant settings. After the U.S. Food and Drug Administration’s approval of an anti–PD-(L)1 agent for a specific indication, 14 trials were launched thereafter, which continued to randomize patients to treatments that were inferior to the best available therapy.
Conclusions
The number of clinical trials investigating anti–PD-(L)1 agents in intrathoracic tumors has experienced a steep increase over the past decade with a notable upward trend for combination trials. To reduce duplicative research efforts and accelerate the development of effective immunotherapeutics, improved coordination among key stakeholders and the adoption of innovative trial designs will be vital.
Keywords: PD-(L)1 axis, NSCLC, SCLC, Mesothelioma, TET, Immunotherapy
Introduction
Antibodies that block the inhibitory programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) pathways, an immunologic checkpoint, have expanded the therapeutic options for an array of solid and hematologic malignancies. Six PD-1/PD-L1 (PD-[L]1) antibodies—nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, and cemiplimab—have been approved by the U.S. Food and Drug Administration (FDA), and the number of approved indications has risen continuously.1 This class of drugs is able to produce a durable response in a fraction of patients and has exhibited either a more favorable adverse effect profile compared with cytotoxic therapies or a modest increase in adverse effects when combined with cytotoxic drugs. An empirical analysis of checkpoint inhibitors found that approximately 43% of U.S. patients with cancer receiving treatment for advanced or metastatic tumors may be eligible for such treatments, and approximately 12% may respond to these therapies.1
The clinical successes seen with anti–PD-(L)1 agents have galvanized the field of oncology, leading to the proliferation of clinical trials evaluating cancer immunotherapy. As of September 2020, there were 4400 clinical trials assessing PD-(L)1 blockade, representing a threefold increase over the preceding 3 years.2 These numbers will certainly grow exponentially as more than 400 immuno-oncologic targets in the global pipeline continue to be evaluated as candidates for treatment.3 To date, there has been no review focusing on clinical trials of anti–PD-(L)1 agents in intrathoracic tumors. Here, we aim to evaluate the landscape and trends in immunotherapy trials involving the PD-(L)1 axis in intrathoracic tumors.
Materials and Methods
We reviewed all publicly available clinical trials involving anti–PD-(L)1 agents on the U.S. National Library of Medicine database (https://clinicaltrials.gov) posted until November 13, 2020 for NSCLC, SCLC, mesothelioma, and thymic epithelial tumor (TET). Eligible agents included antibodies and bispecific antibodies targeting PD-(L)1.
Excluded agents were agents for imaging studies (e.g., 89Zr-atezolizumab), checkpoint inhibitors not targeting the PD-(L)1 axis (e.g., CTLA-4 inhibitors), and cell-based therapy targeting the PD-(L)1 axis (e.g., Pluripotent Killer-PD-1 cell therapy). Specifically, the following agents targeting the PD-(L)1 pathway were used to identify thoracic oncology clinical trials: ABBV-181, AGEN-2034, AK105, AMP-224, AMP-514, atezolizumab, avelumab, BI 754091, CA-170, camrelizumab, CBT-501, cemiplimab, CS1001, CX-072, durvalumab, FAZ053, HLX 10, JNJ-63723283, KN035, KN046, LZM009, LY3300054, M7824, MDX1105-01, MGA-012, MGD013, nivolumab, pembrolizumab, PF-06801591, pidilizumab, prolgolimab, RO7121661, SHR-1316, sintilimab, spartalizumab, tislelizumab, toripalimab, TQB2450, TSR-042. Additional exclusion criteria included “unknown,” “terminated” or “suspended” trial status, trials including more than one extrathoracic tumor type, and nontherapeutic clinical trials.
We then analyzed all available data according to intrathoracic tumor type. We focused particularly on the number of clinical trials using anti–PD-(L)1 monotherapy versus combination approaches, the number of PD-(L)1 agents under investigation, the clinical settings of each trial, the number of trials by start date, and partner drug(s) for combination trials.
To assess redundancy among anti–PD-(L)1 clinical trials, we evaluated how many drugs entered clinical development for the same indication after the FDA approval of any anti–PD-(L)1 agent.
We used Prism GraphPad 8 (version 8.4.2; San Diego, CA), Microsoft Excel 2019 (Version 16.37; Redmond, WA), and Tableau (2020.2.1; Seattle, WA) for data analysis.
