Abstract
Chronic constriction injury (CCI) of infraorbital nerve (IoN) results in whisker pad mechanical allodynia in rats and activation glial cells contributing to the development of orofacial pain. Whisker pad mechanical allodynia (von Frey stimuli) was tested pre and postoperatively and conducted during the treatment time. Photobiomodulation (PBM) and vitamins B complex (VBC) has been demonstrated therapeutic efficacy in ameliorate neuropathic pain. The aim of this study was to evaluate the antinociceptive effect of PBM, VBC or the combined treatment VBC + PBM on orofacial pain due to CCI-IoN. Behavioral and molecular approaches were used to analyses nociception, cellular and neurochemical alterations. CCI-IoN caused mechanical allodynia and cellular alterations including increased expression of glial fibrillary acid protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba-1), administration of VBC (B1/B6/B12 at 180/180/1.8 mg/kg, s.c., 5 times all long 10 sessions) and PBM therapy (904 nm, power of 75Wpico, average power of 0.0434 W, pulse frequency of 9500 Hz, area of the beam 0.13 cm2, 18 s duration, energy density 6 J/cm2, with an energy per point of 0.78 J for 10 sessions) or their combination presented improvement of the nociceptive behavior and decreased expression of GFAP and Iba-1. Additionally, CCI-IoN rats exhibited an upregulation of IL1β, IL6 and TNF-α expression and all treatments prevented this upregulation and also increased IL10 expression. Overall, the present results highlight the pain reliever effect of VBC or PBM alone or in combination, through the modulation of glial cells and cytokines expression in the spinal trigeminal nucleus of rats.
Keywords: Microglia, Astrocytes, Cytokines, Photobiomodulation, B vitamins, Trigeminal pain
Highlights
-
•
Efficacy of PBM and VBC as treatment for orofacial allodynia due to CCI IoN in rats.
-
•
Additional mechanism for the analgesic effect of VBC treatment on orofacial allodynia induced by CCI-IoN.
-
•
VBC or PBM or combined treatment VBC + PBM significantly attenuated CCI-IoN-induced nociceptive response.
1. Introduction
VBC has received a lot of attention due to the probable action in treat painful conditions (Yu et al., 2014), showing important roles in various biological events such as promote nerve regeneration (Sun et al., 2012), relieves thermal hyperalgesia (Yu et al., 2014), alleviate indices of neuropathic pain in diabetic rats (Jolivalt et al., 2009) and presents anti-hyperalgesic results in diverse kinds of neuropathies and pain conditions (Song et al., 2009; Kopruszinski et al., 2012; Yu et al., 2014). VBC therapy shows promise in helping nociception control, it appears to be safe and there are no reports of adverse side effects due the long-term exposure. In this line, non-pharmacological treatments also have gained attention as clinical alternative for treat painful conditions. Photobiomodulation (PBM) has been demonstrating analgesic and anti-inflammatory effects in neuropathic and chronic pain conditions in experimental and clinical studies (Haslerud et al., 2015; de Andrade et al., 2017). Once current therapy for the management of chronic orofacial pain conditions is frequently unsatisfactory and can be limited due to the adverse effects; the search for new protocols that may offer improvements to traditional procedures in the management of chronic orofacial pain is highly required. Recently, because the multiple molecular mechanisms underlying neuropathic pain, several studies with combinate protocols have been performed aiming the newer concept of multi-target therapy.
Studies has been suggested that VBC can protect neurons from certain injuries, additionally exhibit important roles in various biological events to maintain normal neural functions (Hung et al., 2009; Jolivalt et al., 2009; Hobbenaghi et al., 2013) and are clinically administered (Jolivalt et al., 2009). PBM is a non-invasive, therapeutically beneficial and promotes a wide range of biological effects that regulates neuronal function in cell cultures, animal models and clinical conditions (Enwemeka et al., 2004; Medalha et al., 2012; Cotler et al., 2015).
There is accumulating evidence that glial cells (astrocytes and microglia) are involved in the initiation and maintenance of orofacial pain, playing an important role in peripheral and central mechanisms (Chiang et al., 2011; Sessle, 2011; Ji et al., 2013; Zhang et al., 2017). These cells are capable of enhance nociceptive neuron signaling by the release of pro-algesic mediators (Narita et al., 2006; Mika et al., 2013). Studies already demonstrated the critical role of glial cells in the development of neuropathic and orofacial pain (Chacur et al., 2009; Ji et al., 2013; Giardini et al., 2017; Liu et al., 2018), and the increased excitability and sensitization of the spinal trigeminal nucleus complex, especially the subnucleus caudalis (Vc), might be involved in processing orofacial input (Sessle, 2000; Okada-Ogawa et al., 2009; Ren and Dubner, 2011). Glial cells, both in the CNS or in PNS can be activated by many different signals and modulators, including the cytokines (Hanisch and Kettenmann, 2007). Peripheral nerve injury can strongly activate microglia and astrocytes (Xu et al., 2008; Dubovy et al., 2018), which increases the production of different substances, including cytokines like IL1β, IL6 and TNFα (Milligan et al., 2001; Watkins et al., 2003; Johnston et al., 2004). These cytokines mediate allodynia and hyperalgesia, playing an important role in nociception (Ellis et al., 2014). Furthermore, the modulation of glial cells activation by administration of minocycline, pentoxifylline and propentofylline attenuated the hypernociception in rats (Ellis et al., 2014; Mei et al., 2014; Kim et al., 2016; Miyamoto et al., 2017; Abbaszadeh et al., 2018).
Cytokines released from activated glial cells have an important role in glial-neuron communication, contributing to nociceptive transmission (Guo et al., 2007; Ren and Dubner, 2008). IL1β from glial cells contribute to inflammatory temporomandibular joint (TMJ) hypernociception in rats (Zhang et al., 2018), and inhibition of TNF-α or IL1β in trigeminal ganglion by selective inhibitors attenuated trigeminal nerve injury induced mechanical allodynia (Zhang et al., 2016). IL10 is a potent anti-inflammatory cytokine and also has the capacity to inhibit the production of the pro-inflammatory cytokines (Milligan et al., 2012).
The evidence above mentioned suggest that PBM or VCB can be considered effectives to treat painful conditions, and these analgesic effects may be related with the modulation of the inflammatory mediators. Therefore, in this study we also tested the hypothesis that the association of PBM and VBC will improve the allodynic pain threshold and will reduce levels of allogeneic substances.
2. Materials and methods
2.1. Animals
Sixty male Wistar rats, weighing between 200 and 220 g (2 months old, at the beginning of the experimental procedure) were used in all experiments. Animals were divided into six groups: naive, sham, CCI-IoN, CCI-IoN + PBM, CCI-IoN + VBC and CCI-IoN + PBM + VBC (10 animals per group; 5 was used for Western blot and 5 was used for immunohistochemistry assays). They were housed under a 12:12 light–dark cycle with free access to food and water; five rats were housed per cage. All animals were tested during the light cycle at about the same time of the day (8:00 a.m.–12:00 a.m.). All procedures were approved by the Institutional Animal Care Committee of the University of São Paulo (protocol number CEUA 3872071118). All efforts were made to minimize the number of animals used and their suffering (Zimmermann, 1983). To minimize stress, the animals were adapted to the experimental environment for 3 days before the experiments started.
2.2. Chronic constriction injury of infraorbital nerve (CCI-IoN)
Neuropathic pain was induced via chronic constriction injury (CCI) to the right infraorbital nerve (IoN) as described elsewhere (Chichorro et al., 2006). All surgical procedures were performed aseptically on rats anesthetized with ketamine (5 mg/100g body weight, intraperitoneally – i.p.) and xylazine (1 mg/100g body weight, i.p.) administered intraperitoneally. After the anesthesia was established, the facial surface between the eye and whisker pad of the rat was shaved without damaging the whiskers and sterilized with iodine, and 70% isopropyl alcohol was used to remove excess iodine. An incision was made in the skin below the right eye, about 3 mm posterior to the insertion of the whiskers. The muscles upper lip lift and anterior superficial masseter were dissected so that the rostral portion of the infraorbital nerve was exposed, close to the infraorbital fissure. The infraorbital nerve was dissected from adjacent tissues, and two chromic gut ligatures were loosely tied (2 mm apart) around the exposed section. The incision was closed with 4–0 silk sutures. All surgeries were performed by a single investigator to minimize variability. For the sham surgery groups, the IoN was exposed but was not touched or ligated prior to closing the incision. Naive animals, not exposed to CCI-IoN, were used as controls. The animals were put back in the cages and observed until the moment they recover, when they were brought back to the animal facility.
2.3. Mechanical (tactile) stimulation
The region below the eye and caudal to the whisker pad, a region innervated by the second branch of the trigeminal ganglion (Leiser and Moxon, 2006) was tested using traditional von Frey filaments (Stoelting, USA), according of the method previously described (Yonehara et al., 2003; de Oliveira Martins et al., 2013; de Freitas Rodrigues et al., 2019). Briefly, the rats were acclimated, trained and tested for facial mechanical sensitivity three days prior to CCI-IoN (baseline, day 0) and on postoperative days 4, 6, 8, 10, 12, 14, 16, 18, 20 and 22. The threshold intensity of the stiffness stimulus required to elicit a response was determined by the animals’ withdrawing or by their touching or scratching their facial regions after the von Frey filaments were applied. When the animal showed a positive response in two consecutive trials with the same stiffness value, no further von Frey hairs were tested. All the groups in the experiment included 10 animals.
2.4. Photobiomodulation
The treatment was similar to that used in previous study (de Oliveira Martins et al., 2013; Martins et al., 2017a; Martins et al., 2017b; de Freitas Rodrigues et al., 2019). Briefly, rats were irradiated with GaAs laser (Gallium Arsenide, Laserpulse-Laser, Ibramed Brazil) emitting a wavelength of 904 nm, power of 75Wpico, average power of 0.0434 W, pulse frequency of 9500 Hz, area of the beam 0.13 cm2, 18 s duration, energy density 6 J/cm2, with an energy per point of 0.78 J. The treatment with the laser technique was initiated after 3 days of surgery for CCI-IoN injury animals. The laser treatment was performed every other day, involving 10 sessions. After sterilization, the spot was kept without contact with the animal’s skin, directly above the whisker pad. Were irradiated 5 points, with duration of 18 s per point. Each point was irradiated with intervals of 30 s and each session had a total duration of 4 min.
2.5. Vitamins B complex
The vitamins B complex, thiamine, pyridoxine and cyanocobalamin (B1, B6 and B12, respectively) were purchased from Alfa Aesar (Tewksbury, MA, United States), dissolved in sterile 0.9% sodium chloride solution (saline) just before use in doses of 180/180/1.8 mg/ml (subcutaneous - s.c.). The doses of vitamins B were chosen based on literature study (Kopruszinski et al., 2015). The vitamins B were administered immediately after CCI-IoN surgery (day 0), then on days 3, 9, 15 and 21 during the treatment, totaling 5 applications.
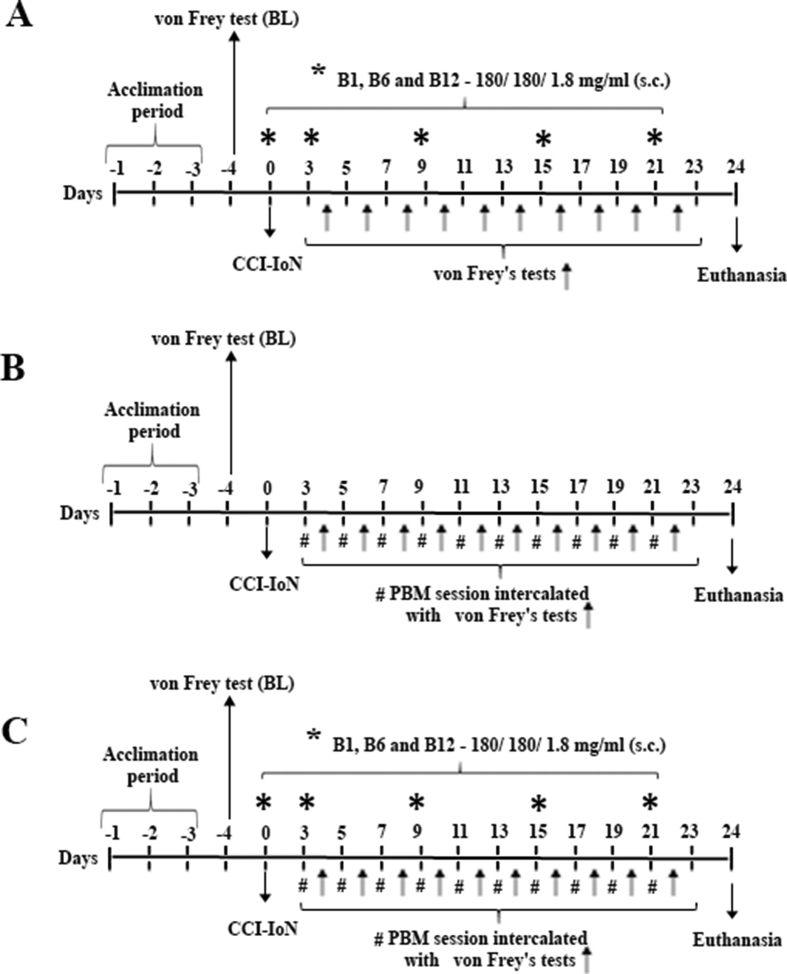
The schematic drawing of the experimental procedures is presented in Fig. 1.
Fig. 1.
Schematic drawing of the experimental procedures and VBC/PBM administration schedule. In A experimental design for rats underwent treatment with VBC alone; in B experimental design for rats underwent treatment with PBM alone and C experimental design for rats underwent treatment with VBC + PBM. Rats were allowed to acclimate to the test chamber for at least 3 days before the first measurement (baseline – BL). Afterwards, was performed the CCI-IoN surgery, and for group of animals treated with vitamins cocktail (VBC and VBC + PBM) was also injected the first administration of VBC cocktail; the animals belonging to the group PBM or VBC + PBM beginning the PBM application 3 days after CCI-IoN and follow every other day; the evaluation of the nociceptive threshold by von Frey test was tested in every other day from day 4 until day 22. All animals were tested during the light cycle at about the same time of the day (8:00 a.m.–12:00 a.m.).
2.6. Western blot assays
Medulla containing the spinal trigeminal nucleus (N = 5 per group) was quickly removed and transferred to a tube containing 200 μL extraction buffer (100 mM Tris, pH 7.4, 10 mM EDTA, 2 mM PMSF, and 10 μg/mL aprotinin) in ice-cold (4 °C) during 30 min. Then they were homogenized using an ultrasonic processor (Sonics & Materials, Newtown, PA). The homogenates were then centrifuged at 12,000 rpm at 4 °C for 20 min and the protein concentration of the supernatant was determined using the Bradford protein assay (Bio-Rad, Melville, NY) (Bradford, 1976). Samples containing 60 μg of protein were loaded on acrylamide gradient gel (Miller et al., 2016) and transferred by electrophoresis to nitrocellulose membranes using a Bio-Rad Trans-Blot Turbo Transfer System during 30-min protocol. After transfer, the membranes were treated for 2 h at room temperature with a blocking solution containing 5% powdered milk, washed and incubated overnight at 4 °C with an anti-GFAP (mouse monoclonal anti-glial fibrillary acidic protein, clone G-A-5; Sigma), anti-Iba 1, rabbit (Ionized calcium binding adaptor molecule 1 for western blotting, Wako Pure Chemicals), anti-IL1β (rabbit polyclonal to IL1β, abcam), anti-IL6 (rabbit polyclonal to IL6, abcam), anti-IL10 (rabbit polyclonal to IL10, abcam) and anti-TNF-α (rabbit polyclonal to TNF-α, abcam) diluted 1:1000. The membranes were then washed and incubated for 2 h at room temperature with peroxidase-conjugated anti-rabbit or anti-mouse (GE Healthcare) secondary antibody, diluted 1:5000. β-actin was used as an internal control (1: 10,000; Sigma). The specifically bound antibody was visualized using a chemiluminescence kit (Amersham Biosciences). The blot was analyzed densitometrically using the NIH-Scion Image 4.0.2, quantified by optical densitometry of the developed autoradiographs (Scion Corporation, USA) and corrected by the optical density for β-actin, whereas samples from control animals were used as the standard for normalization of the results (assuming 100% for naive animals).