Results
Overview of Landscape of PD-(L)1 Inhibitor Therapy in Intrathoracic Tumors
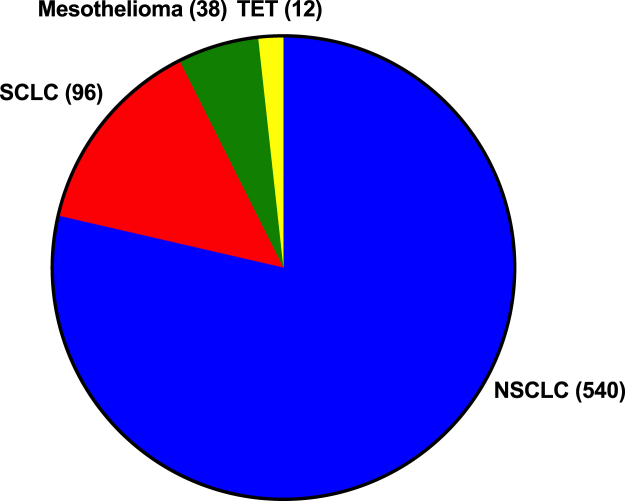
We identified 686 clinical trials investigating anti–PD-(L)1 agents in the treatment of intrathoracic tumors (Fig. 1). Most of these trials focus on NSCLC (n = 540, 78.7%), followed by SCLC (n = 96, 14.0%), mesothelioma (n = 38, 5.5%), and TET (n = 12, 1.7%). Most trials occur in the advanced or metastatic settings (n = 576, 84.0%) (Fig. 2A–D). There were 23 PD-(L)1 agents undergoing investigation (Table 1) with 15 and eight drugs targeting PD-1 and PD-L1, respectively. Two compounds target one additional immunomodulatory protein beyond PD-(L)1 (KN046, M7824). The top four agents by number of trials are pembrolizumab (n = 193), nivolumab (n = 150), durvalumab (n = 109), and atezolizumab (n = 105), which collectively amount to 81.2% of the total trials registered (n = 557) (Fig. 3). Most clinical trials use anti–PD-(L)1 agents in combination with other forms of treatment. (Fig. 4A–D). The number of trials in total and by tumor type continues growing over time (Fig. 5A–D).
Figure 1.
The number of clinical trials investigating PD-(L)1 blockade in thoracic tumors by tumor type. PD-(L)1, PD-1/PD-L1; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TET, thymic epithelial tumor.
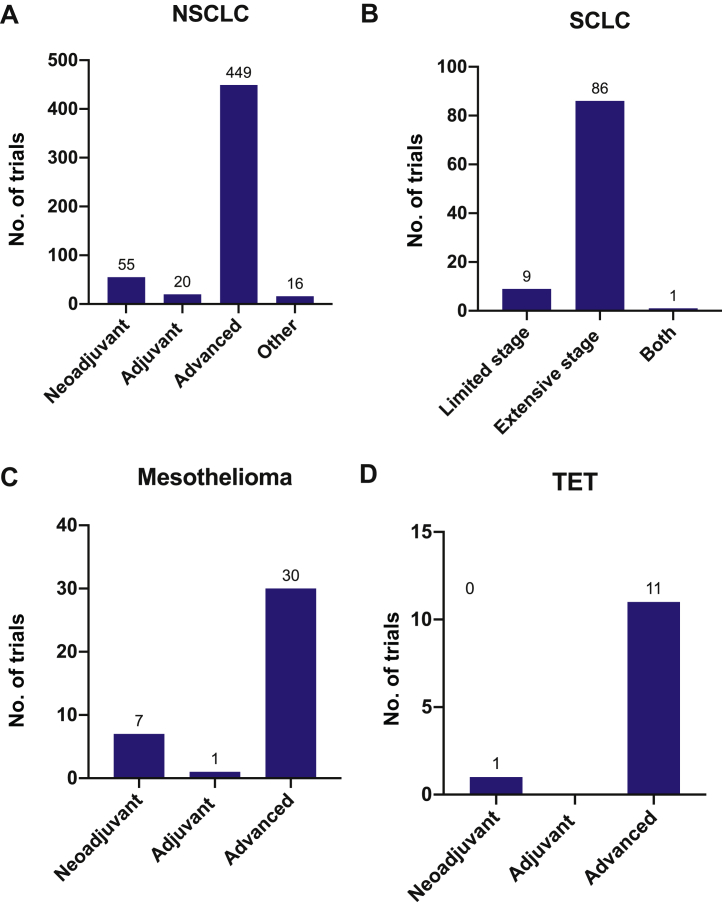
Figure 2.
This figure plots (as bar graphs) the number of trials registered per tumor type in the neoadjuvant, adjuvant, metastatic or advanced, and combined or other (i.e., undefined) settings. (A) NSCLC, (B) SCLC, (C) mesothelioma, (D) thymic malignancy. TET, thymic epithelial tumor.
Table 1.