2.7. Immunohistochemistry
Rats (N = 5 per group) were deep anesthetized with ketamine (5 mg/100g body weight, ip) and xylazine (1 mg/100g body w, ip) and transcardically perfused with 300 ml saline (0.9% NaCl), followed by 300 ml cold (4 °C) 0.1 M phosphate buffer (PB, pH 7.4) containing 4% paraformaldehyde. Then the medulla and upper cervical spinal cord were removed and post-fixed in the same fixative solution for 4 h and then transferred to 30% sucrose in 0.1 M PB for cryoprotection for 48 h at 4 °C. Transverse frozen spinal sections (50 μm thick) of the caudal medulla and upper cervical spinal cord were cut with a sliding microtome adapted for cryosectioning (Leica SM2010 R Sliding Microtome; Heidelberg, Germany) and collected and then stored free floating in 0.01 M PB. All sections were blocked with 2% goat serum in 0.01 M PBS containing 0.3% Triton X-100 for 1 h at room temperature and then incubated overnight at 4 °C with one of the following primary antibodies: anti-Iba1 (1: 500; rabbit polyclonal antibody for immunocytochemistry; Wako Pure Chemicals) and anti-GFAP (1: 500, mouse monoclonal anti-glial fibrillary acidic protein, clone G-A-5; Sigma). The sections were washed three times in 0.01 M PBS (10 min each) and then incubated for 2 h at room temperature with the biotinylated secondary antibodies (goat anti-mouse IgG or goat anti-rabbit IgG, Jackson ImmunoResearch, 1: 200) and then incubated for 1 h 30 min at room temperature with the avidin-biotin complex (1: 100; ABC Elite kit, Vector Laboratories). After washing, the sections were reacted with 0.05% 3, 30-diaminobenzidine and 0.01% hydrogen peroxide in PB. Intensification was conducted with 0.05% osmium tetroxide in water. The sections were mounted on gelatinized slides, dehydrated, cleared, and cover-slipped. The material was analyzed using a Nikon E1000 upright microscope coupled to a Nikon DCM1200 digital camera and digital images were collected. Figures were mounted with Adobe Photoshop CC2018. Manipulation of the images was restricted to threshold and brightness adjustments of the whole image. Controls of the experiments consisted of the omission of primary antibodies, and no staining was observed in these cases (data not shown).
2.8. Statistical analysis
Results are presented as the means ± standard error of the mean. Statistical analyses of data were generated using GraphPad Prism, version 7.00 (Graph-Pad Software Inc., San Diego, CA). Behavioral data were analyzed using two-way repeated measures ANOVA with Tukey’s test comparing groups (treatment x time). Statistical comparison for Western blot data was performed using one-way ANOVA with differences between means were evaluated by the Tukey’s test. In all cases, a value of p ≤ 0.05 was accepted as statistically significant (Snedecor et al., 1946).
3. Results
3.1. Antiallodynic effects of B vitamins and photobiomodulation
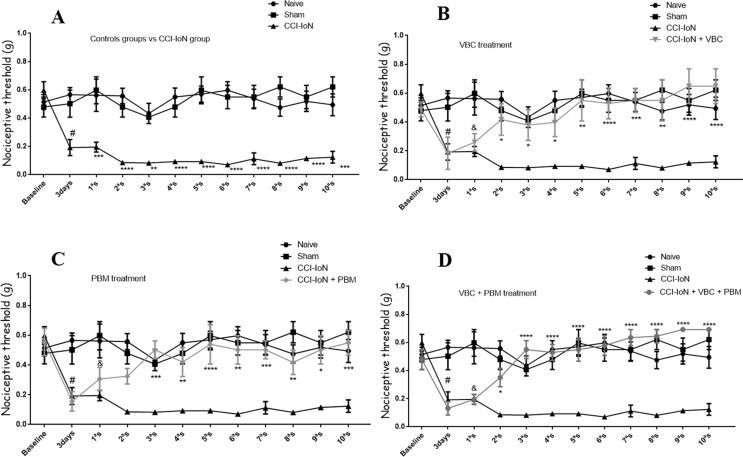
CCI-IoN produced allodynia in rats submitted to the surgery when compared with the naive and sham rats (p ˂ 0.001). Allodynic effects were observed in the rats 3 days after injury and remained for at least 24 days after CCI-IoN, when the experiments were carried out (Fig. 2-A). The statistical analysis carried out indicates that there are significant differences considering the treatment factor F (5, 338) = 57,47, p < 0.0001; time F (11, 338) = 5,693, p < 0.0001; besides evidencing interaction between time and treatment F (55, 338) = 2,68, p < 0.0001.
Fig. 2.
Effect of VBC and PBM on pain threshold induced by chronic constriction injury of Infraorbital nerve (CCI-IoN) in rats. Pain threshold as measured by von Frey test, expressed in grams. Measurements were determined before (baseline), 3 days after the lesion, at which time we also conducted the first session of PBM (3d-1st), and at different intervals after VBC administration and PBM sessions. In A mechanical pain threshold comparing the CCI-IoN group to the baseline value and to the control groups; In B mechanical pain threshold of VBC group compared to the baseline value, control groups and to the CCI-IoN group; In C mechanical pain threshold of PBM group compared to the baseline value, control groups and to the CCI-IoN group and in D mechanical pain threshold of VBC + PBM group compared to the baseline value, control groups and to the CCI-IoN group. The results represent the mean ± SEM of ten animals per group. ***p ˂ 0.004 and ****p ˂ 0.001 for comparison with control groups (naive and sham) and #p ˂ 0.05 for comparison with Baseline (BL).
The application of the Tukey’s test, shows, in particular, that subcutaneous administration of VBC alone attenuates tactile allodynia induced by CCI-IoN, producing a small antiallodynic effect from the 2aS until the 5aS (after two doses of VBC; p ˂ 0.05) and significantly reduced tactile allodynia from the 6aS of treatment (after three doses of VBC; p ˂ 0.001) returning to basal levels and remain until the last session evaluated (Fig. 2-B). PBM, alone, also reduced tactile allodynia from the 3aS (p ˂ 0.002) and remains until the last session evaluated when compared with CCI IoN rats (Fig. 2-C). The combined treatment of VBC plus PBM resulted in significantly reduced tactile allodynia induced due to CCI-IoN from the 3aS (after two doses of VBC and two PBM session, p ˂ 0.001), returning to basal levels, and remain until the last session evaluated (Fig. 2-D). This combination reached the maximal antiallodynic effect in the 7aS (p ˂ 0.0001; compared with CCI-IoN rats) and was more effective that VCB or PBM alone (p ˂ 0.004; compared with CCI-IoN rats, for both). In both cases, for VBC or PBM alone, maximal antiallodynic effect was lower than the maximum effect of the combination of VBC and PBM. Comparing the animals that received any one of treatments (VBC, PBM or VBC + PBM) with the animals taken as control (naive and sham) we did not find differences from 2aS until the last session evaluated. Also, the nociceptive threshold of the CCI-IoN-untreated group did not change significantly during the entire experiment, as shown in Fig. 2.
3.2. Immunoreactivity of GFAP and Iba-1 in the spinal trigeminal nucleus
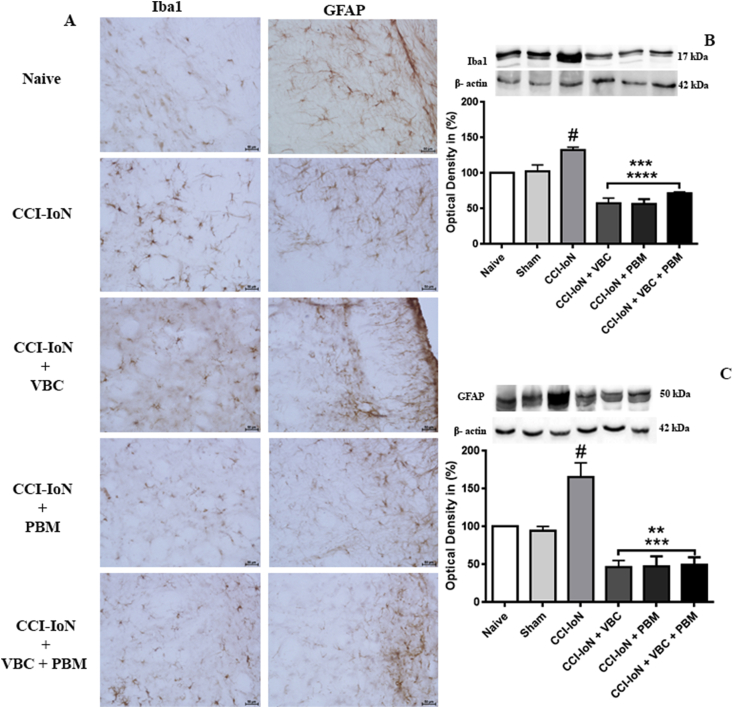
Immunohistochemistry for detection of GFAP, a specific marker of astroglial cells, for normal as well as reactive condition, revealed that in naive and sham animals, there was a baseline expression of GFAP in the spinal trigeminal nucleus. After CCI IoN astroglia showed a significantly higher in GFAP staining, compared to the baseline, with a swollen hypertrophic appearance indicating an activated phenotype (Fig. 3-A). Treatment with VBC, PBM or their combination resulted in a significant reduction in the number of GFAP-positive cells comparing with the injured group.
Fig. 3.
CCI-IoN induces increased expression of glial cells; In A compare the expression of Iba1 and GFAP in the injury ipsilateral side among the five groups, Scale bar, 50 μm. Effect of VBC and PBM on the expression of Iba1 and GFAP in the spinal trigeminal nucleus of chronic constrictive injury of infraorbital nerve rats. In B and C a substantial decrease in Iba1 and GFAP expression was identified in the spinal trigeminal nucleus after VBC and PBM treatments relative to the expression in the CCI-IoN rats (Iba1 ***p ˂ 0.05 and ****p ˂ 0.001; GFAP ***p ˂ 0.001 comparing with the CCI IoN group; #p ˂ 0.05 comparing to the control groups). Data were normalized from naive rats’ GFAP and Iba1 expression. Data are presented as the mean ± SEM of 5 animals per group.
Immunohistochemistry for Iba1, a specific marker of microglial activation, revealed a baseline immunoreactivity of microglia in both naive and sham animals. After CCI-IoN a highest number of activated microglia was observed in the spinal trigeminal nucleus and the treatments also decreased the immunoreactivity of microglial marker Iba1 (Fig. 3-A).
In the absence of the primary antibody only background staining was evident (data not shown).
3.3. Effects of CCI IoN, VBC or PBM on the protein expression of GFAP, Iba1, IL1β IL 6, TNF-α, and IL 10 in the spinal trigeminal nucleus
Protein analysis by western blotting revealed that CCI-IoN increased the expression of the both GFAP and Iba1 comparing with control groups (naive and sham animals) and the treatment of injured rats with VBC, PBM or its combination reduced the Iba1and GFAP expression. For Iba1 expression, the treatments also had the same effect (F (5, 23) = 23,66 for treatment effect, p < 0.0001) when compared with the injured group (Fig. 3-B). For GFAP expression all kinds of treatments showed the same effect with a significant decrease of GFAP expression (F (5, 28) = 16,86 for treatment effect, p < 0.0001) when compared with the CCI-IoN group (Fig. 3-C).
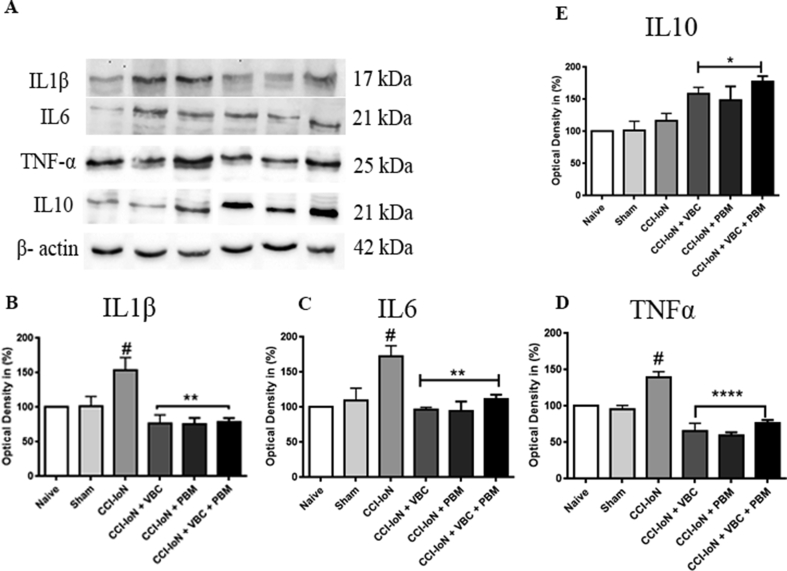
For expression of cytokines Western blot analysis showed an increase of expression of proinflammatory cytokines, IL1β, IL6 and TNF-α in the CCI-IoN group (Fig. 4-B, Fig. 4-C and Fig. 4-D, respectively) in the CCI-IoN group when compared with the control groups (naive and sham, p ˂ 0.05). After the treatments (VBC, PBM and VBC + PBM) we observed a decrease of expression (IL1β, F (5, 23) = 23,66 for treatment effect, p < 0.0001; IL6, F (5, 21) = 6,97 for treatment effect, p = 0.0006 and TNF-α, F (5, 16) = 21,75 for treatment effect, p < 0.0001) when comparing the groups CCI-IoN + VBC, CCI IoN + PBM and CCI-IoN + VBC + PBM to the CCI-IoN group (p ˂ 0.01, p ˂ 0.001 and p ˂ 0.001, respectively). For the anti-inflammatory cytokine interleukin-10 (IL10) we didn’t see alterations after CCI-IoN when compared with the control groups (naive and sham) and after the treatments we saw an increase of IL 10 expression in the groups CCI-IoN + VBC, CCI-IoN + PBM and CCI-IoN + VBC + PBM when compared with control groups and CCI-IoN animals (p ˂ 0.05; Fig. 4-E). CCI-IoN induced a significant increase of proinflammatory proteins. The treatment with VBC and PBM or their combination thus prevented the increased expression of the proinflammatory proteins on the spinal trigeminal nucleus after the infraorbital nerve ligation; and resulted in enhanced expression of the anti-inflammatory cytokine interleukin-10.
Fig. 4.