Table of Anti–PD-(L)1 Agents Undergoing Investigation by Tumor Type
| Agent | Target | NSCLC | SCLC | Mesothelioma | TET | Total |
|---|---|---|---|---|---|---|
| ABBV-181 | PD-1 | 0 | 1 | 0 | 0 | 1 |
| AK105 | PD-1 | 2 | 0 | 0 | 0 | 2 |
| Atezolizumab | PD-L1 | 77 | 22 | 5 | 1 | 105 |
| Avelumab | PD-L1 | 11 | 0 | 1 | 1 | 13 |
| BI 754091 | PD-1 | 1 | 0 | 0 | 0 | 1 |
| Camrelizumab | PD-1 | 20 | 3 | 0 | 0 | 23 |
| Cemiplimab | PD-1 | 4 | 0 | 0 | 0 | 4 |
| CS1001 | PD-L1 | 1 | 1 | 0 | 0 | 2 |
| Durvalumab | PD-L1 | 87 | 18 | 4 | 0 | 109 |
| HLX 10 | PD-1 | 2 | 1 | 0 | 0 | 3 |
| KN046 | PD-L1/CTLA-4 | 3 | 0 | 0 | 1 | 4 |
| M7824 | PD-L1/TGF-β | 4 | 1 | 0 | 1 | 6 |
| MGA-012 | PD-1 | 1 | 0 | 0 | 0 | 1 |
| Nivolumab | PD-1 | 118 | 18 | 12 | 2 | 150 |
| Pembrolizumab | PD-1 | 158 | 13 | 16 | 6 | 193 |
| Prolgolimab | PD-1 | 2 | 0 | 0 | 0 | 2 |
| SHR-1316 | PD-L1 | 2 | 4 | 0 | 0 | 6 |
| Sintilimab | PD-1 | 18 | 5 | 0 | 0 | 23 |
| Spartalizumab | PD-1 | 4 | 0 | 0 | 0 | 4 |
| Tislelizumab | PD-1 | 8 | 2 | 0 | 0 | 10 |
| Toripalimab | PD-1 | 12 | 5 | 0 | 0 | 17 |
| TQB2450 | PD-L1 | 4 | 2 | 0 | 0 | 6 |
| TSR-042 | PD-1 | 1 | 0 | 0 | 0 | 1 |
PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TET, thymic epithelial tumor; TGF-β, transforming growth factor-β.
Figure 3.
The number of clinical trials of PD-(L)1 blockade by agents. The size of the circle corresponds to the number of trials. PD-(L)1, PD-1/PD-L1; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
Figure 4.
The number of clinical trials of single-agent PD-(L)1 blockade versus combination treatment per tumor type. (A) NSCLC, (B) SCLC, (C) mesothelioma, (D) thymic malignancy. PD-(L)1, PD-1/PD-L1; PD-1, programmed cell death protein-1; PD-L1, programmed death-ligand 1; TET, thymic epithelial tumor.
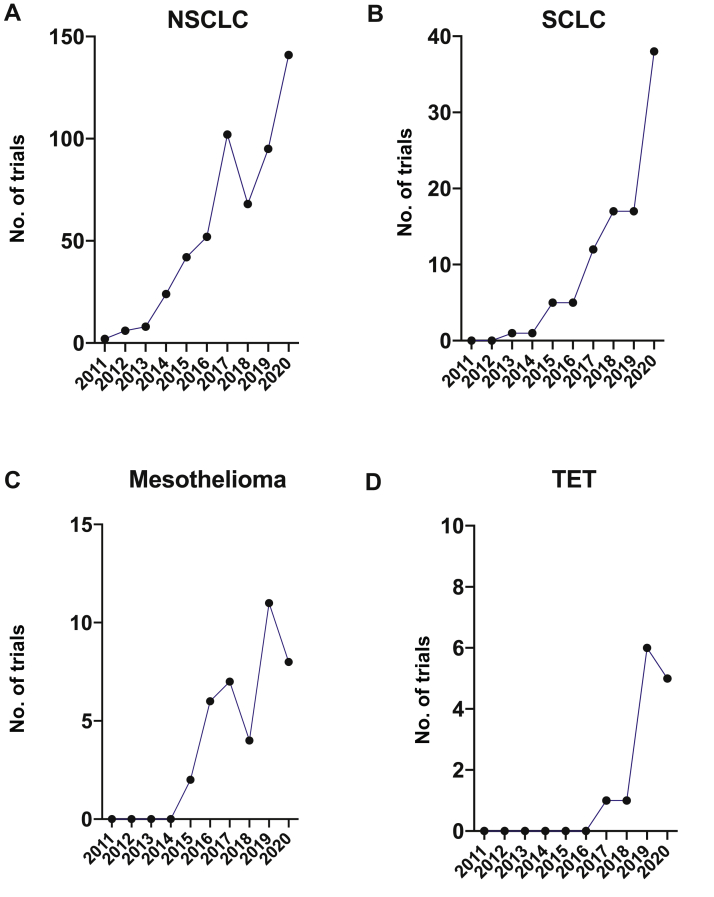
Figure 5.
The number of trials per tumor type per year. (A) NSCLC, (B) SCLC, (C) mesothelioma, (D) thymic malignancy. TET, thymic epithelial tumor.