VBC and PBM attenuates the overexpression of IL1β, IL6 and TNF-α at protein levels induced a significant increase in the expression of IL10. Effect of VBC and PBM on the expression of IL1β, IL6, TNF-α and IL10 in the spinal trigeminal nucleus of chronic constrictive injury of infraorbital nerve rats; Correspondingly, A Western blot analysis demonstrated that IL-1β (B), IL6 (C) and TNF-α (D) levels were increased in the spinal trigeminal nucleus of the CCI-IoN group compared with those in control groups (naive and sham) (#p ˂ 0.05) and decreased in the CCI-IoN + VBC, CCI-IoN + PBM and CCI-IoN + VBC + PBM groups compared with those in CCI-IoN group (**p ˂ 0.01 and ***p ˂ 0.001); in E IL10 level didn’t alter after CCI-IoN compared with the control groups (naive and sham) and increased after the treatments compared with the others groups (p ≤ 0.05). Data were normalized from naive rats’ IL1β, IL6, TNF-α and IL10 expression. Data are presented as the mean ± SEM of 5 animals per group.
4. Discussion
It is well accepted that glial cells actively participate of peripheral sensitization induced by inflammation and peripheral nerve injury (Zhang et al., 2017; Liu et al., 2018), and this process is triggered by a wide range of mediators released by glial cells around the site of tissue damage. The main inflammatory mediators released by glial cells after nerve damage are cytokines (Donegan et al., 2013; Mika et al., 2013). Therefore, relating our behavioral data with the western blotting protein analysis we suggest that IL1β, IL6 and TNF-α expression can be considerate as a marker of pain related behavior, since the increased level of these protein in CCI-IoN group was concomitant with the installation of nociceptive behaviour and the treatments with VBC, PBM or VBC + PBM decreased its expression and improved the nociceptive behaviour. These data reinforce the idea that glial cells play important roles in peripheral and central mechanisms involved in the initiation and maintenance of orofacial pain (Sessle, 2011; Hossain et al., 2017; Fan et al., 2019).
In this study, we explore the antiallodynic effect of VBC, PBM or their combination on CCI-IoN induced pain. CCI-IoN induced astroglial/microglial proliferation and astroglial/microglial activation; we also found a low IL10 protein levels in CCI-IoN rats; however studies using different models of sciatic nerve injury (crush, CCI) already have mentioned a transiently decrease of IL10 after nerve injury (Powell et al., 2000; Okamoto et al., 2001; Stoll et al., 2002; George et al., 2004); and, besides the beneficial effects of IL10, this relative deficiency of local IL10 protein may be essential for effective nerve de- and regeneration (George et al., 2004). Our data indicate that VBC, PBM or VBC + PBM could attenuate the CCI-IoN induced allodynic pain, and VBC + PBM was faster and more effective to ameliorate the pain-related behavior due to CCI-IoN. In addition, the treatments also reduced glial cells (microglial and astrocytes) activation and levels of expression of the proinflammatory IL-1β, IL6 and TNF-α, and also increased the expression of anti-inflammatory cytokine IL10.
Most recent studies suggested administration of vitamin B complex (i.e., B1, B6 and B12) alone or combined as treatment for controlling acute and chronic neuropathic pain following temporary spinal cord ischaemia and CCI-IoN in rats (Yu et al., 2014; Kopruszinski et al., 2015). As demonstrated in this study, VBC could ameliorate the allodynic behavior in CCI-IoN rat model of orofacial pain. Furthermore, we showed an important role in regulate the glial activation, proinflammatory and anti-inflammatory cytokines production, events which may be involved in the development and maintenance of chronic pain (Ji et al., 2013).
Comparing the antiallodynic effect of VBC with others pharmacological and nonpharmacological treatments, the beneficial role of VBC in orofacial pain condition due to nerve injury could be more meaningful in clinical use, due to the absence of significant side effects. The mechanisms underlying this anti-nociceptive effect of VBC are not fully understood. Proinflammatory cytokines can produces central and peripheral sensitization acting in different ways. For example, TNF-α can enhances excitatory synaptic transmission; IL6 inhibits inhibitory synaptic transmission and IL1β can both enhance excitatory synaptic transmission and reduce inhibitory synaptic transmission (Kawasaki et al., 2008). In our study VBC treatment provide a decrease in proinflammatory cytokines, reduces glial cells markers and improve pain-related behavior. These data suggest a potential role of VBC in regulating synaptic plasticity and neuronal excitability contributing for an efficient relief of orofacial pain. Other studies showed that the administration of B vitamins alone or in combination demonstrated antinociceptive and neuroprotective effects in orofacial neuropathic pain models in rats (Kopruszinski et al., 2012; Tamaddonfard et al., 2013). Studies also revealed that the deficiency of vitamin B1 (thiamine) could be associated with neurodegenerative diseases (Jhala and Hazell, 2011) and microglial activation (Wang and Hazell, 2010); furthermore, a synthetic S-acyl derivative of vitamin B1 inhibited inflammatory mediators and enhancing anti-inflammatory factor production in activated microglia (Bozic et al., 2015). Vitamin B6 has anti-inflammatory activity, and supplementation with high doses (100 mg/day) could suppresses plasma IL6 and TNF-α levels (Jankowski and Koerber, 2010). There are also some data showing antinociceptive and anti-inflammatory effects of vitamin B12 (Tamaddonfard et al., 2013). We confirmed that VBC significantly attenuated CCI-IoN-induced allodynia in a rat model of orofacial pain. More interesting, the decreased activation of glial cells was accompanied by a corresponding decreases expression of the pro inflammatory cytokines IL1β, IL6 and TNF-α, pointing out that VBC might be an important antiallodynic mediator inducing analgesic effect through downregulating glial cells activation (astrocytes and microglia) and the synthesis of inflammatory factors which have a well-documented roles in pain facilitation (Watkins et al., 2006).
Previous studies demonstrate that PBM is effective in ameliorate pain related behavior due to nerve injury (de Oliveira Martins et al., 2013; Martins et al., 2017b), inflammation (Neves et al., 2018), after experimental unilateral temporomandibular joint (TMJ) disc injury (de Freitas Rodrigues et al., 2019) and improve morphological recovery of the peripheral nerve (Rocha et al., 2017; Martins et al., 2017a). According to our immunohistochemistry and Western blot results, PBM application attenuated the expression of GFAP, which is up-regulated as astrocytes are activated, and the expression of Iba1, a marker associated with activated microglia in the treated groups when compared to the CCI-IoN group. We found similar results for VBC administration and PBM therapy.
The most interesting result of our study was that the combined treatment didn’t show a significant performance in cellular and molecular assays comparing with the results of with each treatment alone but reaches a better antiallodynic effect as seem in the behavioral tests; suggesting this combination produced a functional synergistic interaction in CCI-IoN rats and produce a complete reduction of tactile allodynia in these animals earlier. To our knowledge, this is the first report about the synergistic interaction between PBM and vitamins B cocktail. While there has publishing’s data about the use of PBM or vitamins B cocktail to treat acute and chronic neuropathic pain, their specifics mechanisms of action remain unclear. Our results clearly demonstrate increased expression of glial cells after CCI-IoN and treatments could prevent this upregulation.
Peripheral nerve injury can strongly activate microglia and astrocytes, these cells can produce chemoattractant, chemokines, and cytokines (Johnston et al., 2004). Main proinflammatory cytokines include IL-1 alpha/beta, TNF-alpha, IL-6, IL-8, IL-17, and IL-18, these cytokines can stimulate the function expression of other inflammatory mediators, leading to exacerbated sensitization, including painful condition (Chiang et al., 2012). Cytokines may also contribute to the reactive oxygen species (ROS) production and to generating oxidative stress (Yamada et al., 2020). It is suggested that PBM act systemically in the oxidative stress markers and cytokine production (in both gene and protein) through glial cell activity, with an effect similar to some kinds of nonsteroidal anti-inflammatory drugs (NSAIDs) (Aimbire et al., 2007; Wu et al., 2014; Yamada et al., 2020). The antinociceptive effect of VBC has been documented, mainly related to their anti-inflammatory effect which may be due to inhibition of action and or synthesis of inflammatory mediators (Xu et al., 2016; Zaringhalam et al., 2016), but can be also by inhibition of nitric oxide synthesis (Moallem et al., 2008).
The inhibition of pro-inflammatory cytokines is essential to reduce pain behavior after nerve injury and this process seems was dependent upon the spinal trigeminal nucleus microglial reactivity. Our finding suggest a direct relationship between glial cells activation, release of inflammatory cytokines and pain related-behavior, here we believe that the treatments, VBC and PBM, may interfere with glial activation, resulting in the rebalancing of proinflammatory and anti-inflammatory cytokines, which can regulate nociceptive neuronal excitability contributing with the antinociceptive effect of PBM and VBC treatments.
There are some limitations to our study. Mainly, we need to further investigate the exact mechanism of regulation of glial cells in our model, as well as whether glial cells inhibition (with specific antagonists) can result in different effects of the treatments in behavioral allodynia and protein expression. Nevertheless, our results suggest an important role for the use of VBC and PBM as a promising candidate for the treatment of pain due to nerve injury. Given that the combination of VBC and PBM used here was better in revert the allodynic behaviour, the use of this protocol may improve the treatment of pain-related to nerve injury and became an inexpensive and safe approach.
In conclusion, our study demonstrated that the treatment with VBC or PBM or combined treatment VBC + PBM significantly attenuated CCI-IoN-induced nociceptive response. Furthermore, Western blot and immunohistochemistry data indicated that the anti-nociceptive effect of VBC and PBM might be mediated through inhibition of glial cells activation and the consequent production of glial-derived inflammatory mediators in the spinal trigeminal nucleus. Collectively, our data suggest an additional implication for anti-nociceptive mechanism of VCB and PBM and it may be dependent on IL10 expression.
Author contribution
All authors made substantial contributions to the following tasks of research: initial conception (Martins D.O., Chacur M.); design (Martins D.O., Venega R.A.G., Marques D.P., Chacur M.); provision of resources (Chacur M.); collection of data (Martins D.O, Venega R.A.G., Marques D.P.); analysis and interpretation of data (Martins D.O.; Chacur M.); writing the first draft of the paper or important intellectual content (Martins D.O, Venega R.A.G.; Marques, D.P.); revision of paper (Martins D.O.; Venega R.A.G., Chacur M.).
Ethics approval and consent to participate
All experimental procedures carried out in this study have been approved by the Institutional Animal Care and Use Committee of the University of São Paulo (protocol number 3872071118) and followed the guidelines for animal care and use set forth by that committee.
Disclosure
This study was supported by the FAPESP (2015/24256-0; 2014/24533-0), CNPq (Brazil). The funding bodies play no role in the design of the study, data collection, analysis, interpretation of the data, or in writing the manuscript.
Declaration of competing interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgements
Martins D.O., was the recipient of a FAPESP Postdoctoral scholarship.
Contributor Information
D.O. Martins, Email: martinsd@usp.br.
D.P. Marques, Email: marquessdaniel_@hotmail.com.
R.A.G. Venega, Email: rafael.venega@usp.br.
M. Chacur, Email: chacurm@icb.usp.br.
References
- Abbaszadeh A., Darabi S., Hasanvand A., Amini-Khoei H., Abbasnezhad A., Choghakhori R., Aaliehpour A. Minocycline through attenuation of oxidative stress and inflammatory response reduces the neuropathic pain in a rat model of chronic constriction injury. Iran. J. Basic Med. Sci. 2018;21(2):138–144. doi: 10.22038/IJBMS.2017.24248.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimbire F., Lopes-Martins R.A., Albertini R., Pacheco M.T., Castro-Faria-Neto H.C., Martins P.S., Bjordal J.M. Effect of low-level laser therapy on hemorrhagic lesions induced by immune complex in rat lungs. Photomed. Laser Surg. 2007;25(2):112–117. doi: 10.1089/pho.2006.1041. [DOI] [PubMed] [Google Scholar]
- Bozic I., Savic D., Laketa D., Bjelobaba I., Milenkovic I., Pekovic S., Nedeljkovic N., Lavrnja I. Benfotiamine attenuates inflammatory response in LPS stimulated BV-2 microglia. PloS One. 2015;10(2) doi: 10.1371/journal.pone.0118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chacur M., Lambertz D., Hoheisel U., Mense S. Role of spinal microglia in myositis-induced central sensitisation: an immunohistochemical and behavioural study in rats. Eur. J. Pain. 2009;13(9):915–923. doi: 10.1016/j.ejpain.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Chiang C.Y., Dostrovsky J.O., Iwata K., Sessle B.J. Role of glia in orofacial pain. Neuroscientist. 2011;17(3):303–320. doi: 10.1177/1073858410386801. [DOI] [PubMed] [Google Scholar]
- Chiang C.Y., Sessle B.J., Dostrovsky J.O. Role of astrocytes in pain. Neurochem. Res. 2012;37(11):2419–2431. doi: 10.1007/s11064-012-0801-6. [DOI] [PubMed] [Google Scholar]
- Chichorro J.G., Zampronio A.R., Souza G.E., Rae G.A. Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugs. Pain. 2006;123(1–2):64–74. doi: 10.1016/j.pain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Cotler H.B., Chow R.T., Hamblin M.R., Carroll J. The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop. Rheumatol. 2015;2(5) doi: 10.15406/mojor.2015.02.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade A.L.M., Bossini P.S., do Canto De Souza A.L.M., Sanchez A.D., Parizotto N.A. Effect of photobiomodulation therapy (808 nm) in the control of neuropathic pain in mice. Laser Med. Sci. 2017;32(4):865–872. doi: 10.1007/s10103-017-2186-x. [DOI] [PubMed] [Google Scholar]
- de Freitas Rodrigues A., de Oliveira Martins D., Chacur M., Luz J.G.C. The effectiveness of photobiomodulation in the management of temporomandibular pain sensitivity in rats: behavioral and neurochemical effects. Laser Med. Sci. 2019 doi: 10.1007/s10103-019-02842-0. In press. [DOI] [PubMed] [Google Scholar]
- de Oliveira Martins D., Martinez dos Santos F., Evany de Oliveira M., de Britto L.R., Benedito Dias Lemos J., Chacur M. Laser therapy and pain-related behavior after injury of the inferior alveolar nerve: possible involvement of neurotrophins. J. Neurotrauma. 2013;30(6):480–486. doi: 10.1089/neu.2012.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan M., Kernisant M., Cua C., Jasmin L., Ohara P.T. Satellite glial cell proliferation in the trigeminal ganglia after chronic constriction injury of the infraorbital nerve. Glia. 2013;61(12):2000–2008. doi: 10.1002/glia.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovy P., Klusakova I., Hradilova-Svizenska I., Joukal M., Boadas-Vaello P. Activation of astrocytes and microglial cells and CCL2/CCR2 upregulation in the dorsolateral and ventrolateral nuclei of periaqueductal gray and rostral ventromedial medulla following different types of sciatic nerve injury. Front. Cell. Neurosci. 2018;12:40. doi: 10.3389/fncel.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis A., Wieseler J., Favret J., Johnson K.W., Rice K.C., Maier S.F., Falci S., Watkins L.R. Systemic administration of propentofylline, ibudilast, and (+)-naltrexone each reverses mechanical allodynia in a novel rat model of central neuropathic pain. J. Pain. 2014;15(4):407–421. doi: 10.1016/j.jpain.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwemeka C.S., Parker J.C., Dowdy D.S., Harkness E.E., Sanford L.E., Woodruff L.D. The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed. Laser Surg. 2004;22(4):323–329. doi: 10.1089/pho.2004.22.323. [DOI] [PubMed] [Google Scholar]