Characteristics of Clinical Trials According to Tumor Type NSCLC
Regarding PD-(L)1 blockade monotherapy, there are multiple other agents that are under development for the treatment of NSCLC, including 16 anti–PD-(L)1 inhibitors (AK105, avelumab, BI 754091, camrelizumab, cemiplimab, CS1001, HLX10, MGA-012, prolgolimab, SHR-1316, sintilimab, spartalizumab, tislelizumab, toripalimab, TQB2450, TSR-042), one bispecific anti–PD-L1/CTLA-4 antibody (KN046), and one bifunctional fusion protein targeting transforming growth factor-β and PD-L1 (M7824) in addition to the already FDA-approved drugs pembrolizumab, nivolumab, atezolizumab, and durvalumab.
PD-(L)1 blockade was most often combined with immunomodulatory therapy (n = 109, 20.2%), followed by chemotherapy (n = 103, 19.1%) and multiple agents/modalities (n = 86, 15.9%) (Fig. 4A). Among the clinical trials of anti–PD-(L)1 therapy in combination with immunomodulatory treatment (n = 111), 27 (24.3%) used anti–CTLA-4 inhibition, 15 (13.5%) utilized vascular endothelial growth factor (VEGF) pathway inhibitors, and 14 (12.6%) combined PD-(L)1 blockade with a tumor vaccine. Among trials using multiple agents in combination with PD-(L)1 blockade (n = 86), the most common regimens included chemotherapy plus VEGF blockade (n = 24) and chemotherapy plus anti–CTLA-4 treatment (n = 12).
Most trials (449 of 540 trials, 83.1%) are being conducted in advanced-stage NSCLC (Fig. 2A). Among clinical trials for advanced-stage NSCLC not amenable to definitive treatment (n = 405), 162 trials (40%) were used in the frontline setting, 201 trials (49.6%) in second-line (and beyond) settings, and 42 trials (10.4%) for any or unclear lines of treatment. A total of 55 neoadjuvant trials (10.2%) and 20 adjuvant trials (3.7%) of PD-(L)1 blockade were identified.
Trials in the First-Line Setting in Advanced NSCLC
In the frontline setting for metastatic NSCLC, avelumab, cemiplimab, durvalumab, M7824, nivolumab, sintilimab, and toripalimab are being assessed as monotherapy in addition to the already FDA-approved pembrolizumab and atezolizumab. Agents that are combined with chemotherapy in the first-line setting included AK105, atezolizumab, camrelizumab, CS1001, durvalumab, HLX10, KN046, MGA-012, pembrolizumab, prolgolimab, sintilimab, tislelizumab, toripalimab, and TSR-042. Among the 44 trials involving these agents, nearly all used platinum-doublet chemotherapy; two trials used single-agent chemotherapy in patients with borderline performance status and in elderly patients (NCT04297605 and NCT04396457, respectively). Immune-modulating agents used in combination with PD-(L)1 blockade included checkpoint inhibitors (anti–CTLA-4 antibodies: tremelimumab, ipilimumab, and quavonlimab; anti-TIGIT antibody: tiragolumab; anti–LAG-antibody: MK-4280), cancer vaccines (TG4010, NEO-PV-01, GRN-1201, and IO102), epigenic modulators (decitabine and tetrahydrouridine), cytokines (AM0010, NKTR-214, and N803), and anti-VEGF inhibitors (bevacizumab and recombinant human endostatin). PARP inhibitors combined with anti–PD-(L)1 therapy included olaparib, rucaparib, and niraparib. Most multiway trials combined anti–PD(L)-1 therapy and chemotherapy as the backbone; agents that were added to the said chemoimmunotherapy backbone included inhibitors with antiangiogenic properties (bevacizumab, lenvatinib, apatinib, famitinib, and recombinant human endostatin), checkpoint inhibitors (relatlimab, ipilimumab, and tremelimumab), immune modulators (oleclumab, canakinumab, and AB928), DNA damage response pathway inhibitors (veliparib and M6620), metabolic modulators (CB-839), and other immune-based therapies (oral restorative microbiota therapy and autologous cytokine-induced killer cell immunotherapy).
Trials in the Second-Line Setting and Beyond in Advanced NSCLC
In addition to the FDA-approved atezolizumab, nivolumab, and pembrolizumab in the second-line setting for NSCLC, avelumab, camrelizumab, durvalumab, prolgolimab, sintilimab, and toripalimab are being evaluated in the second-line setting and beyond. A total of 22 trials combine an anti–PD-(L)1 inhibitor with chemotherapy; 15 trials (68.2%) use single-agent chemotherapy as the partner drug, with four of these 15 trials allowing previous anti–PD-(L)1 therapy (NCT02250326, NCT03977467, NCT04396535, and NCT04480372). Among the 14 clinical trials that use local ablative therapy (13 radiotherapy [RT] and one microwave ablation) in combination with anti–PD-(L)1 inhibitors, two trials specifically enroll patients who progressed on PD-(L)1 blockade (NCT02407171 and NCT03158883). Diverse targeted therapy drugs and immune-modulatory therapies including tyrosine kinase inhibitors, checkpoint blockers, immune modulators, DNA damage response pathway inhibitors, metabolic modulators, and fecal microbial transplantation are being used in combination with anti–PD-(L)1 agents (Supplementary Table 1). Among the 41 trials using immune-modulatory therapy and/or targeted therapy, 36 clinical trials require resistance to the previous PD-(L)1 blockade for eligibility.
Trials in Adjuvant/Neoadjuvant Settings
A total of 71 trials using anti–PD-(L)1 therapy in the neoadjuvant and adjuvant settings (51 neoadjuvant trials and 20 adjuvant trials) were identified (Supplementary Table 2). A total of 10 anti–PD-(L)1 agents being assessed in these settings include atezolizumab, camrelizumab, durvalumab, M7824, nivolumab, pembrolizumab, SHR-1316, sintilimab, tislelizumab, and toripalimab. A total of 19 trials use anti–PD-(L)1 monotherapy, whereas 52 trials use combinatorial approaches, with chemotherapy identified as the most common partner (n = 31). Among the 20 adjuvant trials, 14 trials (70%) use disease-free survival or progression-free survival, and two (10%) use overall survival as a primary outcome measure. Three trials (15%) use decrease or clearance of circulating tumor DNA as a primary outcome measure. Among the 51 neoadjuvant trials, 32 used pathologic response (e.g., major pathologic response, pathologic complete response, pathologic response rate) as a primary outcome measure.
SCLC
A total of 10 anti–PD-(L)1 drugs are being investigated in SCLC (ABBV-181, camrelizumab, CS1001, HLX-10, M7824, SHR-1316, sintilimab, tislelizumab, toripalimab, and TQB2450) in addition to the four FDA-approved drugs (atezolizumab and durvalumab in combination with platinum-doublet chemotherapy; pembrolizumab and nivolumab as monotherapy). Agents used as monotherapy include atezolizumab, nivolumab, pembrolizumab, sintilimab, and toripalimab. ABBV-181, atezolizumab, camrelizumab, CS1001, durvalumab, HLX10, M7824, nivolumab, pembrolizumab, SHR-1316, sintilimab, tislelizumab, toripalimab, and TQB2450 are being assessed in combination trials.
Most trials (n = 87, 90.6%) are underway in extensive-stage SCLC (ES-SCLC), whereas 10 trials (10.4%) are being developed for limited-stage SCLC (LS-SCLC); one trial enrolls both ES-SCLC and LS-SCLC (Fig. 2B).
Chemotherapy was the most frequent partner of PD-(L)1 inhibitor therapy (n = 25, 26.0%), followed by immunomodulatory therapy (n = 19, 19.8%) (Fig. 4B). The most frequency used immunomodulatory therapy was CTLA-4 blockade (n = 10; 52.6%) among trials using such immunomodulation (n = 19).
Trials in ES-SCLC
In terms of anti–PD-(L)1 monotherapy, sintilimab and toripalimab are being assessed as maintenance therapy after completion of chemotherapy in ES-SCLC (NCT03983759 and NCT03971214). In addition to the already FDA-approved atezolizumab and durvalumab for the first-line treatment of ES-SCLC in combination with platinum-doublet chemotherapy, HLX10, nivolumab, pembrolizumab, SHR-1316, tislelizumab, toripalimab, and TQB2450 are being combined with platinum-doublet chemotherapy (NCT04063163, NCT03382561, NCT03066778, NCT03711305, NCT04005716, NCT04012606, and NCT04539977, respectively). Building on the success of frontline chemoimmunotherapy, several trials are assessing the addition of complementary treatments to chemoimmunotherapy: thoracic RT (NCT04402788, NCT04462276, and NCT04472949), tiragolumab (NCT04256421), CDK4/6 inhibitor (NCT03041311), PARP inhibitors (NCT03923270 and NCT03958045), dendritic cell vaccine (NCT04487756), personalized neoantigen vaccine (NCT04397003), multitarget tyrosine kinase inhibitors with antiangiogenesis properties (NCT04313660, NCT04620837, NCT04363255), TLR7 agonist (NCT04101357), and BCL-2 inhibitor (NCT04422210).
Among the 39 studies being performed for refractory or relapsed ES-SCLC, six trials allow previous anti–PD-(L)1 therapy: nivolumab plus ipilimumab (NCT03670056), M7824 plus topotecan or temozolomide (NCT03554473), nivolumab plus temozolomide (NCT03728361), nivolumab plus gemcitabine (NCT03662074), durvalumab plus olaparib in SCLC transformed from EGFR-mutant NSCLC (NCT04538378), nivolumab plus vorolanib (NCT03583086), and durvalumab plus topotecan (NCT04607954).
Trials in LS-SCLC
Several trials also combine anti–PD-(L)1 agents with chemoradiation for LS-SCLC. Atezolizumab, atezolizumab with or without tiragolumab, durvalumab with or without tremelimumab, and toripalimab are being assessed as consolidative therapy after chemoradiation (NCT03540420, NCT04308785, NCT03703297, and NCT04418648). Agents that are given concurrently with chemoradiation include atezolizumab (NCT03811002), durvalumab (NCT03585998), and pembrolizumab (NCT02402920). Sintilimab is given with induction chemotherapy before chemoradiation and continued as maintenance therapy after chemoradiation (NCT04189094).