- Fan W., Zhu X., He Y., Zhu M., Wu Z., Huang F., He H. The role of satellite glial cells in orofacial pain. J. Neurosci. Res. 2019;97(4):393–401. doi: 10.1002/jnr.24341. [DOI] [PubMed] [Google Scholar]
- George A., Kleinschnitz C., Zelenka M., Brinkhoff J., Stoll G., Sommer C. Wallerian degeneration after crush or chronic constriction injury of rodent sciatic nerve is associated with a depletion of endoneurial interleukin-10 protein. Exp. Neurol. 2004;188(1):187–191. doi: 10.1016/j.expneurol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Giardini A.C., Dos Santos F.M., da Silva J.T., de Oliveira M.E., Martins D.O., Chacur M. Neural mobilization treatment decreases glial cells and brain-derived neurotrophic factor expression in the central nervous system in rats with neuropathic pain induced by CCI in rats. Pain Res. Manag. 2017;2017:7429761. doi: 10.1155/2017/7429761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Wang H., Watanabe M., Shimizu K., Zou S., LaGraize S.C., Wei F., Dubner R., Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 2007;27(22):6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Haslerud S., Magnussen L.H., Joensen J., Lopes-Martins R.A., Bjordal J.M. The efficacy of low-level laser therapy for shoulder tendinopathy: a systematic review and meta-analysis of randomized controlled trials. Physiother. Res. Int. 2015;20(2):108–125. doi: 10.1002/pri.1606. [DOI] [PubMed] [Google Scholar]
- Hobbenaghi R., Javanbakht J., Hosseini E., Mohammadi S., Rajabian M., Moayeri P., Aghamohammad Hassan M. Neuropathological and neuroprotective features of vitamin B12 on the dorsal spinal ganglion of rats after the experimental crush of sciatic nerve: an experimental study. Diagn. Pathol. 2013;8:123. doi: 10.1186/1746-1596-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hossain M.Z., Unno S., Ando H., Masuda Y., Kitagawa J. Neuron-Glia crosstalk and neuropathic pain: involvement in the modulation of motor activity in the orofacial region. Int. J. Mol. Sci. 2017;18(10) doi: 10.3390/ijms18102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung K.L., Wang C.C., Huang C.Y., Wang S.J. Cyanocobalamin, vitamin B12, depresses glutamate release through inhibition of voltage-dependent Ca2+ influx in rat cerebrocortical nerve terminals (synaptosomes) Eur. J. Pharmacol. 2009;602(2–3):230–237. doi: 10.1016/j.ejphar.2008.11.059. [DOI] [PubMed] [Google Scholar]
- Jankowski M.P., Koerber H.R. In: Translational Pain Research: from Mouse to Man. Kruger L., Light A.R., editors. 2010. Neurotrophic factors and nociceptor sensitization. (Boca Raton, FL) [Google Scholar]
- Jhala S.S., Hazell A.S. Modeling neurodegenerative disease pathophysiology in thiamine deficiency: consequences of impaired oxidative metabolism. Neurochem. Int. 2011;58(3):248–260. doi: 10.1016/j.neuint.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Ji R.R., Berta T., Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl. 1):S10–S28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I.N., Milligan E.D., Wieseler-Frank J., Frank M.G., Zapata V., Campisi J., Langer S., Martin D., Green P., Fleshner M., Leinwand L., Maier S.F., Watkins L.R. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. 2004;24(33):7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivalt C.G., Mizisin L.M., Nelson A., Cunha J.M., Ramos K.M., Bonke D., Calcutt N.A. B vitamins alleviate indices of neuropathic pain in diabetic rats. Eur. J. Pharmacol. 2009;612(1–3):41–47. doi: 10.1016/j.ejphar.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y., Zhang L., Cheng J.K., Ji R.R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008;28(20):5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.K., Hwang S.H., Lee S.O., Kim S.H., Abdi S. Pentoxifylline ameliorates mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain. Pain Physician. 2016;19(4):E589–E600. [PubMed] [Google Scholar]
- Kopruszinski C.M., Reis R.C., Bressan E., Reeh P.W., Chichorro J.G. Vitamin B complex attenuated heat hyperalgesia following infraorbital nerve constriction in rats and reduced capsaicin in vivo and in vitro effects. Eur. J. Pharmacol. 2015;762:326–332. doi: 10.1016/j.ejphar.2015.05.063. [DOI] [PubMed] [Google Scholar]
- Kopruszinski C.M., Reis R.C., Chichorro J.G. B vitamins relieve neuropathic pain behaviors induced by infraorbital nerve constriction in rats. Life Sci. 2012;91(23–24):1187–1195. doi: 10.1016/j.lfs.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Leiser S.C., Moxon K.A. Relationship between physiological response type (RA and SA) and vibrissal receptive field of neurons within the rat trigeminal ganglion. J. Neurophysiol. 2006;95(5):3129–3145. doi: 10.1152/jn.00157.2005. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhao L., Gu W., Liu Q., Gao Z., Zhu X., Wu Z., He H., Huang F., Fan W. Activation of satellite glial cells in trigeminal ganglion following dental injury and inflammation. J. Mol. Histol. 2018;49(3):257–263. doi: 10.1007/s10735-018-9765-4. [DOI] [PubMed] [Google Scholar]
- Martins D.O., Dos Santos F.M., Ciena A.P., Watanabe I.S., de Britto L.R., Lemos J.B., Chacur M. Neuropeptide expression and morphometric differences in crushed alveolar inferior nerve of rats: effects of photobiomodulation. Laser Med. Sci. 2017;32(4):833–840. doi: 10.1007/s10103-017-2181-2. [DOI] [PubMed] [Google Scholar]
- Martins D.O., Santos F.M., Britto L.R., Lemos J.B., Chacur M. Neurochemical effects of photobiostimulation in the trigeminal ganglion after inferior alveolar nerve injury. J. Biol. Regul. Homeost. Agents. 2017;31(1):147–152. [PubMed] [Google Scholar]
- Medalha C.C., Di Gangi G.C., Barbosa C.B., Fernandes M., Aguiar O., Faloppa F., Leite V.M., Renno A.C. Low-level laser therapy improves repair following complete resection of the sciatic nerve in rats. Laser Med. Sci. 2012;27(3):629–635. doi: 10.1007/s10103-011-1008-9. [DOI] [PubMed] [Google Scholar]
- Mei X.P., Sakuma Y., Xie C., Wu D., Ho I., Kotani J., Xu L.X. Depressing interleukin-1beta contributed to the synergistic effects of tramadol and minocycline on spinal nerve ligation-induced neuropathic pain. Neurosignals. 2014;22(1):30–42. doi: 10.1159/000355071. [DOI] [PubMed] [Google Scholar]
- Mika J., Zychowska M., Popiolek-Barczyk K., Rojewska E., Przewlocka B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013;716(1–3):106–119. doi: 10.1016/j.ejphar.2013.01.072. [DOI] [PubMed] [Google Scholar]
- Miller A.J., Roman B., Norstrom E. A method for easily customizable gradient gel electrophoresis. Anal. Biochem. 2016;509:12–14. doi: 10.1016/j.ab.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Milligan E.D., O’Connor K.A., Nguyen K.T., Armstrong C.B., Twining C., Gaykema R.P., Holguin A., Martin D., Maier S.F., Watkins L.R. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 2001;21(8):2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan E.D., Penzkover K.R., Soderquist R.G., Mahoney M.J. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation. 2012;15(6):520–526. doi: 10.1111/j.1525-1403.2012.00462.x. discussion 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Kume K., Ohsawa M. Role of microglia in mechanical allodynia in the anterior cingulate cortex. J. Pharmacol. Sci. 2017;134(3):158–165. doi: 10.1016/j.jphs.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Moallem S.A., Hosseinzadeh H., Farahi S. A study of acute and chronic anti-nociceptive and anti-inflammatory effects of thiamine in mice. Iran. Biomed. J. 2008;12(3):173–178. [PubMed] [Google Scholar]