Mesothelioma
In October 2020, nivolumab and ipilimumab combination was approved by the FDA for the treatment of unresectable malignant pleural mesothelioma on the basis of CheckMate-743. Other drugs in development include avelumab, atezolizumab, durvalumab, and pembrolizumab. There are seven trials involving atezolizumab, durvalumab, nivolumab, and pembrolizumab in the neoadjuvant adjuvant setting (NCT03228537, NCT02592551, NCT04162015, NCT03918252, NCT02707666, NCT04201145, and NCT03760575). One trial investigates pembrolizumab in the adjuvant setting of mesothelioma (NCT02959463), whereas 30 trials are underway for advanced mesothelioma. Four anti–PD-(L)1 agents—atezolizumab, durvalumab, pembrolizumab, nivolumab—are being tested as monotherapy. In combination trials, PD-(L)1 inhibitors (avelumab, atezolizumab, durvalumab, pembrolizumab and nivolumab) were most frequently combined with immunomodulatory therapy (n = 14, 36.8%), followed by chemotherapy (n = 9, 23.7%) (Fig. 4C). Anti–CTLA-4 inhibition therapy was most often used (n = 6) among trials using immunomodulatory therapy (n = 14).
In terms of frontline chemoimmunotherapy trials, atezolizumab, durvalumab, and pembrolizumab have been combined with platinum-doublet chemotherapy in advanced mesothelioma (NCT03762018, NCT02899195, NCT04334759, NCT02784171, and NCT04153565). In the second-line setting and beyond, anti–PD-(L)1 therapy was combined with mesothelin-targeting agents such as anetumab ravtansine (NCT03126630), LMB-100 (NCT03644550), CRS-207 (NCT03175172), cancer vaccines (NCT04040231, NCT04300244, and NCT03546426), gemcitabine (NCT04480372), PARP inhibitor or CDK4/6 inhibitor (NCT03654833), and RT (NCT03399552 and NCT04166734).
Thymic Epithelial Tumor
Four PD-(L)1 inhibitors are being assessed in clinical trials (pembrolizumab, avelumab, atezolizumab, and nivolumab). KN046 (PD-L1/CTLA-4 inhibitor) and M7824 (PD-L1/transforming growth factor-β inhibitor) are also being investigated as monotherapy. Pembrolizumab had been combined with epacadostat (NCT02364076), but the trial was terminated after the negative results of ECHO-301/KEYNOTE-252 in melanoma. Combination trials of pembrolizumab using sunitinib (NCT03463460) in advanced TET, chemotherapy as neoadjuvant therapy (NCT03858582), and carboplatin and taxane in advanced TET (NCT04554524) are ongoing. Nivolumab is being combined with vorolanib for advanced TET (NCT03583086).
Assessment of Redundancy Among Clinical Trials
We assessed how many clinical trials were initiated after FDA approval of an anti–PD-(L)1 drug for a specific indication (Supplementary Table 3).
For the first-line treatment of advanced NSCLC, pembrolizumab monotherapy was approved on the basis of KEYNOTE-024 on October 24, 2016. Among the five other drugs that have been compared with platinum-doublet chemotherapy for the same indication (atezolizumab, avelumab, durvalumab, nivolumab, and REGN2810) in randomized clinical trials, REGN2810 entered clinical development on March 23, 2017. Head-to-head comparisons between anti–PD-(L)1 agents in this setting are lacking, with the exception of a randomized phase 2 trial comparing sintilimab to pembrolizumab (NCT04252365).
As for first-line chemoimmunotherapy in advanced NSCLC, pembrolizumab plus platinum-doublet chemotherapy was approved for nonsquamous and squamous NSCLC on May 10, 2017 and October 30, 2018, respectively. Eight clinical trials combining PD-(L)1 blockade with platinum-doublet chemotherapy (AK-105, HLX10, CS1001, MGA-012, sintilimab, tislelizumab, toripalimab, prolgolimab) were launched after the approval of pembrolizumab-based regimens; all the trials used platinum-doublet chemotherapy in the control group. There is only one randomized trial that performed a head-to-head comparison between chemoimmunotherapy regimens: TSR-042 (PD-1 inhibitor) plus platinum-doublet chemotherapy versus pembrolizumab plus platinum-doublet chemotherapy (NCT04581824).
Regarding second-line PD-(L)1 blockade for advanced NSCLC, four drugs (camrelizumab, prolgolimab, sintilimab, and tislelizumab) entered clinical development between January 2017 and December 2017 after the approval of pembrolizumab on the basis of KEYNOTE-001 on October 2, 2015.
Atezolizumab in combination with platinum-doublet chemotherapy as first-line treatment for SCLC was approved on the basis of the results of IMpower133. Four clinical trials using HLX10, tislelizumab, toripalimab, and TQB2450 in combination with chemotherapy were launched after the approval of atezolizumab.