- Narita M., Yoshida T., Nakajima M., Miyatake M., Takagi T., Yajima Y., Suzuki T. Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J. Neurochem. 2006;97(5):1337–1348. doi: 10.1111/j.1471-4159.2006.03808.x. [DOI] [PubMed] [Google Scholar]
- Neves L.M.S., Goncalves E.C.D., Cavalli J., Vieira G., Laurindo L.R., Simoes R.R., Coelho I.S., Santos A.R.S., Marcolino A.M., Cola M., Dutra R.C. Photobiomodulation therapy improves acute inflammatory response in mice: the role of cannabinoid receptors/ATP-sensitive K(+) channel/p38-MAPK signalling pathway. Mol. Neurobiol. 2018;55(7):5580–5593. doi: 10.1007/s12035-017-0792-z. [DOI] [PubMed] [Google Scholar]
- Okada-Ogawa A., Suzuki I., Sessle B.J., Chiang C.Y., Salter M.W., Dostrovsky J.O., Tsuboi Y., Kondo M., Kitagawa J., Kobayashi A., Noma N., Imamura Y., Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J. Neurosci. 2009;29(36):11161–11171. doi: 10.1523/JNEUROSCI.3365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Martin D.P., Schmelzer J.D., Mitsui Y., Low P.A. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp. Neurol. 2001;169(2):386–391. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- Powell M.J., Thompson S.A., Tone Y., Waldmann H., Tone M. Posttranscriptional regulation of IL-10 gene expression through sequences in the 3’-untranslated region. J. Immunol. 2000;165(1):292–296. doi: 10.4049/jimmunol.165.1.292. [DOI] [PubMed] [Google Scholar]
- Ren K., Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr. Opin. Anaesthesiol. 2008;21(5):570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K., Dubner R. The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain. Int. Rev. Neurobiol. 2011;97:207–225. doi: 10.1016/B978-0-12-385198-7.00008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha I.R., Ciena A.P., Rosa A.S., Martins D.O., Chacur M. Photobiostimulation reverses allodynia and peripheral nerve damage in streptozotocin-induced type 1 diabetes. Laser Med. Sci. 2017;32(3):495–501. doi: 10.1007/s10103-016-2140-3. [DOI] [PubMed] [Google Scholar]
- Sessle B.J. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit. Rev. Oral Biol. Med. 2000;11(1):57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- Sessle B.J. Peripheral and central mechanisms of orofacial inflammatory pain. Int. Rev. Neurobiol. 2011;97:179–206. doi: 10.1016/B978-0-12-385198-7.00007-2. [DOI] [PubMed] [Google Scholar]
- Snedecor G.W., Sokal R.R., Rohlf F.J. Owa State University Press; New York: 1946. Statistical Methods Biometry. [Google Scholar]
- Song X.S., Huang Z.J., Song X.J. Thiamine suppresses thermal hyperalgesia, inhibits hyperexcitability, and lessens alterations of sodium currents in injured, dorsal root ganglion neurons in rats. Anesthesiology. 2009;110(2):387–400. doi: 10.1097/ALN.0b013e3181942f1e. [DOI] [PubMed] [Google Scholar]
- Stoll G., Jander S., Myers R.R. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J. Peripher. Nerv. Syst. 2002;7(1):13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Sun H., Yang T., Li Q., Zhu Z., Wang L., Bai G., Li D., Wang W. Dexamethasone and vitamin B(12) synergistically promote peripheral nerve regeneration in rats by upregulating the expression of brain-derived neurotrophic factor. Arch. Med. Sci. 2012;8(5):924–930. doi: 10.5114/aoms.2012.31623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaddonfard E., Samadi F., Egdami K. The effects of vitamin B12 and diclofenac and their combination on cold and mechanical allodynia in a neuropathic pain model in rats. Vet. Res. Forum. 2013;4(1):19–24. [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hazell A.S. Microglial activation is a major contributor to neurologic dysfunction in thiamine deficiency. Biochem. Biophys. Res. Commun. 2010;402(1):123–128. doi: 10.1016/j.bbrc.2010.09.128. [DOI] [PubMed] [Google Scholar]
- Watkins L.R., Milligan E.D., Maier S.F. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv. Exp. Med. Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- Watkins L.R., Wieseler-Frank J., Milligan E.D., Johnston I., Maier S.F. Chapter 22 Contribution of glia to pain processing in health and disease. Handb. Clin. Neurol. 2006;81:309–323. doi: 10.1016/S0072-9752(06)80026-6. [DOI] [PubMed] [Google Scholar]
- Wu F., Zhang R., Shen X., Lao L. Preliminary study on pain reduction of monosodium iodoacetate-induced knee osteoarthritis in rats by carbon dioxide laser moxibustion. Evid. base Compl. Alternative Med. 2014;2014:754304. doi: 10.1155/2014/754304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wang W., Zhong X.X., Feng Y., Wei X., Liu X.G. EXPRESS: methylcobalamin ameliorates neuropathic pain induced by vincristine in rats: effect on loss of peripheral nerve fibers and imbalance of cytokines in the spinal dorsal horn. Mol. Pain. 2016;12 doi: 10.1177/1744806916657089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Aita M., Chavkin C. Partial infraorbital nerve ligation as a model of trigeminal nerve injury in the mouse: behavioral, neural, and glial reactions. J. Pain. 2008;9(11):1036–1048. doi: 10.1016/j.jpain.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada E.F., Bobinski F., Martins D.F., Palandi J., Folmer V., da Silva M.D. Photobiomodulation therapy in knee osteoarthritis reduces oxidative stress and inflammatory cytokines in rats. J. Biophot. 2020;13(1) doi: 10.1002/jbio.201900204. [DOI] [PubMed] [Google Scholar]
- Yonehara N., Kudo C., Kamisaki Y. Involvement of NMDA-nitric oxide pathways in the development of tactile hypersensitivity evoked by the loose-ligation of inferior alveolar nerves in rats. Brain Res. 2003;963(1–2):232–243. doi: 10.1016/s0006-8993(02)03983-5. [DOI] [PubMed] [Google Scholar]
- Yu C.Z., Liu Y.P., Liu S., Yan M., Hu S.J., Song X.J. Systematic administration of B vitamins attenuates neuropathic hyperalgesia and reduces spinal neuron injury following temporary spinal cord ischaemia in rats. Eur. J. Pain. 2014;18(1):76–85. doi: 10.1002/j.1532-2149.2013.00390.x. [DOI] [PubMed] [Google Scholar]
- Zaringhalam J., Akbari A., Zali A., Manaheji H., Nazemian V., Shadnoush M., Ezzatpanah S. Long-term treatment by vitamin B1 and reduction of serum proinflammatory cytokines, hyperalgesia, and paw edema in adjuvant-induced arthritis. Basic Clin. Neurosci. 2016;7(4):331–340. doi: 10.15412/J.BCN.03070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Bi R.Y., Gan Y.H. Glial interleukin-1beta upregulates neuronal sodium channel 1.7 in trigeminal ganglion contributing to temporomandibular joint inflammatory hypernociception in rats. J. Neuroinflammation. 2018;15(1):117. doi: 10.1186/s12974-018-1154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Cao D.L., Zhang Z.J., Jiang B.C., Gao Y.J. Chemokine CXCL13 mediates orofacial neuropathic pain via CXCR5/ERK pathway in the trigeminal ganglion of mice. J. Neuroinflammation. 2016;13(1):183. doi: 10.1186/s12974-016-0652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.J., Jiang B.C., Gao Y.J. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell. Mol. Life Sci. 2017;74(18):3275–3291. doi: 10.1007/s00018-017-2513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]