Among the 15 trials that were launched after the FDA approval of PD-(L)1 blockade for a specific indication, 11 trials (73.3%) were being performed in the People’s Republic of China. A total of 14 (93.3%) of these 15 trials were randomized trials, and all the trials compared PD-(L)1–based therapy (either as monotherapy or in combination with chemotherapy) with chemotherapy alone.
Discussion
In this study, we reviewed the current landscape of immunotherapy trials involving the PD-(L)1 axis in intrathoracic tumors. We found that, as of November 13, 2020, there were 686 clinical trials of anti–PD-(L)1 therapy registered on ClinicalTrials.gov for the treatment of intrathoracic tumors. The number of clinical trials investigating immunotherapy in intrathoracic tumors has experienced a steep increase over the past decade, with the first such trials having been registered in 2011 for NSCLC, 2013 for SCLC, 2015 for mesothelioma, and 2017 for TET. It is likely these numbers will continue to rise in the next decade. PD-(L)1 blockade tends to be studied more with combination approaches including chemotherapy, immunomodulatory therapy, RT, targeted therapy, and multiple combinations thereof. This finding mirrors general immuno-oncologic trends in current research and development looking to exploit the immune system at multiple points to best eradicate neoplastic cells.
Whereas the field of thoracic oncology has witnessed a stark change in the treatment of intrathoracic malignancies owing to the introduction of anti–PD-(L)1 therapies, several unmet needs remain. The most combination anti–PD-(L)1 clinical trials are based on empiricism and often lack sound scientific justifications.4 Beyond PD-(L)1 expression, which in themselves are imperfect biomarkers, it is necessary to identify other predictive biomarkers of anti–PD-(L)1 therapy that will aid in the development of rationally designed combination trials. More research must be conducted to understand the mechanisms of primary and acquired resistance to anti–PD-(L)1 therapy because only a subset of patients benefits from the treatment. It is imperative to understand more thoroughly the safety and efficacy of patient populations underrepresented in clinical trials, such as those with autoimmune diseases and those who have undergone organ transplantation. Finally, the duration of anti–PD-(L)1 therapy needs to be better defined.
One of the challenges that the field of immuno-oncology faces is a duplication of efforts.5 A total of 23 anti–PD-(L)1 agents are under clinical development for intrathoracic malignancies. Although a few drugs have novel mechanisms of action (e.g., dual targeting of PD-1/TIM-3, PD-L1/CTLA-4), these drugs largely target the same pathway. Although the unique binding properties of PD-(L)1 antibodies have been reported,6 it is unknown whether the subtle differences in binding profiles translate into clinically meaningful differences in patient outcomes. Some studies suggest that the differences in clinical outcomes in clinical trial results between anti–PD-(L)1 agents are more likely because of trial design–related factors than differences in the mechanism of action or pharmacodynamics of the drugs.7 It can be argued that having several drugs for a specific indication may drive the cost of drugs down through competition, but drug prices sometimes climb over time despite the presence of similar drugs.8 We acknowledge that confirmatory trials are needed to fully establish the safety and efficacy profile of a certain drug class because a single positive clinical trial is insufficient. However, our analysis revealed findings that may suggest duplication of efforts. For example, it should be questioned if conducting 15 randomized clinical trials comparing anti–PD-(L)1 therapy plus platinum-based chemotherapy with chemotherapy alone in treatment-naive advanced NSCLC is the best way to use the finite resources available for clinical research (including the most precious resource, the patients), or if this simply represents the pharmaceutical companies’ intention of developing their own PD-(L)1 therapeutics to gain market share. Similar concerns related to this duplication of immuno-oncology research in NSCLC have been raised by other researchers.9
In addition, we found that 14 trials were launched after the FDA approval of an anti–PD-(L)1 therapy as a therapeutic option in a given tumor type, which continued to randomize patients to treatments that were inferior to the best available therapy. Whereas these studies were conducted mostly outside of the United States, previous work has repeatedly raised the question of the appropriateness of these control arms.10,11
How does one address the challenges the field faces with the fragmented and uncoordinated landscape of the PD-(L)1 therapy? First, innovative trials such as master protocols could be used to study multiple agents in a more coordinated way,12 though potential pitfalls of such protocols include small arm size, issues about reproducibility, and the exploratory nature of the results.13 The role of public and nonprofit organizations is important in facilitating this kind of endeavor.5 One example involves an immunotherapy master protocol called iMATCH, which is being designed by the National Cancer Institute’s clinical trials cooperative groups. iMATCH is expected to provide a platform to assess various biomarker-driven approaches. In addition, more resources should be devoted to rigorously designed trials (e.g., well-designed randomized trials with an appropriate comparator arm or clinical trials with strong translational components). Regulatory agencies such as the National Institutes of Health and FDA will need to develop a unified strategy to promote well-designed immunotherapy studies. The Partnership for Accelerating Cancer Therapies exemplifies a public-private collaboration that enables acceleration of biomarker development and design and the conduct of high-quality clinical trials investigating immunotherapy.14
Our study has several limitations. First, we did not include studies that evaluated more than one extrathoracic tumor type to focus better on intrathoracic tumors. Therefore, it is possible that other drugs targeting the PD-(L)1 pathway in early clinical development might not have been captured. Second, although our analysis was performed using a search of a major clinical trial registry, it is possible some trials were omitted from our analyses.
In conclusion, our analysis provides an overview of the landscape of clinical trials of PD-(L)1 blockade in intrathoracic tumors. Although anti–PD-(L)1 therapy has been found to improve overall survival in several landmark trials, not all patients benefit from the treatment. Neoadjuvant and adjuvant PD-(L)1 blockade in early-stage disease and combination treatment to overcome primary and acquired resistance to PD-(L)1 monotherapy in advanced disease will likely play bigger roles in the future. To develop effective treatment using any anti–PD-(L)1 therapeutic strategy, greater coordination among stakeholders (e.g., pharmaceutical companies, academic centers, public organizations such as the FDA, patient advocacy groups) and adoption of innovative trial designs will be vital.
Acknowledgments
Dr. Kareff contributed to software provision, formal analysis, investigation, provision of resources, writing of the original draft, visualization, and project administration. Dr. Kim contributed to study conceptualization, methodology, software provision, results validation, formal analysis, investigation, provision of resources, data curation, review and editing of the article, and study supervision.
Footnotes
Disclosure: Dr. Samtani reports receiving speaker fees from Roche; advisory and consulting fees from Merck Sharp & Dohme and Roche; and funding for sponsored educational programs (including travel, accommodations, and expenses) from Merck Sharp & Dohme, Roche, and AstraZeneca. Dr. Burotto reports receiving speaker fees from Merck Sharp & Dohme, Roche, Astra Zeneca, BMS, and Novartis; and advisory and consulting fees from Merck Sharp & Dohme, BMS, and Roche. Dr. Kim reports receiving a research grant to the institution from AstraZeneca, BMS, Novartis, Regeneron, Tesaro, Karyopharm, and Debiopharm; and advisory and consulting fees from Novartis and Janssen. The remaining authors declare no conflicts of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100149.
Supplementary Data
References
- 1.Haslam A., Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upadhaya S., Neftelino S.T., Hodge J.P., Oliva C., Campbell J.R., Yu J.X. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials [e-pub ahead of print]. Nat Rev Drug Discov. https://doi.org/10.1038/d41573-020-00204-y accessed November 11, 2020. [DOI] [PubMed]
- 3.Tang J., Pearce L., O’Donnell-Tormey J., Hubbard-Lucey V.M. Trends in the global immuno-oncology landscape [published correction appears in Nat Rev Drug Discov. 2018;17:922] Nat Rev Drug Discov. 2018;17:783–784. doi: 10.1038/nrd.2018.167. [DOI] [PubMed] [Google Scholar]
- 4.Siu L.L., Ivy S.P., Dixon E.L., Gravell A.E., Reeves S.A., Rosner G.L. Challenges and opportunities in adapting clinical trial design for immunotherapies. Clin Cancer Res. 2017;23:4950–4958. doi: 10.1158/1078-0432.CCR-16-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang J., Shalabi A., Hubbard-Lucey V.M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29:84–91. doi: 10.1093/annonc/mdx755. [DOI] [PubMed] [Google Scholar]
- 6.Brown M.E., Bedinger D., Lilov A. Assessing the binding properties of the anti-PD-1 antibody landscape using label-free biosensors. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fessas P., Lee H., Ikemizu S., Janowitz T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol. 2017;44:136–140. doi: 10.1053/j.seminoncol.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad V., De Jesus K., Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14:381–390. doi: 10.1038/nrclinonc.2017.31. [DOI] [PubMed] [Google Scholar]
- 9.Wu D.W., Huang H.Y., Tang Y. Clinical development of immuno-oncology in China. Lancet Oncol. 2020;21:1013–1016. doi: 10.1016/S1470-2045(20)30329-6. [DOI] [PubMed] [Google Scholar]
- 10.Hilal T., Sonbol M.B., Prasad V. Analysis of control arm quality in randomized clinical trials leading to anticancer drug approval by the US Food and Drug Administration. JAMA Oncol. 2019;5:887–892. doi: 10.1001/jamaoncol.2019.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilal T., Gonzalez-Velez M., Prasad V. Limitations in clinical trials leading to anticancer drug approvals by the US Food and Drug Administration. JAMA Intern Med. 2020;180:1108–1115. doi: 10.1001/jamainternmed.2020.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodcock J., LaVange L.M. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377:62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 13.Mazzarella L., Morganti S., Marra A. Master protocols in immuno-oncology: do novel drugs deserve novel designs? J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker R.G., Hoos A., Adam S.J. The partnership for accelerating cancer therapies. Cancer J. 2018;24:111–114. doi: 10.1097/PPO.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.